Abstract
We studied 351 patients with smoldering multiple myeloma (SMM) in whom the underlying primary molecular cytogenetic subtype could be determined based on cytoplasmic immunoglobulin fluorescent in situ hybridization studies. Hundred and fifty-four patients (43.9%) had trisomies, 127 (36.2%) had immunoglobulin heavy chain (IgH) translocations, 14 (4%) both trisomies and IgH translocations, 53 (15.1%) no abnormalities detected and 3 (0.9%) had monosomy13/del(13q) in the absence of any other abnormality. Among 127 patients with IgH translocations, 57 were t(11;14), 36 t(4;14), 11 musculoaponeurotic fibrosarcoma (MAF) translocations, and 23 other or unknown IgH translocation partner. Time to progression (TTP) to symptomatic multiple myeloma was significantly shorter in patients with the t(4;14) compared with patients with t(11;14), median 28 versus 55 months, respectively, P = 0.025. The median TTP was 28 months with t(4;14) (high-risk), 34 months with trisomies alone (intermediate-risk), 55 months with t(11;14), MAF translocations, other/unknown IgH translocations, monosomy13/del(13q) without other abnormalities, and those with both trisomies and IgH translocations (standard-risk), and not reached in patients with no detectable abnormalities (low-risk), P = 0.001. There was a trend to shorter TTP with deletion 17p (median TTP, 24 months). Overall survival from diagnosis of SMM was significantly inferior with t(4;14) compared with t(11;14), median 105 versus 147 months, respectively, P = 0.036.
Keywords: smoldering multiple myeloma, cytogenetics, prognosis, biomarker
INTRODUCTION
Multiple myeloma (MM) is a clonal plasma cell malignancy characterized by osteolytic bone lesions, anemia, hypercalcemia and renal failure.1,2 Despite major advances, most patients eventually relapse and die of the disease.3–6 MM is almost always preceded by an asymptomatic premalignant period that when detected clinically is termed either monoclonal gammopathy of undetermined significance (MGUS) or smoldering MM (SMM) depending on the extent of bone marrow involvement and monoclonal (M) protein levels.7,8 MGUS is present in ~ 3 to 4% of the population above the age of 50, and carries a risk of progression to MM or related malignancy of 1% per year, a rate that makes it difficult to study the impact of biological risk factors.9–11 SMM represents an intermediate stage between MGUS and MM, and has a risk of progression to MM or related malignancy of ~ 10% per year making it well suited for trials of preventive therapy as well as studies of risk factors and biomarkers of disease progression.12–14
The disease phenotypically referred to as MM at a molecular level consists of several distinct subtypes, each of which could be considered a unique disease entity.1,2,15,16 At a high level, MM consists of three main cytogenetic subtypes: trisomies (involving the odd-numbered chromosomes), immunoglobulin heavy chain (IgH) translocations involving chromosome 14q32, and a small subset in which there is evidence of both trisomies and IgH translocations. The IgH translocated subgroup includes several distinct cytogenetic types based on the specific partner chromosome and the oncogene that is dysregulated as a result of the translocation. The most common reciprocal translocations in the IgH translocated subgroup of MM are t(11;14), t(4;14), t(14;16), t(14;20) and t(6;14).17 These abnormalities (trisomies and IgH translocations) are referred to as ‘primary’ cytogenetic abnormalities as they originate at the MGUS stage and are felt to be causally related to the onset of clonal plasma cell proliferation. Some patients have loss of chromosome 13 or deletions involving chromosome 13q, referred to as monosomy13/del(13q), and occasionally this occurs in the absence of any other primary cytogenetic abnormality. Monosomy 13/del(13q) may occur anytime during the disease course from MGUS to MM and is generally not thought to be causally related to the onset of clonal proliferation.15,16,18 Other abnormalities such as deletions involving chromosome 17p, referred to as del(17p), are typically considered late events, indicative of MM rather than premalignancy.
Although several biomarkers have been described to predict risk of progression in MGUS and SMM,19–22 there are no studies investigating risk of progression according to the underlying cytogenetic subtype of the disease. Similarly, although numerous studies have described prognostic differences in patients with MM based on the underlying cytogenetic type,15,16,23,24 there are no data on whether the risk of progression from premalignancy to MM differs based on the primary cytogenetic abnormality. The purpose of this study was to examine the impact of the underlying cytogenetic subtype on the risk of progression to malignancy in a well-defined cohort of patients with SMM.
