Abstract
Current response criteria for light-chain amyloidosis (AL) relegate FLC response to a subsidiary status relative to serum M-protein response. Given that light chains form the substrate for amyloid fibril formation, we hypothesized that changes in FLC might better predict outcome compared to changes in intact immunoglobulin levels. Two patient cohorts were studied, 347 patients who underwent an autologous stem-cell transplant (SCT) and 96 patients treated with melphalan/dexamethasone. We identified the lowest value following therapy for intact serum M-protein and the difference between involved and uninvolved FLC (FLC-diff). We first examined the relative contribution of M-protein and FLC-diff on the overall survival (OS), and found that FLC reduction, rather than M-protein reduction, significantly impacted OS. The median OS was not reached among those with a 50% decrease in FLC-diff compared to 20 months for the remainder. On regression analysis, a 90% reduction in FLC-diff following SCT best predicted being alive at 3 or 5 years. The median OS among those with a 90% decrease was not reached compared to 37.4 months for the rest P < 0.001. The current study supports the notion that FLC response is a more useful measure of hematological response than M-protein response. It also highlights the importance of achieving at least a 90% reduction in the FLC-diff to improve the outcome of patients with light-chain AL. Am. J. Hematol. 86:251–255, 2011.
Introduction
Primary systemic or light-chain amyloidosis (AL) is characterized by clonal proliferation of plasma cells and deposition of immunoglobulin light chain derived amyloid fibrils in various organs [1–3]. Patients with AL, especially in the presence of advanced organ involvement, have a poor outcome [4]. Current treatments for AL are aimed at eradicating the clonal cells in order to reduce the availability of light chains for amyloid formation [5,6]. Effective elimination of the clonal cells has been associated with improvement in organ function and prolongation of survival [6]. The efficacy of treatment can be measured both in terms of the reduction of the clonal plasma cell burden (hematological response) as well as improvement in the organ function (organ response) [5]. The hematological response has traditionally been estimated by the amount of monoclonal protein in the serum or urine while parameters of organ function have been defined for estimating organ response. Given the typically long time interval between hematological response and organ improvement, and studies showing good correlation between degree of hematological response and subsequent organ response, short-term assessment of treatment efficacy has been performed using hematological response as a surrogate for organ response and survival [6–9].
Monoclonal protein in the blood and urine has been traditionally measured using electrophoretic methods, which are technically more suitable for measuring intact monoclonal proteins rather than clonal-free light chains [10]. More recently, the introduction of the serum-free light-chain assay has enabled accurate estimation of kappa and lambda light chains circulating unbound to a heavy chain [7,11,12]. Given that the serum-free light chain forms the substrate for amyloid fibril formation rather than the intact immunoglobulin, we hypothesized that changes in serum-free light chain (sensitive to changes of 1 mg/dL) will be a better predictor of organ improvement and survival outcomes in patients with AL compared to changes in the intact immunoglobulin (sensitive to changes of 50 mg/dL). However, hematological response assessment currently uses the myeloma response criteria, which depend primarily on changes in intact immunoglobulin measured by serum protein electrophoresis. This study was designed to compare changes in serum FLC measurements to changes in SPEP measurements to determine which measurement is a better predictor of outcome in patients with AL. We specifically wanted to examine the impact of free light chain decrease following treatment on outcome among patients with AL, compare serum FLC and serum M-spike responses in terms of eventual outcome, and identify the degree of FLC reduction associated with the best outcome.
Patients and Methods
Study population
Two separate cohorts of patients were included in the current study, 347 patients with AL who underwent an autologous stem-cell transplant (SCT group) and a separate group of 96 patients with AL treated with melphalan and dexamethasone combination (Mel-Dex group), who never underwent a stem-cell transplantation. All patients had biopsy proven light-chain AL. Serum M-protein and FLC measurements (FLC-diff: involved-uninvolved FLC) from baseline and the lowest measurements during follow up, before any other therapy, were collected from medical records and from a prospectively maintained clinical database. The Mayo Foundation Institutional Review Board (IRB) approved the study, and all patients consented to have their medical records reviewed according to IRB practices and Health Insurance Portability and Accountability Act Guidelines.
