Abstract
Background:
The incidence of multidrug resistant enteric fever is increasing alarmingly. This study was planned to determine the rate of isolation of Salmonella spp. and to compare the isolates for their epidemiological parameters and antimicrobial susceptibility patterns at our center.
Methods:
The study was conducted over a span of three years with a total of 8142, 8134, and 8114 blood culture samples processed for the years 2008, 2009, and 2010 respectively. The minimum inhibitory concentration (MIC) for ciprofloxacin and chloramphenicol was determined using an agar dilution method. The MIC for ciprofloxacin was also confirmed by Epsilon-test (E -test) strips.
Results:
Of the total 302 Salmonella spp. isolated, 257 were Salmonella enterica serotype Typhi (85.1%) and 45 (14.9%) were S. enterica serotype Paratyphi A. The majority of the isolates recovered were from the pediatric age group (54.6%) and males (60.6%). Complete susceptibility was observed to chloramphenicol, cefotaxime, ceftriaxone, and azithromycin over the last two years (2009 and 2010), with an increase in resistance to nalidixic acid (100%) and ciprofloxacin (13.6%).
Conclusion:
In our study, we found augmentation of resistance to nalidixic acid and fluoroquinolones and complete sensitivity to ceftriaxone along with reemergence of chloramphenicol sensitivity for Salmonella isolates. This report emphasises the necessity of continuous surveillance of antibiograms of enteric fever isolates in an area.
Keywords: antibiotic resistance, ceftriaxone, ciprofloxacin, enteric fever, Salmonella
Introduction
Enteric fever is a global health problem, especially in the developing countries of the tropics, including India. It is classically caused by Salmonella Typhi (S. Typhi), S. Paratyphi A, and rarely by S. Paratyphi B and C (1). Antimicrobial agents remain the mainstay of treatment for enteric fever; however, indiscriminate use of these drugs has led to the emergence of resistance in Salmonella spp. (2).
Since its introduction in 1948, chloramphenicol has been considered as the gold standard of therapy for typhoid fever. Ampicillin and cotrimoxazole are alternative drugs effective against S. Typhi. However, emergence of multidrug resistant S. Typhi (MDRST) strains showing resistance to ampicillin and cotrimoxazole along with chloramphenicol – especially in South America, the Indian Subcontinent, Africa, and South-east Asia – has led to the use of ciprofloxacin as the mainstay of therapy (3). Rampant use of fluoroquinolones has gradually increased the minimum inhibitory concentrations (MICs) of ciprofloxacin for S. Typhi, causing therapeutic failure, and this has escalated into a worldwide problem of grave concern (4). Nalidixic acid resistance is used as a marker for determining the low level resistance to ciprofloxacin in S. Typhi and is also an indicator of therapeutic failure to ciprofloxacin. Therefore, in cases of nalidixic acid resistance, clinicians are often advised either to change the drug or to increase the dosage. Recently, cephalosporins have gained in importance in the treatment of enteric infections. First and second generation cephalosporins are ineffective but third and fourth generation cephalosporins, like ceftriaxone and cefixime, are highly effective even in nalidixic acid resistant strains (3). Azithromycin is a broad-spectrum azilide that has been found to be efficacious in MDR S. Typhi. As breakpoint concentrations of azithromycin for Salmonella are still not available, laboratories have to be cautious before reporting resistance to it.
The present study was conducted to assess the changing trends in antibiotic susceptibilities of S. Typhi and S. Paratyphi A isolates from blood cultures observed over a period of 3 years (2008–2010) at our tertiary care hospital.
Materials and Methods
The study was conducted in Department of Microbiology over a span of three years in a 750–bed tertiary care hospital in Chandigarh. Blood for culture was collected under strict aseptic conditions. Five milliliters of blood was added to each of two bottles containing liquoid broth and bile broth (HiMedia India Pvt Ltd, Mumbai). Both bottles were incubated aerobically at 37 °C for seven days. Routine subculturing was done on 5% sheep blood agar and MacConkey agar after 24 hours, 48 hours, and then at 7 days. In between these time points, subculturing was done only if turbidity was visible. All the S. Typhi and S. Paratyphi A isolates were identified using standard microbiological techniques and were confirmed by serotyping with specific antisera (based on O and H antigens) procured from the Central Research Institute, Kasauli, India. These isolates were compared for phenotypic traits including epidemiological parameters and antimicrobial susceptibility pattern.
The antimicrobial susceptibility of the isolates was determined by the disc diffusion method of Kirby Bauer (5) on Mueller–Hinton agar using Clinical Laboratory Standards Institute (CLSI) guidelines (6). The antimicrobial agents tested were ampicillin (10 μg), chloramphenicol (30 μg), trimethoprim/sulfamethoxazole (1.25/23.75 μg), nalidixic acid (30 μg), ciprofloxacin (5 μg), ceftriaxone (30μg), cefotaxime (30 μg), and azithromycin (15 μg). However, azithromycin interpretation was based on the disc manufacturer’s recommendations. Minimum inhibitory concentrations were determined for ciprofloxacin and chloramphenicol for all Salmonella isolates using an agar dilution method based on CLSI guidelines. Those isolates showing intermediate or resistant MIC values to ciprofloxacin on the agar dilution method were further confirmed by Epsilon -test strips (E –test strips, BioMerieux, France). All media, antibiotic discs, and powders were procured from HiMedia, Mumbai.
Results
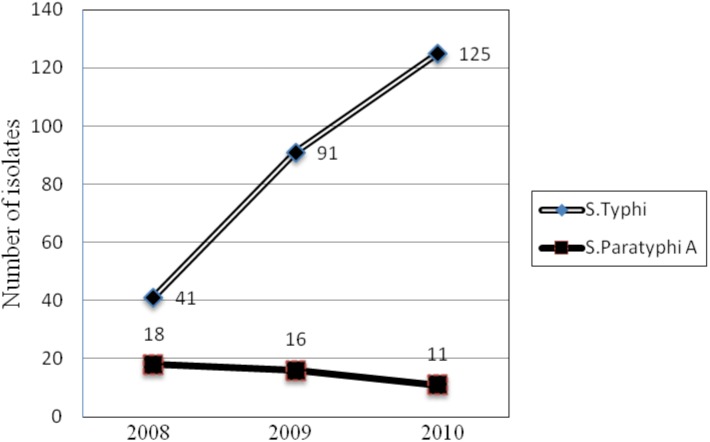
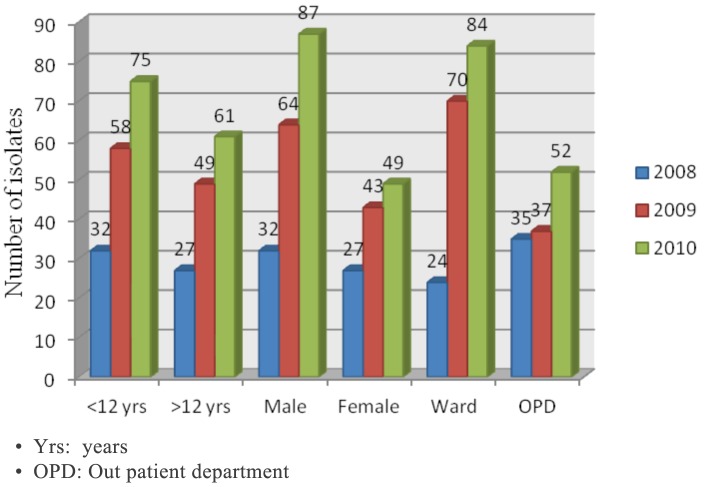
A total of 8142, 8134, and 8114 blood culture samples were processed for the years 2008, 2009, and 2010 respectively, of which 302 samples in all yielded growth of Salmonella spp. The predominant organism was S. Typhi, totaling 257 isolates (85.1%), followed by S. Paratyphi A (45 isolates/14.9%). An increasing prevalence of S. Typhi bacteremia was observed over this three year span (41, 91, 125 isolates in 2008, 2009, 2010 respectively; Figure 1). The majority of the isolates recovered were from the pediatric age group (54.6%) and males (60.6%). The number of patients requiring hospitalisation also increased over the span of 3 years (Figure 2, ward: outpatient department (OPD).
Figure 1:
Prevalence of Salmonella Typhi during 2008-2010 (Increasing trend over the years).
Figure 2:
Age, sex and ward/outpatient department admission wise distribution of all Salmonella isolates over the three year period 2008-2010.
The comparative antibiotic resistance profile of 257 isolates of S. Typhi and 45 S. Paratyphi A to various drugs tested by disc diffusion is shown year-wise in Table 1. Over a 3-year period, the numbers of nalidixic acid resistant S. Typhi (NARST) increased from 90.2% to 100% and the ciprofloxacin resistant isolates increased from 0% to 13.6%, as shown in Table 1. Complete susceptibility was observed to chloramphenicol, cefotaxime, ceftriaxone, and azithromycin. The MICs of all the isolates to chloramphenicol and ciprofloxacin are shown in Tables 2 and 3, respectively. The results obtained with E-test strips were in complete agreement.
Table 1.
Antibiotic resistance pattern of Salmonella spp. during 2008-2010
| *Antimicrobial agent/Isolates | Number of isolates (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ampicillin | Nalidixic acid | Cotrimoxazole | Chloramphenicol | Azithromycin | Ciprofloxacin | Ceftriaxone | Cefotaxime | |
| 2008 | ||||||||
| S. Typhi (41) | 14 (34.1 ) | 37 (90.2) | 4 (9.7) | 1 (2.4) | 0 | 0 | 0 | 0 |
| S. Paratyphi A (18) | 8 (44.4) | 15 (83.3) | 2 (11.1) | 1 (5.5) | 0 | 0 | 0 | 0 |
| 2009 | ||||||||
| S. Typhi (91) | 91 (100) | 91 (100) | 0 | 0 | 0 | 0 | 0 | 0 |
| S. Paratyphi A (16) | 10 (62.5) | 16 (100) | 0 | 0 | 0 | 0 | 0 | 0 |
| 2010 | ||||||||
| S. Typhi (125) | 70 (56) | 125 (100) | 2 (1.6) | 0 | 0 | 17 (13.6) | 0 | 0 |
| S. Paratyphi A | 4 (36.4) | 11 (100) | 0 | 0 | 0 | 0 | 0 | 0 |
Table 2.
Minimum inhibitory concentration of Salmonella spp. to Chloramphenicol
| Year | Number of isolates (%) / Concentration of drug | ||||||
|---|---|---|---|---|---|---|---|
| 1 μg/mL | 2 μg/mL | 4 μg/mL | 8 μg/mL | 16 μg/mL | 32 μg/mL | 64 μg/mL | |
| 2008 | |||||||
| S. Typhi (41) | 7 (17.1) | 23 (56.1) | 7 (17.1) | 3 (7.3) | 0 | 1 (2.4) | 0 |
| S. Paratyphi A (18) | 3 (16.7) | 3 (16.7) | 10 (55.6) | 1 (5.6) | 1 (5.6) | 0 | 0 |
| 2009 | |||||||
| S. Typhi (91) | 34 (37.4) | 48 (52.7) | 5 (5.5) | 3 (3.3) | 1 (1.1) | 0 | 0 |
| S. Paratyphi A (16) | 5 (31.3) | 7 (43.8) | 3 (18.8) | 1 (6.3) | 0 | 0 | 0 |
| 2010 | |||||||
| S. Typhi (125) | 31 (24.8) | 83 (66.4) | 9 (7.2) | 2 (1.6) | 0 | 0 | 0 |
| S. Paratyphi A (11) | 4 (36.4) | 7 (63.6) | 0 | 0 | 0 | 0 | 0 |
Table 3.
Minimum inhibitory concentration of Salmonella spp. to Ciprofloxacin
| Year | Number of isolates (%) / Concentration of drug | ||||||
|---|---|---|---|---|---|---|---|
| 0.125 μg/mL | 0.25 μg/mL | 0.5 μg/mL | 1 μg/mL | 2 μg/mL | 4 μg/mL | 8 μg/mL | |
| 2008 | |||||||
| S. Typhi (41) | 4 (9.8) | 9 (21.9) | 21 (51.2) | 5 (12.2) | 2 (4.9) | 0 | 0 |
| S. Paratyphi A (18) | 2 (11.1) | 14 (77.7) | 1 (5.6) | 1 (5.6) | 0 | 0 | 0 |
| 2009 | |||||||
| S. Typhi (91) | 3 (3.3) | 11 (12.1) | 65 (71.4) | 8 (8.8) | 3 (3.3) | 1 (1.1) | 0 |
| S. Paratyphi A (16) | 3 (18.8) | 11 (68.7) | 2 (12.5) | 0 | 0 | 0 | 0 |
| 2010 | |||||||
| S. Typhi (125) | 4 (3.2) | 9 (7.2) | 67 (53.6) | 22 (17.6) | 16 (12.8) | 5 (4) | 2 (1.6) |
| S. Paratyphi A (11) | 2 (18.2) | 6 (54.5) | 3 (27.3) | 0 | 0 | 0 | 0 |
Discussion
Enteric fever is a growing concern in our country, and the problem is being compounded by the emerging resistance to antibiotics that were previously effective.
In the present study, S. Typhi was the predominant isolate, followed by S. Paratyphi A, which correlates with other studies (7,8). The male to female and pediatric to adult ratios were 1.54:1 and 1.2:1, respectively; these values were similar to those reported in other studies (8,9). The worldwide emergence of multidrug-resistant strains of Salmonella in the last two decades has led to virtual withdrawal of chloramphenicol and its replacement with fluoroquinolones and thirdgeneration cephalosporins for treating enteric fever. However, in the recent past, the efficacy of ciprofloxacin as a first line of treatment for enteric fever has been seriously jeopardised (10). In our study over this three year period, we noted an increase in the number of patients with enteric fever who required hospitalisation. This can be attributed to a commensurate increase in the numbers of nalidixic acid resistant Salmonella Typhi (NARST) and ciprofloxacin-resistant strains associated with treatment failures.
The drug resistance pattern of S. Typhi observed in a previous study (January 2006 to May 2007) at our center was as follows: ampicillin (14.9%), nalidixic acid (95.4%), cotrimoxazole (8.1%), chloramphenicol (4.5%), and ciprofloxacin (0%) (4). Comparison with the present data shows contrasting trends during the period of present study, with a decrease in resistance to chloramphenicol (0%) and cotrimoxazole (1.6%), and an enormous increase in resistance to ampicillin (56%), nalidixic acid (100%), and ciprofloxacin (13.6%). This changing pattern of resistance is also reflected by the increasing MIC values for ciprofloxacin and decreasing MICs for chloramphenicol in S. Typhi as well as S. Paratyphi A when compared to the previous study.
The increasing resistance to fluoroquinolones observed in the present study is perhaps a direct consequence of their indiscriminate prescription not only for typhoid fever but also for other infections. It is also associated with a concomitant reemergence of chloramphenicol susceptibility attributed to its restricted use, which resulted in the withdrawal of selection pressure (11,12).
In the recent past, third generation cephalosporins have gained importance in the treatment of enteric fever. Although complete sensitivity to these drugs (i.e. cefotaxime and ceftriaxone) has been found in our study, some reports have appeared of increasing MICs for even third generation cephalosporins (13–20). Not only are these drugs expensive for routine use in developing nations, but the selection against beta lactamases is also a concern. Further studies will be carried out to monitor rise in MICs to ceftriaxone and also to detect the presence of extended spectrum beta lactamases in this group of organisms.
Conclusion
To conclude, the present study reveals an increase in resistance to fluoroquinolones, complete sensitivity to ceftriaxone, and a reemergence of chloramphenicol sensitivity at our center. Keeping in view the varying geographical patterns over a given time period, the necessity of continuous surveillance of antibiograms of Salmonella isolates in an area is re-emphasized to rationalise enteric fever treatment protocols.
Acknowledgments
None.
Footnotes
Conflict of interest
None.
Funds
None.
Authors’ contributions
Conception and design: VG
Analysis and interpretation of the data: NS
Critical revision of the article for the important intellectual content: NK
Final approval of the article, and dministrative, technical or logistic support: JC
Statistical expertise: NB
Drafting of the article: NS, NB
References
- 1.Harish BN, Menezes GA. Antimicrobial resistance in typhoidal salmonellae. Indian J Med Microbiol. 2011;29(3):223–239. doi: 10.4103/0255-0857.83904. [DOI] [PubMed] [Google Scholar]
- 2.Cooke FJ, Wain J. The emergence of antibiotic resistance in typhoid fever. Travel Med Infect Dis. 2004;2(2):67–74. doi: 10.1016/j.tmaid.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Lakshmi V, Ashok R, Susmita J, Shailaja W. Changing trends in the antibiograms of Salmonella isolates at a tertiary care hospital in Hyderabad. Indian J Med Microbiol. 2006;24(1):45–48. doi: 10.4103/0255-0857.19894. [DOI] [PubMed] [Google Scholar]
- 4.Gupta V, Kaur J, Kaistha N. Re-emerging chloramphenicol sensitivity and emerging low level ciprofloxacin resistance among Salmonella enterica serotype typhi isolates in North India. Trop Doct. 2009;39(1):28–30. doi: 10.1258/td.2008.070452. [DOI] [PubMed] [Google Scholar]
- 5.Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 6.CLSI . Wayne (PA): Clinical and Laboratory Standards Institute; 2009. Performance standards for antimicrobial susceptibility testing, 19th informational supplement, M100-S19. [Google Scholar]
- 7.Manchanda V, Bhalla P, Sethi M, Sharma PK. Treatment of enteric fever in children on the basis of current trends of antimicrobial susceptibility of Salmonella enterica serovar Typhi and Paratyphi A. Indian J Med Microbiol. 2006;24(2):101–106. doi: 10.4103/0255-0857.25182. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Rizvi M, Berry N. Rising prevalence of enteric fever due to multidrug-resistant Salmonella: an epidemiological study. J Med Microbiol. 2008;57(10):1247–1250. doi: 10.1099/jmm.0.2008/001719-0. [DOI] [PubMed] [Google Scholar]
- 9.Chowta MN, Chowta NK. Study of clinical profile and antibiotic response in typhoid fever. Indian J Med Microbiol. 2005;23(2):125–127. doi: 10.4103/0255-0857.16054. [DOI] [PubMed] [Google Scholar]
- 10.Nagshetty K, Channappa ST, Gaddad SM. Antimicrobial susceptibility of Salmonella Typhi in India. J Infect Dev Ctries. 2010;4(2):70–73. doi: 10.3855/jidc.109. [DOI] [PubMed] [Google Scholar]
- 11.Harish BN, Menezes GA. Preserving efficacy of chloramphenicol against typhoid fever in a tertiary care hospital, India. Regional Health Forum, WHO South-East Asia Region. 2011;15(1):92–96. [Google Scholar]
- 12.Indian Network for Surveillance of Antimicrobial Resistance Group Antibiogram of S. enterica serovar Typhi and S. enterica serovar Paratyphi A: a multicentre study from India. WHO South-East Asia Journal of Public Health. 2012;1(2):182–188. doi: 10.4103/2224-3151.206930. [DOI] [PubMed] [Google Scholar]
- 13.Saha SK, Talukder SY, Islam M, Saha S. A highly ceftriaxone resistant Salmonella typhi in Bangladesh. Pediatr Infect Dis J. 1999;18(4):387. doi: 10.1097/00006454-199904000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Capoor MR, Nair D, Hasan AS, Aggarwal P, Gupta B. Narrowing therapeutic options in typhoid fever, India. Southeast Asian J Trop Med Public Health. 2006;37(6):1170–1174. [PubMed] [Google Scholar]
- 15.Capoor MR, Nair D, Deb M, Aggarwal P. Enteric fever perspective in India: emergence of highlevel ciprofloxacin resistance and rising MIC to cephalosporins. J Med Microbiol. 2007;56(8):1131–1132. doi: 10.1099/jmm.0.47170-0. [DOI] [PubMed] [Google Scholar]
- 16.Gokul BN, Menezes GA, Harish BN. ACC-1 beta-Lactamase-producing Salmonella enterica serovar Typhi, India. Emerg Infect Dis. 2010;16(7):1170–1171. doi: 10.3201/eid1607.091643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pokhare BM, Koirala J, Dahal RK, Mishra SK, Khadga PK, Tuladhar NR. Multidrug-resistant and extended-spectrum beta-lactamase (ESBL) producing Salmonella enterica (serotypes Typhi and Paratyphi A) from blood isolates in Nepal: Surveillance of resistance and a search for newer alternatives. Int J Infect Dis. 2006;10(6):434–438. doi: 10.1016/j.ijid.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Rotimi VO, Jamal W, Pal T, Sovenned A, Albert MJ. Emergence of CTX-M-15 type extended-spectrum â-lactamaseproducing Salmonella spp. in Kuwait and the United Arab Emirates. J Med Microbiol. 2008;57(7):881–886. doi: 10.1099/jmm.0.47509-0. [DOI] [PubMed] [Google Scholar]
- 19.Al Naiemi N, Zwart B, Rijnsburger MC, Roosendaal R, Debets-Ossenkopp YJ, Mulder JA, et al. Extendedspectrum-beta lactamase production in a Salmonella enterica serotype Typhi strain from the Philippines. J Clin Microbiol. 2008;46(8):2794–2795. doi: 10.1128/JCM.00676-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sen B, Bhattacharya M, Niyogi SK. In vitro activity of cefpodoxime an expanded spectrum cephalosporin against Salmonella enterica serotype Typhi. Antimicrob Agents Chemother. 2008;52(2):802–803. doi: 10.1128/AAC.00867-07. [DOI] [PMC free article] [PubMed] [Google Scholar]