Abstract
HIV-1 enters the central nervous system early in infection; although HIV-1 does not directly infect neurons, HIV-1 may cause a variety of neurological disorders. Neuronal loss has been found in HIV-1, but synaptodendritic injury is more closely associated with the neurocognitive disorders of HIV-1. The HIV-1 transactivator of transcription (Tat) protein causes direct and indirect damage to neurons. The cysteine rich domain (residues 22–37) of Tat is important for producing neuronal death; however, little is known about the effects of the Tat protein functional domains on the dendritic network. The ability of HIV-1 Tat 1–101 clades B and C, Tat 1–86 and Tat 1–72 proteins, as well as novel peptides (truncated 47–57, 1–72δ31–61, and 1–86 with a mutation at cys22) to produce early synaptodendritic injury (24 hrs), relative to later cell death (48 hrs), was examined using cell culture. Treatment of primary hippocampal neurons with Tat proteins 1–72, 1–86 and 1–101B produced a significant early reduction in F-actin labeled puncta, implicating that these peptides play a role in synaptodendritic injury. Variants with a mutation, deletion, or lack of a cysteine rich region (1–86[Cys22], 1–101C, 1–72δ31–61, or 47–57), did not cause a significant reduction in F-actin rich puncta. Tat 1–72, 1–86, and 1–101B proteins did not significantly differ from one another, indicating the second exon (73–86 or 73–101) does not play a significant role in the reduction of F-actin puncta. Conversely, peptides with a mutation, deletion, or lack of the cysteine rich domain (22–37) failed to produce a loss of F-actin puncta, indicating that the cysteine rich domain plays a key role in synaptodendritic injury. Collectively, these results suggest that for Tat proteins, 1) synaptodendritic injury occurs early, relative to cell death, and 2) the cysteine rich domain of the first exon is key for synaptic loss. Preventing such early synaptic loss may attenuate HIV-1 associated neurocognitive disorders.
Keywords: F-actin, HAND, Primary cell culture, Rat, Clade B, Clade C
Introduction
Human immunodeficiency virus (HIV)-1 infects up to 34 million people worldwide (World Health Organization, 2011). HIV-1 enters the central nervous system early in the infection (Nath, 2002; Resnick et al., 1988), where it has been shown to be present in microglia and in a small percentage of astrocytes (An et al., 1999; Ellis et al., 2007; Gonzalez-Scarano et al., 2005). HIV-1 infection of the central nervous system may result in a spectrum of neurocognitive deficits referred to as HIV-1 associated neurocognitive disorders (HAND) (Antinori et al., 2007). The incidence of the most severe form of HAND, (i.e., HIV-1 associated dementia), has decreased since the implementation of combination anti-retroviral therapy (cART); nevertheless, neurocognitive impairments still affect 40%–70% of HIV-1 infected individuals (Heaton et al., 2011; Letendre, 2011; Lindl et al., 2010).
The HIV genome consists of three major coding regions: core (gag); polymerase (pol); and envelope (env). Regulatory proteins, such as Tat, play a key role in the pathogenesis of HIV infection and are also encoded in the HIV genome (Pavlakis et al., 1990). The HIV-1 transactivator of transcription (Tat) stabilizes the transcription process in order to more efficiently replicate the virus (Dayton et al., 1986; Debaisieux et al., 2012; Gatignol et al., 2000; Pugliese et al., 2005). Tat protein is encoded by two exons with several well-conserved protein domains found on the first exon: the acidic domain (1–21), the cysteine rich domain (22–37), the core/hydrophobic region (38–48), and the basic domain (49–72). Mutations or deletions in residues 22–40 of the first exon are deleterious to transactivation function, whereas modifications are relatively well tolerated within the acidic domain (1–21) and within the glutamine rich region (58–72) found in the basic domain, thereby highlighting the importance of the different domains of the HIV-1 Tat protein (Jeang et al., 1999; Kalantari et al., 2008; Kuppuswamy et al., 1989; Ruben et al., 1989). Most investigations examining the biological effects of Tat protein functional domains have focused on the transactivational ability of the protein (Debaisieux et al., 2012; Hauber et al., 1989; Kalantari et al., 2008; Kubota et al., 1989; Kuppuswamy et al., 1989; Li et al., 2012).
HIV-1 does not directly infect neurons (Gonzalez-Scarano et al., 2005; Nath, 2002). However, HIV-1 Tat is released into the extracellular space of the brain (King et al., 2006; Nath, 2002; Pugliese et al., 2005). Elevated levels of tat mRNA have been found in the brain tissue of individuals with HAD and HIV-1 encephalitis (Cowley et al., 2011; Hudson et al., 2000; Wesselingh et al., 1993; Wiley et al., 1996). HIV-1 Tat has also been detected via immunostaining of brain tissue in individuals with HIV-1 encephalitis (Del et al., 2000; Hudson et al., 2000; Kruman et al., 1999; Liu et al., 2000), and can also be detected in the brains of rhesus macaques infected with a HIV/SIV chimera (Hudson et al., 2000; Kruman et al., 1999). HIV-1 Tat has been shown to initiate neuronal cell death in vitro (Adams et al., 2010; Adams et al., 2012; Aksenov et al., 2009a; Aksenova et al., 2006; Bonavia et al., 2001) and in vivo (Aksenov et al., 2001; Aksenov et al., 2003; Bansal et al., 2000; Fitting et al., 2008a; Fitting et al., 2008b; Fitting et al., 2010a; Kim et al., 2003; Wang et al., 1999). Neuronal cell death has been reported in post-mortem HIV-1 brain tissue (Adle-Biassette et al., 1995; Adle-Biassette et al., 1999); however, cell death correlates poorly with neurocognitive status (Adle-Biassette et al., 1999; Kaul et al., 2001).
In contrast to cell death, dendritic pruning, synaptic loss, and dendritic simplification correlate well with HAND (Adle-Biassette et al., 1999; Everall et al., 1999; Kaul et al., 2001;). Synaptic injury may reflect the early, sometimes asymptomatic, stages of HAND, before extensive neuronal loss occurs (Masliah et al., 1997). The HIV-1 Tat protein has been reported to cause a loss in synaptic density, dendritic pruning, and dendritic simplification both in vivo (Fitting et al., 2010b) as well as in vitro (Kim et al., 2008). Although the role of the functional domains of Tat have been explored in regards to neuronal cell death (Aksenov et al., 2009; Mishra et al., 2008), the role of the Tat protein functional domains in producing early synaptodendritic damage is poorly understood.