PATIENTS AND METHODS
Study cohort
Patients were identified by searching a computerized database and reviewing the medical records of all patients meeting the International Myeloma Working Group definition of SMM in whom the underlying molecular cytogenetic subtype could be determined based on cytoplasmic immunoglobulin fluorescent in situ hybridization (FISH) studies of bone marrow plasma cells.25,26 Patients were seen at the Mayo Clinic from January 1991 through June 2010. All patients had ≥ 10% bone marrow plasma cells and/or serum M protein ≥ 3 g/dl, plus absence of hypercalcemia, renal insufficiency, anemia or lytic bone lesions attributable to a plasma cell disorder. Patients who had received prior chemotherapy or had an existing diagnosis of AL amyloidosis at the time of SMM diagnosis were excluded. Approval for the study was obtained from the Mayo Clinic Institutional Review Board according to federal regulations, and in accordance with the Declaration of Helsinki.
Mayo Clinic electronic medical records, including demographic data; physician notes; laboratory tests; imaging studies; and pathologic reports, such as bone marrow aspirate and biopsy, were reviewed. Relevant laboratory data including bone marrow plasma cell percentage, serum and urine M protein, free light chain (FLC) ratio, hemoglobin, calcium and creatinine were abstracted for analysis.
Molecular cytogenetic classification
All cytoplasmic immunoglobulin FISH studies were performed for clinical purposes at the Mayo Clinic, Rochester, MN, USA, as previously described.27,28 Briefly, aspirate samples were enriched for mononuclear cells using the ACK lyse and cytospin slides were prepared. FISH analysis was performed using the following probes: 3cen (D3Z1), 7cen (D7Z1), 9cen (D9Z1), 15cen (D15Z4), 11q13 (CCND1-XT), 14q32 (IGH-XT), 13q14 (RB1), 13q34 (LAMP1), 14q32 (5′IGH,3′IGH), 17p13.1 (p53) and 17cen (D17Z1). Additional probes as needed were used to detect t(4;14), t(14;16), t(14;20) and other abnormalities based on the results of the initial screen. For the purposes of this study, presence of trisomies of one or more odd-numbered chromosomes was classified as trisomies. A patient was classified into the specific trisomies and IgH translocation categories regardless of when these abnormalities were detected in the course of the disease, including after progression to MM, as these abnormalities are considered primary and present from the initial MGUS stage. Conversely, monosomy13/del(13q) and del(17p) were considered only if they were detected when the patient was in the SMM stage, and at least 6 months or more before any progression event. Patients were initially classified into eight non-overlapping primary cytogenetic groups: trisomies, t(11;14), t(4;14), musculoaponeurotic fibrosarcoma (MAF) translocations (t(14;16) or t(14;20)), unknown/other IgH translocation partner, both trisomies and IgH translocations, monosomy13/del(13q)in the absence of any other primary cytogenetic abnormality, and normal or insufficient plasma cells. Subsequently, groups were pooled together for additional analyses. The prognostic value of monosomy13/del(13q) and del(17p) detected before disease progression was studied separately, comparing patients with and without these abnormalities, regardless of other primary cytogenetic abnormalities.
Statistical analysis
Calculations were performed using SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). Two-sided Fisher exact tests were used to test for differences between categorical variables. Two-sided Wilcoxon rank-sum tests were used to compare continuous variables. Time to progression (TTP) measured from the date of SMM diagnosis until progression to MM was the primary end point. Secondary endpoints studied included TTP from SMM diagnosis until progression to MM or related disorder (AL amyloidosis and plasmacytoma), and overall survival. Kaplan–Meier analysis was performed to generate survival curves. Groups were compared with the two-tailed log-rank test. For univariate and multivariate analysis, bone marrow plasma cell percentage, serum M protein size and serum-FLC ratio were studied as continuous variables. Multivariate analysis was performed using Cox’s proportional hazards model. Median follow-up time was calculated using the reverse Kaplan–Meier method.
RESULTS
Patient characteristics
A total of 351 patients (172 women and 179 men) were studied. Patient characteristics at SMM diagnosis are shown in Table 1. The median follow-up for the cohort was 82 months from diagnosis of SMM. The median age at SMM diagnosis was 63 years (range, 26–90 years). The light chain type was κ in 60%, λ in 37% and biclonal in 3%. The heavy chain type was IgG in 73%, IgA in 16%, 9% other (biclonal, light chain only and IgM), and 1% negative or indeterminate. The serum-FLC level at baseline was available in 246 patients (70%). The FLC ratio was abnormal (reference range <0.26 or >1.65) in 84% of patients (n =206).
Table 1.
Baseline characteristics of patients with smoldering multiple myeloma
| Patients N = 351 | |
|---|---|
| Median age, years (range) | 63 (26–90) |
| Female sex, N (%) | 172 (49) |
| Serum monoclonal protein spike (n = 302), median (range) (g/dl) | 2.15 (0.0–4.9) |
| Urine monoclonal protein spike (n = 258), N (%) | |
| Not present | 125 |
| Detected on immunofixation only | 64 |
| Less than 0.5 g per 24 h | 51 |
| 0.5 grams per 24 h or more | 18 |
| Bone marrow plasma cell percentage (n = 351), median (range) | 20 (4–90) |
| Serum-FLC assay (n = 246), median (range) | |
| Serum-FLC involved/uninvolved ratio, median (range) | 11.6 (1.09–7905.66) |
| Abnormal kappa/lambda FLC ratio (< 0.26 or > 1.65) (%) | 84 |
| Abnormal involved/uninvolved FLC ratio ≥ 8 (%) | 69 |
Abbreviation: FLC, free light chain.