Treatments
Stem-cell collection, conditioning therapy, and supportive care were as previously reported [13]. Briefly, stem cells were collected following priming with G-CSF alone. G-CSF was administered subcutaneously (10 μg/kg) daily until the completion of peripheral blood stem-cell collection with apheresis beginning on the 5th day after starting G-CSF, provided adequate peripheral blood CD34 counts were achieved. All patients undergoing SCT received conditioning with melphalan alone, usually given at 200 mg/m2 divided over 2 days (100 mg/m2 days −2 and −1). In some patients, melphalan was dose reduced to 140 mg/m2 because of advanced age, renal insufficiency, advanced organ involvement, or poor performance status based on treating physician discretion. Melphalan and dexamethasone were given according to previously published regimen [14]. In a proportion of patients, oral melphalan was replaced by intravenous melphalan given at 16 mg/m2 once every month.
Laboratory methods
Serum protein electrophoresis was performed as previously described. Serum FLC values were either obtained from medical records or measured on archived serum samples as part of a previous study [4]. Immunoglobulin FLC quantitation was carried out as previously described using a serum FLC assay (Freelite; The Binding Site Limited) performed on a Siemens BNII nephelometer (Deerfield, IL). The FLC estimation consists of two separate assays: one to detect free-κ and the other to detect free-λ light chains. The reference range for κFLC is 0.33–1.94 mg/dL; for λFLC is 0.57–2.63 mg/dL, and for the κ:λ ratio is 0.26–1.65. The clonal-free light chain is considered the “involved” immunoglobulin-free light chain for the purpose of the analyses (iFLC) and the other light chain is referred to as the “uninvolved” light chain (uFLC).
Statistical analysis
Fisher’s exact test was used to test differences in nominal variables. Differences in continuous variable between groups were compared using Mann–Whitney or Kruskal–Wallis tests. Survival curves were constructed according to the Kaplan–Meier method, and the survival curves were compared using the log rank test. The impact of the change in FLC-diff and M-spike on overall survival (OS) was examined using Cox-proportional hazards; both as continuous variables as well as by dichotomizing with specific cut-offs. Logistic regression was used to identify cutoffs for these measurements that best predicted survival. All analyses were performed using JMP Statistical software (SAS, Cary, NC).
Results
The first cohort of 347 patients undergoing an autologous SCT for AL had a median age of 57 (range, 31–75) years at diagnosis, 199 (57%) were male, and the median (range) duration from diagnosis to SCT was 4 months (1 month to 6 years). The median estimated follow up for the entire cohort was 72 months (95% CI; 64, 75), and 211 (61%) patients were alive at last follow up. The baseline clinical characteristics and transplant related factors are as described in Table I. Thirty-nine patients (11%) died within 100 days of transplant and were not considered evaluable for response assessments.
TABLE I.
Baseline Variables Before Stem-Cell Transplantation and Transplant-Related Factors (N = 347)
| Variable | Median (Range) |
|---|---|
| Age at SCT | 58 years (31–75) |
| Gender: male (%) | 199 (57%) |
| Duration: diagnosis to transplant | 4 months (0.5–75) |
| Serum creatinine | 1.1 mg/dL (0.6–12) |
| Serum albumin | 2.7 g/dL (0.8–4.4) |
| Bone marrow plasma cell % | 7 (0–78) % |
| Number of organs involved | 2 (1–3) |
| Heart | 147 (50%)a |
| Kidney | 240 (69%)a |
| Liver | 52 (15%)a |
| Treatment before SCT | 147 (42%)a |
| Conditioning regimen: reduced dose Melphalan | 123 (35%)a |
| Serum M Protein and free light chain (FLC) measurements | |
| Serum M-Spike (gm/dL) | 0.1 (0–3.9) |
| Serum M-spike ≥ 1.0 gm/dL | 63 (18%)a |
| Serum M-spike ≥ 0.5 gm/dL | 106 (31%)a |
| Serum FLC difference (Involved-uninvolved) (mg/dL) | 14 (0–900) |
| Serum FLC-diff ≥ 10 mg/dL | 167 (57%)a |
| Serum FLC-diff ≥ 7.5 mg/dL | 193 (66%)a |
| Heavy chain present | 52%a |
| Kappa light chain/lambda light chain | 23%a/76%a |
N (%).
