Abstract
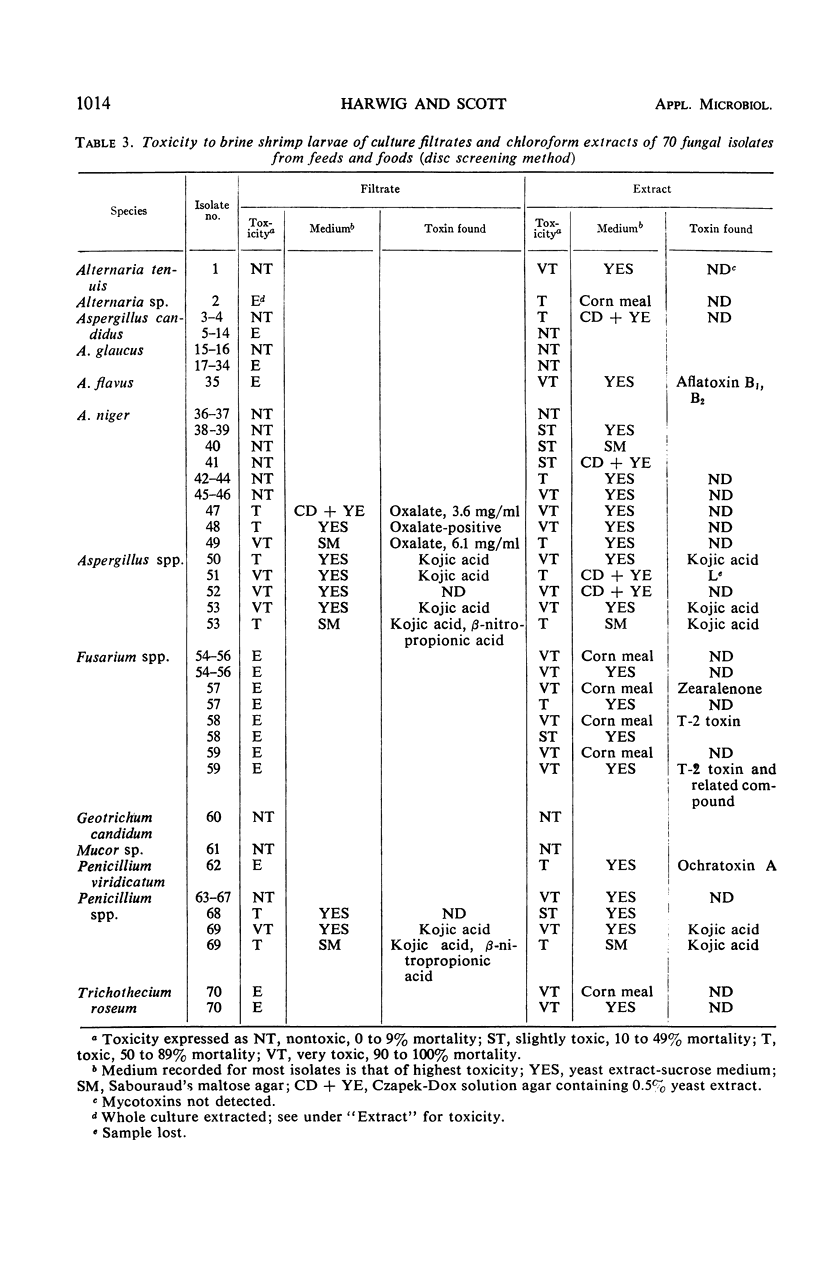
Concentrations resulting in 50% mortality, determined with brine shrimp (Artemia salina L.) larvae exposed to known mycotoxins for 16 hr, were (μg/ml): aflatoxin G1, 1.3; diacetoxyscirpenol, 0.47; gliotoxin, 3.5; ochratoxin A, 10.1; and sterigmatocystin, 0.54. 4-Acetamido-4-hydroxy-2-butenoic acid γ-lactone gave no mortality at 10 μg/ml. Used as a screening system involving discs saturated with solutions of known mycotoxins, the larvae were relatively sensitive to aflatoxin B1, diacetoxyscirpenol, gliotoxin, kojic acid, ochratoxin A, rubratoxin B, sterigmatocystin, stemphone, and T-2 toxin. Quantities of 0.2 to 2 μg/disc caused detectable mortality. The larvae were only moderately sensitive to citrinin, patulin, penicillic acid, and zearalenone which were detectable at 10 to 20 μg/disc. They were relatively insensitive to griseofulvin, luteoskyrin, oxalic acid, and β-nitropropionic acid. The disc screening method indicated that 27 out of 70 fungal isolates from foods and feeds grown in liquid or solid media produced chloroform-extractable toxic material. Examination of toxic extracts by thin-layer chromatography for 17 known mycotoxins showed that the toxicity of eight isolates could be attributed to aflatoxin B1 and B2, kojic acid, zearalenone, T-2 toxin, or ochratoxin A. Nine out of 32 of these fungal isolates grown in four liquid media yielded toxic culture filtrates from at least one medium. Chemical tests for kojic, oxalic, and β-nitropropionic acids showed the presence of one or two of these compounds in filtrates of seven of these nine isolates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. F. The effect of some mycotoxins on the brine shrimp, Artemia salina. J Am Oil Chem Soc. 1969 Feb;46(2):119–119. doi: 10.1007/BF02541223. [DOI] [PubMed] [Google Scholar]
- Buu-Hoï N. P., Chanh P. H. Effect of various types of carcinogens on the hatching of Artemia salina eggs. J Natl Cancer Inst. 1970 Apr;44(4):795–799. [PubMed] [Google Scholar]
- DELCAMBE L. Activités biologiques des actinomycines de Streptomyces S-67. Arch Int Pharmacodyn Ther. 1955 Apr 1;101(3):358–366. [PubMed] [Google Scholar]
- Fishbein L., Falk H. L. Chromatography of mold metabolites. I. Aflatoxins, ochratoxins and related compounds. Chromatogr Rev. 1970 Jan;12(1):42–87. doi: 10.1016/0009-5907(70)80013-8. [DOI] [PubMed] [Google Scholar]
- Grosch D. S. Poisoning with DDT: effect on reproductive performance of Artemia. Science. 1967 Feb 3;155(3762):592–593. doi: 10.1126/science.155.3762.592. [DOI] [PubMed] [Google Scholar]
- Kinosita R., Ishiko T., Sugiyama S., Seto T., Igarasi S., Goetz I. E. Mycotoxins in fermented food. Cancer Res. 1968 Nov;28(11):2296–2311. [PubMed] [Google Scholar]
- Michael A. S., Thompson C. G., Abramovitz M. Artemia salina as a Test Organism for Bioassay. Science. 1956 Mar 16;123(3194):464–464. doi: 10.1126/science.123.3194.464. [DOI] [PubMed] [Google Scholar]
- PHAM-HUU-CHANH, MAMY G. A SIMPLE BIOLOGICAL REAGENT FOR TOXICITY TESTS: THE EGGS OF "ARTEMIA SALINA". Agressologie. 1963 Nov-Dec;4:599–603. [PubMed] [Google Scholar]
- Robinson A. B., Manly K. F., Anthony M. P., Catchpool J. F., Pauling L. Anesthesia of Artemia larvae: method for quantitative study. Science. 1965 Sep 10;149(3689):1255–1258. doi: 10.1126/science.149.3689.1255. [DOI] [PubMed] [Google Scholar]
- Scott P. M., Lawrence J. W. Stemphone, a biologically active yellow pigment produced by Stemphylium sarcinaeforme (Cav.) Wiltshire. Can J Microbiol. 1968 Sep;14(9):1015–1016. doi: 10.1139/m68-170. [DOI] [PubMed] [Google Scholar]
- Scott P. M., Lawrence J. W., van Walbeek W. Detection of mycotoxins by thin-layer chromatography: application to screening of fungal extracts. Appl Microbiol. 1970 Nov;20(5):839–842. doi: 10.1128/am.20.5.839-842.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Walbeek W., Scott P. M., Thatcher F. S. Mycotoxins from food-borne fungi. Can J Microbiol. 1968 Feb;14(2):131–137. doi: 10.1139/m68-022. [DOI] [PubMed] [Google Scholar]
- WILSON B. J., WILSON C. H. Oxalate formation in moldy feedstuffs as a possible factor in livestock toxic disease. Am J Vet Res. 1961 Nov;22:961–969. [PubMed] [Google Scholar]
- van Walbeek W., Scott P. M., Harwig J., Lawrence J. W. Penicillium viridicatum Westling: a new source of ochratoxin A. Can J Microbiol. 1969 Nov;15(11):1281–1285. doi: 10.1139/m69-232. [DOI] [PubMed] [Google Scholar]



