Abstract
Objective
Neuroimaging studies have revealed functional abnormalities in the anterior cingulate cortex in posttraumatic stress disorder (PTSD). The goal of the current research was to determine whether hyperresponsivity of the dorsal anterior cingulate in PTSD is an acquired characteristic or familial risk factor.
Method
Using a case-control twin design, we studied combat-exposed veterans with PTSD (n=12) and their identical combat-unexposed co-twins (n=12), as well as combat-exposed veterans without PTSD (n=14) and their identical combat-unexposed co-twins (n=14). Participants underwent functional magnetic resonance imaging during completion of the Multi-Source Interference Task, which reliably activates the dorsal anterior cingulate.
Results
Combat veterans with PTSD and their co-twins had significantly greater activation in the dorsal anterior cingulate and tended to have larger response time difference scores, as compared to non-PTSD veterans and their co-twins. Dorsal anterior cingulate activation in the exposed twins was positively correlated with their PTSD symptom severity. Dorsal anterior cingulate activation in the unexposed twins was positively correlated with their combat-exposed co-twins’ PTSD symptom severity, but not with depression or alcohol use severity in the combat-exposed co-twins.
Conclusions
Hyperresponsivity in the dorsal anterior cingulate appears to be a familial risk factor for the development of PTSD following psychological trauma.
Keywords: magnetic resonance imaging, limbic system, stress disorders, post-traumatic, twins, monozygotic, gyrus cinguli, Multi-Source Interference Task
Introduction
Several recent neuroimaging studies have reported functional abnormalities in the anterior cingulate cortex in posttraumatic stress disorder (PTSD). The anterior cingulate is a structure in the medial prefrontal cortex that is composed of several functional subdivisions (1,2). Rostral regions of the anterior cingulate activate during emotional states and during tasks that involve interference from emotional stimuli (3–8). In contrast, dorsal regions of the anterior cingulate (2,9) typically activate during a wide variety of tasks that involve interference from non-emotional stimuli (5). The dorsal anterior cingulate is thought to play a role in multiple cognitive processes such as performance monitoring, response selection, error detection, and decision making (5,6,10,11).
In PTSD, rostral portions of the anterior cingulate appear to be hyporesponsive during the presentation of trauma-related and other negative stimuli (12–18), and during emotional interference tasks (19,20). In contrast, dorsal portions appear to show exaggerated responsivity in PTSD during Stroop interference, oddball tasks, fear conditioning, and extinction recall (12,20–25), although not all studies have reported this finding (e.g., 26–29).
Whether functional abnormalities in the anterior cingulate in PTSD are acquired characteristics of the disorder or whether they are familial risk factors for the development of PTSD after trauma is unclear. In order to begin to address this question, we recently used positron emission tomography (PET) and fluorodeoxyglucose (FDG) to study resting regional cerebral metabolic rates for glucose (rCMRglu) in Vietnam combat veterans with and without PTSD, as well as their combat-unexposed identical co-twins without PTSD (30). We found that resting rCMRglu in a region including the dorsal anterior cingulate and midcingulate cortex was elevated in the combat veterans with PTSD and their combat-unexposed co-twins without PTSD, as compared to combat veterans without PTSD and their co-twins. In addition, we found a significant positive correlation between resting rCMRglu values in this region in the unexposed twins and their exposed twins’ PTSD symptom severity scores. Overall, these findings suggest that resting hypermetabolism in the dorsal anterior cingulate is a familial risk factor for the development of PTSD after psychological trauma.
Interpreting the cognitive correlates of rCMRglu abnormalities in a given structure at rest is challenging because the finding is, by definition, unassociated with a specific cognitive task. To help clarify the role of the dorsal anterior cingulate as a possible risk factor for the development of PTSD, in the current study, we examined its function using functional magnetic resonance imaging (fMRI) and the Multi-Source Interference Task, which yields behavioral indicators of cognitive interference and reliably activates the dorsal anterior cingulate in healthy individuals (9,31). Vietnam combat veterans with and without PTSD, as well as their combat-unexposed identical co-twins were studied. According to the logic of the design (32), exaggerated dorsal anterior cingulate activation in the combat veterans with PTSD and in their identical co-twins would be consistent with a familial risk factor. In contrast, exaggerated dorsal anterior cingulate activation in the combat veterans with PTSD and not in their identical co-twins would be consistent with an acquired characteristic of PTSD. Based on previous PET-FDG findings, we hypothesized that combat veterans with PTSD and their identical co-twins would show greater activation in the dorsal anterior cingulate as compared to veterans without PTSD and their identical co-twins. Given minimal previous evidence of significantly elevated response times or error rates on non-emotional Stroop interference tasks in PTSD (19,33–35), we had no directional a priori hypotheses regarding group differences on these behavioral measures.