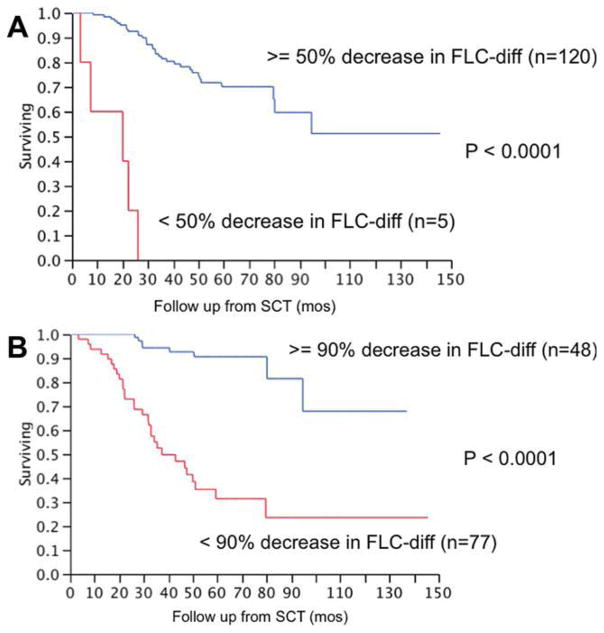
We first examined the impact of the magnitude of decrease in serum M protein (M-protein) and the serum FLC difference (FLC-diff) following therapy on the OS following SCT among patients with serum levels traditionally considered as measurable disease (M-protein > 1.0 g/dL; FLC-diff > 10 mg/dL). In a univariate analysis, using the decrease in M-protein and FLC-diff as continuous variables, we found that the magnitude of decrease in FLC-diff as well as the decrease in M-protein was predictive for longer OS from transplant; however, FLC-diff was associated with a higher hazard ratio (Table II). We then examined both these variables in a multivariate model that included 24 patients who had measurable disease by both criteria and found that changes in FLC-diff were a more powerful predictor of OS following SCT. Given the current response criteria that use a 50% reduction in the M-protein or FLC-diff for a partial response, we repeated the analysis using this cutoff. In univariate analysis, a 50% decrease in either M-protein or FLC-diff was significantly associated with better OS following SCT. In a multivariate analysis that included both the variables, only a 50% reduction in the FLC-dif was predictive of OS (Table III). Among those with baseline FLC-diff ≥ 10 mg/dL (n = 125), the median OS from SCT was not reached for those with at least a 50% decrease in FLC-diff compared to 20 months (95% CI; 3, 22) for the remainder, P < 0.01 (Fig. 1A). When all patients with a baseline FLC-diff > 7.5 mg/dL were included (n = 147), the median OS for those with 50% decrease in FLC-diff was not reached compared to 22 months (95% CI; 3, 26) for the rest (P < 0.01).
TABLE II.
FLC-Diff and M-Spike Response (Lowest/Baseline) as a Continuous Variable for Its Impact on Post-SCT OS
| Variable | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| N | HR | P | N | HR | P | |
| Baseline FLC-diff > 10 mg/dL or/and M-spike > 1.0 gm/dL | ||||||
| FLC-diff | 125 | 1.05 | <0.0001 | 24 | 1.03 | 0.044 |
| M-spike | 50 | 1.03 | 0.005 | 1.002 | 0.9 | |
| Baseline FLC-diff > 7.5 mg/dL or/and M-spike > 0.5 gm/dL | ||||||
| FLC-diff | 147 | 1.05 | <0.0001 | 37 | 1.04 | <0.001 |
| M-spike | 76 | 1.02 | 0.007 | 1.007 | 0.6 | |
TABLE III.
FLC-Diff and M-Spike Response (50% Reduction) for Its Impact on Post-SCT OS
| Variable | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| N | HR | P | N | HR | P | |
| Baseline FLC-diff > 10 mg/dL or/and M-spike > 1.0 g/dL | ||||||
| FLC-diff | 125 | 24 | <0.0001 | 24 | 36 | 0.003 |
| M-spike | 50 | 2.8 | 0.05 | 1.7 | 0.5 | |
| FLC-diff > 7.5 mg/dL or/and M-spike > 0.5 gm/dL | ||||||
| FLC-diff | 147 | 7.8 | <0.0001 | 37 | 29 | <0.0001 |
| M-spike | 76 | 2.02 | 0.07 | 1.1 | 0.9 | |
Figure 1.
Panel A shows the relationship between a 50% decrease in the FLC-diff and the overall survival (OS) from transplant among patients who survived at least 100 days posttransplant and who had a baseline FLC-diff > = 10 mg/dL. The median OS from SCT was not reached among those with a 50% decrease in FLC-diff (n = 120) compared to 20 months (95% CI; 3, 22) for the remaining patients (n = 5). Panel B shows the relationship between a 90% decrease in the FLC-diff and the OS from transplant among patients who survived at least 100 days posttransplant and who had a baseline FLC-diff > = 10 mg/dL. The median OS among those with a 90% decrease in FLC-diff (n = 77) was not reached compared to 37.4 months (95% CI; 32, 58) for the remaining patients (n = 48), P < 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We then performed logistic regression to identify the best reduction of FLC-diff that predicts being alive at 3 and 5 years. The best cutoff was a 90% reduction in FLC-diff following SCT among patients with baseline FLC-diff > = 10 mg/dL (n = 125). The median OS following SCT among those with a 90% decrease in FLC-diff (n = 77) was not reached compared to 37.4 months (95% CI; 32, 58) for the remaining patients (n = 48), P < 0.01 (Fig. 1B). The results were similar when including patients with baseline FLC-diff > = 7.5 mg/dL. When considering this group of patients, median OS following SCT among those with a 90% decrease in FLC-diff (n = 89) was not reached compared to 42.9 months (95% CI; 32, 58) for the remaining patients (n = 58), P < 0.01.
We then validated these endpoints in a cohort of 96 patients who were treated with Melphalan and Dexamethasone combination to examine if these results were applicable in the non-SCT setting. The median age at start of treatment was 64 years (range, 38–82) and 64 (67%) were male (Table IV). The median estimated follow up was 23 months (95% CI; 20, 26) and 36 (40%) were alive at last follow up. Compared to the SCT cohort, only 14% of patients had an M-protein > = 1.0 gm/dL and only 20% had an M-protein > = 0.5 gm/dL. In contrast, nearly three quarters of the patients had a measurable FLC-diff (>10 mg/dL). In fact, only 10 patients had both an M-protein > = 1.0 gm/dL and an FLC-diff > = 10 mg/dL.
TABLE IV.
Baseline Variables Prior to Start of Melphalan and Dexamethasone (N = 96)
| Variable | Median (range) |
|---|---|
| Age at SCT | 64 (38–82) years |
| Gender: male (%) | 64 (67%) |
| Duration: diagnosis to treatment | 1.1 months (0.1–39) |
| Serum creatinine | 1.2 mg/dL (0.5–9.4) |
| Serum albumin | 3.6 gm/dL (1.8–4.2) |
| Bone marrow plasma cell % | 10% (0–65) |
| Number of organs involved | 2 (1–3) |
| Heart | 75 (78%)a |
| Kidney | 57 (59%)a |
| Liver | 29 (30%)a |
| Serum M Protein and free light chain (FLC) measurements | |
| Serum M-spike (gm/dL) | 0.0 (0–4.4) |
| Serum M-spike ≥ 1.0 gm/dL | 13 (14%)a |
| Serum M-spike ≥ 0.5 gm/dL | 19 (20%)a |
| Serum FLC difference (Involved-uninvolved) (mg/dL) | 26 (0.6–433) |
| Serum FLC-diff ≥ 10 mg/dL | 70 (73%)a |
| Serum FLC-diff ≥ 7.5 mg/dL | 78 (81%)a |
N (%).
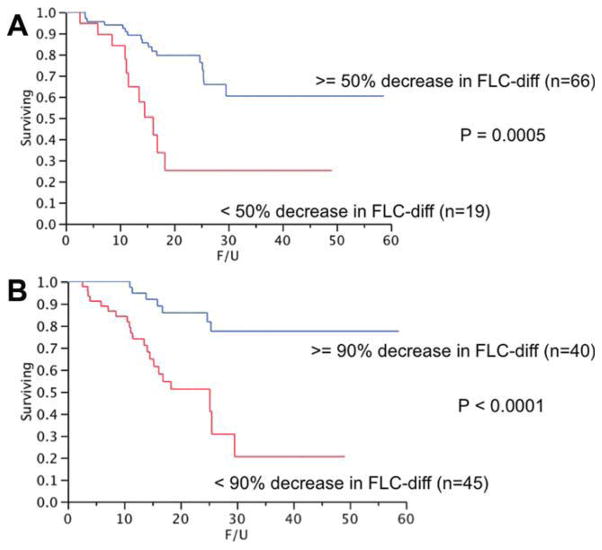
The median OS from start of therapy was not reached for those with > = 50% decrease in FLC-diff compared to 12.2 months (95% CI; 3, 49) for the remainder, P < 0.01 (Fig. 2A). Similarly, the median OS for those attaining a 90% decrease in FLC-diff was not reached compared to 15.3 months (95% CI; 11, 49) for the remaining patients, P < 0.01 (Fig. 2B). Among the patients undergoing SCT, 96% and 38% of the patients surviving beyond day 100 (n = 125) had a 50 and 90% reduction in the FLC-diff, respectively. In comparison, 78% and 47% of patients among the Mel-Dex group receiving at least three cycles of therapy (n = 85) had a 50% and 90% reduction, respectively.
Figure 2.
Panel A shows the relationship between a 50% decrease in the FLC-diff and the overall survival (OS) from start of therapy among patients who received at least three cycles of therapy with melphalan and dexamethasone and who had a baseline FLC-diff > = 10 mg/dL. The median OS was not reached for those with > = 50% decrease in FLC-diff compared to 12.2 months (95% CI; 3, 49) for the rest, P < 0.001. Panel B shows relationship between a 90% decrease in the FLC-diff and the OS from start of therapy among patients who received at least three cycles of therapy with melphalan and dexamethasone and who had a baseline FLC-diff > = 10 mg/dL. The median OS for those attaining a 90% decrease in FLC-diff was not reached compared to 15.3 months (95% CI; 11, 49) for the remaining patients, P < 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
AL represents a plasma-cell proliferative disorder with low-clonal burden in the majority of patients [15,16]. In contrast to multiple myeloma, the immunoglobulin-free light chain secreted by the clonal plasma cells is responsible for the clinical manifestations being the substrate for amyloid fibril formation. Given the current treatment approaches that target the eradication of plasma cell clone, assessing hematological response is the only avenue to determine early, if the treatment is being successful [5]. Organ responses traditionally have been slow to appear and are usually dependent on an adequate hematological response. The traditional approach to hematological response assessment has followed the same guidelines as in patients with myeloma, using serum protein electrophoresis for serial measurement of monoclonal protein, thus limiting its utility mostly to those patients with an intact immunoglobulin of sufficient size [5]. However, much progress has been achieved during the past decade, which makes it imperative that we revise this approach in patients with AL.
The introduction of the serum-free light-chain assay currently allows us to estimate the amount of free immunoglobulin light chains in the serum, which represents the true measurement of the available substrate for amyloid fibril formation [7,11,12,17–22]. Hence an assessment of the changes in the serum FLC is more likely to represent the clinically relevant endpoint than changes in the intact immunoglobulin [23]. A serum M-spike of 1 gm/dL or more has been defined as the minimum amount of M protein in the serum that is required to consider a patient as having measurable disease in the currently used response criteria [10,24]. This number has been based on the precision of the electrophoretic methods currently used for serum M protein estimation. However, as we have seen in the current study as well as previous studies, the proportion of patients with AL who have a measurable level of M-protein is considerably less compared to patients with myeloma, given the low-tumor burden state [18,25]. In contrast, an abnormally high level of free light chain can be seen in the majority of patients with AL. More importantly, in a large proportion of these patients, the difference in the levels between involved and uninvolved light chains is higher than 10 mg/dL, allowing precise estimates of changes with therapy. In addition, changes in free light-chain levels can typically be estimated earlier compared to the intact immunoglobulins, given the normal half-life and turnover rates of the two components. Finally, the newer therapies are associated with better responses and availability of a more sensitive parameter of response assessment is urgently needed [26–30].