Filamentous actin (F-actin) is a major cytoskeletal protein that makes up both pre- and post-synaptic structures (Bleckert et al., 2012; Cingolani et al., 2008), and F-actin is a key target for stabilizing or destabilizing cellular signals which ultimately produce stable synapses (Zhang et al., 2001). In this process, globular actin (G-actin) is polymerized into F-actin, a more stable form of actin (Hotulainen et al., 2009; Johnson et al., 2006; Sekino et al., 2007). Although dendritic spines are some of the most well studied F-actin rich structures, F-actin is a component of a variety of neural structures. For example, F-actin is a major cytoskeletal protein in filopodia (long, headless(Hotulainenstructur et al., 2009; Jacinto et al., 2001; Kaech et al., 2001; Sekino et al., 2007), and patches or “hot sports” along the dendrite appear as F-actin rich puncta (Halpain et al., 1998). F-actin rich patches appear both at the initiation of spinogenesis (Johnson et al., 2006), as well as in non-spiny synapses, which are typically GABA-ergic (Craig et al., 1994; van Spronsen et al., 2010). Phalloidin, a form of phallotoxin isolated from the death cap mushroom (Amanita phalloides) selectively binds to F-actin, but not monomeric G-actin, and has been used to examine the dynamic activity of F-actin in modulating synaptic changes (Halpain et al., 1998; Hotulainen et al., 2009; Kaech et al., 1997; Korobova et al., 2010). In the present study, the term F-actin puncta refers to filopodia, patches, and protruding spines but not growth cones (F-actin rich structures at the most distal ends of the neurite). Quantification of F-actin puncta detects pre- and post-synaptic changes as well as changes in the number of inhibitory and excitatory synapses. Thus, increases or decreases in the quantity of F-actin puncta suggest disruptions in excitatory and inhibitory signaling, which may translate to dysfunctional synaptic communication.
Previous work in our laboratory has shown that neuronal death in vitro occurs after 48 hours of exposure to Tat 1–86 (Aksenova et al., 2009), and moreover, a mutation at cys22 in Tat 1–86 eliminates the neuronal death induced by HIV-1 Tat 1–86 exposure (Aksenov et al., 2009). The present study examines early Tat protein effects on dendritic integrity at a time prior to cell death, and additionally uses synthetic HIV-1 Tat peptides to examine the importance of the highly conserved cysteine rich domain in producing synaptodendritic injury, an important indicator of function in HAND. Full length Tat 1–101, clades B and C, were used to examine specific genetic differences of the Tat protein in producing early synaptic damage which may reflect differences in neurocognitive impairments between Clades B and C. Synthetic peptide lengths 1–72, 1–72 δ 31–61, 1–86,1–86 [Cys 22] and 47–57 were used to partition the capacity of the various Tat protein functional domains for inducing synaptodendritic damage. Our results suggest that synaptic injury due to Tat exposure occurs prior to cell death and is dependent upon key protein residues.
Materials and Methods
Primary hippocampal cell cultures
Primary hippocampal cell cultures were prepared from 18-day-old Sprague-Dawley rat fetuses as previously described (Aksenov et al., 2006; Aksenov et al., 2009b; Aksenova et al., 2006; Aksenova et al., 2009; Bertrand et al., 2011). Procedures were carried out in accordance with the University of South Carolina Institutional Animal Care and Use Committee (animal assurance number: A3049-01). Rat hippocampi were dissected and incubated for 10 min in a solution of 2 mg/ml trypsin in Hank's balanced salt solution (HBSS) buffered with 10 mM HEPES (GIBCO Life Technologies, Grand Island, NY). The tissue was then exposed for 2 min to soybean trypsin inhibitor (1 mg/ml in HBSS) and rinsed 3 times in HBSS. Cells were dissociated by trituration and distributed to poly-L-lysine-coated culture dishes (Costar, Cambridge, MA; MatTek Corporation, Ashland, MA). For cytomorphological studies, hippocampal cells were cultured in 35 mm glass-bottom cell culture dishes (MatTek Corporation, Ashland, MA). For cell viability measurements, hippocampal cell cultures were grown in 96-well plastic plates (Costar, Cambridge, MA). At the time of plating, plates contained DMEM/F12 (GIBCO) supplemented with 100 mL/L fetal bovine serum (Sigma Chemicals, St. Louis, MO). After a 24-hr period, DMEM/F12 was replaced with an equal amount of serum-free Neurobasal medium supplemented with 2% v/v B-27, 2 mM GlutaMAX supplement and 0.5% w/v D-(1) glucose (all ingredients are from GIBCO). Cultures (maintained in the serum-free growth medium) were used for experiments at the age of 14–21 days in vitro (DIV) and were >85–90% neuronal as determined by anti-MAP-2/anti-GFAP/Hoechst fluorescent staining.
Experimental treatment of cultures
The treatment of hippocampal cell cultures with HIV-1 Tat was carried out by the addition of 10 µL of freshly-prepared stock solution of a recombinant Tat variant (Table 1) into the cell culture growth medium. An equal volume of the vehicle was added to control cell cultures. Tat variants were produced using similar recombinant and purification techniques and were determined to be greater than 90% pure and free of endotoxin contamination. Primary hippocampal cells were incubated with 50 nM of each Tat variant for either 24 hours (F-actin) or 48 hours (Live/Dead viability). Investigators were blinded to treatment condition of the cultures.
Table 1.
HIV-1 Tat protein variant lengths, clades, vendors and functional domains
| Peptide Length | Clade | Vendor | Functional Domains |
|---|---|---|---|
| 1–86 | B | Diatheva, Italy | 1st exon + 14 amino acids |
| 1–86[Cys22] | -- | Diatheva, Italy | 1st exon + 14 amino acids; mutation in the cysteine rich domain (Cys22) |
| 1–101 | B | Immunodiagnostic, MA USA | First and second exon; Cysteine rich domain intact |
| 1–101 | C | Prospec, Israel | First and second exon with a mutation in the cysteine rich domain (Cys31) |
| 1–72δ31–61 | -- | U. of Kentucky, USA | 1st exon with a portion of the cysteine rich, the core, the arginine rich, and 4 residues of the glutamine rich domain deleted. |
| 1–72 | B | U. of Kentucky, USA | First exon; Cysteine rich domain intact |
| 47–57 | -- | Anaspec, CA USA | End of the core domain and the arginine rich domain; No cysteine domain present |
Fluorescent labeling and immunocytochemistry – 24 hrs Tat exposure
The fluorescent and immunofluorescent labeling of hippocampal cells were carried out in primary cultures prepared in 35mm glass-bottom cell culture dishes (MatTek Corporation, Ashland, MA). Cultures were fixed with 4% paraformaldehyde and cells were permeabilized in 0.1% Triton X-100 and then incubated for 20 min at room temperature with an F-actin specific probe, Alexa Fluor 488 Phalloidin (0.165µM) (Invitrogen Life Technologies, Grand Island NY). Fixed hippocampal cell cultures were incubated with 10% normal horse serum (NHS) in PBS to block nonspecific binding and then used for labeling the cells with rabbit polyclonal MAP2 (1:1000, Santa Cruz Biotechnology, Inc. Santa Cruz, CA). The secondary antibody for immunofluorescent staining was Alexa Red 594-conjugated goat anti-rabbit IgG (1:500, Invitrogen Life Technologies, Grand Island NY). Blue fluorescent Hoechst dye was used to identify cell nuclei.
Images of F-actin/MAP2 fluorescently co-labeled hippocampal cell cultures were acquired using a high resolution CCD camera attached to the Nikon Eclipse TE2000-E inverted fluorescent computer-controlled microscope (20× objective, 1600 × 2000 pixel image size, 0.17µm/px image resolution at 1X zoom). At least 3 [Green (F-actin; phalloidin staining)/Red (MAP2 immunolabeling)/Blue (Hoechst staining] individual pyramidal neurons with clearly defined dendritic arbors and normal nuclear morphology per culture well were randomly selected and analyzed using the NIS-Elements software package (Nikon). For each neuron selected, F-actin rich synaptic structures were manually counted in several (2–3) digitally magnified dendrites (25–75 µm-long, second order bifurcations). Only those dendritic segments with normal morphology (continuous MAP-2 staining; no beading or varicosities) were included in the analysis. F-actin puncta were identified by using the 518 wavelength emission channel. The low background fluorescence of non-synaptic structures was subtracted (20–60 fluorescence units), allowing clear visualization of puncta. The length of the neurite segment was recorded and the number of puncta were divided by this length and multiplied by 10 yielding puncta per 10µm. F-actin rich structures, or puncta, included for analysis were fine filopodia, spine protrusions, and dendritic F-actin patches. Growth cones (F-actin rich structures located at the most distal dendritic terminus) were excluded from analysis.
Cell viability – 48 hrs Tat exposure
The hippocampal cell viability was assessed in primary neuronal cell cultures prepared in 96-well cell culture plates (Costar, Cambridge, MA) using the microplate reader-formatted variant of the fluorescent calcein AM/ethidium bromide cell labeling assay (Live/Dead kit, Invitrogen, Carlsbad, CA) as described previously (Adams et al., 2010; Adams et al., 2012; Aksenov et al., 2006; Aksenov et al., 2009b; Aksenova et al., 2006; Aksenova et al., 2009). Fluorescence was measured using a Bio-Tek Synergy HT microplate reader (Bio-Tek Instruments, Inc., Winooski, VT). Data are presented in a ratio of calcein fluorescence (live) divided by ethidium fluorescence (dead).
Statistical Analysis
Statistical comparisons were performed with SPSS version 19 (IBM, New York, NY) using a two-way ANOVA. Tukey’s multiple comparison tests were used to determine specific treatment effects. Significant differences were determined with an alpha level set at p≤0.05. Data are presented as mean values ± standard error of the mean (SEM).
Results
Tat 1–86B and Tat 1–101B Produce Similar Synaptodendritic Damage
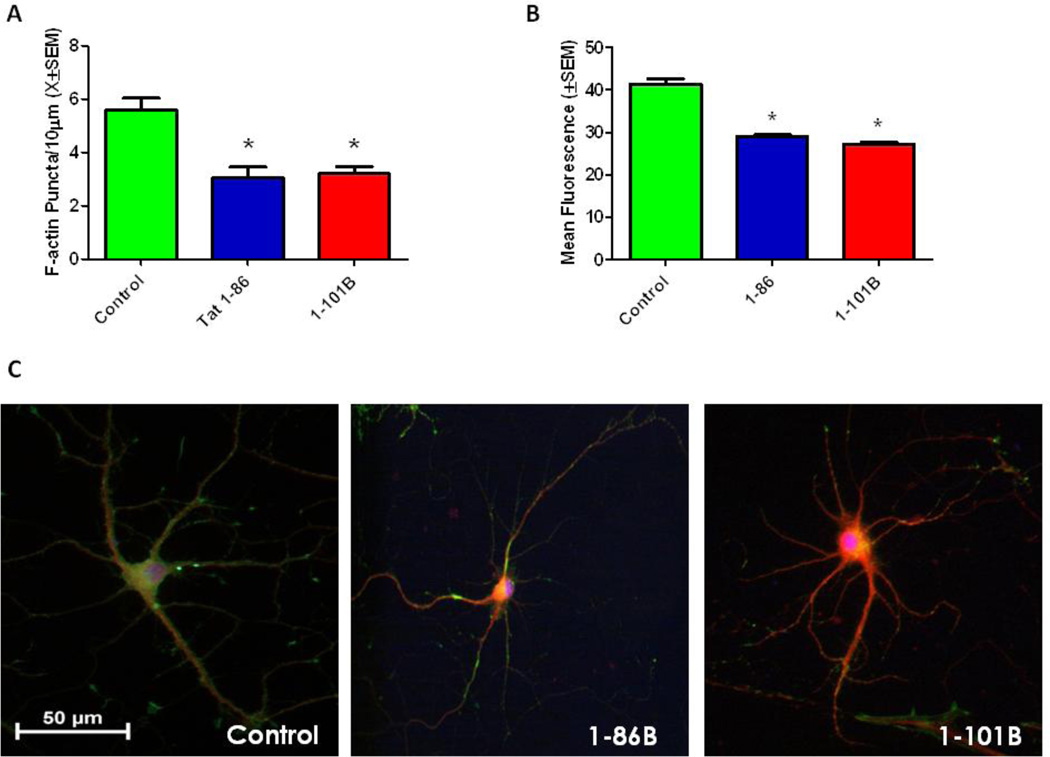
Tat 1–86B and Tat 1–101B are both clade B protein variants differing only in their residue length. The current experiment was performed to evaluate differences, if any, in puncta loss, cell viability and neurite integrity between the two variant lengths. The synaptodendritic integrity of primary hippocampal cells was evaluated by calculating the density of F-actin puncta 24 hours after exposure to Tat 1–101B (50nM) or Tat 1–86 (50nM). There was an overall significant main effect of treatment on puncta loss F(2.24)=13.14,(p<0.001). Tukey’s multiple comparison tests confirmed that Tat 1–101B (p<0.001) and Tat 1–86 (p<0.001) induced significant loss of F-actin puncta when compared to controls, but failed to differ significantly from one another (Fig. 1A).
Figure 1. Tat 1–86 and Tat 1–101B Produce Similar Synaptodendritic Damage.