Cytogenetic classification
The distribution of patients by the various primary cytogenetic categories is given in Table 2. Overall, 154 patients (43.9%) had trisomies, 127 (36.2%) had IgH translocations, 14 (4%) had both trisomies and IgH translocations, 53 (15.1%) had no abnormalities detected (36 normal and 17 insufficient) and 3 patients (0.9%) had monosomy13/del(13q) in the absence of any other abnormality. Among patients with MAF translocations (n =11), eight had translocation t(14;16) and three had t(14;20). Of the patients with both trisomies and IgH translocations (n =14), two had t(4;14), three had t(11;14), one had t(14;20), and the rest had other or unknown partner IgH translocation. Monosomy13/del(13q), with or without primary cytogenetic abnormalities, was detected in 42 patients before disease progression to MM, while del(17p) before disease progression was present in 6 patients.
Table 2.
Distribution of primary cytogenetic categories of smoldering multiple myeloma
| Cytogenetic classification by fluorescent in situ hybridization | Overall (n = 351) No. of patients (%) |
|---|---|
| Trisomy(ies) without IgH translocation | 154 (43.9%) |
| t(11;14)(q13;q32) | 57 (16.2%) |
| t(4;14)(p16;q32) | 36 (10.3%) |
| MAF translocations (t(14;16)(q32;q23) and t(14;20)(q32;q11)) | 11 (3.1%) |
| Other/unknown IgH translocation partner | 23 (6.6%) |
| Both IgH translocation and trisomy (ies) | 14 (4%) |
| Monosomy13/del(13q) in absence of IgH translocation or trisomies | 3 (0.9%) |
| Normal or insufficient | 53 (15.1%) |
Abbreviation: MAF, musculoaponeurotic fibrosarcoma.
Outcomes
During the follow-up period, 219 SMM patients (62.4%) progressed to symptomatic MM. Eight additional patients developed progression to related disorders (AL amyloidosis). The median TTP to MM was 48 months (95% confidence interval, 37–59); TTP to MM or related disorder was 43 months (95% confidence interval, 35–51). On univariate analysis previously established, prognostic markers were significantly associated with risk of progression to MM, including size of the serum M spike (P =0.05), abnormal FLC ratio <0.26 or >1.65 (P =0.001), and bone marrow plasma cell percentage (P =0.001). The median overall survival was 135 months (95% confidence interval, 118–152).
Impact of cytogenetic abnormalities on progression
Patients with t(4;14) had a significantly higher risk of progression compared with t(11;14), median TTP to MM 28 months versus 55 months, respectively, HR, P =0.025, (Figure 1). Corresponding values for median TTP to MM or related disorder were 28 versus 50 months, respectively, P =0.04.
Figure 1.
TTP of SMM to symptomatic MM. The TTP was significantly inferior in patients with the t(4;14) (HR) compared with patients with t(11;14) (SR), median 28 versus 55 months, respectively, P = 0.025.
The risk of progression to SMM was not affected by the presence of monosomy13/del(13q) at baseline, median TTP 48 versus 55 months, respectively, P = 0.9. There were too few patients with del(17p) at baseline (n =6) to make definitive comparisons, but there was a trend to higher risk of progression with a median TTP of 24 months among patients with del(17p) compared with 50 months in patients without del(17p). All six patients with del(17p) had concurrent trisomies.
