Abstract
Cardiovascular diseases are one of the leading causes of mortality. Hypertension (HT) is one of the principal risk factors associated with death. Chronic kidney disease (CKD), which is probably underestimated, increases the risk and the severity of adverse cardiovascular events. It is now recognized that low birth weight is a risk factor for these diseases, and this relationship is amplified by a rapid catch-up growth or overfeeding during infancy or childhood. The pathophysiological and molecular mechanisms involved in the “early programming” of CKD are multiple and partially understood. It has been proposed that the developmental programming of arterial hypertension and chronic kidney disease is related to a reduced nephron endowment. However, this mechanism is still discussed. This review discusses the complex relationship between birth weight and nephron endowment and how early growth and nutrition influence long term HT and CKD. We hypothesize that fetal environment reduces moderately the nephron number which appears insufficient by itself to induce long term diseases. Reduced nephron number constitutes a “factor of vulnerability” when additional factors, in particular a rapid postnatal growth or overfeeding, promote the early onset of diseases through a complex combination of various pathophysiological pathways.
1. Introduction
Cardiovascular diseases ((CVD) hypertension, coronary disease and stroke, and heart failure) are one of the leading causes of mortality in industrialized countries, and the prevalence is increasing in emerging societies. All cardiovascular diseases account for 4.3 million deaths per year in the European Union, and the prevalence of chronic heart failure in the United States of America is approximately 6 million [1, 2]. In industrialized countries, hypertension (HT) affects 25% to 35% of the global population and reaches 60% to 70% of the population aged 60 or more. Hypertension is the principal risk factor of death worldwide [3]. It increases the severity of ischemic vascular diseases and, with obesity and type 2 diabetes, is one of the important risk factors for chronic kidney disease (CKD). Chronic kidney disease is defined as reduced glomerular filtration rate (GFR) up to end-stage renal disease (ESRD), proteinuria, or both. Prevalence of ESRD, estimated to be 0.5–2.5‰ worldwide, is increasing in several countries [4]. In turn, impaired renal factor favors the development of and amplifies the severity of CVD [5–7].
During the last two decades, it has been raised the concept of developmental programming of adult chronic diseases (Developmental Origins of Health and Disease (DOHaD)) [8, 9]. The pathophysiological and molecular mechanisms involved in the early programming of CKD are multiple and partially understood. Reduced nephron endowment has been proposed as playing a determinant role [10–13]. Reduced nephron number is responsible for an adaptive single nephron glomerular hyperfiltration. The consecutive glomerular hypertension may lead over a long time to renal injury, proteinuria, impaired GFR, and hypertension [14]. However, this mechanism is still discussed, and recent experimental studies have failed to show such a link [15–21].
This review discusses factors which influence nephron endowment and the complex relationship between nephron endowment and chronic kidney disease. We hypothesize that the developmental “programming” of chronic kidney disease is a complex phenomenon. It may integrate different factors and pathophysiological pathways. Reduced nephron number constitutes a “factor of vulnerability” which is insufficient by itself when it is moderate. In such a situation, early onset of CKD occurs with additional factors including early growth and nutrition.
2. Developmental Origins of CKD
This concept states that chronic and noncommunicable diseases that are currently observed at adulthood have origins in the fetal and perinatal periods of life. Events or stimulus during particular stages of development can alter permanently the structure and function of various systems. After a silent period, diseases occur at adulthood. David Barker and colleagues discovered in the 1980s', in a cohort of people born in Hertfordshire, UK, at the beginning of the nineteenth century, that the mortality ratio due to coronary heart disease was inversely correlated with birth weight [22, 23]. Low birth weight ((LBW), birth weight ≤ 2500 g) was associated with increased rate of mortality. A number of subsequent epidemiologic and experimental studies confirmed this association and the association with other chronic diseases including hypertension, obesity, insulin-resistance and type 2 diabetes [11, 12, 24–29]. It is of note that low birth weight can be related to either intrauterine growth restriction (IUGR) or preterm birth. Recently, other perinatal factors including maternal obesity, maternal diabetes, fetal exposure to specific drugs and preterm birth have been reported to alter the development of various systems increasing the risk for long term diseases [30, 31].
2.1. Birth Weight and Chronic Kidney Disease
More recently the risk of chronic kidney disease (CKD) has been related to low birth weight [32–35]. In a population-based study, the estimated glomerular filtration rate (eGFR) has been shown to increase of 2.6 to 7 mL/min per each kilogram increase in birth weight [33, 36]. In a case control study, Lackland et al. have shown in a population of South Carolina, USA, that the odds ratio for end-stage renal disease (ESDR) was 1.4 (95% confidence interval, 1.1–1.8) in adults with birth weight below 2.5 kg [32]. Such results have been recently confirmed in a Norwegian study (the Medical Birth Registry and the Norwegian Renal Registry) where patients with birth weight < 10th percentile had a relative risk (RR) for ESRD of 1.7 (95% confidence interval 1.4 to 2.2) [35]. Finally, LBW is associated with a more rapid progression of various kidney diseases such as membranous and IgA nephropathies, nephrotic syndrome, renal cystic diseases, or kidney disease related to obesity and metabolic disorders [37–41]. In animals, maternal diabetes, maternal obesity, and fetal exposure to drugs can alter nephrogenesis and impair renal function on the long term. Such effects have to be demonstrated in humans.
Adults born preterm constitute an emerging population at risk for cardiovascular and renal diseases. Prenatal and postnatal events may influence renal function and structure later on. The Dutch POP study revealed an inverse relationship between birth weight and long term urinary microalbumin/creatinine ratio and plasma creatinin level in young adults born preterm [42]. Increased microalbuminuria and decreased glomerular filtration rate were observed in patients who were born small for gestational age (SGA) [43]. Impaired renal function has been reported in preterm children with previous history of neonatal hypotension and renal dysfunction [44, 45]. Data are scarce regarding renal structure [46, 47]. Recently, Hodgin et al. have reported 6 adults born preterm (mean age of 32 years) with isolated proteinuria and focal segmental glomerular sclerosis [47]. Aside from renal consequences, preterm birth is associated with early markers of cardiovascular disease and higher risk of HT [48–51]. Preterm birth has to be taken into account as a risk factor of CKD since approximately 130 million infants are born preterm worldwide (frequencies vary from 5.5% to more than 12%) and the prevalence is increasing [52]. Moreover, with a significant improvement in perinatal care, the number of preterm infants reaching adulthood is increasing.
2.2. Early Postnatal Growth and the Risk of CKD
While a rapid postnatal growth during childhood and infancy favours the development of cardiovascular diseases, obesity, and type 2 diabetes; the consequences on renal function and structure are relatively unknown in humans [53–57]. The critical period at which the organism is more sensitive to nutrition and growth is still being debated. Faster weight gain during the first 6 months of life, promoted by a high protein diet, favours the accumulation of the metabolic visceral adipose tissue, reduces insulin sensitivity, and increases blood pressure later in life [56–58]. These effects are exacerbated in low birth weight infants related to preterm birth, IUGR, or both [53, 54, 59–62]. In a longitudinal study of a Finnish cohort, Barker et al. showed that adults who developed coronary heart disease or hypertension were born small, grew slowly within the first months of life, and caught up the BMI early in infancy [53–59]. The proportion of hypertension was higher in patients who were born with LBW and were overweight at adulthood. In contrast, breastfeeding and/or slow postnatal growth appear as a protective factor in LBW infants [63, 64]. Indeed, breastfeeding prevents on the long term the development of central adiposity and obesity, a major risk factor of metabolic and cardiovascular diseases [65, 66].
Similar effects have been reproduced in animals. We, and others, have shown that early postnatal overfeeding, obtained by reduction of litter size and limited to the suckling period, induces obesity, cardiovascular, metabolic, and renal diseases in ageing adult rat offspring [67–70]. Such effects were amplified in IUGR offspring [16, 71, 72]. Blood pressure, fasting insulin, and leptin levels are elevated in young adults IUGR rat offspring nourished during the peripubertal period by a hypercaloric diet (applied after the weaning) [72]. The underlying mechanism is complex. Early overfeeding/overgrowth is associated with overactivity of the sympathetic nervous activity, upregulation of the HPA-axis, early hyperinsulinism, and hyperleptinemia. Hyperinsulinism affects endothelial nitric oxide synthase (eNOS), and hyperleptinemia stimulates the sympathetic nervous system activity. Sustained alteration of the control of appetite with leptin resistance and hyperphagia may exacerbate such metabolic, hormonal, and vascular disorders and hence favour the development of cardiovascular and renal diseases [68, 73]. In contrast, adult diseases can be prevented by slow postnatal growth and manipulation of diet early during the development. In rodents, an increase in litter size (a model of neonatal undernutrition) or prolonged maternal gestational low protein diet after birth and during the neonatal period prevents, in normal birth weight and in IUGR offspring, metabolic disorders and adiposity, long term hypertension and glomerular sclerosis [11, 74–77]. In the same way, we have observed that renal function and structure were unaffected in ageing IUGR offspring with a slow postnatal growth [16]. Such a considerable influence of early growth and nutrition on long term blood pressure in rats has been observed in other species, especially in sheep [78, 79]. Finally, adult hypertension and salt sensitive hypertension (52-week-old animals) could be prevented by placing IUGR rat offspring on a low salt diet just 3 weeks after weaning [80].
Altogether, these findings show that early postnatal nutrition (protein/caloric diet, sodium intakes…) and early postnatal growth exert a considerable influence on adult health. Early growth and nutrition can modulate the “fetal programmed” adult chronic diseases. While a rapid postnatal growth and/or overfeeding enhances the “vulnerability state” acquired in utero and accelerates the development of adult diseases (“mismatch hypothesis”), a slow postnatal growth and breastfeeding in particular (possibly through reduced protein and sodium intakes) tend to prevent such diseases.
3. Birth Weight, Nephron Endowment and CKD
3.1. Nephron Number and CKD
It has been proposed, for a long time, that the pathogenesis of hypertension and chronic kidney disease involves a reduction of nephron number [14, 81–84]. According to the scheme proposed by Brenner et al. (based on clinical data and experimental studies), a decrease in the filtration surface area due to reduced nephron number is associated with an adaptive increase in single nephron glomerular filtration rate (SNGFR). Nephrons undergo structural changes with glomerular and tubular enlargement responsible for renal hypertrophy. Glomerular capillaries enlargement affects podocyte physiology and increases glomerular hypertension through exacerbating the transmission of systemic blood pressure into enlarged glomerulus. In parallel, other physiological changes occur including salt retention, higher volume strokes and cardiac output, resetting in pressure-natriuresis mechanisms and elevated peripheral vascular resistance. They contribute to elevate blood pressure levels [14]. Over a long time a vicious circle takes place responsible for glomerular sclerosis, impaired GFR, and systemic hypertension. The hemodynamic adaptive mechanism is accompanied by molecular and biomolecular changes including inflammation, upregulation of the renin angiotensin system (RAS), and the production of nitric oxide and of reactive species which participate to renal injury [85]. Such a renal mechanism has been proposed as a pathophysiological mechanism linking low birth to long term hypertension and chronic kidney disease [11–13].
However, reduced nephron number is not systematically associated with hypertension and impaired GFR, especially when it is moderate. In humans, Hughson et al. did not find this relationship in a group of African-American adults [86]. Several experimental studies failed to demonstrate hypertension and glomerular sclerosis after renal mass resection or congenitally reduced nephron endowment [15–21]. We have recently shown that blood pressure and glomerular sclerosis were unchanged in 22-month-old IUGR ageing males and females rat offsprings with a significant reduction of nephron number (by an average of 25% to 30%) [16]. All these findings suggest that the relationship of reduced nephron number with hypertension and chronic kidney disease is, in fact, more complex and involves various factors. It depends, in part, on the severity of nephron number deficit, the degree of the single nephron glomerular hyperfiltration, or both.
3.2. Birth Weight and Nephron Endowment
The development of the kidney is a complex process in mammalian. The time at which nephrogenesis ends differ according to species: in rodents, nephrogenesis continues after birth up to postnatal days 7 to 10, whereas in sheep, the nephrogenesis is achieved before birth at gestational days 125–130 (the normal duration of gestation is 145–150 days). In human, the nephrogenesis is completed by 34–36th weeks of gestation, that is, before birth. About 60% of the nephrons develop during the third trimester of gestation. The definitive kidney, the metanephros, develops from the specific interaction between the epithelial ureteric bud (UB) and the undifferentiated metanephric mesenchyme (MM). An insignificant event occurring during the early stage of nephrogenesis (the branching morphogenesis) can have dramatic effects on the final nephron number (nephron endowment).
3.2.1. Nephron Number in Human
Nephron number varies widely in the general population and ranges from 2 to more than 10-fold [87–92]. In the Monash series, which included 420 kidneys obtained at autopsy from adults and children from different populations (Aboriginal Australians and white Australians, Senegalese, and white Americans and African Americans), the mean nephron number per kidney was around 900,000 ranging 13-fold from about 210,000 to more than 2,000 000 [88, 90, 91]. Such variability may be explained by genetic and environmental factors, or both. Aboriginal Australians have lower nephron number. Alterations in specific DNA sequences are associated with renal agenesis and hypoplasia [93, 94], and few genetic polymorphisms have been associated to changes in renal volume (a surrogate of nephron mass) [95–97].
The principal factor which determines nephron number is birth weight [90], but it is not the only one. Nephron number can vary 3-fold when birth weight is situated within normal range, that is, 3000 g–3500 g [90]. Low birth weight is associated with reduced nephron number. Intrauterine growth restriction ((IUGR) birth weight <10th percentile for gestational age) decreases the nephron number by an average of 30–35%, whereas the effects of preterm birth are still unknown [87, 98, 99]. In preterm infant, the nephrogenesis has to continue in a potentially unfavourable environment. Reduced kidney size and volume have been reported in children and young adults born preterm and in the ones who had postnatal growth restriction [100–103]. Data from autopsy studies which included kidneys from preterm infants who died during the neonatal period at different gestational and postnatal ages showed signs of accelerated maturation of nephrogenesis with enlarged glomeruli [104, 105]. Similar glomerular hypertrophy was observed in premature baboon (E125/E185), with however a preserved nephron endowment [106]. These findings suggest that postnatal nephrogenesis is altered in sick, preterm infants. Babies included in these studies were likely to be the sickest among the patients and to have suffered a prolonged postnatal “stress” which might compromise the postnatal nephrogenesis. The formation of additional nephrons could be preserved in a part of preterm infants who are less immature and have uncomplicated neonatal care or optimal neonatal growth. More studies are clearly needed to assess factors influencing the postnatal renal development in preterm infants.
3.2.2. Lessons from Experimental Studies
Various factors can alter nephrogenesis [12, 87, 99, 107] (Figure 1). Maternal low protein diet, vitamin A deficiency and maternal iron deficiency, uterine arteries ligation, maternal gestational administration of glucocorticoids, or other drugs (antibiotics) lead in most cases to IUGR and to a reduced nephron number by an average of 20%–50%. Maternal gestational diabetes in rodents can alter fetal nephrogenesis as well [12, 99, 108, 109]. In sheep, chorioamnionitis induced by intra-amniotic injection of lipopolysaccharides (LPS) at embryonic days E121 reduced fetal nephron number by 20% [110]. We showed previously in 20-day-old rat foetuses that maternal low protein diet (MLP) reduced permanently the nephron number by an average of 30% [16, 111].
Figure 1.
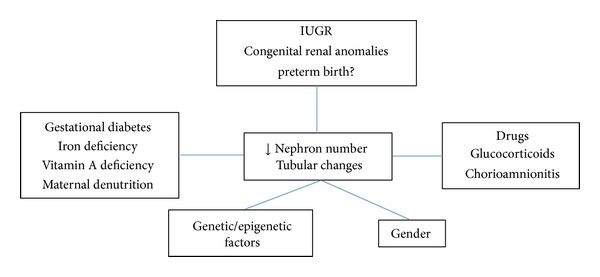
Factors influencing nephron endowment.
The underlying pathophysiological mechanism is incompletely known. Reduction of nephron number may result from an imbalance between pro- and antiapoptotic factors towards apoptosis. Downregulation of the renal renin angiotensin system, fetal overexposure to glucocorticoids, or altered midkine expression has been reported in various IUGR models [99, 112–118]. The expression of specific genes involved in nephrogenesis is altered (Pax2, GDNF) [113, 115]. We found that expression of around 20% of the genome is altered in the fetal kidney of IUGR rat offspring exposed in utero to maternal low protein diet [111, 119]. The expression of genes involved in cell maintenance and signal transduction was decreased, and those belonging to the vascular prothrombotic pathway and to the complement components were considerably overexpressed [111, 119]. The effects of fetal environment on nephron endowment may be epigenetically mediated [119–121]. Hypomethylation of the gene p53 has been associated with reduced nephron number in a rat model of placental insufficiency [121]. In addition, we showed in the kidneys of IUGR fetal offspring changes in the expression of genes coding for specific enzymes involved in epigenetic machinery [119]. Changes in epigenetic marks could be transmitted to the next generation and be responsible for “transmitted” nephron deficit. In rat, offspring (second generation, F2) from parents exposed prenatally to maternal gestational low protein diet (first generation, F1) had normal birth weight but 30% to 40% reduction in nephron number [120]. Additional studies are however needed to confirm and to eventually explain such a transgenerational transmission of acquired phenotype.
Birth weight is not the only predictive factor of the nephron endowment. In animal, the nephron endowment is also characterized by a certain rate of variability. In rodent, despite strictly controlled conditions, the nephron endowment can vary by an average of 10% to 15% for a birth weight situated within normal range [122, 123]. Events that occur during the early stage of nephrogenesis can induce a nephron deficit without affecting birth weight [124–127]. Exposure to maternal low protein diet and administration of a short course of glucocorticoids during the early stage of nephrogenesis, in rodents (E14–17) and in sheep (E80), are sufficient to reduce nephron endowment (−20% to −40%) without inducing low birth weight [124–126]. Interestingly, the nephron endowment can be preserved in IUGR offspring, especially when IUGR is spontaneous or when it occurs late in gestation [123, 128]. In summary, the more the process of IUGR appears early in the gestation, the more the nephrogenesis is affected, and the nephron endowment severely reduced. The early stage of nephrogenesis constitutes a “critical window” when an event can alter profoundly and durably the nephrogenesis.
Postnatal environment can influence nephron endowment in certain situations when the nephrogenesis continues after birth. Recent studies in rodents showed that neonatal undernutrition (−30% to −40%, through increasing litter size) reduced nephron endowment (−20%), but early neonatal overfeeding (through reduction of litter size) enhanced postnatal nephrogenesis (+25%) [70, 129]. However, this last effect was not observed in IUGR offspring (induced by maternal low protein diet). Indeed, while pups displayed a rapid catch-up growth within the first 15 days after birth, the nephron endowment failed to be restored. Interestingly, Wlodek et al. found a relative restoration of nephron endowment when IUGR pups were switch to normal lactating dams [130]. In the last study, IUGR was induced by uterine ligation at embryonic day 17. This discrepancy may result from a marked deficit in nephron precursors observed in fetus exposed to maternal low protein diet at the early stage of fetal development [115, 116].
Such findings emphasize that birth weight is a predictive factor of nephron endowment but is certainly not the only one. Nephron endowment may result from a complex process which integrates the interaction of the fetal environment (or postnatal environment in preterm infants) and the genetic background. Normal birth weight does not always signify a sufficient nephron endowment and low birth weight a severe nephron deficit. The relationship between birth weight and nephron endowment is not so linear and it could be difficult to predict nephron endowment for an individual based only on birth weight.
4. Birth Weight and Chronic Kidney Disease: An “Integrative” Hypothesis
Relationship of birth weight with chronic kidney disease is complex and integrates various factors and pathophysiological mechanisms of which nephron number plays a pivotal role (Figures 2 and 3).
Figure 2.
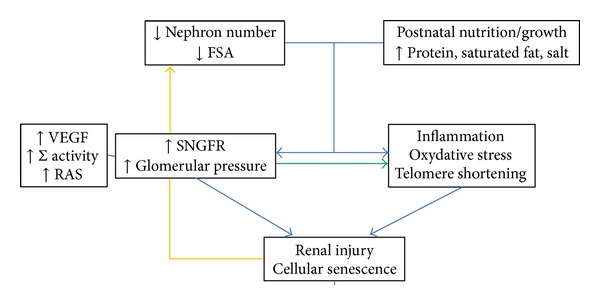
Proposed pathophysiological mechanism of renal injury (glomerular endothelium barrier disruption, podocyte dysfunction, interstitial fibrosis, tubular ischemia). FSA: filtration surface area; GFR: glomerular filtration rate; SNGFR: single nephron glomerular filtration rate; RAS: rennin angiotensin system; VEGF: vascular endothelial growth factor system; ∑: sympathetic nervous system.
Figure 3.
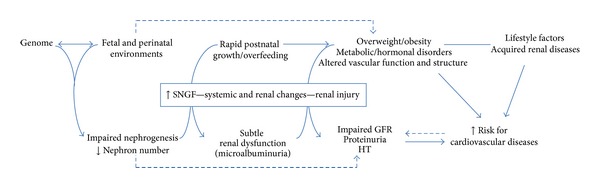
Developmental origin of hypertension and chronic kidney disease: an “integrative hypothesis”.
Predominant factor which determines nephron endowment is birth weight. However, it is not the only one. Nephron endowment, acquired at birth (or after birth for preterm infants), may result from an interaction between genetic background and environmental factors. Environment may alter nephrogenesis through epigenetic pathway, and genetic background may make the kidney less or more sensitive to environmental factors. For example, some mice strains are less sensitive to nephron deficit and glomerular sclerosis induced by maternal gestational administration of aminoglycosides [131]. When nephrogenesis is severely impaired, that is, when the nephron deficit is marked, the risk of early impaired renal function and hypertension is elevated. It is the case of infants born with congenital anomaly of kidney and urinary tract. In most cases, alterations in nephrogenesis are subtle and are probably not responsible by themselves to chronic kidney disease. However, such changes constitute a “factor of vulnerability.”
Various postnatal factors can induce a single nephron glomerular hyperfiltration or glomerular hypertension and together with a reduced filtration surface area may accelerate the occurrence of CKD. Nutrition or growth early in life is one of them. We and others have shown that a rapid postnatal growth and overfeeding (high caloric and protein intakes) early in life induced in young adult IUGR rat offspring a renal hypertrophy and proteinuria (a surrogate of a glomerular hyperfiltration or glomerular endothelium barrier injury) [12, 16, 70, 117, 132]. Protein diet may play an important role since it is known for a long time that high protein intakes in adult animals induce glomerular hyperfiltration, renal hypertrophy, and long term glomerular sclerosis [74]. In another study, twelve-week-old IUGR rat offspring exposed postnatally to a high protein diet (+30%) displayed glomerular hypertrophy, podocyte damage, and early signs of interstitial fibrosis [133]. On the other hand, a slow postnatal growth prevents the development of renal disease in IUGR and normal birth weight offspring. The renal effects of a high protein diet are more marked when the kidney is immature due to its higher capacity than the adults' to adapt renal hemodynamic (with higher sensitivity to the RAS) [134]. This adaptative mechanism is associated with various changes including upregulation of the renal RAS of the VEGF system and overactivity of the sympathetic nervous system. Inflammation and oxidative stress have been demonstrated in these kidneys as well (Figure 2) [135–139]. These changes may initiate a “renal stress,” an infraclinical renal injury. Indeed, kidney of IUGR overfed offspring which displayed a rapid postnatal catch-up growth expressed stress-induced senescence protein markers (p16, p21) and telomere shortening [135–140]. Telomere shortening, related with “oxidative stress,” favours premature cell death (Figure 2). However, it is unknown whether such changes persist on the long term and whether it can be reversed.
Some of these experimental findings have been reported in human. Early high protein diet and rapid growth rate tend to induce a renal hypertrophy. Two recent studies have prospectively evaluated the effects of different diets on renal structure (using ultrasound) in infants born at term with birth weight adapted for gestational age [141, 142]. In the first study, when a group of formula-fed infants was compared to breastfed infants, the authors showed a 25% increase in renal volume at 3 months of age. This effect was transient and was no longer observed at 18 months when all infants were on mixed diet [141]. The second study aimed to investigate the renal effects of two low (1.25 g/dL, average breastfeeding) and high protein (2.05 g/dL, +60%) formula diets in healthy infants [142]. At 6 months of age, while no differences were found between breastfed and low protein formula-fed infants, the kidney volume (and the relative volume of the kidney/body surface area ratio) was 10% higher in high protein formula-fed infants. This renal effect may result from a single nephron glomerular hyperfiltration induced by high protein intakes as demonstrated in experimental studies.
Early nutrition and growth can alter renal function and structure through other pathways. In animal, early postnatal overfeeding is associated with hypertension, obesity, and type 2 diabetes, known as risk factor for CKD. A rapid postnatal catch-up growth and/or overfeeding is associated with hyperleptinemia, hyperinsulinism and insulin-resistance, upregulation of the RAS and HPA-axis, and overactivity of the nervous sympathetic activity (see above). Such effects are responsible for impaired endothelium-dependent vasodilatation, systemic vasoconstriction, oxidative stress, and systemic inflammation which alter in turn vascular structure and arterial stiffness and lead to hypertension. Obesity and hyperglycaemia induce a single nephron glomerular hyperfiltration [143, 144], but it is still unknown if such changes are sufficient by themselves to affect renal structure. Experimentally, hyperglycaemia (administration of streptozocin ± insulin therapy, equivalent of type 1 diabetes) in young adult IUGR offspring induces single nephron glomerular hyperfiltration and proteinuria but does not affect the glomerular structure on the long term (10 mo) [145, 146]. These findings may be explained by the unchanged blood pressure and the associated weight loss which have limited the adverse renal effects of hyperglycaemia. Indeed, blood pressure plays an important role. Comparing two models of obesity-induced renal injury, do Carmo et al. demonstrated the detrimental role of hypertension on renal structure [147]. Finally, in rodents, early overgrowth/overfeeding alters the central control of appetite with sustained hyperphagia. This last effect can have detrimental effects on the kidney. In industrialized countries, a large part of the population is exposed to hypercaloric diet named “western diet.” This diet, characterized by high carbohydrates, salt, protein, and saturated fat contents, is known to increase the risk of atherosclerosis and cardiovascular disease and to promote the development of glomerular sclerosis [148–150]. These effects are mediated by the overactivity of the sympathetic nervous system, inflammation, and oxidative stress (exacerbated in part by angiotensin II) [149]. In human, high protein diet accelerates the deterioration of GFR in adults with low GFR [151]. Other nutrient and are of importance. High salt intakes are known to increase blood pressure levels and the risk of CKD, especially in overweight patients [80, 152, 153]. Such a salt sensitivity may be enhanced in IUGR offspring. In IUGR rat offspring, reduced nephron number is associated with tubular changes including increased expression of the renal tubular Na+ : K+ : 2Cl− cotransporter (NKCC2) and altered the Na+ : K+ ATPase activity responsible for a tendency to sodium retention and a higher sensitivity to high salt intake [80, 154–160]. One can easily understand that this nutritional factor may amplify the vascular and systemic effects of pre-existing type 2 diabetes, obesity, and hypertension and favour the development of CKD.
Altogether these additional factors in combination with “vulnerable” kidneys accelerate the onset of CKD through the increase in the SNGFR, the reinforcing of the exacerbation of the pre-existing “renal stress”, and through the transmission of elevated systemic blood pressure to enlarged glomerular capillaries. Hence, the kidney appears both as the underlying pathophysiological mechanism and as the target organ of developmental programming of CKD.
5. Implications for Followup, Nutrition, and Prevention in Patients at Risk
Early life conditions are of particular importance in the comprehension of chronic kidney disease (CKD). Their importance equals or may exceed that of other later environmental risk factors. Various systems, including the kidney, are permanently altered. Reduced nephron number, with renal tubular changes, constitutes a “factor of vulnerability” when additional factors as the early postnatal growth/nutrition promote early onset of hypertension and CKD through various pathways. Despite this clear pathophysiologic rationale, a number of points still need to be addressed to allow the design of effective preventive strategies. The criteria for a subject being considered at particular risk need to be defined. This is the less easy since a number of epidemiologic and clinical studies show that the long term programming of hypertension and of renal disease does not occur only in well-defined, pathological conditions such as low birth weight, preterm birth, and exposure to maternal diabetes in pregnancy. Even in apparently healthy children, estimated glomerular function has been shown to be correlated with size at birth [161]. Questions such as the optimal nutrition of low birth weight infants, whether due to intrauterine growth restriction, preterm birth or both, the optimal followup of vascular, metabolic, and renal functions, and possible nutritional and pharmacological interventions remain unanswered. Postnatal undergrowth/undernutrition is associated with impaired neurological function and potentially death in certain regions of the world [162]. Future research may aim to clarify early biomarkers and markers of nephron endowment and early renal injury in order to determine optimal perinatal nutrition and the eventual prophylactic measures to be applied to infants at increased risk of developmentally programmed adult diseases. However, simple preventive measures such as promoting breastfeeding (at least 6 mo) and physical activity early in childhood and establishing early program of nutritional education and public nutritional policies (reduced sodium, carbohydrates and saturated fat in ready meals, e.g.) are now feasible and can have significant impact on public health (as suggested by experimental studies).
References
- 1.Allender S, Scarborough P, Peto V, et al. European Cardiovascular Disease Statistics. 2008. [Google Scholar]
- 2.Roger R, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narayan KMV, Ali MK, Koplan JP. Global noncommunicable diseases: were worlds meet. The New England Journal of Medicine. 2010;363(13):1196–1198. doi: 10.1056/NEJMp1002024. [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Journal of the American Medical Association. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 5.Gibson CM, Dumaine RL, Gelfand EV, et al. Association of glomerular filtration rate on presentation with subsequent mortality in non-ST-segment elevation acute coronary syndrome; observations in 13307 patients in five TIMI trials. European Heart Journal. 2004;25(22):1998–2005. doi: 10.1016/j.ehj.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England Journal of Medicine. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 7.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. Journal of the American Society of Nephrology. 2006;17(7):2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 8.Barker DJP, Eriksson JG, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. International Journal of Epidemiology. 2002;31(6):1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. The New England Journal of Medicine. 2008;359(1):6–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiological Reviews. 2005;85(2):571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 11.Simeoni U, Ligi I, Buffat C, Boubred F. Adverse consequences of accelerated neonatal growth: cardiovascular and renal issues. Pediatric Nephrology. 2011;26(4):493–508. doi: 10.1007/s00467-010-1648-1. [DOI] [PubMed] [Google Scholar]
- 12.Vehaskari VM, Woods LL. Prenatal programming of hypertension: lessons from experimental models. Journal of the American Society of Nephrology. 2005;16(9):2545–2556. doi: 10.1681/ASN.2005030300. [DOI] [PubMed] [Google Scholar]
- 13.Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. The American Journal of Kidney Diseases. 1994;23(2):171–175. [PubMed] [Google Scholar]
- 14.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? The American Journal of Hypertension. 1988;1(4):335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 15.Bidani AK, Mitchell KD, Schwartz MM, Navar LG, Lewis EJ. Absence of glomerular injury or nephron loss in a normotensive rat remnant kidney model. Kidney International. 1990;38(1):28–38. doi: 10.1038/ki.1990.163. [DOI] [PubMed] [Google Scholar]
- 16.Boubred F, Daniel L, Buffat C, et al. Early postnatal overfeeding induces early chronic renal dysfunction in adult male rats. The American Journal of Physiology. 2009;297(4):F943–F951. doi: 10.1152/ajprenal.90704.2008. [DOI] [PubMed] [Google Scholar]
- 17.Dickinson H, Walker DW, Wintour EM, Moritz K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. The American Journal of Physiology. 2007;292(1):R453–R461. doi: 10.1152/ajpregu.00481.2006. [DOI] [PubMed] [Google Scholar]
- 18.Griffin KA, Picken MM, Churchill M, Churchill P, Bidani AK. Functional and structural correlates of glomerulosclerosis after renal mass reduction in the rat. Journal of the American Society of Nephrology. 2000;11(3):497–506. doi: 10.1681/ASN.V113497. [DOI] [PubMed] [Google Scholar]
- 19.Hoppe CC, Evans RG, Moritz KM, et al. Combined prenatal and postnatal protein restriction influences adult kidney structure, function, and arterial pressure. The American Journal of Physiology. 2007;292(1):R462–R469. doi: 10.1152/ajpregu.00079.2006. [DOI] [PubMed] [Google Scholar]
- 20.Moritz KM, Mazzuca MQ, Siebel AL, et al. Uteroplacental insufficiency causes a nephron deficit, modest renal insufficiency but no hypertension with ageing in female rats. Journal of Physiology. 2009;587(11):2635–2646. doi: 10.1113/jphysiol.2009.170407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimanyi M, Bertram JF, Black MJ. Does a nephron deficit in rats predispose to salt-sensitive hypertension? Kidney and Blood Pressure Research. 2004;27(4):239–247. doi: 10.1159/000079868. [DOI] [PubMed] [Google Scholar]
- 22.Barker DJP, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. British Medical Journal. 1990;301(6746):259–262. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker DJP, Osmond C, Golding J, Kuh D, Wadsworth MEJ. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. British Medical Journal. 1989;298(6673):564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiological Reviews. 2005;85(2):571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 25.Barker DJP. Adult consequences of fetal growth restriction. Clinical Obstetrics and Gynecology. 2006;49(2):270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94(12):3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 27.Leon DA, Lithell HO, Vågerö D, et al. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15,000 Swedish men and women born 1915-29. British Medical Journal. 1998;317(7153):241–245. doi: 10.1136/bmj.317.7153.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newsome CA, Shiell AW, Fall CHD, Phillips DIW, Shier R, Law CM. Is birth weight related to later glucose and insulin metabolism?: a systematic review. Diabetic Medicine. 2003;20(5):339–348. doi: 10.1046/j.1464-5491.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- 29.Stein CE, Fall CHD, Kumaran K, Osmond C, Cox V, Barker DJP. Fetal growth and coronary heart disease in South India. The Lancet. 1996;348(9037):1269–1273. doi: 10.1016/s0140-6736(96)04547-3. [DOI] [PubMed] [Google Scholar]
- 30.Ligi I, Grandvuillemin I, Andres V, Dignat-George F, Simeoni U. Low birth weight infants and the developmental programming of hypertension: a focus on vascular factors. Seminars in Perinatology. 2010;34(3):188–192. doi: 10.1053/j.semperi.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 31.O'Reilly JR, Reynolds RM. The risk of maternal obesity to the long term health of the offspring. Clinical Endocrinology. 2013;78(1):9–16. doi: 10.1111/cen.12055. [DOI] [PubMed] [Google Scholar]
- 32.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJP. Low birth weights contribute to the high rates of early-onset chronic renal failure in the southeastern United States. Archives of Internal Medicine. 2000;160(10):1472–1476. doi: 10.1001/archinte.160.10.1472. [DOI] [PubMed] [Google Scholar]
- 33.Li S, Chen S-C, Shlipak M, et al. Low birth weight is associated with chronic kidney disease only in men. Kidney International. 2008;73(5):637–642. doi: 10.1038/sj.ki.5002747. [DOI] [PubMed] [Google Scholar]
- 34.White SL, Perkovic V, Cass A, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. The American Journal of Kidney Diseases. 2009;54(2):248–261. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 35.Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birth weight increases risk for end-stage renal disease. Journal of the American Society of Nephrology. 2008;19(1):151–157. doi: 10.1681/ASN.2007020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hallan SI, Vikse BE. Relationship between chronic kidney disease prevalence and end-stage renal disease risk. Current Opinion in Nephrology and Hypertension. 2008;17(3):286–291. doi: 10.1097/MNH.0b013e3282f8b177. [DOI] [PubMed] [Google Scholar]
- 37.Zidar N, Čavić MA, Kenda RB, Ferluga D. Unfavorable course of minimal change nephrotic syndrome in children with intrauterine growth retardation. Kidney International. 1998;54(4):1320–1323. doi: 10.1046/j.1523-1755.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- 38.Rajan T, Barbour SJ, White CT, Levin A. Low birth weight and nephron mass and their role in the progression of chronic kidney disease: a case report on identical twins with Alport disease. Nephrology Dialysis Transplantation. 2011;26(12):4136–4139. doi: 10.1093/ndt/gfr252. [DOI] [PubMed] [Google Scholar]
- 39.Plank C, Östreicher I, Dittrich K, et al. Low birth weight, but not postnatal weight gain, aggravates the course of nephrotic syndrome. Pediatric Nephrology. 2007;22(11):1881–1889. doi: 10.1007/s00467-007-0597-9. [DOI] [PubMed] [Google Scholar]
- 40.Orskov B, Christensen KB, Feldt-Rasmussen B, Strandgaard S. Low birth weight is associated with earlier onset of end-stage renal disease in Danish patients with autosomal dominant polycystic kidney disease. Kidney International. 2012;81(9):919–924. doi: 10.1038/ki.2011.459. [DOI] [PubMed] [Google Scholar]
- 41.Zidar N, Čavić MA, Kenda RB, Koselj M, Ferluga D. Effect of intrauterine growth retardation on the clinical course and prognosis of IgA glomerulonephritis in children. Nephron. 1998;79(1):28–32. doi: 10.1159/000044987. [DOI] [PubMed] [Google Scholar]
- 42.Keijzer-Veen MG, Schrevel M, Finken MJJ, et al. Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. Journal of the American Society of Nephrology. 2005;16(9):2762–2768. doi: 10.1681/ASN.2004090783. [DOI] [PubMed] [Google Scholar]
- 43.Keijzer-Veen MG, Kleinveld HA, Lequin MH, et al. Renal function and size at young adult age after intrauterine growth restriction and very premature birth. The American Journal of Kidney Diseases. 2007;50(4):542–551. doi: 10.1053/j.ajkd.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Abitbol CL, Bauer CR, Montané B, Chandar J, Duara S, Zilleruelo G. Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatric Nephrology. 2003;18(9):887–893. doi: 10.1007/s00467-003-1186-1. [DOI] [PubMed] [Google Scholar]
- 45.Iacobelli S, Loprieno S, Bonsante F, Latorre G, Esposito L, Gouyon JB. Renal function in early childhood in very low birthweight infants. The American Journal of Perinatology. 2007;24(10):587–592. doi: 10.1055/s-2007-992173. [DOI] [PubMed] [Google Scholar]
- 46.Abitbol CL, Chandar J, Rodríguez MM, et al. Obesity and preterm birth: additive risks in the progression of kidney disease in children. Pediatric Nephrology. 2009;24(7):1363–1370. doi: 10.1007/s00467-009-1120-2. [DOI] [PubMed] [Google Scholar]
- 47.Hodgin JB, Rasoulpour M, Markowitz GS, D’Agati VD. Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clinical Journal of the American Society of Nephrology. 2009;4(1):71–76. doi: 10.2215/CJN.01700408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norman M. Preterm birth-an emerging risk factor for adult hypertension? Seminars in Perinatology. 2010;34(3):183–187. doi: 10.1053/j.semperi.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Abitbol CL, Rodriguez MM. The long-term renal and cardiovascular consequences of prematurity. Nature Reviews Nephrology. 2012;8(5):265–274. doi: 10.1038/nrneph.2012.38. [DOI] [PubMed] [Google Scholar]
- 50.Simeoni U, Zetterström R. Long-term circulatory and renal consequences of intrauterine growth restriction. Acta Paediatrica, International Journal of Paediatrics. 2005;94(7):819–824. doi: 10.1111/j.1651-2227.2005.tb01993.x. [DOI] [PubMed] [Google Scholar]
- 51.De Jong F, Monuteaux MC, Van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59(2):226–234. doi: 10.1161/HYPERTENSIONAHA.111.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization. 2010;88(1):31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barker DJP, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. The New England Journal of Medicine. 2005;353(17):1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 54.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. Journal of Hypertension. 2000;18(7):815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 55.Adair LS, Cole TJ. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension. 2003;41(3 I):451–456. doi: 10.1161/01.HYP.0000054212.23528.B2. [DOI] [PubMed] [Google Scholar]
- 56.Leunissen RWJ, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. Journal of the American Medical Association. 2009;301(21):2234–2242. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- 57.Ong K, Loos R. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatrica, International Journal of Paediatrics. 2006;95(8):904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 58.Chomtho S, Wells JCK, Williams JE, Davies PSW, Lucas A, Fewtrell MS. Infant growth and later body composition: evidence from the 4-component model. The American Journal of Clinical Nutrition. 2008;87(6):1776–1784. doi: 10.1093/ajcn/87.6.1776. [DOI] [PubMed] [Google Scholar]
- 59.Eriksson JG, Forsén T, Tuomilehto J, Winter PD, Osmond C, Barker DJP. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. British Medical Journal. 1999;318(7181):427–431. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kerkhof GF, Willemsen RH, Leunissen RW, Breukhoven PE, Hokken-Koelega AC. Health profile of young adults born preterm: negative effects of rapid weight gain in early life. Journal of Clinical Endocrinology and Metabolism. 2012;97(12):4498–4506. doi: 10.1210/jc.2012-1716. [DOI] [PubMed] [Google Scholar]
- 61.Singhal A, Kennedy K, Lanigan J, et al. Nutrition in infancy and long-term risk of obesity: evidence from 2 randomized controlled trials. The American Journal of Clinical Nutrition. 2010;92(5):1133–1144. doi: 10.3945/ajcn.2010.29302. [DOI] [PubMed] [Google Scholar]
- 62.Hack M, Schluchter M, Cartar L, Rahman M. Blood pressure among very low birth weight (<1.5 kg) young adults. Pediatric Research. 2005;58(4):677–684. doi: 10.1203/01.PDR.0000180551.93470.56. [DOI] [PubMed] [Google Scholar]
- 63.Singhal A, Cole TJ, Fewtrell M, Deanfield J, Lucas A. Is slower early growth beneficial for long-term cardiovascular health? Circulation. 2004;109(9):1108–1113. doi: 10.1161/01.CIR.0000118500.23649.DF. [DOI] [PubMed] [Google Scholar]
- 64.Singhal A, Fewtrell M, Cole TJ, Lucas A. Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. The Lancet. 2003;361(9363):1089–1097. doi: 10.1016/S0140-6736(03)12895-4. [DOI] [PubMed] [Google Scholar]
- 65.Owen CG, Whincup PH, Cook DG. Symposium II: infant and childhood nutrition and disease: breast-feeding and cardiovascular risk factors and outcomes in later life: evidence from epidemiological studies. Proceedings of the Nutrition Society. 2011;70(4):478–484. doi: 10.1017/S0029665111000590. [DOI] [PubMed] [Google Scholar]
- 66.Pirilä S, Saarinen-Pihkala UM, Viljakainen H, et al. Breastfeeding and determinants of adult body composition: a prospective study from birth to young adulthood. Hormone Research in Paediatrics. 2012;77(5):281–290. doi: 10.1159/000338334. [DOI] [PubMed] [Google Scholar]
- 67.Boullu-Ciocca S, Dutour A, Guillaume V, Achard V, Oliver C, Grino M. Postnatal diet-induced obesity in rats upregulates systemic and adipose tissue glucocorticoid metabolism during development and in adulthood: its relationship with the metabolic syndrome. Diabetes. 2005;54(1):197–203. doi: 10.2337/diabetes.54.1.197. [DOI] [PubMed] [Google Scholar]
- 68.Plagemann A, Harder T, Rake A, et al. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome X-like alterations in adulthood of neonatally overfed rats. Brain Research. 1999;836(1-2):146–155. doi: 10.1016/s0006-8993(99)01662-5. [DOI] [PubMed] [Google Scholar]
- 69.Velkoska E, Cole TJ, Dean RG, Burrell LM, Morris MJ. Early undernutrition leads to long-lasting reductions in body weight and adiposity whereas increased intake increases cardiac fibrosis in male rats. Journal of Nutrition. 2008;138(9):1622–1627. doi: 10.1093/jn/138.9.1622. [DOI] [PubMed] [Google Scholar]
- 70.Boubred F, Buffat C, Feuerstein J-M, et al. Effects of early postnatal hypernutrition on nephron number and long-term renal function and structure in rats. The American Journal of Physiology. 2007;293(6):F1944–F1949. doi: 10.1152/ajprenal.00141.2007. [DOI] [PubMed] [Google Scholar]
- 71.Jennings BJ, Ozanne SE, Dorling MW, Hales CN. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Letters. 1999;448(1):4–8. doi: 10.1016/s0014-5793(99)00336-1. [DOI] [PubMed] [Google Scholar]
- 72.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. The American Journal of Physiology. 2000;279(1):E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 73.Coupé B, Grit I, Hulin P, Randuineau G, Parnet P. Postnatal growth after intrauterine growth restriction alters central leptin signal and energy homeostasis. PLoS ONE. 2012;7(1) doi: 10.1371/journal.pone.0030616.e30616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. The New England Journal of Medicine. 1982;307(11):652–659. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- 75.Mccance RA. Food, growth, and time. The Lancet. 1962;280(7258):671–676. doi: 10.1016/s0140-6736(62)90499-3. [DOI] [PubMed] [Google Scholar]
- 76.Petry CJ, Jennings BJ, James LA, Hales CN, Ozanne SE. Suckling a protein-restricted rat dam leads to diminished albuminuria in her male offspring in adult life: a longitudinal study. BMC Nephrology. 2006;7, article 14 doi: 10.1186/1471-2369-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tarry-Adkins JL, Joles JA, Chen J-H, et al. Protein restriction in lactation confers nephroprotective effects in the male rat and is associated with increased antioxidant expression. The American Journal of Physiology. 2007;293(3):R1259–R1266. doi: 10.1152/ajpregu.00231.2007. [DOI] [PubMed] [Google Scholar]
- 78.De Matteo R, Stacy V, Probyn M, Desai M, Ross M, Harding R. The perinatal development of arterial pressure in sheep: effects of low birth weight due to twinning. Reproductive Sciences. 2008;15(1):66–74. doi: 10.1177/1933719107307716. [DOI] [PubMed] [Google Scholar]
- 79.Mühle A, Mühle C, Amann K, et al. No juvenile arterial hypertension in sheep multiples despite reduced nephron numbers. Pediatric Nephrology. 2010;25(9):1653–1661. doi: 10.1007/s00467-010-1512-3. [DOI] [PubMed] [Google Scholar]
- 80.Stewart T, Ascani J, Craver RD, Vehaskari VM. Role of postnatal dietary sodium in prenatally programmed hypertension. Pediatric Nephrology. 2009;24(9):1727–1733. doi: 10.1007/s00467-009-1196-8. [DOI] [PubMed] [Google Scholar]
- 81.Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. The New England Journal of Medicine. 2003;348(2):101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 82.Garg AX, Muirhead N, Knoll G, et al. Proteinuria and reduced kidney function in living kidney donors: a systematic review, meta-analysis, and meta-regression. Kidney International. 2006;70(10):1801–1810. doi: 10.1038/sj.ki.5001819. [DOI] [PubMed] [Google Scholar]
- 83.Wikstad I, Celsi G, Larsson L, Herin P, Aperia A. Kidney function in adults born with unilateral renal agenesis or nephrectomized in childhood. Pediatric Nephrology. 1988;2(2):177–182. doi: 10.1007/BF00862585. [DOI] [PubMed] [Google Scholar]
- 84.Zucchelli P, Cagnoli L. Proteinuria and hypertension after unilateral nephrectomy. The Lancet. 1985;2(8448):p. 212. doi: 10.1016/s0140-6736(85)91523-5. [DOI] [PubMed] [Google Scholar]
- 85.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: Guyton revisited. European Heart Journal. 2005;26(1):11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 86.Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney International. 2006;69(4):671–678. doi: 10.1038/sj.ki.5000041. [DOI] [PubMed] [Google Scholar]
- 87.Merlet-Benichou C, Gilbert T, Vilar J, Moreau E, Freund N, Lelievre- Pegorier M. Nephron number: variability is the rule: causes and consequences. Laboratory Investigation. 1999;79(5):515–527. [PubMed] [Google Scholar]
- 88.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatric Nephrology. 2011;26(9):1529–1533. doi: 10.1007/s00467-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 89.Hoy WE, Hughson MD, Bertram JF, Douglas-Denton R, Amann K. Nephron number, hypertension, renal disease, and renal failure. Journal of the American Society of Nephrology. 2005;16(9):2557–2564. doi: 10.1681/ASN.2005020172. [DOI] [PubMed] [Google Scholar]
- 90.Hughson M, Farris AB, III, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney International. 2003;63(6):2113–2122. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 91.McNamara BJ, Diouf B, Douglas-Denton RN, Hughson MD, Hoy WE, Bertram JF. A comparison of nephron number, glomerular volume and kidney weight in Senegalese Africans and African Americans. Nephrology Dialysis Transplantation. 2010;25(5):1514–1520. doi: 10.1093/ndt/gfq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anatomical Record. 1992;232(2):194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 93.Faa G, Gerosa C, Fanni D, et al. Morphogenesis and molecular mechanisms involved in human kidney development. Journal of Cellular Physiology. 2012;227(3):1257–1268. doi: 10.1002/jcp.22985. [DOI] [PubMed] [Google Scholar]
- 94.Song R, Yosypiv IV. Genetics of congenital anomalies of the kidney and urinary tract. Pediatric Nephrology. 2011;26(3):353–364. doi: 10.1007/s00467-010-1629-4. [DOI] [PubMed] [Google Scholar]
- 95.El Kares R, Manolescu DC, Lakhal-Chaieb L, et al. A human ALDH1A2 gene variant is associated with increased newborn kidney size and serum retinoic acid. Kidney International. 2010;78(1):96–102. doi: 10.1038/ki.2010.101. [DOI] [PubMed] [Google Scholar]
- 96.Quinlan J, Lemire M, Hudson T, et al. A common variant of the PAX2 gene is associated with reduced newborn kidney size. Journal of the American Society of Nephrology. 2007;18(6):1915–1921. doi: 10.1681/ASN.2006101107. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Z, Quinlan J, Hoy W, et al. A common RET variant is associated with reduced newborn kidney size and function. Journal of the American Society of Nephrology. 2008;19(10):2027–2034. doi: 10.1681/ASN.2007101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hinchliffe SA, Lynch MRJ, Sargent PH, Howard CV, van Velzen D. The effect of intrauterine growth retardation on the development of renal nephrons. British Journal of Obstetrics and Gynaecology. 1992;99(4):296–301. doi: 10.1111/j.1471-0528.1992.tb13726.x. [DOI] [PubMed] [Google Scholar]
- 99.Lelièvre-Pégorier M, Merlet-Bénichou C. The number of nephrons in the mammalian kidney: environmental influences play a determining role. Experimental Nephrology. 2000;8(2):63–65. doi: 10.1159/000020649. [DOI] [PubMed] [Google Scholar]
- 100.Drougia A, Giapros V, Hotoura E, Papadopoulou F, Argyropoulou M, Andronikou S. The effects of gestational age and growth restriction on compensatory kidney growth. Nephrology Dialysis Transplantation. 2009;24(1):142–148. doi: 10.1093/ndt/gfn431. [DOI] [PubMed] [Google Scholar]
- 101.Keijzer-Veen MG, Devos AS, Meradji M, Dekker FW, Nauta J, van der Heijden BJ. Reduced renal length and volume 20 years after very preterm birth. Pediatric Nephrology. 2010;25(3):499–507. doi: 10.1007/s00467-009-1371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schmidt IM, Chellakooty M, Boisen KA, et al. Impaired kidney growth in low-birth-weight children: distinct effects of maturity and weight for gestational age. Kidney International. 2005;68(2):731–740. doi: 10.1111/j.1523-1755.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 103.Bacchetta J, Harambat J, Dubourg L, et al. Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney International. 2009;76(4):445–452. doi: 10.1038/ki.2009.201. [DOI] [PubMed] [Google Scholar]
- 104.Rodríguez MM, Gómez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatric and Developmental Pathology. 2004;7(1):17–25. doi: 10.1007/s10024-003-3029-2. [DOI] [PubMed] [Google Scholar]
- 105.Sutherland MR, Gubhaju L, Moore L, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. Journal of the American Society of Nephrology. 2011;22(7):1365–1374. doi: 10.1681/ASN.2010121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ. Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. The American Journal of Physiology. 2009;297(6):F1668–F1677. doi: 10.1152/ajprenal.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boubred F, Vendemmia M, Garcia-Meric P, Buffat C, Millet V, Simeoni U. Effects of maternally administered drugs on the fetal and neonatal kidney. Drug Safety. 2006;29(5):397–419. doi: 10.2165/00002018-200629050-00004. [DOI] [PubMed] [Google Scholar]
- 108.Nehiri T, van Huyen J-PD, Viltard M, et al. Exposure to maternal diabetes induces salt-sensitive hypertension and impairs renal function in adult rat offspring. Diabetes. 2008;57(8):2167–2175. doi: 10.2337/db07-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Simeoni U, Barker DJ. Offspring of diabetic pregnancy: long-term outcomes. Seminars in Fetal and Neonatal Medicine. 2009;14(2):119–124. doi: 10.1016/j.siny.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 110.Galinsky R, Moss TJM, Gubhaju L, Hooper SB, Jane Black M, Polglase GR. Effect of intra-amniotic lipopolysaccharide on nephron number in preterm fetal sheep. The American Journal of Physiology. 2011;301(2):F280–F285. doi: 10.1152/ajprenal.00066.2011. [DOI] [PubMed] [Google Scholar]
- 111.Buffat C, Boubred F, Mondon F, et al. Kidney gene expression analysis in a rat model of intrauterine growth restriction reveals massive alterations of coagulation genes. Endocrinology. 2007;148(11):5549–5557. doi: 10.1210/en.2007-0765. [DOI] [PubMed] [Google Scholar]
- 112.Dickinson H, Walker DW, Wintour EM, Moritz K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. The American Journal of Physiology. 2007;292(1):R453–R461. doi: 10.1152/ajpregu.00481.2006. [DOI] [PubMed] [Google Scholar]
- 113.Abdel-Hakeem AK, Henry TQ, Magee TR, et al. Mechanisms of impaired nephrogenesis with fetal growth restriction: altered renal transcription and growth factor expression. The American Journal of Obstetrics and Gynecology. 2008;199(3):e1–e7. doi: 10.1016/j.ajog.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singh RR, Moritz KM, Bertram JF, Cullen-McEwen LA. Effects of dexamethasone exposure on rat metanephric development: in vitro and in vivo studies. The American Journal of Physiology. 2007;293(2):F548–F554. doi: 10.1152/ajprenal.00156.2007. [DOI] [PubMed] [Google Scholar]
- 115.Welham SJM, Riley PR, Wade A, Hubank M, Woolf AS. Maternal diet programs embryonic kidney gene expression. Physiological Genomics. 2005;22:48–56. doi: 10.1152/physiolgenomics.00167.2004. [DOI] [PubMed] [Google Scholar]
- 116.Welham SJM, Wade A, Woolf AS. Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney International. 2002;61(4):1231–1242. doi: 10.1046/j.1523-1755.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- 117.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatric Research. 2001;49(4):460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 118.Vilar J, Lalou C, van Huyen J-PD, et al. Midkine is involved in kidney development and in its regulation by retinoids. Journal of the American Society of Nephrology. 2002;13(3):668–676. doi: 10.1681/ASN.V133668. [DOI] [PubMed] [Google Scholar]
- 119.Vaiman D, Gascoin-Lachambre G, Boubred F, et al. The intensity of IUGR-induced transcriptome deregulations is inversely correlated with the onset of organ function in a rat model. PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0021222.e21222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harrison M, Langley-Evans SC. Intergenerational programming of impaired nephrogenesis and hypertension in rats following maternal protein restriction during pregnancy. British Journal of Nutrition. 2009;101(7):1020–1030. doi: 10.1017/S0007114508057607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. The American Journal of Physiology. 2003;285(5):R962–R970. doi: 10.1152/ajpregu.00201.2003. [DOI] [PubMed] [Google Scholar]
- 122.Bertram JF, Soosaipillai MC, Ricardo SD, Ryan GB. Total numbers of glomeruli and individual glomerular cell types in the normal rat kidney. Cell and Tissue Research. 1992;270(1):37–45. doi: 10.1007/BF00381877. [DOI] [PubMed] [Google Scholar]
- 123.Jones SE, Nyengaard JR, Flyvbjerg A, Bilous RW, Marshall SM. Birth weight has no influence on glomerular number and volume. Pediatric Nephrology. 2001;16(4):340–345. doi: 10.1007/s004670000559. [DOI] [PubMed] [Google Scholar]
- 124.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuña G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatric Research. 2005;58(3):510–515. doi: 10.1203/01.PDR.0000179410.57947.88. [DOI] [PubMed] [Google Scholar]
- 125.Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney International. 2001;59(5):1663–1669. doi: 10.1046/j.1523-1755.2001.0590051663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41(2):328–334. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lloyd LJ, Foster T, Rhodes P, Rhind SM, Gardner DS. Protein-energy malnutrition during early gestation in sheep blunts fetal renal vascular and nephron development and compromises adult renal function. Journal of Physiology. 2012;590(2):377–393. doi: 10.1113/jphysiol.2011.220186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mitchell EKL, Louey S, Cock ML, Harding R, Black MJ. Nephron endowment and filtration surface area in the kidney after growth restriction of fetal sheep. Pediatric Research. 2004;55(5):769–773. doi: 10.1203/01.PDR.0000120681.61201.B4. [DOI] [PubMed] [Google Scholar]
- 129.Schreuder MF, Nyengaard JR, Remmers F, van Wijk JAE, Delemarre-Van de Waal HA. Postnatal food restriction in the rat as a model for a low nephron endowment. The American Journal of Physiology. 2006;291(5):F1104–F1107. doi: 10.1152/ajprenal.00158.2006. [DOI] [PubMed] [Google Scholar]
- 130.Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM. Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. Journal of the American Society of Nephrology. 2007;18(6):1688–1696. doi: 10.1681/ASN.2007010015. [DOI] [PubMed] [Google Scholar]
- 131.Schwedler SB, Gilbert T, Moreau E, Striker LJ, Merlet-Bénichou C, Striker GE. Nephrotoxin exposure in utero reduces glomerular number in sclerosis-prone but not in sclerosis-resistant mice. Kidney International. 1999;56(5):1683–1690. doi: 10.1046/j.1523-1755.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- 132.Nwagwu MO, Cook A, Langley-Evans SC. Evidence of progressive deterioration of renal function in rats exposed to a maternal low-protein diet in utero . British Journal of Nutrition. 2000;83(1):79–85. [PubMed] [Google Scholar]
- 133.Chen J, Xu H, Shen Q, Guo W, Sun L. Effect of postnatal high-protein diet on kidney function of rats exposed to intrauterine protein restriction. Pediatric Research. 2010;68(2):100–104. doi: 10.1203/PDR.0b013e3181e5bc33. [DOI] [PubMed] [Google Scholar]
- 134.Chevalier RL. Reduced renal mass in early postnatal development. glomerular dynamics in the guinea pig. Biology of the Neonate. 1983;44(3):158–165. doi: 10.1159/000241710. [DOI] [PubMed] [Google Scholar]
- 135.Jennings BJ, Ozanne SE, Dorling MW, Hales CN. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Letters. 1999;448(1):4–8. doi: 10.1016/s0014-5793(99)00336-1. [DOI] [PubMed] [Google Scholar]
- 136.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41(3):457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 137.Correa-Rotter R, Hostetter TH, Rosenberg ME. Effect of dietary protein on renin and angiotensinogen gene expression after renal ablation. The American Journal of Physiology. 1992;262(4):F631–F638. doi: 10.1152/ajprenal.1992.262.4.F631. [DOI] [PubMed] [Google Scholar]
- 138.Schrijvers BF, Rasch R, Tilton RG, Flyvbjerg A. High protein-induced glomerular hypertrophy is vascular endothelial growth factor-dependent. Kidney International. 2002;61(5):1600–1604. doi: 10.1046/j.1523-1755.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 139.Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney International. 2005;68(5):2180–2188. doi: 10.1111/j.1523-1755.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 140.Luyckx VA, Compston CA, Simmen T, Mueller TF. Accelerated senescence in kidneys of low-birth-weight rats after catch-up growth. The American Journal of Physiology. 2009;297(6):F1697–F1705. doi: 10.1152/ajprenal.00462.2009. [DOI] [PubMed] [Google Scholar]
- 141.Schmidt IM, Damgaard IN, Boisen KA, et al. Increased kidney growth in formula-fed versus breast-fed healthy infants. Pediatric Nephrology. 2004;19(10):1137–1144. doi: 10.1007/s00467-004-1567-0. [DOI] [PubMed] [Google Scholar]
- 142.Escribano J, Luque V, Ferre N, et al. Increased protein intake augments kidney volume and function in healthy infants. Kidney International. 2011;79(7):783–790. doi: 10.1038/ki.2010.499. [DOI] [PubMed] [Google Scholar]
- 143.Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. The American Journal of Physiology. 2008;294(4):F685–F696. doi: 10.1152/ajprenal.00324.2007. [DOI] [PubMed] [Google Scholar]
- 144.Reese PP, Simon MK, Stewart J, Bloom RD. Medical follow-up of living kidney donors by 1 year after nephrectomy. Transplantation Proceedings. 2009;41(9):3545–3550. doi: 10.1016/j.transproceed.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jones SE, White KE, Flyvbjerg A, Marshall SM. The effect of intrauterine environment and low glomerular number on the histological changes in diabetic glomerulosclerosis. Diabetologia. 2006;49(1):191–199. doi: 10.1007/s00125-005-0052-z. [DOI] [PubMed] [Google Scholar]
- 146.Lim K, Lombardo P, Schneider-Kolsky M, Hilliard L, Denton KM, Jane Black M. Induction of hyperglycemia in adult intrauterine growth-restricted rats: effects on renal function. The American Journal of Physiology. 2011;301(2):F288–F294. doi: 10.1152/ajprenal.00564.2010. [DOI] [PubMed] [Google Scholar]
- 147.do Carmo JM, Tallam LS, Roberts JV, et al. Impact of obesity on renal structure and function in the presence and absence of hypertension: evidence from melanocortin-4 receptor-deficient mice. The American Journal of Physiology. 2009;297(3):R803–R812. doi: 10.1152/ajpregu.00187.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Heidemann C, Schulze MB, Franco OH, Van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation. 2008;118(3):230–237. doi: 10.1161/CIRCULATIONAHA.108.771881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Odermatt A. The western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. The American Journal of Physiology. 2011;301(5):F919–F931. doi: 10.1152/ajprenal.00068.2011. [DOI] [PubMed] [Google Scholar]
- 150.Zarraga IGE, Schwarz ER. Impact of dietary patterns and interventions on cardiovascular health. Circulation. 2006;114(9):961–973. doi: 10.1161/CIRCULATIONAHA.105.603910. [DOI] [PubMed] [Google Scholar]
- 151.Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Annals of Internal Medicine. 2003;138(6):460–I51. doi: 10.7326/0003-4819-138-6-200303180-00009. [DOI] [PubMed] [Google Scholar]
- 152.du Cailar G, Ribstein J, Mimran A. Dietary sodium and target organ damage in essential hypertension. The American Journal of Hypertension. 2002;15(3):222–229. doi: 10.1016/s0895-7061(01)02287-7. [DOI] [PubMed] [Google Scholar]
- 153.Verhave JC, Hillege HL, Burgerhof JGM, et al. Sodium intake affects urinary albumin excretion especially in overweight subjects. Journal of Internal Medicine. 2004;256(4):324–330. doi: 10.1111/j.1365-2796.2004.01390.x. [DOI] [PubMed] [Google Scholar]
- 154.Kett MM, Denton KM. Renal programming: cause for concern? The American Journal of Physiology. 2011;300(4):R791–R803. doi: 10.1152/ajpregu.00791.2010. [DOI] [PubMed] [Google Scholar]
- 155.Simonetti GD, Raio L, Surbek D, Nelle M, Frey FJ, Mohaupt MG. Salt sensitivity of children with low birth weight. Hypertension. 2008;52(4):625–630. doi: 10.1161/HYPERTENSIONAHA.108.114983. [DOI] [PubMed] [Google Scholar]
- 156.Manning J, Beutler K, Knepper MA, Matti Vehaskari V. Upregulation of renal BSC1 and TSC in prenatally programmed hypertension. The American Journal of Physiology. 2002;283(1):F202–F206. doi: 10.1152/ajprenal.00358.2001. [DOI] [PubMed] [Google Scholar]
- 157.Moritz KM, de Matteo R, Dodic M, et al. Prenatal glucocorticoid exposure in the sheep alters renal development in utero: implications for adult renal function and blood pressure control. The American Journal of Physiology. 2011;301(2):R500–R509. doi: 10.1152/ajpregu.00818.2010. [DOI] [PubMed] [Google Scholar]
- 158.Ojeda NB, Johnson WR, Dwyer TM, Alexander BT. Early renal denervation prevents development of hypertension in growth-restricted offspring. Clinical and Experimental Pharmacology and Physiology. 2007;34(11):1212–1216. doi: 10.1111/j.1440-1681.2007.04754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53(2):404–408. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sherman RC, Langley-Evans SC. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clinical Science. 2000;98(3):269–275. [PubMed] [Google Scholar]
- 161.Lopez-Bermejo A, Sitjar C, Cabacas A, et al. Prenatal programming of renal function: the estimated glomerular filtration rate is influenced by size at birth in apparently healthy children. Pediatric Research. 2008;64(1):97–99. doi: 10.1203/PDR.0b013e31817282db. [DOI] [PubMed] [Google Scholar]
- 162.Lutter CK, Lutter R. Fetal and early childhood undernutrition, mortality, and lifelong health. Science. 2012;337(6101):1495–1499. doi: 10.1126/science.1224616. [DOI] [PubMed] [Google Scholar]