A. Tat 1–86 or Tat 1–101B treatment (50 nM) for 24 hours produced significant loss of F-actin puncta. Second order dendritic branches were selected from 20X images of F-actin/MAP2/Hoechst-stained Tat-treated and control pyramidal hippocampal neurons for puncta quantification. Results are presented as mean F-actin labeled puncta per 10µm of neuronal dendrite ± SEM. *- indicates a significant (p<0.05) difference relative to control.
B. Tat 1–86 and Tat 1–101B treatment (50nM) for 48 hours produced significant cell death. Fluorescent units were determined in hippocampal cell cultures using Live/Dead assays. Results are presented as mean fluorescence values ± SEM. *- indicates a significant (p<0.05) difference relative to control.
C. Control, Tat 1–86 (50nM) and Tat 1–101B (50nM) treated neurons labeled with F-actin(Green)/MAP-2(Red)/Hoescht(Blue). The hippocampal pyramidal neuron in the control condition demonstrated robust F-actin labeling (green) and complex branching patterns. Both of the Tat treated neurons had greatly diminished F-actin staining (green), and decreased dendritic branching (red) relative to controls. Scale bar = 50 microns
Cell viability of primary hippocampal cell cultures was evaluated 48 hours after exposure to 50nM Tat 1–86B or 50nM Tat 1–101B was assessed using a live/dead assay. There was an overall significant main effect of treatment on viability F(2,24)=70.24,(p<0.001). Post hoc analyses multiple comparison test determined that Tat 1–101B (p<0.001) and Tat 1–86 (p<0.001) produce significant cell death, 34% and 29% respectively, when compared to controls, but failed to differ significantly from one another (Fig. 1B).
At 24 hours, neurons displayed a normal pyramidal morphology (Fig. 1C). However, relative to control neurons, which had a robust F-actin staining and complex dendritic branching patterns, both Tat 1–86B and Tat 1–10B treated neurons demonstrated decreased branching, decreased F-actin and an overall simplification of the neuronal network.
Tat 1–86 [Cys22] and Tat 1–10C Do Not Produce Damage
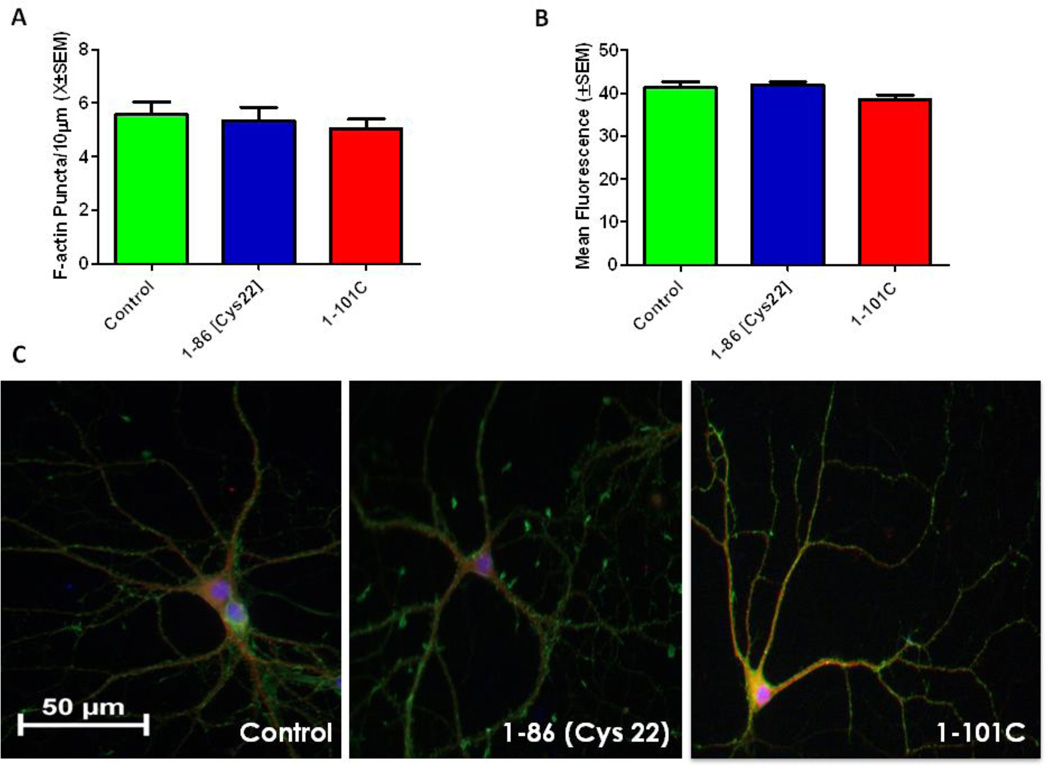
Tat 1–101C is a naturally occurring variant of the Tat protein with a mutation at Cys31, whereas Tat 1–86[Cys22] is a synthetic peptide with a mutation at Cys22. Our previous work demonstrated that a mutation in the cysteine rich domain attenuates decreases in cell viability at 48 hours after Tat 1–86 exposure (Aksenov et al., 2009). The present experiment was undertaken to determine if changes in puncta loss, viability or neuronal morphology were residue specific, or could be generalized to the cysteine rich domain. The synaptodendritic integrity of primary hippocampal cells was evaluated by calculating the density of F-actin puncta 24 hours after exposure to either Tat 1–101C (50nM) or Tat 1–86 [Cys22] (50nM). There was no significant overall main effect of treatment on F-actin puncta (Fig. 2A). Cell viability of primary hippocampal cell cultures was evaluated 48 hours after exposure to 50nM Tat 1–101C or 50nM Tat 1–86 [Cys22]. There was no overall significant main effect of treatment on cell viability, indicating that neither Tat 1–101C nor 1–86 [Cys22] produced neuronal cell death (Fig. 2B). The morphologic appearance of the hippocampal pyramidal cells (Fig. 2C), did not differ from controls, with all groups displaying intact dendrites, complex branching patterns and abundant F-actin staining.
Figure 2. Tat 1–101C and Tat 1–86 (Cys22) do not produce synaptodendritic injury.
A. Tat 1–86 (Cys22) or Tat 1–101 Clade C treatment (50nM) for 24 hours did not produce significant loss of F-actin puncta relative to controls. Second order dendritic branches were selected from 20X images of F-actin/MAP2/Hoechst-stained Tat-treated and non-treated control pyramidal hippocampal neurons for puncta quantification. Results are presented as mean F-actin labeled puncta per 10µm of neuronal dendrite ± SEM.
B. Tat 1–101 Clade C or Tat 1–86 (Cys22) treatment (50nM) for 48 hours did not produce cell death. Fluorescent units were determined in hippocampal cell cultures using Live/Dead assays. Results are presented as mean values ± SEM.
C. Control, Tat 1–86 (Cys22) (50nM) and Tat 1–101 Clade C (50nM) treated neurons labeled with F-actin(Green)/MAP-2(Red)/Hoescht(Blue). The hippocampal pyramidal neuron in the control condition demonstrates robust F-actin labeling (green), complex branching patterns, with extensive fine dendritic network. Tat 1–86 (Cys22) and 1–101 Clade C neurons also presented strong F-actin labeling and complex dendritic branching patterns, suggesting that treatment with these peptides does not produce significant changes in dendritic integrity. Scale bar = 50 microns
The Cysteine Rich Region of Tat is Critical for Synaptodendritic Damage
The prior experiments determined that the cysteine region was vital to the loss of F-actin puncta and cell death. This experiment examined the effects of the cysteine region relative to the core and basic domains of the Tat protein. Tat 1–72 was used to determine if the second exon was essential for either the puncta loss, cell death or dendritic integrity. The Tat protein deletion mutation, Tat 1–72δ31–61, further assessed the relative importance of the cysteine rich domain and the arginine rich domain. However, as a small portion of the cysteine rich domain was present in the deletion mutant, the short peptide Tat 47–57 was also examined to assess the importance of internalization of the protein to puncta loss and cell death.
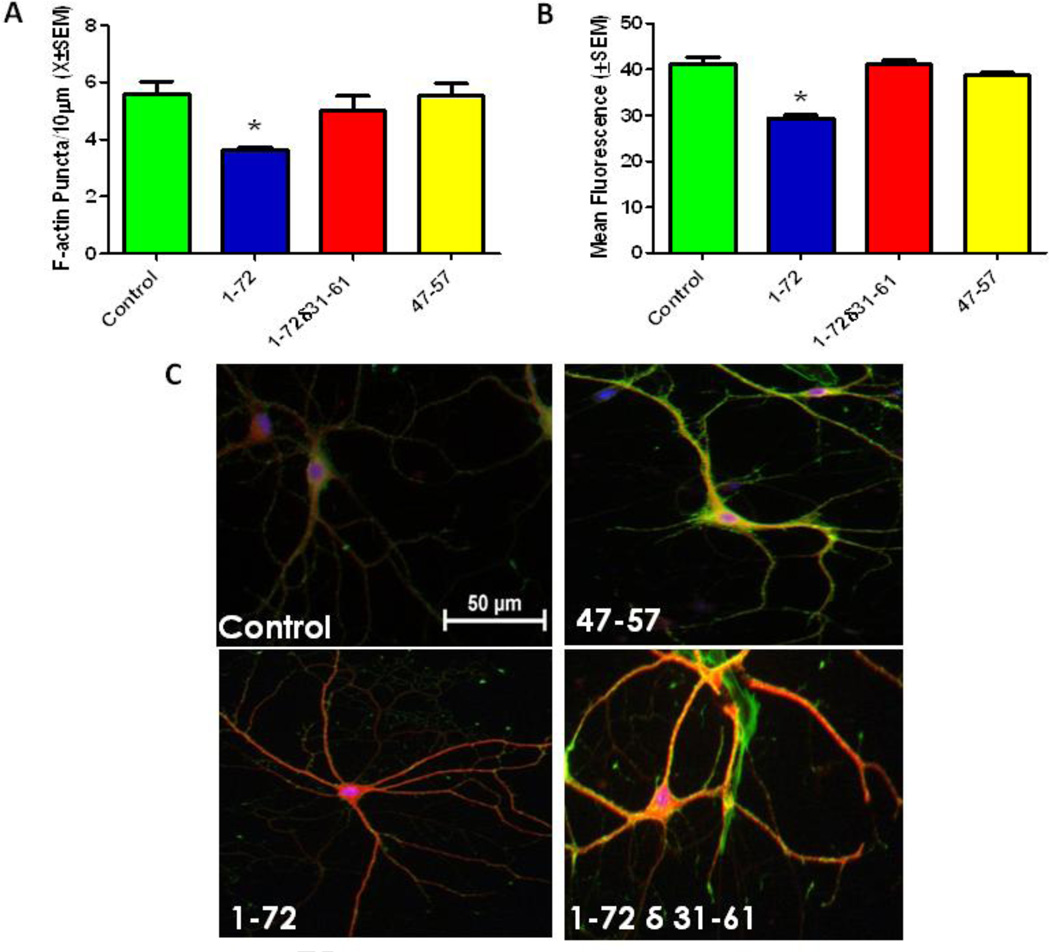
The synaptodendritic integrity of primary hippocampal cells was evaluated by calculating the density of F-actin puncta 24 hours after exposure Tat 1–72 (50nM), Tat 1–72δ31–61(50nM) or Tat 47–57 (50nM). There was a significant overall effect of treatment on puncta density F(3,32)=4.91(p<0.006). Tukey’s multiple comparisons confirmed that Tat 1–72 produced significant puncta loss (p<0.05), but Tat 1–72δ31–61 and Tat 47–57 failed to produce significant puncta loss compared to controls (Fig 3A).
Figure 3. The cysteine rich domain of Tat protein is critical for producing synaptodendritic injury.
A. Tat 1–72 treatment (50nM) for 24 hours produced significant loss of puncta relative to controls; however, Tat 1–72 (δ31–61) or Tat 47–57 treatment (50nM) for 24 hours did not produce significant loss of F-actin puncta. Second order dendritic branches were selected from 20X images of F-actin/MAP2/Hoechst-stained Tat-treated and non-treated control pyramidal hippocampal neurons for puncta quantification. Results are presented as mean F-actin labeled puncta per 10µm of neuronal dendrite ± SEM. *- indicates a significant (p<0.05) difference relative to control.
B. Tat 1–72 treatment (50nM) for 48 hours produced significant cell death; however, Tat 1–72 δ31–61 (50nM) or Tat 47–57 treatment (50nM) for 48 hours did not produce cell death. Fluorescent units were determined in hippocampal cell cultures using Live/Dead assays. Results are presented as mean values ± SEM. *- indicates a significant (P<0.05) difference relative to control.
C. Control, Tat (47–57) (50nM), Tat 1–72 (50nM), and Tat 1–72 δ 31–61(50nM) treated neurons labeled with F-actin(Green)/MAP-2(Red)/Hoescht(Blue). The hippocampal pyramidal neuron in the control condition, Tat (47–57), and Tat 1–72δ31–61 show robust F-actin labeling (green), and complex branching (red). Treatment with Tat 1–72 resulted in diminished F-actin staining (loss of green) and decreased dendritic branching. Scale bar = 50 microns
Cell viability of hippocampal cell cultures was evaluated 48 hours after exposure to Tat 1–72 (50nM),Tat 1–72δ31–61(50nM), or Tat 47–57 (50nM). There was a significant overall main effect of treatment on cell viability F(3,32)= 42.11 (p<0.001). Tukey’s multiple comparison test confirmer that Tat 1–72 produced significant cell death (29% cell death; p<.001); however, Tat 1–72δ31–61 and Tat 47–57 failed to produce significant cell death compared to controls (Fig 3B).
The control, Tat 1–72δ31–61, and Tat 47–57 treated pyramidal neurons all displayed complex branching patterns, extensive fine networks and strong F-actin labeling (Fig. 3C). In contrast, neurons treated with Tat 1–72 produced less branching, simplification of the fine network and reduced F-actin staining.
Discussion
In the current study, synthetic HIV-1 Tat peptides of varying lengths with specific mutations or deletions were used to examine the effects of the Tat functional domains on synaptodendritic injury. We found that Tat variants with an intact cysteine rich region (residues 22–37) in the first exon, regardless of protein length, produced significant early damage to the synaptic network and later cell death. Conversely, Tat protein variants with a mutation, deletion, or a lack of a cysteine rich domain, failed to induce synaptic damage or later cell death. These results suggest that the cysteine rich domain plays a role in the early neuropathological event of synaptodendritic injury. Synaptic injury is currently the best neuropathologic correlate of cognitive decline in HAND (Ellis et al., 2007). Synaptic dysfunction and neurocognitive impairments are generally evident long before neurons die in neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease (Coleman et al., 2004; van Spronsen et al., 2010). Although the direct relationship between specific synaptic dysfunction(s) and HAND remains unclear, the identification of viral attributes capable of producing synaptic damage/dysfunction (one being the presence of specific Tat protein domains) are key to understanding HAND.
F-actin is a key marker for synapse stability (Zhang et al., 2001). Our F-actin stained puncta structurally include excitatory synapses (Hotulainen et al., 2009; Jacinto et al., 2001), inhibitory synapses (Craig et al., 1994), and both pre-/post-synaptic structures (Bleckert et al., 2012; Cingolani et al., 2008), allowing for the overall assessment of synaptic integrity. We observed a reduction of F-actin puncta and general dendritic field simplification in response to Tat peptides with the cysteine rich region intact (Tat 1–72, Tat 1–86, Tat 1–101B). Extending the peptide length (from 72 to 86 and 101 aa) did not significantly affect the pronounced loss of puncta, suggesting that the second exon does not play a key role in Tat-induced synaptodendritic injury. Tat 1–72 is not a naturally occurring human isolate; however, Tat 1–101 (Rosen et al., 1986) and Tat 1–86 (Barre-Sinoussi et al., 1983) have both been reported in human plasma following HIV-1 infection. Although the neural uptake of Tat 1–86 has been shown to be more efficient than that of Tat 1–72 (Ma et al., 1997), the present results suggest that protein uptake may not be necessary for puncta loss and dendritic network degradation.
Additionally, Tat 1–72, Tat 1–86, and Tat 1–101B have previously been shown to cause cell death at later time points in cell culture models (Aksenov et al., 2009a; Aksenov et al., 2009b; Aksenova et al., 2009; King et al., 2006; Mishra et al., 2008). In the present studies we confirmed these original reports for the individual proteins (cell death at 48 hours), and further demonstrate that these protein variants do not significantly differ in their ability to decrease viability. Moreover, we now report that these recombinant proteins (1–86[Cys22], 1–101C, 1–72δ31–61), from the same manufacturers and produced under similar conditions, did not produce damage, thereby eliminating the concern of endotoxin contamination confounding the synaptodendritic damage and cell death profile for these particular Tat protein variants.
The Tat protein is subject to genetic variations and modifications (Li et al., 2012). HIV-1 Tat is encoded by two exons, and has several distinct functional domains which are essential for viral replication and are responsible for cell death (Karn, 1999; Kuppuswamy et al., 1989). The first three domains: the acidic domain (1–21), the cysteine rich domain (22–37), and the core/hydrophobic region (38–48) represent the minimal number of residues required for the transactivation capabilities of Tat (Debaisieux et al., 2012; Kuppuswamy et al., 1989). The basic domain (49–72) can be broken down into two subdomains: the arginine-rich domain (49–57) and the glutamine-rich domain (58–72) (Li et al., 2012). Modifications and mutations are relatively well- tolerated within the acidic domain (1–21), but mutations in residues 22–40 are deleterious to transactivation function (Jeang et al., 1999; Kalantari et al., 2008; Kuppuswamy et al., 1989; Ruben et al., 1989) and have been shown to attenuate Tat induced neuronal cell death (Aksenov et al., 2009b; Campbell et al., 2011).
The present study used peptides with mutations at Cys 22 (1–86[Cys22]), and Cys 31 (Tat 1–101C), and demonstrated that these mutations do not cause significant puncta loss. Additionally, we used Tat 47–57 which includes the last two residues of the core domain (38–48) and the arginine rich domain (49–57) - the region responsible for binding to cell membranes and for uptake into adjacent cells (Debaisieux et al., 2012; Jeang et al., 1999; Kuppuswamy et al., 1989; Ruben et al., 1989) - but lacks the cysteine rich domain (22–37). Tat 47–57 did not produce loss of F-actin puncta, suggesting neuronal uptake alone is not responsible for Tat effects. Indeed, Tat 1–72 δ 31–61 (the first exon with the end of the cysteine rich domain, the arginine rich domain, and part of the glutamine rich region deleted), did not produce significant loss of F-actin puncta, accentuating the importance for the cysteine rich domain for induction of puncta loss, and moreover, that neuronal uptake of the Tat peptide is not necessary for puncta loss.
HIV-1 neurocognitive related deficits have been reported as more common in clade B prevalent areas (North America and Europe) relative to clade C areas (Sub-Saharan Africa and Asia) (Satishchandra et al., 2000; however, see Gupta et al., 2007). HIV-1 Tat C, which has a mutation at cys31, has been shown to be less neurotoxic than clade B Tat (Aksenov et al, 2009; Mishra et al, 2008; Ranga et al, 2004). The present study confirms that Tat 1–101C does not produce significant cell death, and adds that Tat 1–101C also does not produce a significant loss of F-actin puncta. Although Tat may not mediate neurocognitive deficits in the clade C population, there are other HIV-1 proteins, such as gp120, known to cause neuronal death (Bansal et al., 2000;Kaul) and synapse loss (Kim et al., 2011). Thus, elucidating further differences in HIV-1 proteins between the two clades remains an important question and may shed light on the relationship between synaptic dysfunction and HAND.
Conclusions
In the present study, F-actin puncta provided a broad assessment of early synaptodendritic damage produced by variants of HIV-1 Tat protein. An intact cysteine rich domain of the Tat protein exon 1 is critical for producing synaptodendritic injury; however, Tat protein uptake into the neurons is not necessary for damaging synapses. Greater understanding of the cellular mechanisms of synaptodendritic damage and dysfunction may aid in the treatment of HIV-1 associated neurocognitive disorders. Although the direct relationship between specific excitatory/inhibitory synaptic dysfunction(s) and HAND remains unclear, the identification of viral attributes capable of producing synaptic damage/dysfunction (e.g., the presence/absence of specific Tat protein alterations) are key to understanding HAND.
Highlights.
HIV-1 Tat 1–86 and Tat 1–101 Clade B both produce synaptodendritic damage.
The cysteine rich region of HIV-1 Tat is required for damage to the neuronal network.
Tat 1–101 Clade C and Tat 1–86 [Cys22] failed to produce neurotoxicity.
Acknowledgements
The authors acknowledge the untimely passing of Dr. Michael Y. Aksenov and his contributions to the work described. This work was supported, in part, by a Biomedical Behavioral T32 training program, a University of South Carolina Research Foundation grant and NIH Grants # DA013137, DA031604, HD043680.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. ER-beta mediates 17beta-estradiol attenuation of HIV-1 Tat-induced apoptotic signaling. Synapse. 2010;64:829–838. doi: 10.1002/syn.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Soy Isoflavones Genistein and Daidzein Exert Anti-Apoptotic Actions via a Selective ER-mediated Mechanism in Neurons following HIV-1 Tat(1–86) Exposure. PLoS. One. 2012;7:e37540. doi: 10.1371/journal.pone.0037540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol. Appl. Neurobiol. 1999;25:123–133. doi: 10.1046/j.1365-2990.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- Adle-Biassette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, Gray F. Neuronal apoptosis in HIV infection in adults. Neuropathol. Appl. Neurobiol. 1995;21:218–227. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. Attenuated neurotoxicity of the transactivation-defective HIV-1 Tat protein in hippocampal cell cultures. Exp. Neurol. 2009;219:586–590. doi: 10.1016/j.expneurol.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27:217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci. Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Adams SM, Mactutus CF, Booze RM. Neuronal survival and resistance to HIV-1 Tat toxicity in the primary culture of rat fetal neurons. Exp. Neurol. 2009;215:253–263. doi: 10.1016/j.expneurol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci. Lett. 2006;395:235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J. Neuropathol. Exp. Neurol. 1999;58:1156–1162. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bertrand SJ, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Endogenous amyloidogenesis in long-term rat hippocampal cell cultures. BMC. Neurosci. 2011;12:38. doi: 10.1186/1471-2202-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckert A, Photowala H, Alford S. Dual pools of actin at presynaptic terminals. J. Neurophysiol. 2012;107:3479–3492. doi: 10.1152/jn.00789.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavia R, Bajetto A, Barbero S, Albini A, Noonan DM, Schettini G. HIV-1 Tat causes apoptotic death and calcium homeostasis alterations in rat neurons. Biochem. Biophys. Res. Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- Campbell GR, Watkins JD, Loret EP, Spector SA. Differential induction of rat neuronal excitotoxic cell death by human immunodeficiency virus type 1 clade B and C tat proteins. AIDS Res. Hum. Retroviruses. 2011;27:647–654. doi: 10.1089/aid.2010.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- Coleman P, Federoff H, Kurlan R. A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurol. 2004;63:1155–1162. doi: 10.1212/01.wnl.0000140626.48118.0a. [DOI] [PubMed] [Google Scholar]
- Cowley D, Gray LR, Wesselingh SL, Gorry PR, Churchill MJ. Genetic and functional heterogeneity of CNS-derived tat alleles from patients with HIV-associated dementia. J. Neurovirol. 2011;17:70–81. doi: 10.1007/s13365-010-0002-5. [DOI] [PubMed] [Google Scholar]
- Craig AM, Blackstone CD, Huganir RL, Banker G. Selective clustering of glutamate and gamma-aminobutyric acid receptors opposite terminals releasing the corresponding neurotransmitters. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12373–12377. doi: 10.1073/pnas.91.26.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton AI, Sodroski JG, Rosen CA, Goh WC, Haseltine WA. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986;44:941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- Debaisieux S, Rayne F, Yezid H, Beaumelle B. The ins and outs of HIV-1 Tat. Traffic. 2012;13:355–363. doi: 10.1111/j.1600-0854.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- Del VL, Croul S, Morgello S, Amini S, Rappaport J, Khalili K. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J. Neurovirol. 2000;6:221–228. doi: 10.3109/13550280009015824. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat. Rev. Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Differential long-term neurotoxicity of HIV-1 proteins in the rat hippocampal formation: a design-based stereological study. Hippocampus. 2008a;18:135–147. doi: 10.1002/hipo.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Dose-dependent long-term effects of Tat in the rat hippocampal formation: a design-based stereological study. Hippocampus. 2010a;20:469–480. doi: 10.1002/hipo.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal injection of the HIV-1 proteins gp120 and Tat: differential effects on behavior and the relationship to stereological hippocampal measures. Brain Res. 2008b;1232:139–154. doi: 10.1016/j.brainres.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, Knapp PE, Hauser KF. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am. J. Pathol. 2010b;177:1397–1410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatignol A, Jeang KT. Tat as a transcriptional activator and a potential therapeutic target for HIV-1. Adv. Pharmacol. 2000;48:209–227. doi: 10.1016/s1054-3589(00)48007-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat. Rev. Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gupta JD, Satishchandra P, Gopukumar K, Wilkie F, Waldrop-Valverde D, Ellis R, Ownby R, Subbakrishna DK, Desai A, Kamat A, Ravi V, Rao BS, Satish KS, Kumar M. Neuropsychological deficits in human immunodeficiency virus type 1 clade C-seropositive adults from South India. J. Neurovirol. 2007;13:195–202. doi: 10.1080/13550280701258407. [DOI] [PubMed] [Google Scholar]
- Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J. Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J, Malim MH, Cullen BR. Mutational analysis of the conserved basic domain of human immunodeficiency virus tat protein. J. Virol. 1989;63:1181–1187. doi: 10.1128/jvi.63.3.1181-1187.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P, Llano O, Smirnov S, Tanhuanpaa K, Faix J, Rivera C, Lappalainen P. Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J. Cell Biol. 2009;185:323–339. doi: 10.1083/jcb.200809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J. Neurovirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- Jacinto A, Wolpert L. Filopodia. Curr. Biol. 2001;11:R634. doi: 10.1016/s0960-9822(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Jeang KT, Xiao H, Rich EA. Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J. Biol. Chem. 1999;274:28837–28840. doi: 10.1074/jbc.274.41.28837. [DOI] [PubMed] [Google Scholar]
- Johnson OL, Ouimet CC. A regulatory role for actin in dendritic spine proliferation. Brain Res. 2006;1113:1–9. doi: 10.1016/j.brainres.2006.06.116. [DOI] [PubMed] [Google Scholar]
- Kaech S, Fischer M, Doll T, Matus A. Isoform specificity in the relationship of actin to dendritic spines. J. Neurosci. 1997;17:9565–9572. doi: 10.1523/JNEUROSCI.17-24-09565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Parmar H, Roelandse M, Bornmann C, Matus A. Cytoskeletal microdifferentiation: a mechanism for organizing morphological plasticity in dendrites. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7086–7092. doi: 10.1073/pnas.111146798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantari P, Narayan V, Natarajan SK, Muralidhar K, Gandhi UH, Vunta H, Henderson AJ, Prabhu KS. Thioredoxin reductase-1 negatively regulates HIV-1 transactivating protein Tat-dependent transcription in human macrophages. J. Biol. Chem. 2008;283:33183–33190. doi: 10.1074/jbc.M807403200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J. Tackling Tat. J. Mol. Biol. 1999;293:235–254. doi: 10.1006/jmbi.1999.3060. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Martemyanov KA, Thayer SA. Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. J. Neurosci. 2008;28:12604–12613. doi: 10.1523/JNEUROSCI.2958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Shin AH, Thayer SA. Activation of cannabinoid type 2 receptors inhibits HIV-1 envelope glycoprotein gp120-induced synapse loss. Mol. Pharmacol. 2011;80:357–366. doi: 10.1124/mol.111.071647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes. Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol. Biol. Cell. 2010;21:165–176. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Nath A, Maragos WF, Chan SL, Jones M, Rangnekar VM, Jakel RJ, Mattson MP. Evidence that Par-4 participates in the pathogenesis of HIV encephalitis. Am. J. Pathol. 1999;155:39–46. doi: 10.1016/S0002-9440(10)65096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Endo S, Maki M, Hatanaka M. Role of the cysteine-rich region of HIV tat protein on its trans-activational ability. Virus Genes. 1989;2:113–118. doi: 10.1007/BF00315255. [DOI] [PubMed] [Google Scholar]
- Kuppuswamy M, Subramanian T, Srinivasan A, Chinnadurai G. Multiple functional domains of Tat, the trans-activator of HIV-1, defined by mutational analysis. Nucleic Acids Res. 1989;17:3551–3561. doi: 10.1093/nar/17.9.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top. Antivir. Med. 2011;19:137–142. [PMC free article] [PubMed] [Google Scholar]
- Li L, Dahiya S, Kortagere S, Aiamkitsumrit B, Cunningham D, Pirrone V, Nonnemacher MR, Wigdahl B. Impact of Tat Genetic Variation on HIV-1 Disease. Adv. Virol. 2012;2012:123605. doi: 10.1155/2012/123605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J. Neuroimmune. Pharmacol. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat. Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J. Virol. 1997;71:2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann. Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Mishra M, Vetrivel S, Siddappa NB, Ranga U, Seth P. Clade-specific differences in neurotoxicity of human immunodeficiency virus-1 B and C Tat of human neurons: significance of dicysteine C30C31 motif. Ann. Neurol. 2008;63:366–376. doi: 10.1002/ana.21292. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J. Infect. Dis. 2002;186(Suppl 2):S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Pavlakis GN, Felber BK. Regulation of expression of human immunodeficiency virus. New Biol. 1990;2:20–31. [PubMed] [Google Scholar]
- Pugliese A, Vidotto V, Beltramo T, Petrini S, Torre D. A review of HIV-1 Tat protein biological effects. Cell Biochem. Funct. 2005;23:223–227. doi: 10.1002/cbf.1147. [DOI] [PubMed] [Google Scholar]
- Ranga U, Shankarappa R, Siddappa NB, Ramakrishna L, Nagendran R, Mahalingam M, Mahadevan A, Jayasuryan N, Satishchandra P, Shankar SK, Prasad VR. Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J. Virol. 2004;78:2586–2590. doi: 10.1128/JVI.78.5.2586-2590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick L, Berger JR, Shapshak P, Tourtellotte WW. Early penetration of the blood-brain-barrier by HIV. Neurology. 1988;38:9–14. doi: 10.1212/wnl.38.1.9. [DOI] [PubMed] [Google Scholar]
- Rosen CA, Sodroski JG, Goh WC, Dayton AI, Lippke J, Haseltine WA. Post-transcriptional regulation accounts for the trans-activation of the human T-lymphotropic virus type III. Nature. 1986;319:555–559. doi: 10.1038/319555a0. [DOI] [PubMed] [Google Scholar]
- Ruben S, Perkins A, Purcell R, Joung K, Sia R, Burghoff R, Haseltine WA, Rosen CA. Structural and functional characterization of human immunodeficiency virus tat protein. J. Virol. 1989;63:1–8. doi: 10.1128/jvi.63.1.1-8.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satishchandra P, Nalini A, Gourie-Devi M, Khanna N, Santosh V, Ravi V, Desai A, Chandramuki A, Jayakumar PN, Shankar SK. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989–96) Indian. J. Med. Res. 2000;111:14–23. [PubMed] [Google Scholar]
- Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem. Int. 2007;51:92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Shin AH, Kim HJ, Thayer SA. Subtype selective NMDA receptor antagonists induce recovery of synapses lost following exposure to HIV-1 Tat. Br. J. Pharmacol. 2012;166:1002–1017. doi: 10.1111/j.1476-5381.2011.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias KF, Duman JG, Um K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog. Neurobiol. 2011;94:133–148. doi: 10.1016/j.pneurobio.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spronsen M, Hoogerraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol., Neurosci. Rep. 2010;10:207–214. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Barks JD, Silverstein FS. Tat, a human immunodeficiency virus-1-derived protein, augments excitotoxic hippocampal injury in neonatal rats. Neuroscience. 1999;88:585–597. doi: 10.1016/s0306-4522(98)00242-5. [DOI] [PubMed] [Google Scholar]
- Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, Farber JM, Griffin JW, Griffin DE. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann. Neurol. 1993;33:576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Baldwin M, Achim CL. Expression of HIV regulatory and structural mRNA in the central nervous system. AIDS. 1996;10:843–847. doi: 10.1097/00002030-199607000-00007. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World Health Organization; 2011. Global Summary of the HIV/AIDS Epidemic. Ref Type: Electronic Citation. [Google Scholar]
- Zhang W, Benson DL. Stages of synapse development defined by dependence on F-actin. J. Neurosci. 2001;21:5169–5181. doi: 10.1523/JNEUROSCI.21-14-05169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]