Abstract
Just over a century ago, animal responses to injections of jellyfish extracts unveiled the phenomenon of anaphylaxis. Yet, until very recently, understanding of jellyfish sting toxicity has remained limited. Upon contact, jellyfish stinging cells discharge complex venoms, through thousands of barbed tubules, into the skin resulting in painful and, potentially, lethal envenomations. This review examines the immunological and toxinological responses to stings by prominent species of jellyfish including Physalia sp. (Portuguese Man-o-War, Blue-bottle), Cubozoan jellyfish including Chironex fleckeri, several Carybdeids including Carybdea arborifera and Alatina moseri, Linuche unguiculta (Thimble jellyfish), a jellyfish responsible for Irukandji syndrome (Carukia barnesi) and Pelagia noctiluca. Jellyfish venoms are composed of potent proteinaceous porins (cellular membrane pore-forming toxins), neurotoxic peptides, bioactive lipids and other small molecules whilst the tubules contain ancient collagens and chitins. We postulate that immunologically, both tubular structural and functional biopolymers as well as venom components can initiate innate, adaptive, as well as immediate and delayed hypersensitivity reactions that may be amenable to topical anti-inflammatory-immunomodifier therapy. The current challenge for immunotoxinologists is to deconstruct the actions of venom components to target therapeutic modalities for sting treatment.
Keywords: Jellyfish, envenomation, sting, allergy, toxin, immunology
INTRODUCTION
The stings of jellyfish and related cnidarians represent a unique therapeutic challenge. Cutaneous deposition of potent bio-active toxin molecules [1] and of foreign stinging cell tubules may activate innate and adaptive cellular and humoral immune responses. The challenge for immunotoxinologists is to deconstruct the actions of toxins and to target therapeutic modalities for stings and general drug discovery. The exploration of other fascinating aspects of jellyfish biology and chemistry, such as the 2008 Nobel-prize winning story of Green Fluorescent Proteins [2] and the nature of basal metazoan innate immune repertoire, evident through the study of extant cnidarian genomics [3], is beyond the scope of this paper.
It may have been forgotten that the most recognised immune response to foreign material, “anaphylaxis”, originated from experiments with jellyfish extracts in which toxicity was under investigation. Two French biologists, Portier and Richet, having demonstrated that the toxicity of the Atlantic jellyfish Physalia physalis (Portuguese Man-O’-War) in dogs was dose related, sought to demonstrate the same phenomenon by another species (Actinies) from the phylum Cnidaria (Class Anthozoa, Order Actiniaria sea anemones). However, after observing that the toxin from the sea anemone proved to be less potent than that from the jellyfish, they sought to re-use their animals for further experiments with sea anemone toxin but discovered to their astonishment that a second injection of the toxin, even at lesser doses than the first, caused death. They reasoned that this phenomenon be named anaphylaxis [4, 5], a term in Greek signifying that the second dose, in contrast to protection (phylaxis), conveyed not protection but susceptibility. For this discovery they were awarded the 1913 Nobel Prize in Physiology or Medicine.
In subsequent years, numerous clinical observations and experiments have established that the toxins of numerous jellyfish species provoke a variety of immunological responses. From the hundreds of recognised species of jellyfish, this review differentiates the toxic and immunological responses to stings by reference to some prominent species. It also proposes a new synthesis of both issues that suggests new paths for investigation and therapeutic interventions.
JELLYFISH AND THEIR MECHANISM OF STINGING
The word “jellyfish” refers to the free-floating medusal gelatinous lifecycle stage of members of the phylum Cnidaria (see Fig. 1). A defining feature of this ancient phylum is the cnidae, a remarkably specialized, explosive organelle elaborated by the Golgi apparatus and comprised of a collagen-walled capsule containing a rapidly eversible penetrant or non-penetrant tubule (see Fig. 2). Specialised cnidae producing penetrant “stinging cells” termed nematoblasts, each synthesize a singular nematocyst containing a micron-diameter eversible spine-laden tubule of approximately 200 to 800 micron length [6, 7] allowing the deposition of capsular contents or “venom” for the purposes of defence and capture of prey [6, 8]. Classes of animals in the phylum Cnidaria include not only jellyfish but also sea anemones and corals.
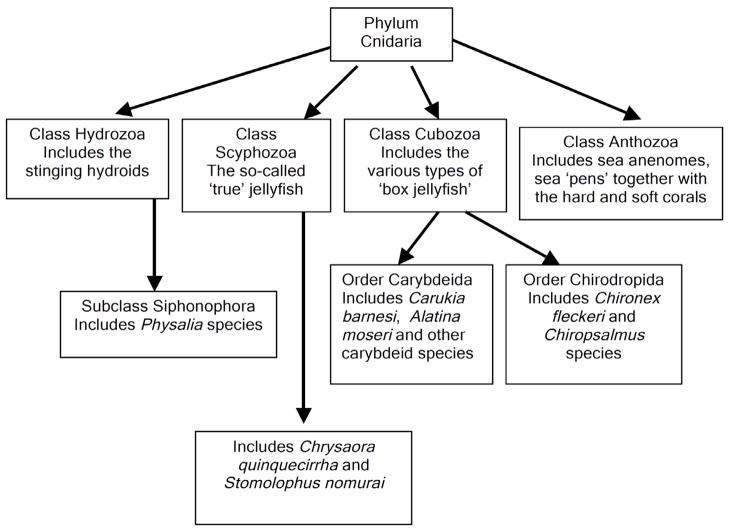
Fig. 1.
Taxonomic overview of medically and toxinologically significant jellyfish, placed in the context of the other major Cnidarian classes. This phylum is one of the oldest living animal groups with major classes extant in Precambrian times. Cnidarians are characterised by the presence of radial symmetry, two cell layers (ecto- and endoderm) with a single body cavity. They are primarily predatory organisms possessing cnidae, an intracellular capsule elaborated by the Golgi apparatus. Specialised cnidae (nematoblasts) elaborate a “stinging cell” (nematocyst) for the purposes of defence and capture of prey. It is these nematocysts that contain diverse and potent venoms.
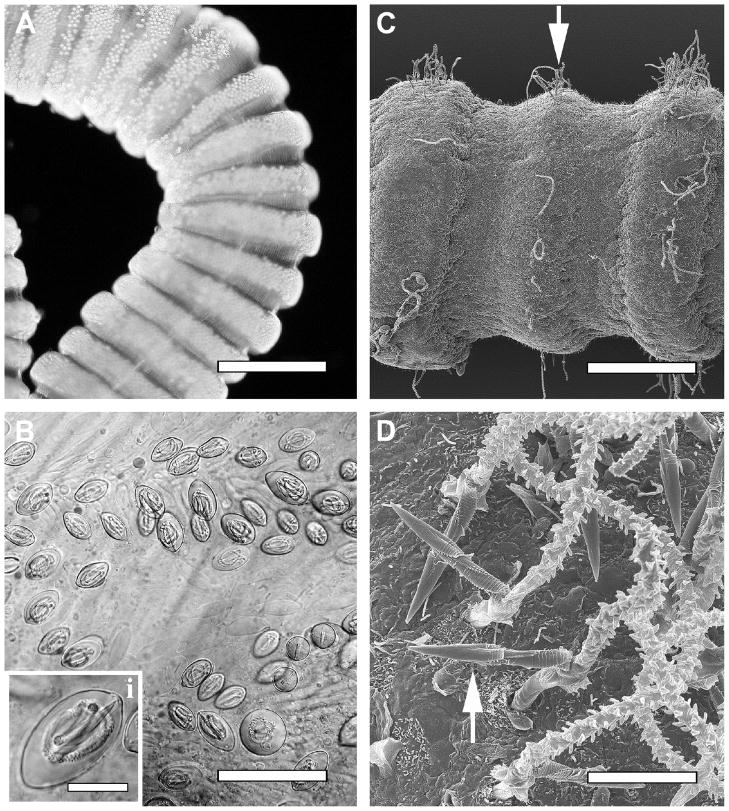
Fig. 2.
(A–D) Light microscopic and scanning electron microscopic images of the Hawaiian box jellyfish Carybdea alata tentacles and nematocysts – prototype examples of the venom injection apparatus in the cubozoa. (A) Light micrograph of contracted proximal tentacle of C. alata exhibiting bands of packed nematocysts in the tentacle tissue. (B) Light micrograph of higher magnification of nematocyst band showing the orientation of undischarged nematocysts in rows. Inset shows the predominant nematocyst type (heterotrichous microbasic euryteles) found in the tentacles of adult C. alata. (C) Low-magnification scanning electron micrograph of distal C. alata tentacle. Discharged heterotrichous microbasic eurytele tubules are evident in raised bands on the outer surface of the tentacle (arrow). (D) High-magnification scanning electron micrograph of C. alata tentacle surface exhibiting discharged heterotrichous microbasic euryteles. Bars 1.5 μm (A), 65 μm (B), 15 μm (B, inset), 400 μm (C), 10 μm (D).
In jellyfish, bands or buttons of thousands of densely packed nematocysts line the epithelial surfaces of tentacles and, in some species, the medusal bell of the animal. Upon physical contact, the capsules of the nematocysts (“spring-loaded syringes”) fire a barbed arrow-like tubule within 700 ns of physical contact at high velocity (18.6 m/sec) and acceleration (5.4 × 106 g) creating a pressure of 7.7 GPa at the site of impact [9]. Upon contact with human skin or other surface (e.g., cornea), thousands of tubules transporting toxins are deposited per square centimetre of the epidermis and dermis. The combined physical impalement by barbed tubules and deposition of potent venom toxins quickly immobilize and kill prey. In humans, toxins cause local and systemic injury and may also provoke immunological responses. The length of the penetrant tubules of some species renders possible the direct deposition of venom toxin into pierced capillaries [10] thus explaining the rapid onset of toxicity in humans.
In addition to envenomation, stings embed spine-laden tubules which are composed of ancient mini-collagens, glycoproteins and polysaccharides [9]. We postulate that these substances may separately trigger antigenic, allergenic or innate immune responses. This concept is supported by disparate lines of evidence. Whilst the nematocyst-derived venom of the edible jellyfish Nemopilema (Stomolophus) nomurai causes cardiovascular depression in experimental animals [11] and may cause death in humans [12], the collagen extracted from the exumbrella of this jellyfish enhanced IgM, IgG, interferon and tumour necrosis factor production by human lymphocytes [13, 14]. This source of collagen also enhanced inflammatory cytokine secretion, antibody secretion and population changes in immune cells [15]. Interestingly, these inflammatory effects were no more marked than those stimulated by bovine collagen leading the investigators to conclude that jellyfish collagen, being free of risk of bovine spongiform encephalopathy, could be used safely as a polymer scaffold [15]. That remains to be determined. Together, these observations suggest that the collagenous structural elements of tubules may indeed be immunogenic, but the similarities and differences between these collagens and those from different parts of a particular jellyfish and those of different species are unknown.
Likewise, jellyfish structural carbohydrates such as chitin may play a role in triggering immune responses to jellyfish stings. Recent work investigating the pathogenesis of airway inflammation and asthma has revealed the importance of this pathway in triggering such reactions, with human genotypes associated with impaired processing of chitin by chitinase-like proteins, having higher rates of such diseases [16]. Studies of immune responses to nematode chitins in mice have also demonstrated a pivotal role for these ubiquitous molecules in the tissue accumulation of IL-4 expressing innate immune cells, unrelated to Toll-like receptor tissue recognition [17]. As chitin is present in cnidarian tubule spines (AY unpublished data), it seems plausible that these molecules together with the genetic heterogeneity of dermal chitinases, will contribute to the outcome of any given cnidarian sting. One would anticipate that, analogous to airway inflammation and parasite responses, those with impaired chitin clearance, such as due to certain polymorphisms in chitinase-related genes [18], may display more severe outcomes after jellyfish stings.
CUTANEOUS TOXIC AND IMMUNE RESPONSES TO STINGING
The deposition of the complex mixture of nematocyst constituents, venom, carried by jellyfish tubules probably sets off a complicated system of cellular and cytokine interactions analogous to that described on entry of pathogens or allergens into human skin [19]. Although little is known about the effects of purified venom components in the skin, it is speculated that the immune response to them is like that to any potential allergen or antigen with keratinocytes, tissue macrophages, dendritic cells (DC) and mast cells being the key cellular mediators (see Fig. 3). Although keratinocytes are the front line protective defence against physical incursion into the skin, they also have another role which is to release thymic stromal lymphopoietin which activates T-cells to produce cytokines, known to be prominent in allergic dermatitis. Dendritic cells, a heterogeneous population of lympho-myeloid origin critical to the initiation of immune responses, capture and present antigens to T-cells or migrate to regional lymph nodes evoking immune or delayed hypersensitivity allergic reactions. Combinations of pathogen pattern recognition receptors such as mannose-binding lectins and DC-expressed Toll-like receptor heterodimers contribute to innate immune response pathways and may also contribute to the immune responses to jellyfish venoms and associated structural molecules. Given the prominent necrosis induced by the sting of multiple cnidarians species, particularly those of chirodropids, the recently identified DC-activating necrosis receptors, such as CLEC9A (also known as Dendritic cell NK lectin Group Receptor-1 (DNGR-1) [20], may also be contribute to the response to tissue injury.
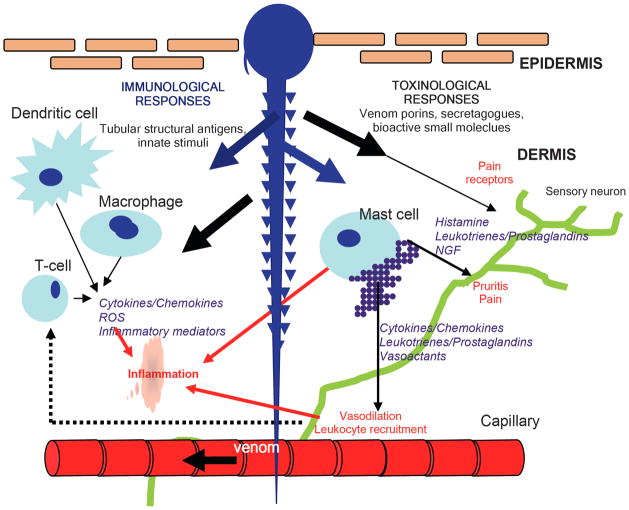
Fig. 3.
Schematic representation of the immunological and toxic responses to nematocyst penetration of the skin. Thousands of nematocyst tubules (~1 × 250 microns) covered with mineralized chitinous spines perforate and are left embedded in the skin. Rapid/sustained release of venom into skin: Specific venom porin proteins, peptides, lipids and small molecules activate keritinocytes, dermal mast cells and nocioceptors in skin (including TRPV1 receptors). Venom-activated mast cells explosively degranulate to release factors which elicit rapid immune cell recruitment (extravasation) to the dermal site with resultant swelling, pain and redness. Venom porins, secretagogues, and small molecules directly activate various extravasated immunocytes further initiating inflammatory cytokine responses as well as probable effects secondary to toxin-induced cell damage through the dendritic cell activating necrosis receptors. Chitinous spines and tubule collagen induce sustained activation of innate immunological activation of pro-inflammatory cells, Langerhans dendritic cells, macrophages and mast cells.
Mast cells are potent drivers of inflammation, releasing biogenic amines such as histamine and other substances including platelet activating factor, prostaglandins, leukotrienes, proteases and cytokines into their tissue environment when stimulated. Dermal mast cells respond to three types of stimulus that are likely to be relevant in nematocyst envenomation. First, primary envenomation may activate mast cells directly through the introduction of porins, secretagogues (bioactive lipids, amines) in the venom itself [21, 22]. Second components of the nematocyst tubule or venom may activate innate or pattern recognition receptors on mast cells. Third physical changes (hypo-osmolarity, acidification, reactive oxygen species) at the sting site may activate mast cells directly. In the case of repeated stings, classical IgE dependent allergy to toxin or tubule components may play a role in mast cell activation and mediator release in response to specific IgE bound to their surfaces.
PHYSALIA
The two known species of this jellyfish, Physalia physalis [“Portuguese Man-O’-War” and Physalia utriculus [Blue bottle”], inhabit the Atlantic and Indo-Pacific oceans respectively. These brightly blue-coloured species float on the water surface, sometimes as members of a vast armada. Although the medusal floats are clearly visible, tentacles trail many metres unseen beneath the surface and represent a threat to unwary swimmers, and their nematocysts can still discharge if handled out of water. The stings of these species, which resemble a “string of beads”, cause sharp pain which may extend beyond the site of the immediate lesion (see Fig. 4). The pain usually subsides quickly and the sting fades within hours to days. Occasionally however, the wound may blister and a systemic illness consisting of headache, vomiting, abdominal pain and diarrhoea is provoked. The few deaths following stings in Atlantic waters [23, 24] have been attributed to cardiovascular toxicity. The toxins appear to be able to form pores in the plasma membranes of cells [25] leading to influx of calcium, osmotic swelling and subsequent cellular lysis.
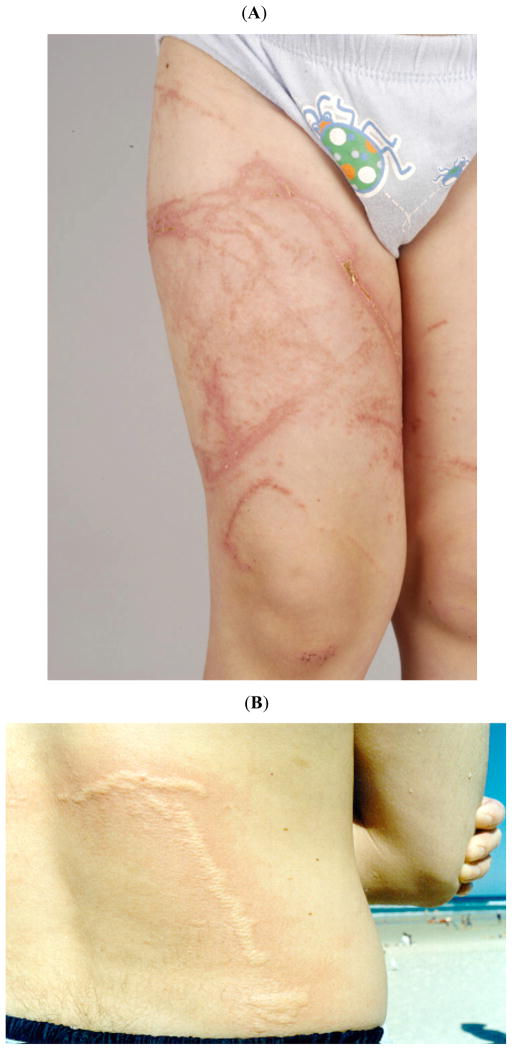
Fig. 4.
(A, B) Examples of the acute and delayed effects of jellyfish stings on skin. (A) This photographs shows the more delayed effects of a severe chirodropid sting to a child in Thailand. It was taken nine days after the sting and show multiple areas of skin necrosis as well as delayed erythematous urticarial reactions. (B) This photograph shows the acute effects (minutes) of a Blue bottle, Physalia utriculus, sting on a young adult in Australia (Courtesy of Stephen Leahy). Photograph courtesy of Andrew Jones.
Some evidence exists that stings by this jellyfish may provoke immediate and delayed hypersensitivity responses. A case of rapid collapse (unconsciousness, pulselessness and unreactive dilated pupils) after a sting by P. utriculus has been considered circumstantially an allergic phenomenon [26]. In another case, features of immediate hypersensitivity (urticaria, oedma and bronchospasm) responded quickly to treatment with epinephrine, volume expansion and steroids but one week later a pruritic vesicular eruption occurred on skin initially stung [27]. In a series of 66 patients who had exhibited cutaneous, extracutaneous or anaphylactoid reactions to stings by either Physalia physalis or Chrysaora quinquecirrha (Sea nettle), the majority had developed IgG antibodies, some had developed IgE antibodies to the toxins of the two jellyfish and some patients developed cross-reacting antibodies to toxins of both jellyfish [28]. Perhaps the last phenomenon occurred because toxins or embedded tubule antigens of both species are similar, resembling the cross-reactivity observed between bee and some wasp toxins, or because the structural elements of their embedded tubules are similar. The phenomena of recurrent cutaneous eruptions with evidence of humoural and cellular immunological responses may follow single envenomations by jellyfish including Physalia and Chrysaora species [29] possibly reflecting the long lived presence of glycosylated tubule proteins with the ability to trigger innate immune responses in the skin. No specific therapy exists for Physalia stings and even first-aid is controversial. A randomised trial of immersion in hot water versus application of cold packs favoured hot water treatment [30, 31] but the mechanism and clinical significance is debatable.
SEABATHER’S ERUPTION (LINUCHE UNGUICULTA)
This pruritic papulopustular dermatosis has occurred among bathers in waters in the Bahamas, the Western Caribbean or off southern Florida. The affliction is often confined to areas of skin which had been covered by clothing. The agent responsible is any of the three free-swimming or larval stages (ephyrae, medusae, planulae) of the minute “thimble jellyfish” Linuche unguiculata [32] which are easily trapped in or beneath clothing causing nematocyst discharge. Contact with the creature is not painful but a prickling or stinging sensation may be experienced while the victim is in the water followed later by severe itching. This toxic component of the illness is followed by an immunological component consisting of an urticarial eruption and a distressing dermatitis which may last many days to several weeks. In victims, sera contained high titers of IgG antibody against antigens of the jellyfish [32–34] and against other jellyfish P. physalia and C. quinquecirrha [33]. Histopathological examination of lesions reveals superficial and deep perivascular and interstitial infiltrate of lymphocytes, neutrophils and eisinophils [33]. There is no specific treatment for seabather’s eruption. Local antipruritic agents and analgesic agents afford relief but opinions differ concerning the efficacy of topical and systemic steroids [32, 33] and antihistamines are opined not useful [32]. Interestingly, a similar if not the same jellyfish, Linuche aquila, inhabits the Indo-Pacific region but no reports of a similar illness from this region exist.
CUBOZOANS
The class Cubozoa, or “box jellyfish”, is comprised of two orders (Carybdeidae and Chirodropidae – see Fig. 1) [35]. The most notorious of the later is the relatively massive, kilogram sized, multi-tentacled Chironex fleckeri which often bears over 48, 2 meter long 5 mm wide ribbon like tentacles and inhabits waters of northern Australia and south-east Asia. Stings are extremely painful and have caused numerous deaths [23] probably by rapid cardiovascular collapse [36, 37] sometimes within minutes of a sting (see Fig. 4). The toxic components of venoms act directly on muscle and nerve tissue [38–40]. For over 40 years scientists have attempted to identify the toxins but only recently has a measure of success been achieved. Two toxin proteins of molecular weight 43 and 45 kDa have been sequenced [41, 42] and share considerable homology with three other known lethal haemolytic proteins from other Chirodropidae Chironex yamaguchii, (reported as Chiropsalmus quadrigatus), as well as the Carybdeidae, Carybdea arborifera (reported as Carybdea rastoni) and Alatina moseri (reported as Carybdea alata). Additional larger cytolytic proteins are present in the venom [43] and phospholipase A2 activity is present in tentacles [44]. Some identified protein toxins are antigenic [41, 43]. The venom may act by creating pores in myocytic membranes [45] as has been shown for toxins of Physalia [25]. Reports of human envenomations thus far have not suggested an immunological component although delayed cutaneous eruptions are suspicious [46] (see Fig. 4).
An ovine-derived antivenom is available for use but the rapidity of onset of envenomation in severe cases may render it useless. This antivenom does not appear to be useful against carydbeid venoms [47, 48]. Calcium channel blockade has been proposed as treatment for Chironex fleckeri stings but this is not prudent. Although calcium entry into cardiac myocytes cells has been observed after experimental application of C. fleckeri venom, the use of calcium channel blockade does not prevent calcium influx [38, 45] and did not prevent acute cardiovascular collapse [36, 37]. Moreover, since calcium channel blockers cause hypotension and are not used for this reason in treatment of cardiac dysrhythmias in cardiopulmonary resuscitation, their use is likely to ensure death of a stung victim in the circumstance of acute cardiovascular collapse. If pore formation is indeed the action of toxins in this species as has been observed [45], calcium channel blockade would not only be harmful but also futile. We speculate that use of extracorporeal life support combined with good quality cardiopulmonary resuscitation may offer the only realistic hope of recovery of an envenomated victim in cardiac arrest.
IRUKANDJI SYNDROME
A distinct jellyfish envenomation syndrome, called the Irukandji syndrome, characterised by sweating, anxiety, muscle spasm, severe hypertension and potentially late hypotension and cardiac failure, has been attributed to certain species of smaller, four-tentacled box jellyfish [49–52]. This includes, but is not limited to, the carybdeid species Carukia barnesi [53–56] The deaths in 2002 of two tourists in Far North Queensland, Australia, highlighted the potential risk and severity of this syndrome [57, 58]. It is now appreciated to occur elsewhere in the Indo-Pacific and the Caribbean [59–61].
Typically, and unlike larger multi-tentacled box jellyfish stings, the Irukandji sting site usually has minimal local reaction and the systemic effects take 20–30 minutes to become evident with the syndrome evolving over hours-days. Current understanding of the underlying pathophysiology is limited. Amongst suspected Irukandji species, only the venom of Carukia barnesi and Alatina mordens, has been studied to any extent [48, 62, 63]. Clinically, some features of Irukandji syndrome resemble that of catecholamine excess, such as that seen in phaeochromocytoma [48]. Accordingly, elevated serum adrenaline and noradrenaline levels have been found in experimentally envenomated animals [48, 62]. It is notable that some scorpion envenomations may also exhibit cardiovascular features similar to this syndrome [64, 65], with catecholamine excess and initial hypertension followed by late hypotension. Animal experiments and human clinical studies have implicated both cytokines (IL-1alpha, IL-6, IL8, IFN-gamma, GM-CSF and TNF alpha) as well as nitric oxide release as an underlying mechanism [64, 65].
The most effective therapy for Irukandji syndrome seems to be an intravenous infusion of magnesium (MgSO4 or MgCl2) [66, 67]. The rationale for the use of magnesium therapy is based on the in vivo evidence of catecholamine excess, sympathetic and parasympathetic involvement [68]. Anecdotal evidence suggests that magnesium is highly successful in reducing both hypertension and pain in Irukandji syndrome [66, 67].
The pharmacological mechanism underlying the severe pain of Irukandji syndrome remains to be determined. Theories include ischaemia due to vasoconstriction of arterioles as a result of excess catecholamines, or sodium channel-dependent activation of afferent pain pathways [66]. Relevant to this may be the observations that some Cnidarian venoms (Chironex fleckeri, Aiptasia pulchella, Cyanea capillata and Physalia physalis) appear to activate TRPV1, a non-selective cation channel expressed in nociceptive neurones [40]. This mechanism is comparable to that of capsaicin and may explain the immediate burning pain victims of these species experience [40]. Whether TRPV1 channels are implicated in Irukandji syndrome is uncertain. Indeed, although Irukandji stings may cause local pain, the characteristic severe muscular pain of delayed onset cannot be explained by the involvement of local nociceptive effects alone. Indeed, compared to the pronounced local, and immediate pain associated with those jellyfish venoms tested [40], Irukandji syndrome pain is very different. Hence a distinct mechanism would be predicted.
PELAGIA NOCTILUCA
This pelagic jellyfish known as the “Little Mauve Stinger” inhabits all oceans. Its sting is painful and appears as irregular shaped wheals. Pruritus and wheezing have been reported [23]. No deaths have been reported but a convincing case of anaphylaxis after a jellyfish sting, surmised to be P. noctiluca, attests that allergic reactions are possible. In that case [69], the victim’s basophils (blood stage mast cells) immediately released histamine in response to exposure to a crude extract of venom derived from tentacles of another jellyfish Chrysaora quinquecirrha, suggesting the presence of mast cell secretagogues in the venom, or the development of a classical FcεRI/IgE-mediated allergic response to a venom-derived antigen. The latter seems likely given that serum transferred sensitivity. Interestingly, the victim may have been previously exposed and developed IgEs to a Pelagia antigen that is conserved in Chrysaora from either tubule or venom. Even if the offending species was not P. noctiluca, this phenomenon illustrates the propensity for immunological reactions or cross reactions to jellyfish venoms or to their tubular elements, or both. Recurrent cutaneous eruptions without re-envenomation attributed to P. noctiluca also suggest an immunological process [70] again, possibly reflecting long term residency of antigenic or innately immuno-active tubule components in the victim’s skin.
OTHER JELLYFISH SPECIES AND HUMAN ENVENOMATION
A multitude of diverse illnesses and effects distant to the cutaneous lesions have been reported after jellyfish stings [24]. The features of some illnesses or their responses to treatment suggest immunological involvement. Moreover, either the location of the illness or the duration separating the sting and manifestation of illness or both are also more suggestive of an immunological process than a toxin-related process. For examples, allergic dermatitis has been reported after contact with Carybdea arborifera (reported as Carybdea rastoni) [71], persistent cutaneous hypersensitivity after contact with Atatina moseri (reported as Carybdea alata) [72] and delayed vesicular eruptions occur after contact with Rhopilema nomadica [73] while persistent dermatitis responded to topical applications of tacrolimus [74] and of pimecrolimus [75] which inhibit activation of T-cells by blocking transcription of cytokines. Guillain-Barré syndrome has occurred after sting by Pelagis noctiluca [76].
CONCLUSIONS
Little is known about the mechanistic basis of inflammatory responses to jellyfish envenomation. When deposited in the skin of sting victims, these complex venoms some components of which are cytolytic proteins, cause local pain, skin lesions and distal effects in many human organ systems although rarely fatal. Only a few venom proteins such as those of the Box jellyfish (Chironex fleckeri) have been characterised and sequenced. Some venom components are also antigenic and may trigger an acute hypersensitivity immune response from which acute toxic responses are difficult to differentiate. Delayed hypersensitivity responses may also be promoted and these manifested in the skin as persistent, recurrent, vesicular or pruritic dermatitis for which newer agents such as the topical immunomodulator pimecrolimus may be beneficial. The numerous other illnesses following jellyfish stings may be either toxin or immune-based, or both. Jellyfish stings also deposit in the skin foreign structural biopolymers including chitin and mini-collagens that we speculate may contribute to the resultant host immune response.
In conclusion, it would seem fitting to quote Thomas Henry Huxley, famous as Darwin’s ‘Bulldog’, but less well known as the man who named the Phylum Nematophora [Huxley], now Cnidaria, and who undertook pioneering studies of jellyfish anatomy and systematics. These words were written near the end of his Voyage on the HMS Rattlesnake regarding his approach to the study of jellyfish –
“… I paid comparatively little attention to the collection of new species, caring rather to come to some clear and definite idea as to the structure of those which had been indeed long known, but very little understood. Unfortunately for science, but fortunately for me, this method appears to have been somewhat novel with observers of these animals, and consequently everywhere new and remarkable facts were to be had for the picking up” [77].
Applied to the study of jellyfish and their venoms, Huxley’s observations remain just as true today.
Acknowledgments
Dr. Ken Winkel is supported by funding from the Australian Government Department of Health and Ageing, and Snowy Nominees.
Drs. Angel Yanagihara and Helen Turner are funded by the Hawaii Community Foundation, Victoria and Bradley Geist Foundation for projects entitled “Potent Inflammatory Effector Molecules in Hawaiian Box Jellyfish Envenomation” and NIH/NIDA (1 R21 DA024444-01) “Novel TRPV Pharmacophores from Cnidarian Venom”.
References
- 1.Šuput D. In vivo effects of cnidarian toxins and venoms. Toxicon. 2009;54(8):1190–1200. doi: 10.1016/j.toxicon.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Zimmer M. GFP: from jellyfish to the Nobel prize and beyond. Chem Soc Rev. 2009;38(10):2823–32. doi: 10.1039/b904023d. [DOI] [PubMed] [Google Scholar]
- 3.Miller DJ, Hemmrich G, Ball EE, Hayward DC, Khalturin K, Funayama N, Agata K, Bosch TCG. The innate immune repertoire in cnidaria - ancestral complexity and stochastic gene loss. Genome Biol. 2007;8(4):R59.1–R59.13. doi: 10.1186/gb-2007-8-4-r59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richet G. La découverte de l’anaphylaxie brève mais triomphale rencontre de deux physiologistes. His Des Sci Méd. 2003;37(4):463–469. [PubMed] [Google Scholar]
- 5.Portier MM, Richet C. De l’action anaphylactique de certains venins. Compt Rend Soc Biol (Paris) 1902;54:170–172. [Google Scholar]
- 6.Yanagihara AA, Kuroiwa JMY, Oliver L, Kunkel DD. The ultrastructure of nematocysts from the fishing tentacle of the Hawaiian bluebottle, Physalia utriculus (Cnidaria, Hydrozoa, Siphonophora) Hydrobiologia. 2002;489(1–3):139–150. [Google Scholar]
- 7.Yanagihara AA, Kuroiwa JMY, Oliver LM, Chung JJ, Kunkel DD. Ultrastructure of a novel eurytele nematocyst of Carybdea alata Reynaud (Cubozoa, Cnidaria) Cell Tiss, Res. 2002;308(2):307–318. doi: 10.1007/s00441-002-0545-8. [DOI] [PubMed] [Google Scholar]
- 8.Fautin DG. Structural diversity, systematics, and evolution of cnidae. Toxicon. 2009;54(8):1054–1064. doi: 10.1016/j.toxicon.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Özbek S, Balasubramanian PG, Holstein TW. Cnidocyst structure and the biomechanics of discharge. Toxicon. 2009;54(8):1038–1045. doi: 10.1016/j.toxicon.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Rifkin J, Endean R. The structure and function of the nematocysts of Chironex fleckeri Southcott. Cell Tiss Res. 1983;233(3):563–577. doi: 10.1007/BF00212225. [DOI] [PubMed] [Google Scholar]
- 11.Kim E, Lee S, Kim JS, Yoon WD, Lim D, Hart AJ, Hodgson WC. Cardiovascular effects of Nemopilema nomurai (Scyphozoa: Rhizostomeae) jellyfish venom in rats. Toxicol Lett. 2006;167(3):205–211. doi: 10.1016/j.toxlet.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Shede Q. Stomolophus nomurai in China. In: Williamson JA, Fenner PJ, Burnett JW, Rifkin JF, editors. Venomous and Poisonous Marine Animals. University of New South Wales Press; Sydney: 1996. pp. 214–216. [Google Scholar]
- 13.Suguhara T, Ueno M, Goto Y, Shiraishi R, Doi M, Akiyama K, Yamauchi S. Immunostimulation effect of jellyfish collagen. Biosci Biotechnol Biochem. 2006;70(9):2131–2137. doi: 10.1271/bbb.60076. [DOI] [PubMed] [Google Scholar]
- 14.Nishimoto S, Goto Y, Morishige H, Shiraishi R, Doi M, Akiyama S, Sugahara T. Mode of action of the immunostimulatory effect of collagen from jellyfish. Biosci Biotechnol Biochem. 2008;72(11):2806–2814. doi: 10.1271/bbb.80154. [DOI] [PubMed] [Google Scholar]
- 15.Song E, Kim SY, Chun T, Byun HJ, Lee YM. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials. 2006;27(15):2951–2961. doi: 10.1016/j.biomaterials.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, Radford S, Parry RR, Heinzmann A, Deichmann KA, Lester LA, Gern JE, Lemanske RF, Jr, Nicolae DL, