Abstract
Proline plays important roles in protein synthesis and structure, metabolism (particularly the synthesis of arginine, polyamines, and glutamate via pyrroline-5-carboxylate), and nutrition, as well as wound healing, antioxidative reactions, and immune responses. On a pergram basis, proline plus hydroxyproline are most abundant in collagen and milk proteins, and requirements of proline for whole-body protein synthesis are the greatest among all amino acids. Therefore, physiological needs for proline are particularly high during the life cycle. While most mammals (including humans and pigs) can synthesize proline from arginine and glutamine/glutamate, rates of endogenous synthesis are inadequate for neonates, birds, and fish. Thus, work with young pigs (a widely used animal model for studying infant nutrition) has shown that supplementing 0.0, 0.35, 0.7, 1.05, 1.4, and 2.1% proline to a proline-free chemically defined diet containing 0.48% arginine and 2% glutamate dose dependently improved daily growth rate and feed efficiency while reducing concentrations of urea in plasma. Additionally, maximal growth performance of chickens depended on at least 0.8% proline in the diet. Likewise, dietary supplementation with 0.07, 0.14, and 0.28% hydroxyproline (a metabolite of proline) to a plant protein-based diet enhanced weight gains of salmon. Based on its regulatory roles in cellular biochemistry, proline can be considered as a functional amino acid for mammalian, avian, and aquatic species. Further research is warranted to develop effective strategies of dietary supplementation with proline or hydroxyproline to benefit health, growth, and development of animals and humans.
Keywords: Proline, Nutrition, Biochemistry, Health, Growth
Introduction
Proline and its metabolite (hydroxyproline) are unique amino acids (AA) both chemically and biochemically (Hu et al. 2008; Kaul et al. 2008). They constitute one-third of AA in the collagen proteins which comprise approximately 30% of body proteins. On a per-gram basis, the requirement of proline for whole-body protein synthesis is the highest among all AA. However, the current edition of Nutrient Requirements of Swine (NRC 1998) concluded that L-proline (the physiological isomer) is not needed in the diets for gestating, neonatal, growing, finishing pigs or boars to achieve their maximal production performance. Likewise, the National Research Council (1998) does not provide data on proline contents in feed ingredients commonly used to formulate swine diets. Furthermore, proline is not considered a nutritionally essential or conditionally essential AA for humans without burns or injury (Elango et al. 2009). This is very unfortunate and reflects a lack of knowledge about proline biochemistry and nutrition in mammals.
Recent years have witnessed increasing interest in research on proline metabolism and nutrition (Phang et al. 2010; Srivastava et al. 2010; Wang et al. 2009a; Watford 2008), as well as metabolic diseases (Pérez-Arellano et al. 2010). Growing evidence shows that proline is a key regulator of multiple biochemical and physiological processes in cells. For example, proline is a signaling molecule, a sensor of cellular energy status, and a source of pyrroline-5-carboxylate (P5C) and superoxide anion (a free radical) participating in redox reactions in humans and animals (Phang et al. 2008, 2010). Additionally, proline plays an important role in differentiation of cells (including embryonic stem cells; Pistollato et al. 2010), as well as conceptus (fetus and associated extraembryonic membranes) growth and development (Wu et al. 2008). Furthermore, the seminal discovery that proline serves as a major AA for the synthesis of polyamines (key regulators of DNA and protein synthesis, as well as cell proliferation and differentiation) in the small intestine (Wu et al. 2000) and placenta (Kwon et al. 2003a; Wu et al. 2005) led to reconsideration of its crucial role in fetal and neonatal nutrition (Wu 2009). New developments in proline metabolism are shaping the science and practice of animal and human nutrition. The major objective of this article is to review recent advances in this exciting area of AA research.
Chemical structures and functions of proline and hydroxyproline
Proline and hydroxyproline contains an α-imino group and, therefore, they are α-imino acids (Fig. 1). However, because proline is a substrate for protein synthesis like a-AA and hydroxyproline is its post-translational metabolite, they are loosely referred to as AA in biochemistry. Proline and hydroxyproline are major AA in the collagen proteins which contain three chains of polypeptides (two α1 chains and one α2 chain) and are major extracellular components in connective tissues (e.g., skin, tendon, cartilage, vessels of the vascular system, and bone). The helical region of collagen comprises the repeat of Gly–X–Y, where proline can be in the X or Y position and hydroxyproline occurs only in the Y position (Krane 2008). The unique ring structure of proline and hydroxyproline distinguishes them from other AA in terms of rigidity, chemical stability, and biochemical reactions.
Fig. 1.
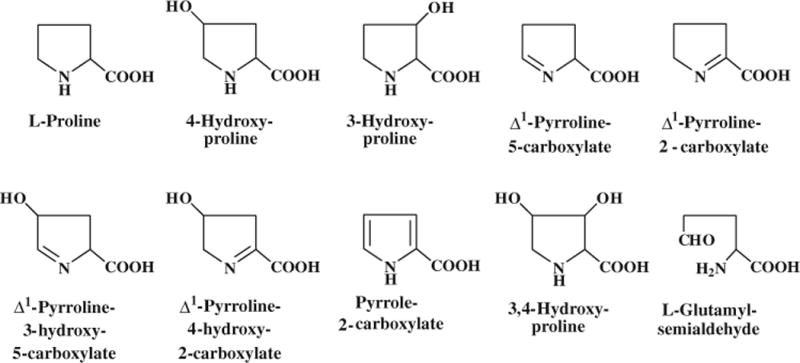
Structures of proline and related metabolites. All of these proline metabolites occur in animals, with 4-hydroxyproline being the most abundant
Proline residues in the collagen proteins can be hydroxylated in the endoplasmic reticulum by collagen prolyl 4-hydroxylase or prolyl 3-hydroxylase in the presence of oxygen, ascorbic acid, α-ketoglutarate, and Fe2+ (Gorres and Raines 2010). The ratio of 4-hydroxyproline to 3-hydroxyproline in collagen proteins is approximately 100:1. Other prolyl 4-hydroxylases, including hypoxia-inducible transcription factor α, act on non-collagen proteins (Krane 2008). The hydroxylation of proline is a post-translational event because the hydroxylated residues appear after the collagen polypeptides are synthesized. This reaction also occurs in other proteins to regulate cellular oxygen sensing and physiological responses to hypoxia (Chandel 2010; Wenger and Hoogewijs 2010). Free hydroxyproline and 3-hydroxyproline are generated from the degradation of collagens or other proteins containing 4-hydroxylprolyl and 3-hydroxylprolyl residues, respectively (Phang et al. 2008, 2010).
Proline plays versatile roles in cell metabolism and physiology (Table 1). For example, proline is a major nitrogenous substrate for the synthesis of polyamines in the small intestine of neonatal pigs (Wu et al. 2000) as well as the placentae of gestating pigs (Wu et al. 2005) and sheep (Wu et al. 2008). This discovery is significant because both tissues are characterized by high rates of protein synthesis and cell proliferation. Pathways exist for the synthesis of polyamines from proline via proline oxidase and ornithine decarboxylase (Wu et al. 2005, 2008). Additionally, proline and its metabolite (P5C) are now known to regulate gene expression and cellular signaling pathways that are crucial to health and disease (Hu et al. 2008). Interestingly, proline can scavenge free radicals (Kaul et al. 2008), and this antioxidant property of proline may explain why its concentrations increase markedly in response to cellular oxidative stress (Verbruggen and Hermans 2008). Furthermore, results of recent findings suggest that proline may play a role in regulating the mammalian target of rapamycin (mTOR) activation pathway (van Meijl et al. 2010), which integrates signals from nutrients (glucose and AA), cellular energy status, growth factors, and various stress factors to affect cell growth and function (Li et al. 2009b; Liao et al. 2008). Therefore, proline acts in concert with arginine, glutamine, and leucine (activators of mTOR and regulators of polyamine production) to enhance protein synthesis in cells and tissues (e.g., the small intestine and skeletal muscle) (Wu et al. 2010a).
Table 1.
Functions of proline in cellular metabolism and physiology
| Regulation of gene expression and cell differentiation |
| mTOR activation (integrating nutrient and growth factor signaling in cells) |
| Proline signaling via pyrroline-5-carboxylate, superoxide anion (a free radical), and cellular redox reactions |
| Polyamines glutamate and protein syntheses |
| Hydroxyproline generation |
| Arginine synthesis in mammals (particularly important for milk-fed neonates) |
| Scavenging oxidants |
Although hydroxyproline has been traditionally considered to have little nutritional significance, it is now recognized as a substrate for the synthesis of glycine (an essential AA for chickens), pyruvate, and glucose (particularly important for neonates and ruminants) (Fig. 2). Hydroxyproline may also scavenge oxidants and regulate the redox state of cells (Phang et al. 2008, 2010). Furthermore, hydroxyproline may greatly impact the nutrition of birds which cannot sufficiently synthesize glycine from other AA.
Fig. 2.
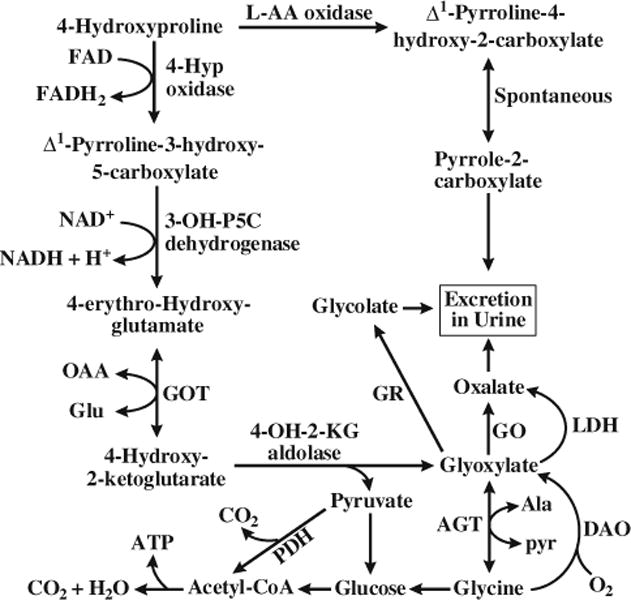
Metabolism of 4-hydroxyproline in animals. AA amino acid, AGT alanine-glyoxylate aminotransferase, Ala alanine, DAO D-amino acid oxidase, Glu glutamate, GO glycolate oxidase, GOT glutamate oxaloacetate transaminase, GR glyoxylate reductase, 4-Hyp 4-hydroxy-proline, 4-OH-2-KG 4-hydroxy-2-ketoglutarate, 3-OH-P5C Δ1-pyrro-line-3-hydroxy-5-carboxylate, LDH lactate dehydrogenase, OAA oxaloacetate, PDH pyruvate dehydrogenase, Pyr pyruvate
Developmental changes in proline and hydroxyproline in body proteins and physiological fluids
Concentrations of proline and hydroxyproline in tissue proteins increase markedly during fetal growth and development. For example, in the fetal pig, hydroxyproline increases from 0.84 to 3.64 g per 100 g protein between days 40 and 114 (term) of gestation (Wu et al. 1999). Proline and hydroxyproline account for 12% (wt/wt) of proteins in the animal body at birth and postnatally, with the ratio of proline to hydroxyproline being 2.25:1. Thus, 31% of protein-bound proline is hydroxylated after the polypeptide is synthesized. This indicates that requirements of proline for protein accretion substantially increase during both prenatal and postnatal periods (Wu et al. 2010b).
There are also marked changes in concentrations of free proline in the conceptus during pregnancy (Wu et al. 1995a, 2008). For example, between days 30 and 60 of gestation, concentrations of proline in porcine amniotic fluid increase by 141%, but those in porcine allantoic fluid decrease by 82%. Similarly, concentrations of proline in ovine amniotic fluid increased from 89 to 172 μM between days 30 and 60 of pregnancy (Kwon et al. 2003a). Interestingly, in contrast to gilts, concentrations of proline in ovine allantoic fluid increased by 172% during the same period. This reflects the differences in proline metabolism and requirements among animals or during the life cycle within the same species.
Proline metabolism
Proline synthesis
Proline synthesis from glutamine, glutamate, arginine, and ornithine in animals is cell-, tissue-, and species-specific (Wu et al. 2008). All mammals can synthesize proline from arginine via arginase (both type I and type II), ornithine aminotransferase, and P5C reductase, with the mammary tissue, small intestine (postweaning animals), liver, and kidneys being quantitatively the most active tissues (Wu et al. 2008). In mammary tissue, the major products of arginine catabolism are proline, ornithine, and urea (O’Quinn et al. 2002). Because proline oxidase activity is absent from mammary tissue, there is no degradation of arginine-derived proline in the lactating gland. This ensures maximal net production of proline from arginine by the lactating mammary gland. Because P5C synthase is also absent from mammary tissue, there is no formation of proline from glutamine or glutamate by this tissue (O’Quinn et al. 2002). Thus, arginase plays an important role in proline synthesis by lactating mammary tissue. Interestingly, the activity of P5C reductase is at least 50-fold greater than that of P5C dehydrogenase in lactating mammary tissue, thereby favoring the conversion of arginine-derived P5C into proline rather than into glutamate and glutamine (O’Quinn et al. 2002). The synthesis of proline from arginine helps to prevent an irreversible loss of arginine carbons in lactating porcine mammary tissue. These findings also provide a biochemical explanation for the observation that the output of proline in sow’s milk greatly exceeds the uptake of plasma proline by the lactating mammary gland, whereas the output of arginine in sow’s milk is much lower than the uptake of plasma arginine by the lactating mammary gland (Wu and Morris 1998). Because of extensive catabolism of arginine for proline synthesis via the arginase pathway and the lack of proline catabolism in lactating porcine mammary tissue, there is a relative enrichment of proline but a relative deficiency of arginine in milk proteins (Davis et al. 1994; Wu and Knabe 1994).
The small intestine of postweaning pigs degrades approximately 40% of arginine in the enteral diet, with proline being a major product (Wu et al. 2007a, b). Additionally, both glutamate and glutamine in the enteral diet are almost completely degraded by the small intestine, with proline being a significant product (Reeds and Burrin 2001; Watford 2008). In the postabsorptive state, one-third of glutamine in arterial blood is extracted by the pig small intestine (Wu et al. 1994). Products of glutamate and glutamine degradation in enterocytes include not only proline but also ornithine, citrulline, arginine, and alanine (Wu et al. 1995b). Studies with jejunum-cannulated young pigs demonstrated net release of proline from the small intestine of food-deprived piglets (Wu et al. 1994). De novo synthesis and the hydrolysis of small peptides in enterocytes and the intestinal lumen may be sources of this gut-derived proline. Glucocorticoids are major hormones that regulate proline synthesis from arginine and glutamine in cells and tissues (Flynn et al. 2009).
In contrast to mammals, birds have low arginase activity in tissues and, therefore, a limited ability to convert arginine into proline (Austic 1976). Therefore, proline is a nutritionally essential AA for avian species, including chickens (Graber and Baker 1971). Additionally, carnivores (e.g., cats and ferrets) lack P5C synthase in enterocytes and other cell types, and cannot convert glutamine and glutamate into proline in the body (Wu and Morris 1998). Thus, arginine is the only substrate for proline synthesis in these species. Owing to a high demand for dietary arginine for multiple synthetic processes and the lack of its endogenous synthesis, arginine is a nutritionally essential AA for carnivores. Dietary supplementation with proline may compensate for some arginine in these animals due to an inhibition of arginase by proline-derived ornithine.
Proline degradation
Except for mammary tissue, most tissues express proline oxidase activity (Wu et al. 2008). A byproduct of this mitochondrial enzyme is superoxide anion (O2−), which can be converted into H2O2 and other reactive oxygen species (Phang et al. 2008). In tissues and cells (e.g., porcine placenta and enterocytes of neonatal pigs) that do not contain arginase activity, proline is the only substrate for the synthesis of ornithine and, therefore, polyamines (putrescine, spermidine, and spermine) (Wu et al. 2000, 2005). This is of enormous importance in both nutrition and physiology because (1) polyamines are key molecules regulating DNA and protein synthesis, as well as cell proliferation, differentiation, and migration; (2) both placentae and neonatal small intestine grow very rapidly. In ruminants, placentae contain both arginase and proline oxidase, which helps to compensate for relatively low concentrations of proline in maternal blood (Kwon et al. 2003b).
Although all cells can recycle P5C into proline by P5C reductase and convert P5C into ornithine by ornithine aminotransferase, the utilization of P5C for the synthesis of citrulline is highly cell- and tissue-specific (Wu et al. 2008). Of particular note, only mammalian enterocytes are capable of synthesizing citrulline from P5C, indicating a unique role for the small intestine in proline metabolism (Wu et al. 2009). Although the mammalian liver can convert P5C into ornithine via the urea cycle, there is no net synthesis of arginine in this organ because exceedingly high arginase activity rapidly hydrolyzes arginine into ornithine and urea (Wu and Morris 1998). In liver and kidneys, P5C can be oxidized completely to CO2 via the formation of α-ketoglutarate by P5C dehydrogenase. However, in placentae and enterocytes with limited P5C dehydrogenase activity, oxidation of proline to CO2 is negligible (Chen et al. 2009; Wu et al. 2000, 2005). This prevents an irreversible loss of proline carbons and maximizes the availability of P5C for the synthesis of polyamines. Compelling evidence shows that polyamines play an important role in intestinal growth, function, and health during the neonatal period (Wu et al. 2000).
Because the entire molecule of P5C is incorporated into citrulline via ornithine aminotransferase and ornithine carbamoyltransferase in enterocytes, proline provides its nitrogen and carbon skeleton for citrulline and arginine synthesis in the small intestine which expresses these two enzymes and P5C synthase (Wu and Morris 1998). A lack of knowledge or misunderstanding of these basic biochemical reactions can lead researchers to make erroneous conclusions regarding the contribution of proline carbons to endogenous synthesis of arginine. Such errors, which have recently occurred with glutamine studies, will surely not advance the field of mammalian arginine metabolism but rather will result in much misleading confusion in literature.
The arginine–proline cycle between mother and neonate
As noted previously, arginine is actively utilized to form proline in the lactating mammary gland, resulting in a deficiency of arginine and an abundance of proline in milk protein relative to needs by neonates (Wu et al. 2004). Concentrations of free arginine in milk are also relatively low (Wu and Knabe 1994). It is possible that arginine is a major factor limiting maximal milk production by mammals (Mateo et al. 2008), but much experimental data are required to test this hypothesis. Interestingly, milk-derived proline is a major precursor for the synthesis of citrulline (the precursor of arginine) in enterocytes of postnatal pigs (Dillon et al. 1999; Wu 1997). Thus, there is an arginine–proline “cycle” between mother and neonate. Although intestinal synthesis of citrulline and arginine partially compensates for an arginine deficiency in sow’s milk, one must wonder why there is extensive catabolism of arginine by the lactating mammary gland? There are several possible answers to this intriguing question. First, the uptake of proline from maternal blood by the lactating mammary gland may be inadequate for milk protein synthesis. Thus, arginine catabolism may be necessary to provide sufficient proline for maximizing protein synthesis by the lactating porcine mammary gland. Second, through the NADPH-dependent conversion of P5C into proline, arginine may regulate the cellular redox state and pentose cycle activity. The pentose cycle functions to provide NADPH and ribose-5-phosphate for a variety of metabolic processes. For example, NADPH is required for fatty acid synthesis, whereas ribose-5-phosphate is essential for purine synthesis and cell proliferation. This notion is consistent with the finding that dietary arginine supplementation to sows increases production of milk lipids (Kirchgessner et al. 1991) and piglet growth (Mateo et al. 2008). Third, arginine is the common substrate for both arginase and nitric oxide synthase, and thus, arginase may play an important role in regulating nitric oxide and polyamine synthesis by the lactating mammary gland. Although nitric oxide is quantitatively a minor product of arginine catabolism, it may play a crucial role in the regulation of mammary gland blood flow and thus the uptake of nutrients from blood by the lactating mammary gland (Kim and Wu 2009). Likewise, polyamines produced by mammary tissue regulate lactogenesis (Oka and Perry 1974) and greatly contribute to their abundance in sow’s milk (Motyl et al. 1995). There is little arginase activity in the small intestine of neonates, and yet, polyamines are essential for cell proliferation and differentiation (Wu 1998). Thus, milk-borne polyamines may be of nutritional importance for growth and development of the neonatal intestine. Finally, because the neonatal pig has a low capacity to synthesize proline (an essential AA for young pigs) (Ball et al. 1986), arginine catabolism via the arginase pathway in the lactating mammary gland will ensure an adequate supply of proline to suckling piglets to support tissue protein synthesis and extracellular matrix formation. Thus, through the arginine–proline cycle between mother and neonate, the mother sustains a capacity for milk synthesis and provides both proline and polyamines to her offspring, whereas the neonate can synthesize arginine and have both exogenous and endogenous polyamines required for protein synthesis and cell growth (Wu et al. 2009).
Proline and hydroxyproline nutrition
Composition of proline and hydroxyproline in postnatal animals and feedstuffs
The content of proline and hydroxyproline is 12 and 5.3 g/kg wet tissue in young pigs, respectively (Wu et al. 2010b). Proline represents 12% of proteins in the milk of mammals, including sows and cows (Davis et al. 1994; Wu et al. 1994). Indeed, proline is the most abundant AA in milk proteins (e.g., 30 g/kg dry matter in sow’s milk), followed by glutamate, leucine, and glutamine (Haynes et al. 2009; Kim and Wu 2004). The abundance of proline in milk protein is consistent with a high requirement of proline for neonatal growth and development (Wu et al. 2010b). Notably, the content of proline is even higher in meat and bone meal, and poultry by-product meal than in milk (Table 2). Indeed, proline and hydroxyproline are most abundant in meat and bone meal, poultry by-product meal, and salmon proteins. Therefore, these animal products are excellent sources of proline and hydroxyproline for post-weaning animals and post-hatching birds. In general, animal proteins contain 3–6-fold greater amounts of proline than plant proteins per gram of feedstuff. Among plant proteins, proline is the second most abundant AA in barley, wheat, and wheat middlings, and the third most abundant AA in corn (grain) and sorghum (Table 2).
Table 2.
Composition of dry matter (DM), crude protein (CP), and amino acids in feedstuffs (%, as-fed basis)
| Nutrient | Barleya | Corn, graina | Cotton seed mealb | Fish mealc | Rice protein concentrated | Salmon protein hydrolysated | Soy bean meale | Spray dried plasma proteind | Sorghuma | Wheat, graina | Wheat middlingsa |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | 91.4 | 90.8 | 93.9 | 91.9 | 92.7 | 91.4 | 87.7 | 90.9 | 90.8 | 89.9 | 91.6 |
| CP | 13.9 | 9.60 | 43 | 60.1 | 67.5 | 92.7 | 44.0 | 78.0 | 10.3 | 12.4 | 16.9 |
| EAA | |||||||||||
| Arg | 0.70 | 0.54 | 4.35 | 3.78 | 5.26 | 5.47 | 3.23 | 4.57 | 0.42 | 0.61 | 1.15 |
| His | 0.31 | 0.29 | 1.12 | 1.23 | 1.65 | 1.59 | 1.14 | 2.61 | 0.24 | 0.29 | 0.46 |
| Ile | 0.48 | 0.35 | 1.11 | 2.51 | 2.91 | 2.16 | 2.07 | 2.90 | 0.40 | 0.40 | 0.52 |
| Leu | 0.95 | 1.14 | 2.14 | 4.41 | 5.31 | 3.97 | 3.41 | 7.51 | 1.36 | 0.81 | 1.04 |
| Lys | 0.44 | 0.32 | 1.62 | 4.46 | 2.21 | 5.05 | 2.80 | 6.90 | 0.24 | 0.35 | 0.64 |
| Met | 0.16 | 0.21 | 0.66 | 1.69 | 1.77 | 1.89 | 0.59 | 0.69 | 0.20 | 0.20 | 0.23 |
| Phe | 0.78 | 0.47 | 2.07 | 2.38 | 3.52 | 2.10 | 2.29 | 4.38 | 0.54 | 0.58 | 0.68 |
| Thr | 0.45 | 0.36 | 1.17 | 2.54 | 2.12 | 2.62 | 1.78 | 4.33 | 0.29 | 0.35 | 0.53 |
| Trp | 0.11 | 0.05 | 0.43 | 0.50 | 0.81 | 0.48 | 0.50 | 1.38 | 0.07 | 0.11 | 0.16 |
| Val | 0.67 | 0.48 | 1.62 | 2.96 | 4.13 | 2.78 | 2.18 | 5.20 | 0.54 | 0.54 | 0.78 |
| NEAA | |||||||||||
| Ala | 0.51 | 0.72 | 1.34 | 4.62 | 3.47 | 5.93 | 1.97 | 4.18 | 0.98 | 0.44 | 0.77 |
| Aspf | 0.77 | 0.67 | 3.37 | 6.70 | 5.39 | 6.18 | 5.15 | 7.35 | 0.70 | 0.60 | 1.09 |
| Cys | 0.22 | 0.22 | 0.70 | 0.62 | 1.45 | 0.42 | 0.69 | 2.73 | 0.20 | 0.28 | 0.34 |
| Glug | 3.87 | 1.85 | 7.76 | 9.15 | 10.9 | 10.0 | 8.26 | 11.5 | 2.20 | 3.78 | 3.66 |
| Gly | 0.55 | 0.41 | 1.39 | 6.04 | 2.77 | 12.0 | 1.95 | 2.76 | 0.36 | 0.54 | 0.90 |
| Pro | 1.79 | 0.82 | 1.33 | 3.91 | 2.94 | 6.17 | 2.88 | 4.44 | 0.87 | 1.28 | 1.22 |
| Ser | 0.58 | 0.46 | 1.56 | 2.57 | 2.36 | 2.60 | 2.26 | 3.98 | 0.46 | 0.57 | 0.73 |
| Tyr | 0.42 | 0.39 | 1.14 | 2.19 | 3.32 | 1.32 | 1.70 | 4.04 | 0.44 | 0.38 | 0.49 |
EAA nutritionally essential amino acids, NEAA nutritionally nonessential amino acids
Glandless cottonseed meal (LaRue et al. 1985)
Knabe et al. (1989) for essential amino acids; values for nonessential amino acids were determined by authors of the present article using HPLC (Li et al. 2009c)
Hansen et al. (1993). Proline was determined using HPLC (Wu 1993)
Aspartate plus asparagine
Glutamate plus glutamine
Digestion and absorption of proline
The circulating proline in plasma is derived from the diet, intracellular protein degradation, extracellular protein degradation, and endogenous synthesis (Wu et al. 2008). Peptide-bound proline is hydrolyzed by proteases in the luminal fluids of the stomach (pepsin) and small intestine (trypsin, chymotrypsin, elastase, carboxypeptidases A and B, and aminopeptidase) to yield dipeptides or tripeptides (Wu and Self 2005) (Fig. 3). The mucosa of the small intestine secretes proline peptidase (prolidase) that specifically hydrolyzes proline-containing dipeptides (Sjostrom et al. 1973). Prolidase also hydrolyzes hydroxy-proline-containing dipeptides. These small peptides (containing proline or hydroxyproline) in the lumen of the small intestine can be directly transported into enterocytes (absorptive epithelial cells) by H+ gradient-driven peptide transporters, whereas free proline is taken up into cells primarily by the Na+-dependent system IMINO transporter and system NBB transporter (present on the brush border for the transport of neutral AA), as well as the Na+-independent system L transporter (Brandsch 2006). The entry of proline into enterocytes represents the first step for its utilization as an essential substrate for synthetic pathways (including synthesis of proteins and ornithine). Because proline is extensively degraded by enterocytes and luminal microorganisms (Bergen and Wu 2009), only ~60% of dietary proline enters the portal circulation (Wu et al. 2008).
Fig. 3.
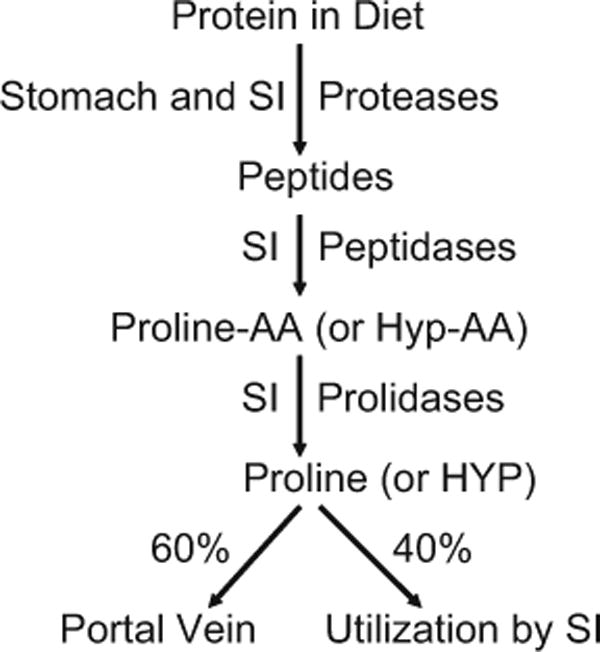
Digestion of dietary protein-bound proline or hydroxyproline (Hyp) in the gastrointestinal tract of the small intestine. Proteases and peptidases in the lumen of the small intestine hydrolyze proteins and large peptides, respectively, to eventually form proline- or hydroxyproline-containing dipeptides. These dipeptides are hydrolyzed by specific proline peptidases (prolidases) to yield free proline or hydroxyproline. Approximately 40% of luminal proline is catabolized by the mammalian small intestine, and the responsible cell types include enterocytes (Wu 1997) and bacteria (Dai et al. 2010). SI small intestine
Proline as a nutritionally essential AA for poultry, young mammals, and fish
There are remarkable differences in proline metabolism among species. Proline is a nutritionally essential AA for poultry (Baker 2009; Graber et al. 1970), young mammals (including piglets) (Ball et al. 1986), as well as wounded animals and humans (Barbul 2008) because of inadequate endogenous synthesis via the arginase and P5C synthase pathways relative to needs. Additionally, endogenous synthesis of proline from glutamate cannot meet the requirements for proline by many species of fish (Li et al. 2009a). Therefore, proline is now considered as a conditionally essential AA for fish in both early life and adult stages (Li et al. 2009a; Zhang et al. 2006). Interestingly, dietary supplementation with 0.07, 0.14, and 0.28% hydroxyproline (a metabolite of proline) to a plant protein-based diet enhanced weight gains of salmon (Aksnes et al. 2006). It is possible that hydroxyproline may spare proline by reducing proline catabolism or stimulate tissue protein synthesis through multiple signaling pathways.
The essential requirement for proline as a nutrient for poultry, young mammals, and wounded subjects is supported by several lines of experimental evidence. First, supplementing 0.0, 0.2, 0.4, and 0.8% proline to a chemically purified diet containing 1% arginine and 10% glutamate dose-dependently increased daily weight gains (from 11.88 to 13.38 g/day) of young chickens without affecting their feed intake (an average of 114 g/chick) (Fig. 4). Second, supplementing 0, 0.35, 0.7, 1.05, 1.4, and 2.1% proline to a proline-free chemically defined diet containing 0.48% arginine and 2% glutamate dose-dependently improved daily weight gains (from 342 to 411 g/day) (Fig. 5) and feed efficiency (gram feed/gram gain, from 1.66 to 1.35) of young pigs, while reducing concentrations of urea in plasma by one-half (Kirchgessner et al. 1995). Notably, increasing the dietary content of proline from 0.0 and 2.1% enhanced daily nitrogen retention from 1.27 to 1.53 g/kg bodyweight0.75. Similarly, we found that supplementing 1% proline to a corn- and soybean meal-based diet enhanced villus height, small-intestinal weight, and growth performance in weanling pigs (Table 3). Third, dietary proline is necessary for promoting tissue repair and nitrogen balance in both animals and humans with wounds and burns (Barbul 2008). These findings have important implications for proline as a dietary essential nutrient in humans and animals under certain physiological and pathological conditions.
Fig. 4.
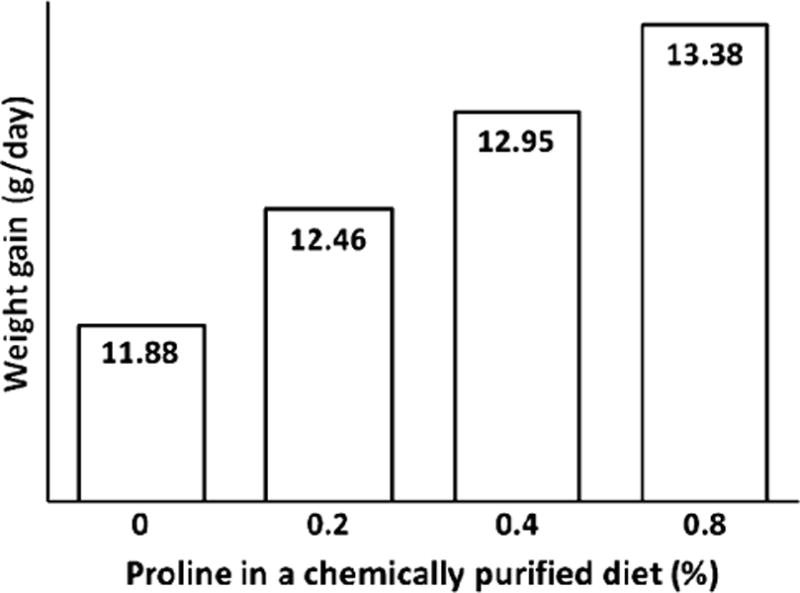
Effect of supplementing proline to a chemically purified diet on growth performance of young chickens. Data are taken from Graber et al. (1970). Chicks (the cross of New Hampshire males to Columbian females) were fed a purified diet supplemented with 0, 0.2, 0.4, and 0.8% L-proline for 6 days. The basal diet contained the following amino acids (% of diet): L-arginine–HCl, 1.21; L-histidine–HCl·H2O, 0.41; L-lysine–HCl, 1.19; L-tyrosine, 0.45; L-tryptophan, 0.15; L-phenylalanine, 0.50; DL-methionine, 0.35; L-cystine, 0.35; L-threonine, 0.65; L-leucine, 1.20; L-isoleucine, 0.60; L-valine, 0.82; glycine, 1.20; and l-glutamate, 10.00. Supplementing L-proline to the basal diet resulted in a linear increase (P < 0.01) in weight gain
Fig. 5.
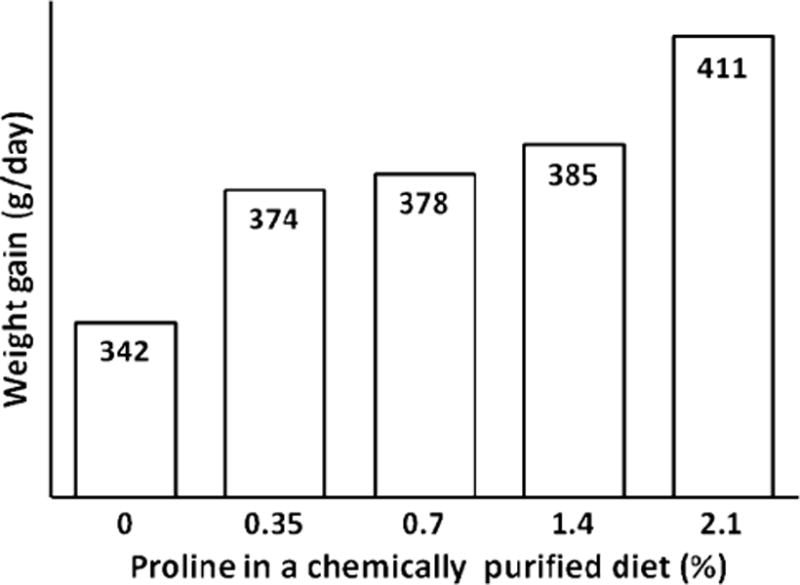
Effect of supplementing proline to a chemically purified diet on growth performance of young pigs. Data are taken from Kirchgessner et al. (1995). Young pigs were fed a purified diet supplemented with 0, 0.35, 0.7, 1.4, and 2.1% L-proline. The basal diet contained the following amino acids (% of diet): L-lysine–HCl, 1.82; DL-methionine, 0.48; L-cystine, 0.44; L-threonine, 1.06; L-tryptophan, 0.26; L-isoleucine, 0.86; L-leucine, 1.58; L-phenylalanine, 0.86; L-tyrosine, 0.86%; L-histidine, 0.56; L-valine, 1.08; L-alanine, 1.67; L-arginine, 0.48; L-aspartate, 3.61; L-glutamate, 2.02; glycine, 1.54; and L-serine, 2.24. Supplementing L-proline to the basal diet resulted in a linear increase (P < 0.01) in weight gain
Table 3.
Effects of proline supplementation on growth performance of weanling piglets
| Treatment | Dry matter intake Days 21–35 [g/(kg bodyweight × day)] |
Plasma proline (mM) | Bodyweight
|
Bodyweight gain Days 21–35 (g/d) |
Jejunal villus heighta (μm) | Small intestinal weight (g) | |
|---|---|---|---|---|---|---|---|
| Day 21 (kg) |
Day 35 (kg) |
||||||
| Alanine | 32.7 ± 1.9 | 0.32 ± 0.02 | 5.82 ± 0.05 | 8.04 ± 0.08 | 159 ± 3.8 | 274 ± 6.1 | 291 ± 4.2 |
| Proline | 33.1 ± 2.1 | 0.41 ± 0.03† | 5.85 ± 0.06 | 8.39 ± 0.09† | 181 ± 4.5† | 319 ± 7.7† | 323 ± 5.0† |
Values are means ± SEM, n = 18 for feed intake, jejunal villus height, and small-intestinal weight; n = 36 for other parameters. Pigs were weaned at 21 days of age to a corn- and soybean meal-based diet (Wu et al. 1996) supplemented with 0.775% L-alanine (isonitrogenous control) or 1% L-proline. There were two pigs per pen. Amino acids were added to the basal diets at the expense of cornstarch. On day 35 of age, blood samples were obtained from jugular vein 1 h after feeding, and plasma was obtained for proline analysis (Wu 1993)
P < 0.05 versus the Alanine group
A different study was conducted to determine jejunal villus height on day 7 postweaning (Wu et al. 1996) as described above, except that pigs were euthanized on this day to obtain the jejunum. The depth of lamina propria was 240 ± 5 and 253 ± 6 μm, respectively, for the alanine and proline groups
It should be borne in mind that effects of dietary supplementation with any AA depend on its supplemental dose and the content of other AA in the diet (Ferreira et al. 2010; Stipanuk et al. 2009; Tan et al. 2009a, b). For example, when the basal diet contained no glutamine, proline supplementation had no effect on piglet growth (Chung and Baker 1993). Thus, optimal nutritional conditions for proline supplementation must be identified to realize its potential for improving growth and reproductive performance of animals. In this regard, supplemental proline may play an important role in fetal survival, growth, and development (Wu et al. 2008). Because of its regulatory roles in metabolism and physiology, proline can be considered as a functional AA in nutrition (Li et al. 2007; Wu et al. 2007b). We must recognize that the traditional view that proline is a nonessential AA for the adult mammal is solely based on nitrogen balance studies. Whether proline is a nutritionally essential AA for animals should be reevaluated through careful design of experiments and use of meaningful criteria (including functional needs such as maximal growth performance, fertility, embryonic/fetal survival and growth, and immunity) (Wu 2009).
Conclusion and perspectives
Proline and hydroxyproline are unique AA for maintaining cell structure and function. There are remarkable species differences in proline metabolism and requirements among vertebrate animals during the life cycle. However, emerging evidence consistently points to proline as an important regulator of cell metabolism and physiology. Therefore, proline can be considered as a functional AA for humans, livestock species, poultry, and fish. This promising role of proline is expected to be translated into enhanced efficiency of nutrient utilization and improved health in organisms. Although much is known about proline needs for wound healing in humans (Barbul 2008), there is a paucity of information about roles for proline in growth and development of the fetus and neonate, as well as lactation performance of mothers. Indeed, there are few experimental data for the essentiality of requirements of dietary proline by mammals. Given the significant problem of intrauterine growth retardation (IUGR) in both humans and livestock species (Wu et al. 2004, 2006), it is surprising that there are no data in literature regarding (1) impacts of IUGR on postnatal proline metabolism and dietary requirements in offspring; (2) a potential role for alterations of IUGR-associated proline metabolism in the pathogenesis of chronic disease (e.g., hypertension, cancer, obesity, and diabetes) in adults; or (3) effects of supplementing proline to IUGR offspring on their growth and health. These questions can be addressed using animal models, such as pigs (Li et al. 2010; Rhoads and Wu 2009; Suryawan et al. 2009), sheep (Reynolds et al. 2006; Satterfield et al. 2010; Wang et al. 2010), and rodents (Blachier et al. 2010; Zeng et al. 2008). Additionally, multidisciplinary research is necessary to identify optimal conditions for dietary supplementation with proline to mammals (particularly neonates with IUGR), birds, and fish to improve their health, reproductive performance, growth, and development. Such endeavors can be greatly facilitated by using recent advanced tools, such as genomics (Jobgen et al. 2009; Palii et al. 2009), transcripteomics (Mutch et al. 2005), proteomics (Wang et al. 2009b), metabolomics (He et al. 2009), and bioinformatics (Fu et al. 2010). Collectively, elucidation of the complex mechanisms responsible for the actions of proline on cells is expected to expand its applications to solve major practical problems in livestock production and human medicine.
Acknowledgments
This work was supported, in part, by grants from National Institutes of Health (1R21 HD049449), National Research Initiative Competitive Grants (2008-35206-18764, 2008-35203-19120, and 2009-35206-05211) from the USDA Cooperative State Research, Education, Texas AgriLife Research (H-8200), North Carolina Agricultural Experiment Station, and the Thousand-People-Talent program at China Agricultural University. We thank graduate students, postdoctoral fellows, technicians, and many collaborators for their important contributions to the work described in this article.
Abbreviations
- AA
Amino acid
- IUGR
Intrauterine growth retardation
- mTOR
Mammalian target of rapamycin
- NRC
National Research Council
- P5C
Pyrroline-5-carboxylate
Contributor Information
Guoyao Wu, Email: g-wu@tamu.edu, Department of Animal Science and Faculty of Nutrition, Texas A&M University, College Station, TX 77843, USA; State Key Laboratory of Animal Nutrition, China Agricultural University, Beijing 100193, China.
Fuller W. Bazer, Department of Animal Science and Faculty of Nutrition, Texas A&M University, College Station, TX 77843, USA
Robert C. Burghardt, Department of Veterinary Integrative Biosciences, Texas A&M University, College Station, TX 77843, USA
Gregory A. Johnson, Department of Veterinary Integrative Biosciences, Texas A&M University, College Station, TX 77843, USA
Sung Woo Kim, Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA.
Darrell A. Knabe, Department of Animal Science and Faculty of Nutrition, Texas A&M University, College Station, TX 77843, USA
Peng Li, National Renderers Association, Alexandria, VA 22314, USA.
Xilong Li, Department of Animal Science and Faculty of Nutrition, Texas A&M University, College Station, TX 77843, USA.
Jason R. McKnight, Department of Animal Science and Faculty of Nutrition, Texas A&M University, College Station, TX 77843, USA
M. Carey Satterfield, Department of Animal Science and Faculty of Nutrition, Texas A&M University, College Station, TX 77843, USA.
Thomas E. Spencer, Department of Animal Science and Faculty of Nutrition, Texas A&M University, College Station, TX 77843, USA
References
- Aksnes A, Mundheim H, Toppe J, et al. The effect of dietary hydroxyproline supplementation on salmon (Salmo salar L.) fed high plant protein diets. Aquaculture. 2006;275:242–249. [Google Scholar]
- Austic RE. Nutritional and metabolic interrelationships of arginine, glutamic acid and proline in the chicken. Fed Proc. 1976;35:1914–1916. [PubMed] [Google Scholar]
- Baker DH. Advances in protein-amino acid nutrition of poultry. Amino Acids. 2009;37:29–41. doi: 10.1007/s00726-008-0198-3. [DOI] [PubMed] [Google Scholar]
- Ball RO, Atkinson JL, Bayley HS. Proline as an essential amino acid for the young pig. Br J Nutr. 1986;55:659–668. doi: 10.1079/bjn19860072. [DOI] [PubMed] [Google Scholar]
- Barbul A. Proline precursors to sustain mammalian collagen synthesis. J Nutr. 2008;138:2021S–2024S. doi: 10.1093/jn/138.10.2021S. [DOI] [PubMed] [Google Scholar]
- Bergen WG, Wu G. Intestinal nitrogen recycling and utilization in health and disease. J Nutr. 2009;139:821–825. doi: 10.3945/jn.109.104497. [DOI] [PubMed] [Google Scholar]
- Blachier F, Lancha AH, Jr, Boutry C, et al. Alimentary proteins, amino acids and cholesterolemia. Amino Acids. 2010;38:15–22. doi: 10.1007/s00726-009-0239-6. [DOI] [PubMed] [Google Scholar]
- Brandsch M. Transport of L-proline, L-proline-containing peptides and related drugs at mammalian epithelial cell membranes. Amino Acids. 2006;31:119–136. doi: 10.1007/s00726-006-0307-0. [DOI] [PubMed] [Google Scholar]
- Chandel NS. Mitochondrial regulation of oxygen sensing. Adv Exp Med Biol. 2010;661:339–354. doi: 10.1007/978-1-60761-500-2_22. [DOI] [PubMed] [Google Scholar]
- Chen LX, Li P, Wang JJ, et al. Catabolism of nutritionally essential amino acids in developing porcine enterocytes. Amino Acids. 2009;37:143–152. doi: 10.1007/s00726-009-0268-1. [DOI] [PubMed] [Google Scholar]
- Chung TK, Baker DH. A note on the dispensability of proline for weanling pigs. Anim Prod. 1993;56:407–408. [Google Scholar]
- Dai ZL, Zhang J, Wu G, et al. Utilization of amino acids by bacteria from the pig small intestine. Amino Acids. 2010 doi: 10.1007/s00726-010-0556-9. [DOI] [PubMed] [Google Scholar]
- Davis TA, Nguyen HV, Garciaa-Bravo R, et al. Amino acid composition of human milk is not unique. J Nutr. 1994;124:1126–1132. doi: 10.1093/jn/124.7.1126. [DOI] [PubMed] [Google Scholar]
- Dillon EL, Knabe DA, Wu G. Lactate inhibits citrulline and arginine synthesis from proline in pig enterocytes. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1079–G1086. doi: 10.1152/ajpgi.1999.276.5.G1079. [DOI] [PubMed] [Google Scholar]
- Elango R, Ball RO, Pencharz PB. Amino acid requirements in humans: with a special emphasis on the metabolic availability of amino acids. Amino Acids. 2009;37:19–27. doi: 10.1007/s00726-009-0234-y. [DOI] [PubMed] [Google Scholar]
- Ferreira AG, Lima DD, Delwing D, et al. Proline impairs energy metabolism in cerebral cortex of young rats. Metab Brain Dis. 2010;25:161–168. doi: 10.1007/s11011-010-9193-y. [DOI] [PubMed] [Google Scholar]
- Flynn NE, Bird JG, Guthrie AS. Glucocorticoid regulation of amino acid and polyamine metabolism in the small intestine. Amino Acids. 2009;37:123–129. doi: 10.1007/s00726-008-0206-7. [DOI] [PubMed] [Google Scholar]
- Fu WJ, Stromberg AJ, Viele K, et al. Statistics and bioinformatics in nutritional sciences: analysis of complex data in the era of systems biology. J Nutr Biochem. 2010;21:561–572. doi: 10.1016/j.jnutbio.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorres KL, Raines RT. Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol. 2010;45:106–124. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlob RO, DeRouchey JM, Tokach MD, et al. Amino acid and energy digestibility of protein sources for growing pigs. J Anim Sci. 2006;84:1396–1402. doi: 10.2527/2006.8461396x. [DOI] [PubMed] [Google Scholar]
- Graber G, Baker DH. Ornithine utilization by the chick. Proc Soc Exp Biol Med. 1971;138:585–588. doi: 10.3181/00379727-138-35946. [DOI] [PubMed] [Google Scholar]
- Graber G, Allen NK, Scott HM. Proline essentiality and weight gain. Poul Sci. 1970;49:692–697. doi: 10.3382/ps.0490692. [DOI] [PubMed] [Google Scholar]
- Hansen JA, Knabe DA, Burgoon KG. Amino acid supplementation of low-protein sorghum-soybean meal diets for 20- to 50-kilogram swine. J Anim Sci. 1993;71:442–451. doi: 10.2527/1993.712442x. [DOI] [PubMed] [Google Scholar]
- Haynes TE, Li P, Li XL, et al. L-Glutamine or L-alanyl-L-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids. 2009;37:131–142. doi: 10.1007/s00726-009-0243-x. [DOI] [PubMed] [Google Scholar]
- He QH, Kong XF, Wu GY, et al. Metabolomic analysis of the response of growing pigs to dietary l-arginine supplementation. Amino Acids. 2009;37:199–208. doi: 10.1007/s00726-008-0192-9. [DOI] [PubMed] [Google Scholar]
- Hu CA, Khalil S, Zhaorigetu S, et al. Human Δ1-pyrroline-5-carboxylate synthase: function and regulation. Amino Acids. 2008;35:665–672. doi: 10.1007/s00726-008-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobgen W, Fu WJ, Gao H, et al. High fat feeding and dietary L-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino Acids. 2009;37:187–198. doi: 10.1007/s00726-009-0246-7. [DOI] [PubMed] [Google Scholar]
- Kaul S, Sharma SS, Mehta IK. Free radical scavenging potential of L-proline: evidence from in vitro assays. Amino Acids. 2008;34:315–320. doi: 10.1007/s00726-006-0407-x. [DOI] [PubMed] [Google Scholar]
- Kim SW, Wu G. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr. 2004;134:625–630. doi: 10.1093/jn/134.3.625. [DOI] [PubMed] [Google Scholar]
- Kim SW, Wu G. Regulatory role for amino acids in mammary gland growth and milk synthesis. Amino Acids. 2009;37:89–95. doi: 10.1007/s00726-008-0151-5. [DOI] [PubMed] [Google Scholar]
- Kirchgessner VM, Rader G, Roth-Maier DA. Influence of an oral arginine supplementation on lactation performance of sows. J Anim Physiol Anim Nutr. 1991;66:38–44. [Google Scholar]
- Kirchgessner M, Fickler J, Roth FX. Effect of dietary proline supply on N-balance of piglets. 3. Communication on the importance of nonessential amino acids for protein retention. J Anim Physiol Anim Nutr. 1995;73:57–65. [Google Scholar]
- Knabe DA, LaRue DC, Gregg EJ, et al. Apparent digestibility of nitrogen and amino acids in protein feedstuffs by growing pigs. J Anim Sci. 1989;67:441–458. doi: 10.2527/jas1989.672441x. [DOI] [PubMed] [Google Scholar]
- Krane SM. The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of colla-gens. Amino Acids. 2008;35:703–710. doi: 10.1007/s00726-008-0073-2. [DOI] [PubMed] [Google Scholar]
- Kwon H, Spencer TE, Bazer FW, et al. Developmental changes of amino acids in ovine fetal fluids. Biol Reprod. 2003a;68:1813–1820. doi: 10.1095/biolreprod.102.012971. [DOI] [PubMed] [Google Scholar]
- Kwon H, Wu G, Bazer FW, et al. Developmental changes in polyamine levels and synthesis in the ovine conceptus. Biol Reprod. 2003b;69:1626–1634. doi: 10.1095/biolreprod.103.019067. [DOI] [PubMed] [Google Scholar]
- LaRue DC, Knabe DA, Tanskley TD., Jr Commercially processed glandless cottonseed meal for starter, grower and finisher swine. J Anim Sci. 1985;60:495–502. [Google Scholar]
- Li P, Yin YL, Li DF, et al. Amino acids and immune function. Br J Nutr. 2007;98:237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- Li P, Mai KS, Trushenski J, et al. New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids. 2009a;37:43–53. doi: 10.1007/s00726-008-0171-1. [DOI] [PubMed] [Google Scholar]
- Li XL, Bazer FW, Gao HJ, et al. Amino acids and gaseous signaling. Amino Acids. 2009b;37:65–78. doi: 10.1007/s00726-009-0264-5. [DOI] [PubMed] [Google Scholar]
- Li P, Kim SW, Li XL, et al. Dietary supplementation with cholesterol and docosahexaenoic acid affects concentrations of amino acids in tissues of young pigs. Amino Acids. 2009c;37:709–716. doi: 10.1007/s00726-008-0196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL, Bazer FW, Johnson GA, et al. Dietary supplementation with 0.8% L-arginine between days 0 and 25 of gestation reduces litter size in gilts. J Nutr. 2010;140:1111–1116. doi: 10.3945/jn.110.121350. [DOI] [PubMed] [Google Scholar]
- Liao XH, Majithia A, Huang XL, et al. Growth control via TOR kinase signaling, an intracellular sensor of amino acids and energy availability, with crosstalk potential to proline metabolism. Amino Acids. 2008;35:761–770. doi: 10.1007/s00726-008-0100-3. [DOI] [PubMed] [Google Scholar]
- Lin FD, Knabe DA, Tanksley TD., Jr Apparent digestibility of amino acids, gross energy and starch in corn, sorghum, wheat, barley, oat groats and wheat middlings for growing pigs. J Anim Sci. 1987;64:1655–1663. doi: 10.2527/jas1987.6461655x. [DOI] [PubMed] [Google Scholar]
- Mateo RD, Wu G, Moon HK, et al. Effects of dietary arginine supplementation during gestation and lactation on the performance of lactating primiparous sows and nursing piglets. J Anim Sci. 2008;86:827–835. doi: 10.2527/jas.2007-0371. [DOI] [PubMed] [Google Scholar]
- Motyl T, Ploszaj T, Wojtasik A, et al. Polyamines in cow’s and sow’s milk. Comp Biochem Physiol B. 1995;111:427–433. doi: 10.1016/0305-0491(95)00010-6. [DOI] [PubMed] [Google Scholar]
- Mutch DM, Wahli W, Williamson G. Nutrigenomics and nutrigenetics: the emerging faces of nutrition. FASEB J. 2005;19:1602–1616. doi: 10.1096/fj.05-3911rev. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) Nutrient requirements of swine. 10. National Academy Press; Washington, DC: 1998. [Google Scholar]
- O’Quinn PR, Knabe DA, Wu G. Arginine catabolism in lactating porcine mammary tissue. J Anim Sci. 2002;80:467–474. doi: 10.2527/2002.802467x. [DOI] [PubMed] [Google Scholar]
- Oka T, Perry JW. Arginase affects lactogenesis through its influence on the biosynthesis of spermidine. Nature. 1974;250:660–661. doi: 10.1038/250660a0. [DOI] [PubMed] [Google Scholar]
- Palii SS, Kays CE, Deval C, et al. Specificity of amino acid regulated gene expression: analysis of gene subjected to either complete or single amino acid deprivation. Amino Acids. 2009;37:79–88. doi: 10.1007/s00726-008-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Arellano I, Carmona-Alvarez F, Martínez AI, et al. Pyrroline-5-carboxylate synthase and proline biosynthesis: from osmotolerance to rare metabolic disease. Protein Sci. 2010;19:372–382. doi: 10.1002/pro.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phang JM, Donald SP, Pandhare J, et al. The metabolism of proline, as a stress substrate, modulates carcinogenic pathways. Amino Acids. 2008;35:681–690. doi: 10.1007/s00726-008-0063-4. [DOI] [PubMed] [Google Scholar]
- Phang JM, Liu W, Zabirnyk O. Proline metabolism and microenvironmental stress. Annu Rev Nutr. 2010;30:441–463. doi: 10.1146/annurev.nutr.012809.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistollato F, Persano L, Rampazzo E, et al. L-Proline as a modulator of ectodermal differentiation in ES cells. Am J Physiol Cell Physiol. 2010;298:C979–C981. doi: 10.1152/ajpcell.00072.2010. [DOI] [PubMed] [Google Scholar]
- Reeds PJ, Burrin DG. Glutamine and the bowel. J Nutr. 2001;131:2505S–2508S. doi: 10.1093/jn/131.9.2505S. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Caton JS, Redmer DA, et al. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572:51–58. doi: 10.1113/jphysiol.2005.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads JM, Wu G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids. 2009;37:111–122. doi: 10.1007/s00726-008-0225-4. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Bazer FW, Spencer TE, Wu G. Sildenafil citrate treatment enhances amino acid availability in the conceptus and fetal growth in an ovine model of intrauterine growth restriction. J Nutr. 2010;140:251–258. doi: 10.3945/jn.109.114678. [DOI] [PubMed] [Google Scholar]
- Sjostrom H, Noren O, Josefsson L. Purification and specificity of pig intestinal prolidase. Biochim Biophys Acta. 1973;327:457–470. doi: 10.1016/0005-2744(73)90429-4. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Zhu W, Johnson WH, Jr, et al. The structure of the proline utilization a proline dehydrogenase domain inactivated by N-propargylglycine provides insight into conformational changes induced by substrate binding and flavin reduction. Biochemistry. 2010;49:560–569. doi: 10.1021/bi901717s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanuk MH, Ueki I, Dominy JE, et al. Cysteine dioxygenase: a robust system for regulation of cellular cysteine levels. Amino Acids. 2009;37:55–63. doi: 10.1007/s00726-008-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawan A, O’Connor PMJ, Bush JA, et al. Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids. 2009;37:97–104. doi: 10.1007/s00726-008-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BE, Li XG, Kong XF, et al. Dietary L-arginine supplementation enhances the immune status in early-weaned piglets. Amino Acids. 2009a;37:323–331. doi: 10.1007/s00726-008-0155-1. [DOI] [PubMed] [Google Scholar]
- Tan BE, Yin YL, Liu ZQ, et al. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids. 2009b;37:169–175. doi: 10.1007/s00726-008-0148-0. [DOI] [PubMed] [Google Scholar]
- van Meijl LE, Popeijus HE, Mensink RP. Amino acids stimulate Akt phosphorylation, and reduce IL-8 production and NF-kappaB activity in HepG2 liver cells. Mol Nutr Food Res. 2010 doi: 10.1002/mnfr.200900438. [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- Wang W, Qiao S, Li D. Amino acids and gut function. Amino Acids. 2009a;37:105–110. doi: 10.1007/s00726-008-0152-4. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Ou DY, Yin JD, et al. Proteomic analysis reveals altered expression of proteins related to glutathione metabolism and apoptosis in the small intestine of zinc oxide-supplemented piglets. Amino Acids. 2009b;37:209–218. doi: 10.1007/s00726-009-0242-y. [DOI] [PubMed] [Google Scholar]
- Wang J, Ma H, Tong C, et al. Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J. 2010;24:2066–2076. doi: 10.1096/fj.09-142315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford M. Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J Nutr. 2008;138:2003S–2007S. doi: 10.1093/jn/138.10.2003S. [DOI] [PubMed] [Google Scholar]
- Wenger RH, Hoogewijs D. Regulated oxygen sensing by protein hydroxylation in renal erythropoietin-producing cells. Am J Physiol Renal Physiol. 2010;298:F1287–F1296. doi: 10.1152/ajprenal.00736.2009. [DOI] [PubMed] [Google Scholar]
- Wu G. Determination of proline by reversed-phase high performance liquid chromatography with automated pre-column o-phthaldialdehyde derivatization. J Chromatogr. 1993;641:168–175. doi: 10.1016/s0021-9673(01)90673-9. [DOI] [PubMed] [Google Scholar]
- Wu G. Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1382–G1390. doi: 10.1152/ajpgi.1997.272.6.G1382. [DOI] [PubMed] [Google Scholar]
- Wu G. Intestinal mucosal amino acid catabolism. J Nutr. 1998;128:1249–1252. doi: 10.1093/jn/128.8.1249. [DOI] [PubMed] [Google Scholar]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- Wu G, Knabe DA. Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr. 1994;124:415–424. doi: 10.1093/jn/124.3.415. [DOI] [PubMed] [Google Scholar]
- Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Self JT. Proteins. In: Pond WG, Bell AW, editors. Encyclopedia of animal science. Marcel Dekker; New York: 2005. pp. 757–759. [Google Scholar]
- Wu G, Borbolla AG, Knabe DA. The uptake of glutamine and release of arginine, citrulline and proline by the small intestine of developing pigs. J Nutr. 1994;124:2437–2444. doi: 10.1093/jn/124.12.437. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Tuo W. Developmental changes of free amino acid concentrations in fetal fluids of pigs. J Nutr. 1995a;125:2859–2868. doi: 10.1093/jn/125.11.2859. [DOI] [PubMed] [Google Scholar]
- Wu G, Knabe DA, Yan W, et al. Glutamine and glucose metabolism in enterocytes of the neonatal pig. Am J Physiol Regulatory Integr Comp Physiol. 1995b;268:R334–R342. doi: 10.1152/ajpregu.1995.268.2.R334. [DOI] [PubMed] [Google Scholar]
- Wu G, Meier SA, Knabe DA. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr. 1996;126:2578–2584. doi: 10.1093/jn/126.10.2578. [DOI] [PubMed] [Google Scholar]
- Wu G, Ott TL, Knabe DA, et al. Amino acid composition of the fetal pig. J Nutr. 1999;129:1031–1038. doi: 10.1093/jn/129.5.1031. [DOI] [PubMed] [Google Scholar]
- Wu G, Flynn NE, Knabe DA. Enhanced intestinal synthesis of polyamines from proline in cortisol-treated piglets. Am J Physiol Endocrinol Metab. 2000;279:E395–E402. doi: 10.1152/ajpendo.2000.279.2.E395. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Cudd TA, et al. Maternal nutrition and fetal development. J Nutr. 2004;134:2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Hu J, et al. Polyamine synthesis from proline in the developing porcine placenta. Biol Reprod. 2005;72:842–850. doi: 10.1095/biolreprod.104.036293. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Wallace JM, et al. Intrauterine growth retardation: Implications for the animal sciences. J Anim Sci. 2006;84:2316–2337. doi: 10.2527/jas.2006-156. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Cudd TA, et al. Pharmacokinetics and safety of arginine supplementation in animals. J Nutr. 2007a;137:1673S–1680S. doi: 10.1093/jn/137.6.1673S. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Davis TA, et al. Important roles for the arginine family of amino acids in swine nutrition and production. Livest Sci. 2007b;112:8–22. [Google Scholar]
- Wu G, Bazer FW, Datta S, et al. Proline metabolism in the conceptus: Implications for fetal growth and development. Amino Acids. 2008;35:691–702. doi: 10.1007/s00726-008-0052-7. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Davis TA, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–168. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Burghardt RC, et al. Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J Anim Sci. 2010a;88:E195–E204. doi: 10.2527/jas.2009-2446. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Burghardt RC. Functional amino acids in swine nutrition and production. In: Doppenberg J, et al., editors. Dynamics in animal nutrition. Wageningen Academic Publishers; The Netherlands: 2010b. pp. 69–98. [Google Scholar]
- Zeng XF, Wang FL, Fan X, et al. Dietary arginine supplementation during early pregnancy enhances embryonic survival in rats. J Nutr. 2008;138:1421–1425. doi: 10.1093/jn/138.8.1421. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dabrowski K, Hliwa P, et al. Indispensable amino acid concentrations decrease in tissues of stomachless fish, common carp in response to free amino acid- or peptide-based diets. Amino Acids. 2006;31:165–172. doi: 10.1007/s00726-006-0345-7. [DOI] [PubMed] [Google Scholar]