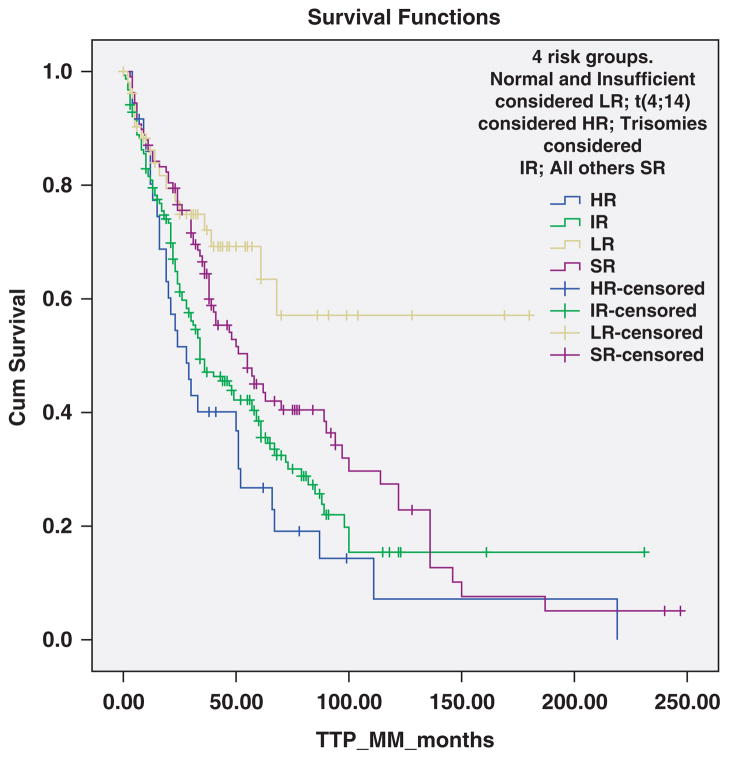
Patients were categorized into four cytogenetically distinct groups based on risk of progression (Figure 2). Patients with t(4;14) were considered high-risk (HR); trisomies alone were considered intermediate-risk; those with t(11;14), MAF translocations, other/unknown 14q32 translocations, monosomy13/del(13q) alone without other abnormalities, and those with both trisomies and IgH translocations considered standard-risk; and patients with normal FISH or insufficient plasma cells were considered low-risk. The risk of progression to MM was significantly different between the four groups, with median TTP to MM 28, 34, 55 months, and not reached, respectively, P = 0.001 (Figure 2). The corresponding values for median TTP to MM or related disorder were 28, 34, 50 and 101 months, respectively, P = 0.002. The median TTP to MM for the trisomies group and the overall P-value (P = 0.001) were unchanged when the analysis was repeated after removing the six patients with del(17p).
Figure 2.
TTP of SMM to symptomatic MM according to four risk groups defined by primary cytogenetic abnormalities. The median TTP was 28 months with the t(4;14) (HR), 34 months with trisomies alone (IR), 55 months with t(11;14), MAF translocations, other/unknown 14q32 translocations, monosomy13/del(13q) without other abnormalities, and those with both trisomies and IgH translocations (SR), and not reached in patients with no detectable abnormalities (LR), P = 0.001.
As patients with del(17p) had a median TTP to MM of 24 months, the analysis by the four risk groups was performed after amending the HR group to include del(17p). In this modified analysis, the two patients with t(4;14) who also had trisomies (one progressed after 13 months and one was progression free at 21 months) were also considered HR (Table 3). The risk of progression to MM was significantly different between the revised four groups, with median TTP to MM of 24, 34, 55 months, and not reached, respectively, P = 0.001 (Figure 3).
Table 3.
Cytogenetically defined risk-based classification of SMM
| Risk | Cytogenetic class | No. of patients (%)a | Median TTP to myeloma (months)* | Median TTP to myeloma or related disorder (months)** | Median OS from SMM diagnosis (months)*** | Median OS from MM diagnosis (months)****,b |
|---|---|---|---|---|---|---|
| High-riska | t(4;14), del(17p) | 44 (12.5%) | 24 | 24 | 105 | 60 |
| Intermediate- risk | Trisomy (ies) without IgH translocation | 148 (42.2%) | 34 | 34 | 135 | 77 |
| Standard-risk | t(11;14) MAF translocations, t14;16 or t(14;20) Other/unknown IgH translocation partner Both trisomies and IgH translocation except t(4;14) Monosomy13/del(13q) in absence of IgH translocation or trisomies |
106 (30.2%) | 55 | 54 | 147 | 86 |
| Low-risk | No abnormalities (normal or insufficient) | 53 (15.1%) | Not reached | 101 | 135 | 112 |
Abbreviations: IgH, immunoglobulin heavy chain; MAF, musculoaponeurotic fibrosarcoma; MM, multiple myeloma; OS, overall survival; SMM, smoldering multiple myeloma; TTP, time to progression.
P = 0.001.
P = 0.002.
P = 0.12 (global); P = 0.02 (high-risk versus standard-risk).
P = 0.04.
Modified to include del(17p) and t(4;14) with concurrent trisomies.
N = 219 patients.
Figure 3.
TTP of SMM to symptomatic MM according to modified cytogenetically defined risk-based classification. The median TTP was 24 months with the t(4;14)/del(17p) (HR), 34 months with trisomies alone (IR), 55 months with t(11;14), MAF translocations, other/unknown 14q32 translocations, monosomy13/del(13q) without other abnormalities, and those with both trisomies and IgH translocations except t(4;14) (SR), and not reached in patients with no detectable abnormalities (LR), P = 0.002.
On multivariate analysis, the increased risk of progression associated with t(4;14) remained significant (P = 0.01) in a model that included bone marrow plasma cell percentage (P = 0.001), but was not independent of the serum-FLC ratio (P-value 0.44 and 0.021, respectively). Similarly, the four-group risk model retained significance (P<0.001) in a model that included bone marrow plasma cell percentage (P = 0.001); but was not independent of the serum-FLC ratio. In a subset of 68 patients with serum M protein ≥ 3 g/dl and bone marrow plasma cell percentage ≥ 10%, the TTP to MM was 28, 21 and 55 months in the revised HR, IR and SR groups, respectively, P=0.03; there were only two patients with LR in this subset, and thus they were excluded from the survival comparisons.