Method
Participants
Participants were drawn from a pool of identical male twins who had participated in a previous study (36). Twenty-six pairs participated. Eighteen of these pairs participated in our previous PET-FDG study (30). Each combat-exposed twin had served in the Vietnam combat theater, whereas his combat-unexposed co-twin had not. Of the exposed twins, 12 developed current combat-related PTSD, and 14 never did, as determined by the Clinician Administered PTSD Scale (CAPS)(37) using criteria from the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV). Thus, there were four participant groups: (1) combat-exposed veterans with current, combat-related PTSD (n=12) and (2) their combat-unexposed co-twins (n=12); (3) combat-exposed veterans who never had combat-related PTSD (n=14) and (4) their combat-unexposed co-twins (n=14). The study was approved by the Partners Healthcare System Institutional Review Board. All participants gave written informed consent.
Demographics and Psychometrics
Forty-nine participants were right-handed, and 3 (1 combat-exposed with PTSD; 2 combat-exposed without PTSD) were left-handed. None of the participants reported a history of major head injury involving loss of consciousness for more than ten minutes, tumor, epilepsy, cerebrovascular accident, or other neurological disorder.
According to the Structured Clinical Interview for DSM-IV (38), participants in the combat-exposed group with PTSD met criteria for the following current comorbid diagnoses: major depression (n=4), dysthymia (n=2), panic disorder (n=3), social phobia (n=1), specific phobia (n=2), and cannabis dependence (n=1). Combat-unexposed co-twins of the PTSD group met criteria for the following current diagnoses: major depression (n=1), bipolar disorder (n=1), specific phobia (n=3), panic disorder (n=1), alcohol dependence (n=1), and civilian-related PTSD (n=1). Analyses were conducted both with and without the latter participant and his co-twin. Combat-exposed veterans who never had PTSD met criteria for current dysthymia (n=1) and specific phobia (n=1), and two of their combat-unexposed co-twins met criteria for current dysthymia.
Eight participants were taking antidepressants at the time of study: combat-exposed participants with PTSD (n=3) and their unexposed co-twins (n=1); combat-exposed participants without PTSD (n=1) and their unexposed co-twins (n=3). Six participants were taking other types of medications. In the combat-exposed group with PTSD: benzodiazepine (n=1) antipsychotic (n=1), sympatholytic (n=1), and opiate (n=2). One co-twin of a combat-exposed participant without PTSD was taking a sympatholytic.
Participants completed the Beck Depression Inventory (BDI) (39), the Michigan Alcohol Screening Test (MAST) (40), the Childhood Trauma Questionnaire (CTQ) (41), and a measure of the severity of combat exposure (42). (See Table 1.)
Table 1.
Group means and standard deviations of combat-exposed Vietnam veterans with and without PTSD and their combat-unexposed, identical co-twins
| PTSD Pairs* | Non-PTSD Pairs† | Mixed Model ANOVA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed | Unexposed | Exposed | Unexposed | Diagnosis | Exposure | Interaction | ||||||||
| n=12 | n=12 | n=14 | n=14 | F(1,24) | p | F(1,24) | p | F(1,24) | p | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||||
| Age (years) | 55.0 | 2.9 | 55.0 | 2.9 | 56.4 | 2.2 | 56.4 | 2.2 | 2.1 | .16 | -- | -- | -- | -- |
| Education (years) | 12.8 | 3.1 | 13.3 | 3.1 | 13.6 | 1.7 | 13.6 | 2.0 | 0.4 | ns | 0.5 | ns | 0.2 | ns |
| MAST# | 8.6 | 4.4 | 8.3 | 4.9 | 6.0 | 2.1 | 4.8 | 1.8 | 4.5a | 0.051 | 1.0a | ns | 0.4a | ns |
| Combat Severity¶ | 8.7 | 1.5 | -- | -- | 3.6 | 2.7 | -- | -- | 31.2b | <.0001 | -- | -- | -- | -- |
| CAPS§-Current | 61.7 | 22.3 | -- | -- | 7.6 | 10.2 | -- | -- | 66.3 | <.0001 | -- | -- | -- | -- |
| BDI¥ | 11.5 | 9.0 | 4.5 | 5.5 | 3.1 | 4.6 | 3.5 | 4.6 | 3.4c | 0.08 | 6.6c | .02 | 8.3c | .011 |
| CTQv | 60.4 | 8.7 | 59.3 | 9.9 | 63.4 | 6.2 | 62.2 | 3.6 | 1.4b | ns | 0.56b | ns | 0.003b | ns |
| Birth Weight (oz) | 89.5 | 14.0 | 97.0 | 10.0 | 88.5 | 24.4 | 82.8 | 23.6 | 0.85d | ns | 0.05d | ns | 2.4d | ns |
As determined by the presence of current, combat-related PTSD in the combat-exposed twin
As determined by the absence of current or past, combat-related PTSD in the combat-exposed twin
Michigan Alcoholism Screening Test (range 0–25)
18-item measure (range 0–18)
Clinician-Administered PTSD Scale (range 0–136), combat-related
Beck Depression Inventory (range 0–63)
Childhood Trauma Questionnaire
Due to missing data:
df=1,15;
df=1,23;
df=1,16,
df=1,18
Task Procedures
In the Multi-Source Interference Task (31), participants viewed sets of three numbers or letters on a computer screen projected into the magnet via a tilted mirror. They were told that one number would always be different from the other two items. Participants were asked to report via button-press the identity of the number that was different from the others, respond as quickly as possible while minimizing errors, and complete 48 practice trials before beginning the experiment.
The task consisted of two conditions presented in separate alternating blocks. In the Control (C) condition, the identity of the target number (i.e., the one that was different from the other two) always matched its position on the screen/button-press. In the Interference (I) condition, the identity of the target number never matched its position (see Supplemental Data for sample stimuli). The task began and ended with 30 seconds of Fixation (F), which consisted of a white dot presented in the middle of the screen.
Stimuli were presented via a Macintosh Powerbook computer and MacStim 3. Each stimulus remained on the screen for 1.5 seconds with .25 seconds between stimuli. Each block consisted of 24 stimuli. Each run consisted of 10 blocks of alternating conditions in a fixed order (i.e., FCICICICIF) and lasted a total of 6 min, 36 seconds. Participants completed up to three runs of the Multi-Source Interference Task. Because response time differences between conditions (IvC) and dorsal anterior cingulate activation can decline with each additional run in this task, we decided a priori to analyze data from the first run only (9). (For second run results, see Supplemental Data.)
fMRI Procedures
We used a Symphony/Sonata 1.5 Tesla high-speed imaging device (Siemens Medical Systems, Iselin NJ) with a 3-axis gradient head coil. After shimming, high-resolution structural MRI images (3D-MPRAGE; TR/TE/flip angle=2.73sec/3.31msec/7°) with a 1.3 mm slice thickness were collected. Functional MRI blood oxygenation level dependent (BOLD) images were acquired using a gradient echo T2*-weighted sequence (TR/TE/flip angle=1.5sec/40msec/90°) in 16 coronal slices perpendicular to the AC-PC line (thickness=5mm, 1mm-skip).
Data Analysis
Behavioral Analyses
Response times from only correct trials were averaged within each condition and run for each participant. Errors were expressed for each participant as a percentage of the total number of trials on which the participant responded within each condition and run. Difference scores were created by subtracting the average response time in the Control condition from the average response time in the Interference condition. Difference scores were also calculated for error rates (I-C).
fMRI Analyses
We conducted two types of analyses on the fMRI data: whole-brain voxelwise comparisons, and then analysis of variance (ANOVA) of fMRI data that were extracted from the dorsal anterior cingulate in the voxelwise maps of individual subjects. In both types of analyses, we treated exposed versus unexposed co-twins as a repeated measure (i.e., main effect of Exposure). In addition, we treated the twin pairs in which the combat-exposed twin had PTSD as a separate group from the twin pairs in which the exposed twin never had PTSD. A significant difference between these two groups of twin pairs (i.e., a significant main effect of PTSD Diagnosis) would be consistent with a familial risk factor (as long as there was also no interaction between PTSD Diagnosis and Exposure). This finding would indicate that the combat-exposed twins with PTSD have the same functional abnormality as their unexposed co-twins without PTSD. A significant PTSD Diagnosis x Exposure interaction that reflected an abnormality in only the exposed twins with PTSD would indicate an acquired sign of PTSD. Lastly, a significant main effect of Exposure (i.e., a significant difference between all combat-exposed twins as compared to all combat-unexposed twins collapsing across PTSD diagnosis) in the absence of an interaction would suggest that the functional abnormality is associated with exposure to combat and not PTSD.
Voxelwise Analyses
Statistical parametric mapping analysis of the imaging data was conducted using SPM2. Each participant’s functional images were motion corrected and coregistered to his high-resolution structural MRI image. The resulting images were spatially normalized in a standard stereotactic space (Montreal Neurological Institute, MNI) and then smoothed (8mm-FWHM). At each voxel, the BOLD data were fit to a linear statistical model by the method of least squares. Hypotheses were tested as contrasts in which linear compounds of the model parameters were evaluated using t statistics, which were then transformed to z-scores.
We used an approach that consisted of two hierarchical levels of analysis, in which the second level’s random-effects analysis absorbed the random effects from the first level. First, IvC contrast images were generated for each participant. For the purpose of examining the main effect of PTSD Diagnosis, the IvC contrast images of the combat-exposed and combat-unexposed participants were averaged (first level), and then the PTSD and non-PTSD pairs were compared (second level). For the purpose of examining the PTSD Diagnosis x Exposure interaction, the IvC contrast images of the combat-exposed and combat-unexposed participants were contrasted (first level), and then the PTSD and non-PTSD pairs were compared (second level). Then for the purpose of examining the main effect of combat Exposure, the IvC contrast images of the combat-exposed and combat-unexposed subjects were compared in a two-group t-test.
The statistical parametric maps resulting from the above analyses were inspected for main effects and their interaction in the dorsal anterior cingulate, which was defined as the portion of the anterior cingulate that is superior to the corpus callosum, between y=0 and y=+30mm (43). Given our strong a priori hypotheses, we applied a significance threshold of p≤.001 one-tailed, uncorrected (z-score ≥3.09) to activations in the dorsal anterior cingulate. For regions about which we had no a priori prediction, we applied a more conservative constant significance threshold of p≤.00002, two-tailed, uncorrected (z-score≥4.27)(29,30).
Region of Interest Analyses
We extracted fMRI data from the dorsal anterior cingulate activation at the maximum voxel within each subject following previously established methods (9). (Data could not be extracted for 4 subjects (2 combat-exposed twins with PTSD, 1 combat-exposed twin without PTSD and 1 of their co-twins) because the maximum voxel value of their I-C activations did not fall within the dorsal anterior cingulate proper.) We then further analyzed these data for the main effects of PTSD Diagnosis and combat Exposure and their interaction using a mixed model that treated combat Exposure as a within-pairs repeated measure, PTSD Diagnosis as a between-pairs measure, and twin pairs as a random effect (44). We also performed correlational analyses with the extracted data in order to determine whether dorsal anterior cingulate activation in all combat-exposed twins correlated with their own CAPS scores, as well as whether dorsal anterior cingulate activation in the combat-unexposed twins correlated with their combat-exposed twins’ CAPS scores and other clinical measures. All p-values are two-tailed unless otherwise indicated.
Results
Behavioral Results
Response time and error rate difference scores (I-C) were submitted to separate 2 (PTSD Diagnosis: PTSD, non-PTSD) x 2 (Exposure: exposed, unexposed) repeated measures ANOVAs. Regarding response time difference scores, there was a trend for a main effect of PTSD Diagnosis, F(1,24)=3.61, p=.07. The combat-exposed participants with PTSD and their co-twins tended to have larger response time difference scores than the combat-exposed participants without PTSD and their co-twins. (See Table 2.) No other effect was significant (ps>.44). When one PTSD twin pair was temporarily removed due to civilian PTSD in the co-twin, the main effect of PTSD Diagnosis became significant, F(1,23)=5.30, p=.03.
Table 2.
Response time and error rate data
| PTSD Pairs | Non-PTSD Pairs | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposed (n=12) | Unexposed (n=12) | Exposed (n=14) | Unexposed (n=14) | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Mean Response Times (msec): | ||||||||
| Interference | 1066 | 117 | 1064 | 136 | 983 | 95 | 998 | 100 |
| Control | 661 | 84 | 665 | 103 | 633 | 114 | 669 | 98 |
| Difference Score | 405 | 129 | 399 | 93 | 350 | 70 | 329 | 81 |
| Mean Error Rates (%): | ||||||||
| Interference | 6.0 | 4.5 | 5.7 | 6.0 | 5.7 | 5.6 | 4.3 | 4.1 |
| Control | 0.5 | 0.9 | 1.1 | 2.0 | 1.0 | 1.7 | 0.3 | 0.9 |
| Difference Score | 5.5 | 4.7 | 4.6 | 5.5 | 4.7 | 4.4 | 4.0 | 4.3 |
Regarding error rate difference scores, no effects were significant (all ps >.41), even when the PTSD pair mentioned above was temporarily removed from the analysis.
FMRI Results
Voxelwise Analyses
There was a significant main effect of PTSD Diagnosis in the dorsal anterior cingulate: MNI coordinates [+10,+6,+46], z=3.17, k at p<.001=1. Combat-exposed veterans with PTSD and their co-twins exhibited greater BOLD signal changes in the IvC contrast than combat-exposed veterans without PTSD and their co-twins. When we ran a small volume correction based on the volume of right dorsal anterior cingulate in the current sample, the false discovery rate (FDR) p-value for the main effect of PTSD diagnosis was p=.05. The small volume correction based on the volume of bilateral dorsal anterior cingulate yielded an FDR p=.08. Contrasts between the subgroups revealed a similar pattern (Table 3).
Table 3.
Results of voxelwise fMRI analyses
| Region | Z-score | MNI Coordinates (x, y, z) | |
|---|---|---|---|
|
|
|||
| Main Effect of Diagnosis: | |||
| PTSD Pairs > non- PTSD Pairs | dorsal anterior cingulate | 3.17 2.79 |
10, 6, 46 −2, 2, 40 |
| Non-PTSD Pairs > PTSD Pairs | none | ||
|
|
|||
| Exposed Twins: | |||
| PTSD> non-PTSD | dorsal anterior cingulate | 2.71 | 10, 6, 42 |
| Non-PTSD> PTSD | none | ||
|
|
|||
| Unexposed Twins: | |||
| PTSD> non-PTSD | dorsal anterior cingulate | 3.22 | 2, 2, 40 |
| Non-PTSD> PTSD | none | ||
Note: Interference vs. Control contrast images were used in the above analyses.
MNI = Montreal Neurological Institute. L = left, R = right.
No regions exhibited significantly lower BOLD signal changes in the PTSD twin pairs relative to the non-PTSD twin pairs. No brain regions met significance thresholds for a main effect of Exposure or a Diagnosis x Exposure interaction.
Region of Interest Analyses
Data from the dorsal anterior cingulate activation in individual subjects were extracted and further analyzed. The main effect of PTSD Diagnosis was significant, F(1,20)=8.9, p=.007 (Figure 1), and remained significant after removing one PTSD pair in which the combat-unexposed co-twin had civilian-related PTSD, F(1,19)=7.9, p=.01.
Figure 1.
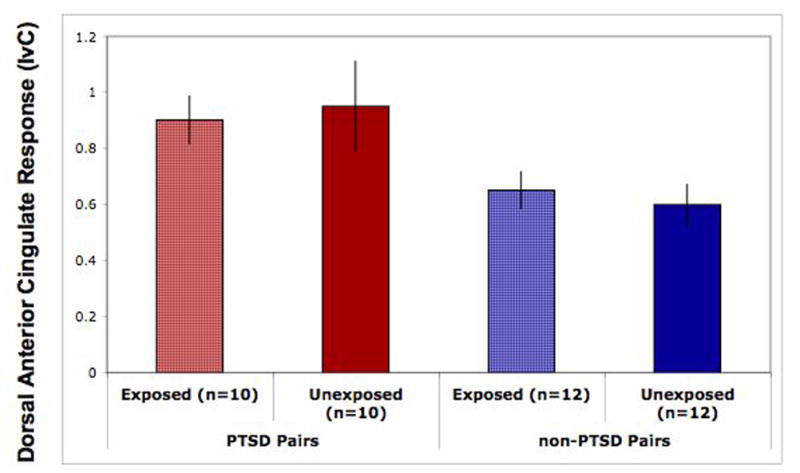
Functional MRI data extracted from the maximum voxels in the dorsal anterior cingulate of individual subjects and displayed per group. These data show a main effect of PTSD Diagnosis, F(1, 20)=8.9, p=.007, consistent with the whole-brain voxelwise analyses. Error bars represent standard error of the mean.
The following covariates were tested as potential confounders of the main effect of PTSD Diagnosis by examining their association with the dependent measure using a screening threshold of p<0.20: birth weight, age, total score on the CTQ, years of education, BDI score, MAST score, MDD, psychiatric medication use, left-handedness, and severity of combat exposure. Only age met this threshold. Adjusted for age, the main effect of PTSD Diagnosis was F(1,19)=4.9, p=.04.
BOLD signal changes in the dorsal anterior cingulate in the combat-exposed twins were positively correlated with their own current CAPS scores, r(21)=.37, p=.043, one-tailed (see Supplemental Data). BOLD signal changes in the dorsal anterior cingulate in the combat-unexposed twins were positively correlated with their combat-exposed twins’ current CAPS scores, r(23)=.34, p=.05, one-tailed (see Supplemental Data), and combat exposure scores, r(22)= .38, p=.03, one-tailed. However, these correlations became non-significant when a potential outlier was removed, r(22)=.26, p = .12, one-tailed and r(21)=.30, p = .09, one-tailed, respectively. BOLD signal changes in the dorsal anterior cingulate in the combat-unexposed twins were not significantly correlated with their combat-exposed twins’ BDI scores, r(16)=.13, p=.31, MAST scores, r(15)= −.04, p=.45, or response time difference scores, r(23)=.02, p=.46 (all ps one-tailed for comparison). In a post-hoc analysis, response time difference scores (IvC) in the combat-unexposed twins were not significantly correlated with their combat-exposed twins’ CAPS scores r(24)=.29, p=.15, two-tailed.
Discussion
Vietnam combat veterans with PTSD and their identical co-twins showed greater fMRI activation in the dorsal anterior cingulate during a non-emotional interference task as compared to Vietnam combat veterans without PTSD and their identical co-twins. In addition, dorsal anterior cingulate activation in the combat-exposed twins was positively correlated with their own CAPS scores. Furthermore, dorsal anterior cingulate activation in the combat-unexposed twins was positively correlated with their combat-exposed twins’ CAPS and combat exposure scores, but not with other clinical measures. These results suggest that dorsal anterior cingulate hyperresponsivity is a familial risk factor for PTSD rather than an acquired characteristic of PTSD.
The finding of exaggerated activation in dorsal anterior cingulate in PTSD is consistent with a few previous findings in PTSD singletons (12,20–22,24,25)(but see also 26–29). Two previous studies found exaggerated dorsal anterior cingulate activation during interference tasks in combat veterans with PTSD (20,25). In those previous studies, the exaggerated activation in PTSD was not accompanied by a significant behavioral effect. That is, the response time difference scores were not significantly greater in PTSD than in the trauma-exposed comparison group. This could be attributable to small sample sizes of the previous studies. In the current study, with a larger sample size the behavioral effect was nearly significant (p=.07) with all subjects included, and became significant (p=.03) when one PTSD pair was removed due to civilian PTSD in the combat-unexposed co-twin. Interestingly, although dorsal anterior cingulate activation in the combat-unexposed twins correlated with their combat-exposed twins’ CAPS scores, response time difference scores in the combat-unexposed twins did not. This suggests that dorsal anterior cingulate activation might be a more sensitive measure of vulnerability than behavioral measures of interference.
The current findings are consistent with those of our previous PET-FDG twin study (which included 18 of the same pairs studied here)(30). Thus, PTSD twin pairs exhibited increased rCMRglu in the dorsal anterior cingulate at rest, as well as greater activation during an interference task involving non-emotional information. Moreover, the locations of these two findings are similar (PET-FDG study: +10,+2,+42; current fMRI study: +10,+6,+46). Although these findings await replication in a new sample, it appears that the functional abnormality in the dorsal anterior cingulate in PTSD can be elicited using either technique. With fMRI, radioisotopes are not required and the implementation of a specific cognitive task helps to pinpoint the cognitive correlates of the functional abnormality.
Exaggerated dorsal anterior cingulate activation in the PTSD group and their identical co-twins could reflect increased cognitive interference and/or response selection, an interpretation that is consistent with the trend for greater response time differences in those groups. Exaggerated dorsal anterior cingulate activation in the PTSD group and their co-twins is not likely attributable to error monitoring as all groups had similar error rate difference scores. Because our task was presented in blocks, we were unable to remove fMRI data from individual trials on which errors occurred. Exaggerated dorsal anterior cingulate activation could also reflect autonomic arousal or its regulation during task performance (22,45,46), although we do not have psychophysiologic measures to support this possibility. However, previous behavioral studies have found that individuals with PTSD are not more physiologically responsive during Stroop interference tasks or other cognitive stressors as compared to control groups (19,33,47). Finally, given that the dorsal anterior cingulate is activated during the expression of conditioned fear responses in healthy humans (46) and is hyperresponsive during extinction recall in PTSD (23), the exaggerated activation observed in PTSD in the present study may reflect a general hyperresponsivity of this brain region that could be related to the exaggerated fear responses observed in PTSD. The positive correlation found between dorsal anterior cingulate activation and PTSD symptom severity is consistent with this speculation.
All participants in this study were male, which may limit the generalizability of the findings. Other limitations include the presence of disorders other than PTSD and medication use in some of the twins. Another possible limitation is the presence of civilian PTSD in one co-twin. However, civilian PTSD in this co-twin was not driving the main effect of diagnosis because the hyperactivation remained even when this twin pair was removed. Given that we did not include combat-exposed subjects who had past or partial PTSD, our findings may not generalize to those conditions. Future studies might implement event-related designs and psychophysiologic monitoring in order to further clarify the role of dorsal anterior cingulate function as a potential familial risk factor for the development of PTSD following psychological trauma. In addition, future studies should seek to determine whether hyperresponsivity of the dorsal anterior cingulate during interference tasks in PTSD is associated with specific genotypes, such as the presence of a short allele of the serotonin transporter polymorphism, which has been associated with increased risk of PTSD after exposure to traumatic events (48).
Supplementary Material
Acknowledgments
This work was supported by USPHS Grant #R01MH54636 to Dr. Pitman. The U.S. Department of Veterans Affairs provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Through their support of the VET Registry, numerous other U.S. organizations also provided invaluable assistance, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Institutes of Health; National Opinion Research Center; National Research Council, National Academy of Sciences; and Institute for Survey Research, Temple University. The authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry, and of the other participants, without whose contribution this research would not have been possible. The authors also thank Mary Foley, and Lawrence White for technical assistance.
Footnotes
Disclosures: Dr. Rauch received funded research through MGH for Brain Stimulation Therapy from Medtronics, Inc.; funded research through MGH for VNS from Cyberonics; and funded research through MGH on anxiolytic action from Cephalon. He also received honoraria from Novartis for consultation on emerging treatments; Neurogen for his participation as a consultant on emerging trends in anxiety associated with insomnia; Sepracor for his consultation on fear/conditioning/extinction; Primedia for his participation in developing a CE activity; and Medtronics, Inc for his attendance of the Advisory Board meeting on the Anatomy and Neuroscience of anxiety and depression. Although no direct conflict is anticipated, the financial disclosures of Dr. Bush are as follows: Grant or general research support has been provided over the past decade by the National Institutes of Mental Health, the National Science Foundation, the Mental Illness and Neuroscience Discovery (MIND) Institute, the Benson-Henry Institute for Mind-Body Medicine, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the Centers for Disease Control, the David Judah Fund, the McIngvale Fund, the Johnson and Johnson Center for the Study of Psychopathology, McNeil Pharmaceuticals, Pfizer Pharmaceuticals, Eli Lilly & Co. and the Center for Functional Neuroimaging Technologies (P41RR14075). Dr. Bush has, or has had in the past, a relationship with one or more organizations listed below as follows: former advisory board member and speaker’s honoraria from Eli Lilly and Company and Novartis Pharmaceuticals; and has received speaker’s honoraria from Shire U.S. Inc., Janssen Pharmaceuticals, Johnson & Johnson and McNeil Pharmaceuticals. Dr. Bush has served as a judge for Intel Corporation science competition for which he received an honorarium. Dr. Bush does not now, and has not at any time, had a financial interest in any of these entities. All other authors have no competing financial interests.
References
- 1.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–43. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 2.Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–44. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 4.Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum Brain Mapp. 1998;6:270–82. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush G, Luu P, Posner MI. Cognitive and emotional influence in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 6.Mohanty A, Engels AS, Herrington JD, Heller W, Ho MH, Banich MT, Webb AG, Warren SL, Miller GA. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–51. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 7.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 8.Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–28. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 9.Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, Makris N, Surman C, Aleardi M, Mick E, Biederman J. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry. 2008;65:102–14. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- 10.Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–66. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- 11.Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- 12.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–40. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158:1920–2. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 15.Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63:184–92. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- 16.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999;156:575–84. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 17.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 18.Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, Gordon E, Bryant RA. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29:347–57. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 19.Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. 2004;55:612–20. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–42. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 21.Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C, Gordon E, Williams LM. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:111–8. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Felmingham KL, Williams LM, Kemp AH, Rennie C, Gordon E, Bryant RA. Anterior cingulate activity to salient stimuli is modulated by autonomic arousal in Posttraumatic Stress Disorder. Psychiatry Res: Neuroimaging. 2009;173:59–62. doi: 10.1016/j.pscychresns.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pannu Hayes J, Labar KS, Petty CM, McCarthy G, Morey RA. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Res. 2009;172:7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin LM, Bush G, Whalen PJ, Handwerger K, Cannistraro PA, Wright CI, Martis B, Macklin ML, Lasko NB, Orr SP, Pitman RK, Rauch SL. Dorsal anterior cingulate function in posttraumatic stress disorder. J Trauma Stress. 2007;20:701–12. doi: 10.1002/jts.20231. [DOI] [PubMed] [Google Scholar]
- 26.Falconer E, Bryant R, Felmingham KL, Kemp AH, Gordon E, Peduto A, Olivieri G, Williams LM. The neural networks of inhibitory control in posttraumatic stress disorder. J Psychiatry Neurosci. 2008;33:413–22. [PMC free article] [PubMed] [Google Scholar]
- 27.Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry. 2003;53:204–10. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- 28.Moores KA, Clark CR, McFarlane AC, Brown GC, Puce A, Taylor DJ. Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Res. 2008;163:156–70. doi: 10.1016/j.pscychresns.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Marzol Peters P, Metzger L, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–76. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 30.Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP, Goetz JM, Fischman AJ, Rauch SL, Pitman RK. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1099–107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: validation study with fMRI in individual subjects. Mol Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- 32.Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, Shenton ME, Yehuda R, Orr SP. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann N Y Acad Sci. 2006;1071:242–54. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litz BT, Weathers FW, Monaco V, Herman DS, Wulfsohn M, Marx B, Keane TM. Attention, arousal, and memory in posttraumatic stress disorder. J Trauma Stress. 1996;9:497–519. doi: 10.1007/BF02103661. [DOI] [PubMed] [Google Scholar]
- 34.Stein MB, Kennedy CM, Twamley EW. Neuropsychological function in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:1079–88. doi: 10.1016/s0006-3223(02)01414-2. [DOI] [PubMed] [Google Scholar]
- 35.Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- 36.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY, Pitman RK. Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: association with posttraumatic stress disorder. Arch Gen Psychiatry. 2003;60:283–8. doi: 10.1001/archpsyc.60.3.283. [DOI] [PubMed] [Google Scholar]
- 37.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 38.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. New York: New York State Psychiatric Institute, Biometrics Research Department; 1995. [Google Scholar]
- 39.Beck AT, Steer RA. Manual for the revised Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 40.Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–8. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 42.Janes GR, Goldberg J, Eisen SA, True WR. Reliability and validity of a combat exposure index for Vietnam era veterans. J Clin Psychol. 1991;47:80–6. doi: 10.1002/1097-4679(199101)47:1<80::aid-jclp2270470112>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 43.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Littlell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- 45.Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–52. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 46.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–4. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 47.Orr SP, Meyerhoff JL, Edwards JV, Pitman RK. Heart rate and blood pressure resting levels and responses to generic stressors in Vietnam veterans with posttraumatic stress disorder. J Trauma Stress. 1998;11:155–64. doi: 10.1023/A:1024421502881. [DOI] [PubMed] [Google Scholar]
- 48.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–9. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


