Abstract
Background:
Hippocampal volume is affected by several psychiatric illnesses of old age, as well as by normal aging. It is important to have a normal data in a population to assist in diagnosis. The aim of this study is to determine hippocampal volume in normal Malay people aged 50 years old and older.
Methods:
This was a cross-sectional study of the normal Malay population aged 50 to 77 years. We included 43 participants, representing 19 men and 24 women. Magnetic resonance imaging (MRI) was performed using a GE Signa Horizon LX 1.0 Tesla. Oblique coronal images of temporal lobes were obtained and hippocampal volumetry was done manually and normalised with intracranial volume.
Results:
Mean right and left hippocampal volumes (HCVs) were 3.43 cm³ (SD 0.32) and 3.26 cm³ (SD 0.34), with a significant difference between them (P < 0.001). Total mean HCVs exhibited no significant difference between men and women (P = 0.234). The means of the normalised right and left HCVs were 3.42 cm³ (SD 0.31) and 3.26 cm³ (SD 0.32).
Conclusion:
The mean right and left hippocampal volumes were significantly different in this study. Men had slightly larger mean HCVs but the difference was not statistically significant. It was found that normalisation further reduces the mean volume difference between the genders.
Keywords: magnetic resonance imaging, hippocampus, normal value, Malay, aged, 50 and over
Introduction
The brain and its related structures are subjected to physiological shrinkage in parallel with the aging process. However, whether physiological shrinkage occurs in the hippocampus remains controversial in the majority of magnetic resonance (MR) volumetry studies. Several authors have shown that hippocampal volume (HCV) is well preserved throughout adulthood in men and women, with no significant correlation with age (1–3).
HCV is affected by several neurodegenerative and psychiatric diseases. Alzheimer disease (AD) is the most common disorder associated with reduced hippocampal volume. Others, such as mild cognitive impairment and Parkinson’s disease, which also occur at the elderly age group, are associated with reduced volume to a lesser degree. Since the presentations of all these illnesses are similar, it is important to differentiate in the management of these neurodegenerative diseases.
Alzheimer’s disease can occur at any age starting from as young as 40 years old. However, it is very rare among those 40–50 years old, increases between 60 and 65 years old and becomes very common after 80 years old (4). Parkinson’s disease is rare under the age of 50 years and the mean age of onset is 60 to 69 years old (5).
Several studies have revealed significant volumetric differences in HCV between AD and control groups; however, it has been difficult to judge whether these differences were due to pathologic processes or physiological atrophy. Thus, it is important to define a range of normal values for the hippocampal formation, the amygdala and the temporal horn amongst healthy subjects in different age groups (6).
There are wide variations of HCVs in the available literature, mainly due to the different protocols for anatomical boundaries. In Malaysia, there is currently no reference value for normal HCV. The aim of this study is to have normative values of HCV, specifically the population 50 years old and above.
On MR images, HCV can be estimated by visual assessment or objectively measured. Volume measurements are found to be significantly more sensitive than visual inspection alone. The sensitivity of volume measurement has been reported to be 92% compared to 56% on visual inspection of hippocampal atrophy in temporal lobe epilepsy (7). Visual inspection of the hippocampus is subjective and is neither sensitive nor specific for the detection of hippocampal atrophy; therefore, a more objective measurement is needed before any diagnosis is concluded (8). The main drawbacks that limit widespread clinical application of manual hippocampal volumetry are time consumption and special training requirements.
HCV measurement can be carried out using manual, semiautomatic or completely automatic computer segmentation methods for sequential MR images. Although the automated methods can reliably detect hippocampal sclerosis, manual hippocampal volumetry is still the gold standard. Some researchers reported in a review that 90% of the 118 protocols in their database (consisting of 423 papers) used manual tracing for hippocampal volumetry (9).
Materials and Methods
A cross-sectional study was conducted at Hospital Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia from July 2010 to August 2011. Participants 50 years old and above were recruited when they scored a minimum of 24 out of 30 on the mini mental state examination (MMSE) test. Exclusion criteria were focal neurological deficit, history of psychiatric disorders, epilepsy, substance abuse, significant head trauma with documented intracranial haemorrhage and abnormal MR imaging (MRI) brain findings (e.g. tumours).