In the current study, using a cohort of uniformly treated patients, we have demonstrated that change in the FLC-diff is a better predictor of OS outcome compared to the change in the intact immunoglobulin. Whether the comparison was performed using these variables as a continuous variable or using the traditional cutoff of 50% decrease, the FLC change allowed better prediction of outcome. We only included the patients surviving at least 100 days after transplant to allow adequate time for the development of a response to therapy. More importantly, we found that a 90% decrease in the FLC-diff is a better predictor of long-term survival compared to current definition of 50% decrease for assessing partial response. Although this is similar to the VGPR response in the current criteria used for myeloma, the 90% reduction in FLC-diff is a better goal in patients with AL for several reasons [10]. The majority of patients in the current study achieved a 50% decrease in the FLC-diff providing little discriminatory value for this cutoff in terms of any treatment decisions. In contrast, a 90% decrease was seen only in 38% of patients and predicted a superior outcome with a 90% survival at 5 years, thus allowing clinicians to adapt therapy in patients failing to achieve this goal. These findings are consistent with the previous reports suggesting the best outcomes with achievement of a complete response determined by current criteria. In addition, we were also able to confirm the prognostic value of this degree of FLC reduction in a separate cohort of patients not undergoing SCT, underscoring that this goal is independent of the type of therapy. It is not surprising that a profound reduction in the amyloid substrate is more likely to translate into eventual organ improvement and improved survival.
In conclusion, we recommend that the serum-free light chain be used as the primary marker for hematological response assessment in patients with light-chain AL, and the intact immunoglobulin be used only in the few patients where a measurable level of FLC-diff is not available and a serum M-protein meets the measurable disease criteria (> = 1 gm/dL). We also recommend that a 90% decrease in the serum FLC-diff be considered as the minimum for defining hematological response in these patients. We propose that for study purposes a lower level of FLC-diff of 7.5 mg/dL be the lowest level considered measurable for response.
Acknowledgments
Contract grant sponsor: Hematologic Malignancies Program, Paul Calabresi K12 grant (SKK).
Contract grant sponsor: National Cancer Institute; Contract grant numbers: CA62242, CA93842, CA10080.
Contract grant sponsor: National Institutes of Health.
Contract grant sponsor: the Department of Health and Human Services.
Footnotes
Conflict of interest: SKK was involved in design of concept, data collection, analysis, and writing the paper, AD, MQL, SRH, SRZ, FKB, NL, RAK, SVR, and MAG were involved in writing the manuscript. AD, Honoraria from Binding site for lecture. RAK, Honoraria from Binding site for lecture. SKK, MQL, SRH, SRZ, FKB, NL, SVR, and MAG none relevant to this manuscript.
References
- 1.Kyle RA, Bayrd ED. “Primary” systemic amyloidosis and myeloma. Discussion of relationship and review of 81 cases. Arch Intern Med. 1961;107:344–353. doi: 10.1001/archinte.1961.03620030032004. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA. Primary systemic amyloidosis: Clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59. [PubMed] [Google Scholar]
- 3.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR. Amyloidosis. Best Pract Res Clin Haematol. 2005;18:709–727. doi: 10.1016/j.beha.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Dispenzieri A, Gertz MA, Kyle RA, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: A staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22:3751–3757. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79:319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 6.Gertz MA, Lacy MQ, Dispenzieri A, et al. Effect of hematologic response on outcome of patients undergoing transplantation for primary amyloidosis: Importance of achieving a complete response. Haematologica. 2007;92:1415–1418. doi: 10.3324/haematol.11413. [DOI] [PubMed] [Google Scholar]
- 7.Sanchorawala V, Seldin DC, Magnani B, et al. Serum free light-chain responses after high-dose intravenous melphalan and autologous stem cell transplantation for AL (primary) amyloidosis. Bone Marrow Transpl. 2005;36:597–600. doi: 10.1038/sj.bmt.1705106. [DOI] [PubMed] [Google Scholar]
- 8.Gertz MA, Kyle RA, Greipp PR. Response rates and survival in primary systemic amyloidosis. Blood. 1991;77:257–262. [PubMed] [Google Scholar]
- 9.van G, II, van Rijswijk MH, Bijzet J, et al. Histological regression of amyloid in AL amyloidosis is exclusively seen after normalization of serum free light chain. Haematologica. 2009;94:1094–1100. doi: 10.3324/haematol.2008.004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 11.Abraham RS, Katzmann JA, Clark RJ, et al. Quantitative analysis of serum free light chains. A new marker for the diagnostic evaluation of primary systemic amyloidosis. Am J Clin Pathol. 2003;119:274–278. doi: 10.1309/LYWM-47K2-L8XY-FFB3. [DOI] [PubMed] [Google Scholar]
- 12.Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215–224. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- 13.Gertz MA, Lacy MQ, Dispenzieri A, et al. Stem cell transplantation for the management of primary systemic amyloidosis. Am J Med. 2002;113:549–555. doi: 10.1016/s0002-9343(02)01208-1. [DOI] [PubMed] [Google Scholar]
- 14.Palladini G, Perfetti V, Obici L, et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood. 2004;103:2936–2938. doi: 10.1182/blood-2003-08-2788. [DOI] [PubMed] [Google Scholar]
- 15.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR. Amyloidosis: Diagnosis and management. Clin Lymphoma Myeloma. 2005;6:208–219. doi: 10.3816/CLM.2005.n.048. [DOI] [PubMed] [Google Scholar]
- 16.Dispenzieri A, Merlini G, Comenzo RL. Amyloidosis: 2008 BMT Tandem Meetings (February 13–17, San Diego) Biol Blood Marrow Transpl. 2008;14:6–11. doi: 10.1016/j.bbmt.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradwell AR, Carr-Smith HD, Mead GP, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47:673–680. [PubMed] [Google Scholar]
- 18.Katzmann JA, Abraham RS, Dispenzieri A, et al. Diagnostic performance of quantitative kappa and lambda free light chain assays in clinical practice. Clin Chem. 2005;51:878–881. doi: 10.1373/clinchem.2004.046870. [DOI] [PubMed] [Google Scholar]
- 19.Akar H, Seldin DC, Magnani B, et al. Quantitative serum free light chain assay in the diagnostic evaluation of AL amyloidosis. Amyloid. 2005;12:210–215. doi: 10.1080/13506120500352339. [DOI] [PubMed] [Google Scholar]
- 20.Bochtler T, Hegenbart U, Heiss C, et al. Evaluation of the serum-free light chain test in untreated patients with AL amyloidosis. Haematologica. 2008;93:459–462. doi: 10.3324/haematol.11687. [DOI] [PubMed] [Google Scholar]
- 21.Lachmann HJ, Gallimore R, Gillmore JD, et al. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol. 2003;122:78–84. doi: 10.1046/j.1365-2141.2003.04433.x. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda M, Yamada T, Gono T, et al. Serum levels of free light chain before and after chemotherapy in primary systemic AL amyloidosis. Intern Med. 2005;44:428–433. doi: 10.2169/internalmedicine.44.428. [DOI] [PubMed] [Google Scholar]
- 23.Dispenzieri A, Lacy MQ, Katzmann JA, et al. Absolute values of immunoglobulin free light chains are prognostic in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2006;107:3378–3383. doi: 10.1182/blood-2005-07-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 25.Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: Relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437–1444. [PubMed] [Google Scholar]
- 26.Sanchorawala V, Skinner M, Quillen K, et al. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem-cell transplantation. Blood. 2007;110:3561–3563. doi: 10.1182/blood-2007-07-099481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seldin DC, Anderson JJ, Skinner M, et al. Successful treatment of AL amyloidosis with high-dose melphalan and autologous stem cell transplantation in patients over age 65. Blood. 2006;108:3945–3947. doi: 10.1182/blood-2006-06-029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wechalekar AD, Goodman HJ, Lachmann HJ, et al. Safety and efficacy of risk-adapted cyclophosphamide, thalidomide, and dexamethasone in systemic AL amyloidosis. Blood. 2007;109:457–464. doi: 10.1182/blood-2006-07-035352. [DOI] [PubMed] [Google Scholar]
- 29.Wechalekar AD, Lachmann HJ, Offer M, et al. Efficacy of bortezomib in systemic AL amyloidosis with relapsed/refractory clonal disease. Haematologica. 2008;93:295–298. doi: 10.3324/haematol.11627. [DOI] [PubMed] [Google Scholar]
- 30.Kastritis E, Wechalekar AD, Dimopoulos MA, et al. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J Clin Oncol. 2010;28:1031–1037. doi: 10.1200/JCO.2009.23.8220. [DOI] [PubMed] [Google Scholar]