Impact of cytogenetic abnormalities on overall survival in SMM
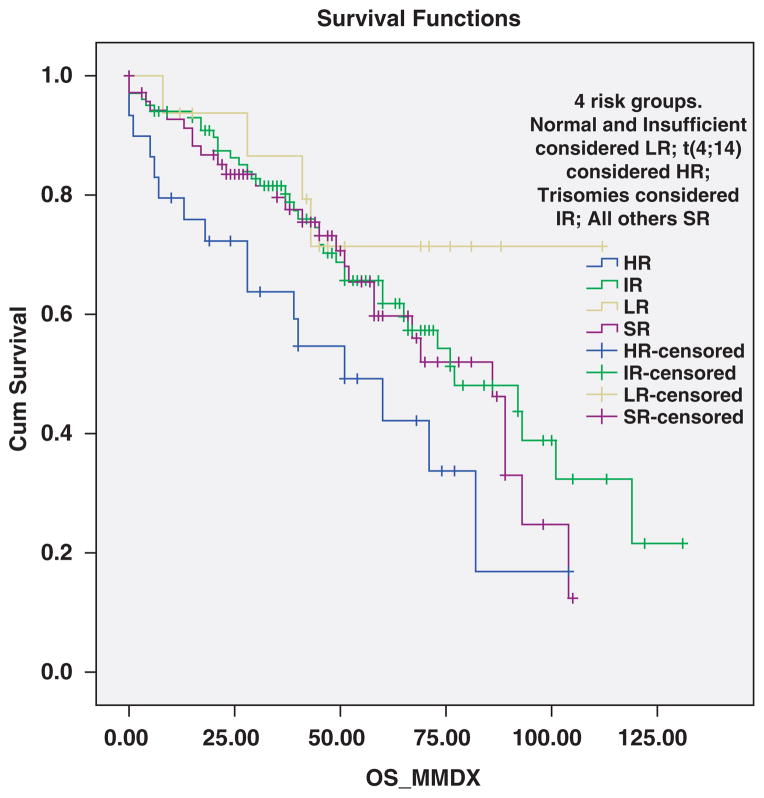
Median survival of the cohort from time of SMM diagnosis was 135 months; median survival from MM diagnosis was 77 months. The t(4;14) SMM was associated with significantly inferior overall survival measured from diagnosis of SMM compared with patients with t(11;14) SMM, 105 versus 147 months, respectively, P = 0.036 (Figure 4). When the analysis was restricted to patients with eventual progression to MM, median survival was 89 versus 141 months, respectively, P = 0.06. Overall survival measured from diagnosis of SMM appeared similar among the other cytogenetic groups of SMM; thus median survival for the four groups classified according to risk of progression was 105, 135, 141 and 135 months in the high, intermediate, standard and low-risk groups, respectively, P = 0.25. However, survival times measured from time of MM diagnosis was as expected significantly different across the four groups with median times of 51, 77, 86 and 112 months, respectively, P = 0.04 (Figure 5). The outcomes by the revised risk groups in which del(17p) patients and patients with t(4;14) and trisomies are considered HR is shown in Table 3.
Figure 4.
Overall survival from time of diagnosis of SMM. Survival was significantly inferior in patients with the t(4;14) (HR) compared with patients with t(11;14) (SR), median 105 versus 147 months, respectively, P = 0.036.
Figure 5.
Overall survival from diagnosis of symptomatic MM according to the four cytogenetically defined risk groups. Survival was 51 months with t(4;14) (HR), 77 months with trisomies alone (IR), 86 months with t(11;14), MAF translocations, other/unknown 14q32 translocations, monosomy13/del(13q) without other abnormalities, and those with both trisomies and IgH translocations (SR), and 112 months in patients with no detectable abnormalities (LR), P = 0.04.
DISCUSSION
In this study, we show for the first time the significant impact of primary cytogenetic abnormalities on progression to MM. Several studies have shown that primary cytogenetic abnormalities have a significant impact on prognosis in patients with clonal plasma cell disorders once progression to malignancy occurs.15,16 However, despite the fact that these abnormalities are present at the MGUS and SMM stage,27,29 the impact of the various cytogenetic subtypes on risk of malignant transformation is not known. Studies in this regard have been limited by the fact that sensitive cytogenetic testing for accurate classification has only recently been available and sufficient follow-up is required to capture risk of progression.
Our study shows that patients with t(4;14) have a significantly higher risk of progression to MM compared with the t(11;14) and other cytogenetic categories. This subgroup also had shortened overall survival from SMM diagnosis as well as after progression to MM. Patients with del(17p) at diagnosis of SMM may have a high risk of progression, but there were few cases to make any definitive conclusions. Trisomies that are associated with a favorable prognosis from time of diagnosis of MM were associated with a risk of progression that is intermediate between t(4;14) and t(11;14). This intermediate risk persisted in analysis that excluded six patients with del(17p) all of whom had trisomies. However, in the subset of patients with high M protein levels (≥ 3 g/dl), the TTP to MM with trisomies was similar to HR patients.
Patients with SMM can be broadly classified based on four cytogenetically defined groups as shown in Figure 2. Patients with no detectable abnormality (normal results or low tumor burden reflected as insufficient plasma cells for FISH analysis) had the best outcome. It is felt that all patients with clonal plasma cell disorders have underlying cytogenetic abnormalities provided more sensitive detection methods are used.16 However, the improved outcome in these patients was not fully explained by lower levels of bone marrow plasma cell involvement alone, and thus more study is needed to determine the cytogenetic nature of this subgroup of patients. Although there were limited numbers of patients with t(14;16), t(14;20) and other/unknown 14q32 translocations, these patients had a risk of progression to MM resembling t(11;14). Similarly, as a group, patients with both trisomies and IgH translocations also resembled patients with t(11;14); however, there were only two patients with t(4;14) who also had trisomies to determine whether patients with t(4;14) with concurrent trisomies have a lower risk of progression than patients without trisomies. In a revised model (Figure 3 and Table 3), patients with del(17p) and patients with t(4;14) with concurrent trisomies were also considered HR. This led to an improvement in assessment of risk, and is the model that we propose for clinical prognostic purposes.
Monosomy13/del(13q) had no impact on risk of progression. Initially it was thought that monosomy13/del(13q) was a significant adverse prognostic marker in MM, but later studies showed that even in MM the prognostic effect was seen only in karyotypic studies where the abnormality probably functions as a surrogate marker for hypodiploidy, IgH translocations or proliferation rather than being a true driver of risk.28,30–32 However, the frequent occurrence of this abnormality in early stages of plasma cell dyscrasias despite lack of effect on outcome needs further study.
We found that the adverse impact of t(4;14) was not related to bone marrow plasma cell involvement, as both factors remained independently significant on multivariate analysis. However, the adverse effect of t(4;14) was not independent of an abnormal FLC ratio. We have previously shown that an abnormal FLC ratio is a significant risk factor for progression in SMM.22,33 We have also shown that patients with t(4;14) MM often have markedly high FLC ratios.34 The mechanism by which a high FLC ratio is associated with higher risk of progression is not clear, and is only partly related to renal failure from cast nephropathy. The result of this study suggests that part of the prognostic effect of the FLC ratio (as a predictor of risk of progression in SMM) may in part derive from the fact that it serves as a surrogate biomarker for HR t(4;14) subtype SMM.
Except in rare instances, treatment is not recommended for SMM outside the setting of a clinical trial.14,35 The recommended clinical monitoring for SMM is serial follow-up every 3–4 months with the initiation of treatment upon symptomatic progression.36 As patients with t(4;14) myeloma appear to spend a shorter duration of time in the premalignant stage, and have a worse outcome following diagnosis of MM, the type of follow-up and indications for therapy in this subgroup may need to be adjusted.
Our study provides clear evidence that clinically defined MM consists of several cytogenetically distinct diseases, with variations not just in clinical presentation and prognosis, but also the risk of progression from premalignancy to malignant disease. Patients with t(4;14) translocation have the highest risk of progression followed by patients with trisomies. Patients with del(17p) are also likely in the HR group. So far, therapy has not been recommended for SMM patients,37 and trials with thalidomide,38,39 bisphosphonates,40,41 anakinra (IL-1 receptor antagonist)42 and curcumin43 have not demonstrated a clear benefit of early intervention. However, as evidence favoring early intervention accumulates with newer approaches,44 data on the effect of therapy according to the underlying molecular classification of the disease will be of critical importance.
Acknowledgments
This work was supported by National Cancer Institute grants CA168762, CA 107476, CA 100707, CA90297052 and CA 83724. Also supported in part by ECOG CA 21115T, the Jabbs Foundation (Birmingham, UK), the Henry J. Predolin Foundation, USA, Mayo Clinic Cancer Center and the Mayo Foundation. Rafael Fonseca is a Clinical Investigator of the Damon Runyon Cancer Research Fund.
Footnotes
CONFLICT OF INTEREST
Dr Fonseca has received a patent for the prognostication of MM based on genetic categorization of the disease. He has received consulting fees from Medtronic, Otsuka, Celgene, Genzyme, BMS, Lilly, Onyx, Binding Site, Millenium and AMGEN. He also has sponsored research from Cylene and Onyx. The remaining authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
SVR and SKK designed the research, analyzed the data, wrote and edited the manuscript. VG, RF, AD, WIG, DL, RPK, JAL and RAK participated in data interpretation, reviewed the manuscript and provided critical comments. All authors reviewed and approved the final manuscript.
References
- 1.Rajkumar SV. Treatment of multiple myeloma. Nature Rev Clin Oncol. 2011;8:479–491. doi: 10.1038/nrclinonc.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajkumar SV. Multiple myeloma: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol. 2013;88:225–235. doi: 10.1002/ajh.23390. [DOI] [PubMed] [Google Scholar]
- 3.Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha S, Lacy M, Mikhael J, Hayman S, Buadi F, Detweiler-Short K, et al. Response to salvage therapies and outcome in patients with multiple myeloma relapsing after pomalidomide therapy. Leukemia. 2012;26:839–841. doi: 10.1038/leu.2011.279. [DOI] [PubMed] [Google Scholar]
- 5.Mohty B, El-Cheikh J, Yakoub-Agha I, Avet-Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and ‘retreatment’ approaches in the era of novel agents. Leukemia. 2012;26:73–85. doi: 10.1038/leu.2011.310. [DOI] [PubMed] [Google Scholar]
- 6.Short KD, Rajkumar SV, Larson D, Buadi F, Hayman S, Dispenzieri A, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25:906–908. doi: 10.1038/leu.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113:5418–5422. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis of monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 10.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg AJ, Vachon CM, Rajkumar SV. Disparities in the prevalence, pathogenesis and progression of monoclonal gammopathy of undetermined significance and multiple myeloma between blacks and whites. Leukemia. 2012;26:609–614. doi: 10.1038/leu.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar SV, Merlini G, San Miguel JF. Redefining myeloma. Nature Rev Clin Oncol. 2012;9:494–496. doi: 10.1038/nrclinonc.2012.128. [DOI] [PubMed] [Google Scholar]
- 14.Blade J, Dimopoulos M, Rosinol L, Rajkumar SV, Kyle RA. Smoldering (asymptomatic) multiple myeloma: current diagnostic criteria, new predictors of outcome, and follow-up recommendations. J Clin Oncol. 2010;28:690–697. doi: 10.1200/JCO.2009.22.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonseca R, Barlogie B, Bataille R, Bastard C, Bergsagel PL, Chesi M, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546–1558. doi: 10.1158/0008-5472.can-03-2876. [DOI] [PubMed] [Google Scholar]
- 16.Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar SK, Mikhael JR, Buadi FK, Dingli D, Dispenzieri A, Fonseca R, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) Consensus Guidelines. Mayo Clinic Proc. 2009;84:1095–1110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann H, Ackermann J, Baldia C, Nosslinger T, Wieser R, Seidl S, et al. Both IGH translocations and chromosome 13q deletions are early events in monoclonal gammopathy of undetermined significance and do not evolve during transition to multiple myeloma. Leukemia. 2004;18:1879–1882. doi: 10.1038/sj.leu.2403518. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi G, Kyle RA, Larson DR, Witzig TE, Kumar S, Dispenzieri A, et al. High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia. 2012;27:680–685. doi: 10.1038/leu.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kastritis E, Terpos E, Moulopoulos L, Spyropoulou-Vlachou M, Kanellias N, Eleftherakis-Papaiakovou E, et al. Extensive bone marrow infiltration and abnormal free light chain ratio identifies patients with asymptomatic myeloma at high risk for progression to symptomatic disease. Leukemia. 2012 doi: 10.1038/leu.2012.309. advance online publication 27 November 2012. [DOI] [PubMed] [Google Scholar]
- 21.Katzmann JA, Clark R, Kyle RA, Larson DR, Therneau TM, Melton LJ, III, et al. Suppression of uninvolved immunoglobulins defined by heavy/light-chain pair suppression is a risk factor for progression of MGUS. Leukemia. 2012;27:208–212. doi: 10.1038/leu.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen JT, Kumar SK, Dispenzieri A, Kyle RA, Katzmann JA, Rajkumar SV. Serum free light chain ratio as a biomarker for high-risk smoldering multiple myeloma. Leukemia. 2012 doi: 10.1038/leu.2012.296. advance online publication 27 November 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avet-Loiseau H, Magrangeas F, Moreau P, Attal M, Facon T, Anderson K, et al. Molecular heterogeneity of multiple myeloma: pathogenesis, prognosis, and therapeutic implications. J Clin Oncol. 2011;29:1893–1897. doi: 10.1200/JCO.2010.32.8435. [DOI] [PubMed] [Google Scholar]
- 24.Avet-Loiseau H, Soulier J, Fermand JP, Yakoub-Agha I, Attal M, Hulin C, et al. Impact of high-risk cytogenetics and prior therapy on outcomes in patients with advanced relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone. Leukemia. 2010;24:623–628. doi: 10.1038/leu.2009.273. [DOI] [PubMed] [Google Scholar]
- 25.The International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 26.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonseca R, Bailey RJ, Ahmann GJ, Rajkumar SV, Hoyer JD, Lust JA, et al. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 2002;100:1417–1424. [PubMed] [Google Scholar]
- 28.Fonseca R, Harrington D, Oken MM, Dewald GW, Bailey RJ, Van Wier SA, et al. Biological and prognostic significance of interphase fluorescence in situ hybridization detection of chromosome 13 abnormalities in multiple myeloma: an Eastern Cooperative Oncology Group Study. Cancer Res. 2002;62:715–720. [PubMed] [Google Scholar]
- 29.Fonseca R, Aguayo P, Ahmann GJ, Jalal SM, Rajkumar SV, Kyle RA, et al. Translocations at 14q32 are common in patients with the monoclonal gammopathy of undetermined significance (MGUS) and involve several partner chromosomes. Blood. 1999;94(Suppl 1):663a (A 2943). [Google Scholar]
- 30.Tricot G, Barlogie B, Jagannath S, Bracy D, Mattox S, Vesole DH, et al. Poor prognosis in multiple myeloma is associated only with partial or complete deletions of chromosome 13 or abnormalities involving 11q and not with other karyotype abnormalities. Blood. 1995;86:4250–4256. [PubMed] [Google Scholar]
- 31.Avet-Louseau H, Daviet A, Sauner S, Bataille R. Intergroupe Francophone du M. Chromosome 13 abnormalities in multiple myeloma are mostly monosomy 13. Br J Haematol. 2000;111:1116–1117. doi: 10.1046/j.1365-2141.2000.02488.x. [DOI] [PubMed] [Google Scholar]
- 32.Shaughnessy J, Jr, Tian E, Sawyer J, McCoy J, Tricot G, Jacobson J, et al. Prognostic impact of cytogenetic and interphase fluorescence in situ hybridization-defined chromosome 13 deletion in multiple myeloma: early results of total therapy II. Br J Haematol. 2003;120:44–52. doi: 10.1046/j.1365-2141.2003.03948.x. [DOI] [PubMed] [Google Scholar]
- 33.Dispenzieri A, Kyle RA, Katzmann JA, Therneau TM, Larson D, Benson J, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2007 Oct 17;111:785–789. doi: 10.1182/blood-2007-08-108357. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S, Zhang L, Dispenzieri A, Van Wier S, Katzmann JA, Snyder M, et al. Relationship between elevated immunoglobulin free light chain and the presence of IgH translocations in multiple myeloma. Leukemia. 2010;24:1498–1505. doi: 10.1038/leu.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–1127. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyle RA, Durie BGM, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–1127. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexanian R, Barlogie B, Dixon D. Prognosis of asymptomatic multiple myeloma. Arch Intern Med. 1988;148:1963–1965. [PubMed] [Google Scholar]
- 38.Rajkumar SV, Gertz MA, Lacy MQ, Dispenzieri A, Fonseca R, Geyer SM, et al. Thalidomide as initial therapy for early-stage myeloma. Leukemia. 2003;17:775–779. doi: 10.1038/sj.leu.2402866. [DOI] [PubMed] [Google Scholar]
- 39.Witzig TE, Laumann KM, Lacy MQ, Hayman SR, Dispenzieri A, Kumar S, et al. A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia. 2012;27:220–225. doi: 10.1038/leu.2012.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barlogie B, van Rhee F, Shaughnessy JD, Jr, Epstein J, Yaccoby S, Pineda-Roman M, et al. Seven-year median time to progression with thalidomide for smoldering myeloma: partial response identifies subset requiring earlier salvage therapy for symptomatic disease. Blood. 2008;112:3122–3125. doi: 10.1182/blood-2008-06-164228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musto P, Petrucci MT, Bringhen S, Guglielmelli T, Caravita T, Bongarzoni V, et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008;113:1588–1595. doi: 10.1002/cncr.23783. [DOI] [PubMed] [Google Scholar]
- 42.Lust JA, Lacy MQ, Zeldenrust SR, Dispenzieri A, Gertz MA, Witzig TE, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84:114–122. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golombick T, Diamond TH, Manoharan A, Ramakrishna R. Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: a randomized, double-blind placebo-controlled cross-over 4g study and an open-label 8g extension study. Am J Hematol. 2012;87:455–460. doi: 10.1002/ajh.23159. [DOI] [PubMed] [Google Scholar]
- 44.Mateos M, Lopez-Corral L, Hernandez M, Giraldo P, De la Rubia J, De Arriba F, et al. Smoldering multiple myeloma (SMM) at high-risk of progression to symptomatic disease: a phase III, randomized, multicenter trial based on lenalidomide-dexamethasone (Len-Dex) as induction therapy followed by maintenance therapy with Len alone vs no treatment. Blood. 2011;118:3996. [Google Scholar]