MRI protocol
This study employed a Signa Horizon LX 1.0 Tesla MR unit (General Electric, Milwaukee, Wisconsin). The MR brain protocol series was performed first on sagittal T1 and on axial T1, T2 and fluid attenuation inversion recovery (FLAIR) with a 5 mm slice thickness and a 2 mm gap. Brain images were reviewed and if normal, temporal lobe series were carried out with coronal acquisition perpendicular to the long axis of hippocampus (LAH). The series included coronal inversion recovery IR; T2; FLAIR with a 4 mm thickness and a 1 mm gap; and 3D coronal spoiled gradient echo (SPGR) with a 2 mm thickness. and a 1 mm gap.
The image matrix size was 256 × 256 pixels. With our 1.0 Tesla MR machine, a 4 mm slice thickness was used to produce the optimal signal-to-noise ratio, and a 1 mm gap was applied to reduce cross-talk artefacts. Volumetry was performed on IR coronal temporal lobe images and the parameters (TE, TR, FOV and NEX) were 11 ms, 420 ms, 20 × 20 cm and 2.0.
Volumetric measurement
Hippocampal volumetry was performed using a manual tracing method with open source software, specifically Osirix ver. 3.7.1 (Pixmeo Sarl). This study followed the anatomical hippocampal boundaries described by Obenaus et al., (10). The subicular complex, hippocampus proper, dentate gyrus, alveus, and hippocampal fimbria were included for measurement of hippocampal body and tail. Measurement excluded the amygdala, parahippocampal gyrus, isthmus of the cingulated gyrus, and the crus of fornix.
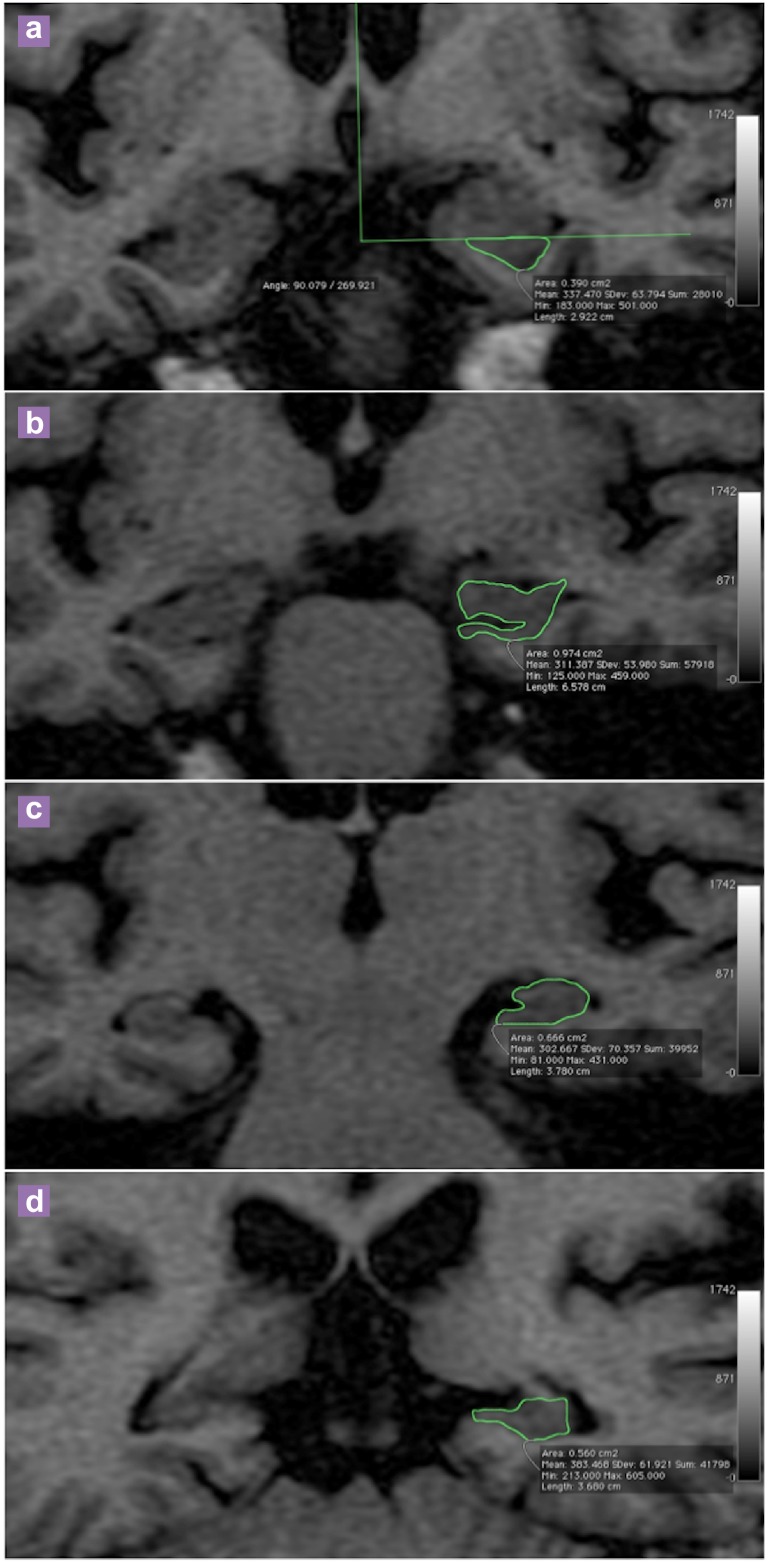
Using the Osirix software, the hippocampal boundary was manually traced three times in each slice and the average area of the hippocampus was obtained. Measurement was performed on a slice- by-slice basis to visualise the anatomic landmark for segmentation (Figure 1). The volume of the hippocampal complex (cm³) was obtained by multiplying the total area with the slice thickness (0.5 cm).
Figure 1:
Representative images in coronal plane of T1WI (TE = 11 msec, TR = 440 msec) of a 63 years old male subject showing the manual tracing of left hippocampus. (a) No clear boundary identified as alvelus is not visualised, therefore the head is separated from the overlying amygdala by drawing an arbitrary line below the uncal recess; (b) The alveus is included in the mesurement; (c) The first slice of the body is identified when choroidal fissure is visualised; (d) First slice of the tail is identified when the crus of fornix is in profile.
Intracranial volumetry was performed using the anatomical boundaries and alternate slices method described by Eritaia et al., (11). Manual tracings were done on T1-weighted images, and sagittal slices (5 mm slice thickness, 2 mm gap) were used. A standard grey-level threshold of 33% was applied to images in order to help delineate the outer border of the dura more clearly. Measurement was carried out three times for each slice and the average was taken. The total intracranial area was obtained by summing the total surface area of all the alternate slices (odd numbers). Intracranial volume (ICV) was obtained by multiplying the total surface area (∑A) by an effective slice thickness of 1.4 cm (2 slices).
For the normalisation of hippocampal volume measurements, the covariance method as introduced by Jack et al. (4), was used with intracranial volume as the correction factor. The formula for the covariance method was as follows:
NV =
OV – Grad (CMi – CMmean)
NV: Corrected hippocampal volume
OV: Original hippocampal volume Grad: Gradient of the regression line between the HCV and ICV
CMi: Appropriate cerebral measurement for that subject
CMmean: Mean value of all subjects
Data analysis
Data entry and analysis were performed using Predictive Analytics SoftWare (PASW)® Statistics 18 (SPSS-Statistical Package for the Social Sciences, Chicago IL). Mean, standard deviation, and distribution of HCV were obtained. The paired t test was applied to compare the right and left hippocampal volumes, and the independent t test was used for comparison of hippocampal volumes between males and females. In this study, the level of significance, denoted by alpha (α), was set at 0.05. The simple linear regression test was used to determine the relationship between intracranial and hippocampal volumes.
Results
A total of 44 adult Malay participants were recruited. However, one subject was excluded from the study due to an incidental finding of intracranial neoplasm on MRI. There were 19 men and 24 women of aged 50 to 77 years (men: 61.9 (SD 7.3) years; women: 58.2 (SD 4.9) years). Normal distributions of the age were observed in both groups. All the subjects underwent a mental state examination done using the MMSE test; a median score of 28 (IqR = 2) was obtained.
The mean and standard deviation of the original right and left HCVs for all subjects were 3.43 cm³ (SD 0.32) and 3.26 cm³ (SD 0.34), respectively, with a total mean HCV of 6.69 cm³ (SD 0.64). Most of our participants (95%) had a larger right HCV, with only two subjects exhibiting a larger left side. The paired t test showed a statistically significant difference between the right and left HCVs, at P < 0.001 (Table 1). The independent t test showed a statistically non- significant mean difference for original total HCV between male and female subjects (P > 0.05) subjects. The mean difference was 0.24 cm³ (SD –0.16, 0.63) (Table 2).
Table 1.
Comparison between right and left hippocampal volumes in all subjects
| Group (n) | Mean (SD) (cm³) | Mean Difference (cm³) (95% CI) | t test (df) | P valuea |
|---|---|---|---|---|
| Right hippocampus (43) | 3.43 (0.32) | 0.18 (0.13, 0.22) | 7.47 (42) | < 0.001 |
| Left hippocampus (43) | 3.26 (0.34) |
a Paired t test, df.
Table 2.
Comparison of total original total hippocampal volume between genders
| Group (n) | Mean (SD) (cm³) | Mean Difference (cm³) (95% CI) | t test (df) | P valuea |
|---|---|---|---|---|
| Male (19) | 6.82 (0.68) | 0.24 (-0.16, 0.63) | 1.21 (41) | 0.234 |
| Female (24) | 6.58 (0.59) |
a Independent t test.
The mean and standard deviation of ICVs for all subjects was 1332.54 cm³ (SD 142.9), with a range of 1101.88 cm³ to 1725.81 cm³. The male subjects had higher mean ICVs at 1435.75 cm³ (SD 124.7). Normalised HCVs were obtained using the co-variance method as previously described. The gradient of the regression line between HCVs and ICVs, obtained using simple linear regression, was 0.001. This result indicated that when ICV increases by 1 cm³, the total hippocampal volume will be larger by 0.001. The results of normalised right and left HCVs in male and female subjects are summarised in Table 3.
Table 3.
Normalised right and left hippocampal volume
| Group (n) | Mean (SD) (cm³) | ||
|---|---|---|---|
| Right | Left | Total | |
| Male (19) | 3.39 (0.33) | 3.23 (0.34) | 6.61 (0.66) |
| Female (24) | 3.47 (0.29) | 3.28 (0.31) | 6.75 (0.57) |
| All subjects (43) | 3.42 (0.31) | 3.26 (0.32) | 6.69 (0.61) |
Discussion
Original right and left hippocampal volumes
There have been several studies conducted to measure normal hippocampal volume for different age groups. Table 4 compares the right and left HCVs in the present study against the other published data and the MR results obtained. Our volumes were generally larger compared to those found in the studies Zipursky et al. (5), Bigler et al. (12), and Jack et al. (4). On the other hand, our results showed smaller volumes compared to research performed in Canada by Watson et al. (7), and almost identical results to the studies done in the United Kingdom (UK) by Cook et al. (6) and Van Paesschen et al. (8). In Van Paesschen et al. (8) study, there were some technical differences in relation to our research. They used a 1.5 Tesla Siemens MR machine and employed 1 in 3 slice measurement strategy, with a slice thickness of 1 mm and a 0.25 mm gap, resulting in an effective slice thickness of 3.75 mm. This resulted in an increase in the volume averaging effect, contributing to the slightly larger volumes obtained.
Table 4.
Comparisons of our HCV values against published studies
| Reference | MR scanner | Hippocampal volume (cm³) | |
|---|---|---|---|
| Right | Left | ||
| Zipursky et al., (5) | GE 1.5T | 2.07 | 1.99 |
| Bigler et al.,(12) | GE 1.5T | 2.47 | 2.35 |
| Jack et al., (4) | GE 1.5T | 2.80 | 2.40 |
| Cook et al., (6) | GE 1.5T | 3.23 | 3.19 |
| Van Paesschenet et al., (8) | Siemens 1.5T | 3.33 | 3.32 |
| Our study Original HCV | GE 1.0T | 3.43 | 3.26 |
| Normalised HCV | 3.42 | 3.26 | |
| Soininen et al., (23) | Siemens 1.5T | 3.71 | 3.35 |
| Watson et al., (7) | Philips 1.5T | 5.26 | 4.90 |
The main reasons for wide range of variation in HCV were probably variation in anatomical boundaries and MR acquisition. In the review by Geuze et al. (9), the smallest HCV was discovered by Zipursky et al. (5). In this research, the most anterior region of the hippocampal body and the most posterior region of the tail were excluded. The largest HCV value was obtained by Watson et al. (7), likely due to the inclusion of part of the crus of fornix in hippocampal tail portion. In contrast, our study excluded the crus of fornix. Beyond the anatomical boundaries, the imaging planes were also different in some studies using the sagittal plane perpendicular to the Sylvian fissure.
A large study done in China with 619 healthy volunteers of 40 to 90 years of age revealed smaller volumes than were obtained in our results. In this study, the means and standard deviations of HCV were 2.76 cm² (SD 0.08) for 40–60 year olds, 2.40 cm² (SD 0.08) for 61–70 years old and 2.24 cm² (SD 0.08) for 71–80 years old (13). The researchers used a 1.5-T MR unit, similar anatomical boundaries and the same imaging plane to our study; they employed a 2 mm slice thickness without a gap.
The main drawback of using a 1.0 Tesla MR machine is its lower contrast resolution. In particular, it is difficult to delineate the head of the hippocampus anteriorly, where it is fused to the amygdala. In higher resolution images, it is clearly visualised as a distinct shape with a wavy superior portion. The superior boundary of the hippocampus is defined when the amygdala can be distinguished from the hippocampus in the anterior-most slices. In most of our subjects, we needed to draw an arbitrary line below the uncal recess in order to separate these two structures, as the alveus was not visible in this region for demarcation purposes, as described in the protocol used (10).
Most recent clinical studies involving HCV used 1.5 Tesla MR machines (9). However, Bartzokis et al. (14), compared the volumetry of different brain structures at 0.5 and 1.5 Tesla and reported good intra-scanner and inter-scanner reliability. Even though our volumes were generally larger, good correlation with the higher resolution images is expected and the magnitude of right-to-left discrepancies should be similar.
Laakso et al. (15), analysed the volumes of the hippocampus using different slice thicknesses. They employed a 1.5 Tesla Siemens scanner and obtained oblique coronal images of the hippocampus. The slices obtained were reformatted to contiguous slices with thicknesses of 1, 3, and 5 mm similarly oriented perpendicularly to the LAH. The study found no significant difference in using different thicknesses.
Our study utilised the 4 mm slice thickness in the coronal oblique plane. A gap of 1 mm was necessary to reduce cross-talk artefacts in the images and to obtain better tissue contrast resolution with an optimum signal-to-noise ratio. This is also a standard practice for 1.0 Tesla MR machines. Usage of thick slices would not necessarily introduce systematic bias to the volumetric measurement. A thicker slice allows a shorter duration of scan and better signal-to = noise ratio. There were also several sources of measurement errors related to MR images, including differences in the number of movement artefacts, alterations in magnetic field inhomogeneity, machine-dependent image- to-image variation in MR image intensity scale, and voxel size variations due to drifts in imager calibration (16).
Right and left hippocampal volume discrepancies
Our results revealed that both the original and normalised HCV volumes were significantly higher on the right side, with differences of 0.17 cm³ and 0.16 cm³, respectively, which is consistent with a meta-analysis published by Pedraza et al., (17). A meta-analysis involving 82 studies from 1990 to 2002 consisting of 3564 normal adult participants concluded that hippocampi are persistently asymmetrical structures in normal adults, with a larger right side. The authors described a difference of 0.21 cm³, which is comparable to our study. They concluded that differences in MRI magnetic field strength and slice thickness might contribute to right and left HCV asymmetry. Nonetheless, their analysis revealed that studies using the 1.0 Tesla machine have a comparable degree of volumetric asymmetry.
Comparison of original hippocampal volumes between males and females
Currently, there is no consensus regarding the effect of sex difference on HCV. There was no statistically significant difference of HCV between men and women in our study. In a larger study involving 61 normal subjects of 6 to 82 years old, Li et al. (18), found no statistically significance differences in either original or normalised HCVs among gender groups in a Chinese population. Several other researchers found similar results (19,20).
On the other hand, several studies have demonstrated a different result. Free et al.’s, study involving 32 subjects revealed that men have significantly larger hippocampi than women (P < 0.01) (21). Another Chinese study by Cheon et al. involving 30 control subjects revealed that female subjects had slightly larger hippocampi, but this was not statistically significant (P > 0.05) (22).
Normalised right and left hippocampal volumes
There was no statistically significant difference in the mean normalised total HCVs between men and women in our study. Normalised HCVs have the advantage of reducing discrepancies in volumes between genders. This was reflected in our results, which showed a reduction in mean difference between male and female HCVs. The difference was 0.24 cm³ in the original HCVs and 0.14 cm³ in the normalised data. The correction via the covariance method in our study also produced a reduction in standard deviation for the hippocampal volume, giving more consistent data. Our constellation of findings is consistent with those of previous studies (7,8).
Conclusion
The use of MR volumetry is not widespread due to being time consuming and because of the need to practice to become familiar with the anatomical boundaries. However, with the increasing aging population, it is becoming important in the early detection and management of AD. HCV has a wide range of values due to regional differences and technical variation. Our study results could be helpful in diagnosing and managing of these patients in Malaysia.
Footnotes
Conflict of interest
None
Funds
This study was done under the Research University grant (1001/PSKBP/8120197) and approved by the Ethics committee with the reference number of USMKK/PPP/JEPeM [209.3.(01)].
Authors’ contributions
Conception and design: MFE, RY, MSA, WM
Analysis and interpretation of the data, collection and assembly of data: MFE
Drafting of the article: MFE, RY
Critical revision of the article for the important intellectual content: MFE, AHAK, WM
Final approval of the article, provision of study materials or patient, obtaining of funding: WM
Statistical expertise: AKG
References
- 1.Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol Aging. 2005;26(7):1093–1098. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Jack CR Jr, Dickson DW, Parisi JE, Xu YC, Cha RH, O'Brien PC, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58(5):750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19(12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology. 1989;172(2):549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- 5.Zipursky RB, Marsh L, Lim KO, DeMent S, Shear PK, Sullivan EV, et al. Volumetric MRI assessment of temporal lobe structures in schizophrenia. Biol Psychiatry. 1994;35(8):501–516. doi: 10.1016/0006-3223(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 6.Cook MJ, Fish DR, Shorvon SD, Straughan K, Stevens JM. Hippocampal volumetric and morphometric studies in frontal and temporal lobe epilepsy. Brain. 1992;115(4):1001–1015. doi: 10.1093/brain/115.4.1001. [DOI] [PubMed] [Google Scholar]
- 7.Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42(9):1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 8.Van PW, Connelly A, King MD, Jackson GD, Duncan JS. The spectrum of hippocampal sclerosis: a quantitative magnetic resonance imaging study. Ann Neurol. 1997;41(1):41–51. doi: 10.1002/ana.410410109. [DOI] [PubMed] [Google Scholar]
- 9.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 1. Review of methodologies currently employed. Mol Psychiatry. 2005;10(2):147. doi: 10.1038/sj.mp.4001580. [DOI] [PubMed] [Google Scholar]
- 10.Obenaus A, Yong-Hing CJ, Tong KA, Sarty GE. A reliable method for measurement and normalization of pediatric hippocampal volumes. Pediatr Res. 2001;50(1):124–32. doi: 10.1203/00006450-200107000-00022. doi: 10.1203/00006450-200107000-00022 . [DOI] [PubMed] [Google Scholar]
- 11.Eritaia J, Wood SJ, Stuart GW, Bridle N, Dudgeon P, Maruff P, et al. An optimized method for estimating intracranial volume from magnetic resonance images. Magn Reson Med. 2000;44(6):973–977. doi: 10.1002/1522-2594(200012)44:6<973::aid-mrm21>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, et al. Hippocampal volume in normal aging and traumatic brain injury. AJNR Am J Neuroradiol. 1997;18(1):11–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Qiwen M, Jingxia X, Zongyao W, Yaqin W, Zhang S. A Quantitative MR Study of the Hippocampal Formation, the Amygdala, and the Temporal Horn of the Lateral Ventricle in Healthy Subjects 40 to 90 Years of Age. AJNR Am J Neuroradiol. 1999;20(2):207–211. [PMC free article] [PubMed] [Google Scholar]
- 14.Bartzokis G, Mintz J, Marx P, Osborn D, Gutkind D, Chiang F, et al. Reliability of in vivo volume measures of hippocampus and other brain structures using MRI. Magn Reson Imaging. 1993;11(7):993–1006. doi: 10.1016/0730-725x(93)90218-3. [DOI] [PubMed] [Google Scholar]
- 15.Laakso MP, Juottonen K, Partanen K, Vainio P, Soininen H. MRI volumetry of the hippocampus: the effect of slice thickness on volume formation. Magn Reson Imaging. 1997;15(2):263–265. doi: 10.1016/s0730-725x(96)00390-6. [DOI] [PubMed] [Google Scholar]
- 16.Whitwell JL, Crum WR, Watt HC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR Am J Neuroradiol. 2001;22(8):1483–1489. [PMC free article] [PubMed] [Google Scholar]
- 17.Pedraza O, Bowers D, Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J Int Neuropsychol Soc. 2004;10(5):664–678. doi: 10.1017/S1355617704105080. [DOI] [PubMed] [Google Scholar]
- 18.Li YJ, Ga SN, Huo Y, Li SY, Gao XG. Characteristics of hippocampal volumes in healthy Chinese from MRI. Neurol res. 2007;29(8):803–806. doi: 10.1179/016164107X223557. [DOI] [PubMed] [Google Scholar]
- 19.Yucel K, Hakyemez B, Parlak M, Oygucu IH. Morphometry of some elements of limbic system in normal population: a quantitative MRI study. Neuroanatomy. 2002;1:15–21. [Google Scholar]
- 20.Zou L X.J, Zhoul X, Sun C Y X. Hippocampal formations, amygdala and anterior temporal lobes: normative volumetric measurement from MR imaging in normal adults of China. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34(4):719–722. [PubMed] [Google Scholar]
- 21.Free SL, Bergin PS, Fish DR, Cook MJ, Shorvon SD, Stevens JM. Methods for normalization of hippocampal volumes measured with MR. AJNR Am J Neuroradiol. 1995;16(4):637–643. [PMC free article] [PubMed] [Google Scholar]
- 22.Cheon J, Chang K, Kim H, Han M, Hong S, Seong S, et al. MR of hippocampal sclerosis: comparison of qualitative and quantitative assessments. AJNR Am J Neuroradiol. 1998;19(3):465–468. [PMC free article] [PubMed] [Google Scholar]
- 23.Soininen HS, Partanen K, Pitkanen A, Vainio P, Hanninen T, Hallikainen M, et al. Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology. 1994;44(9):1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]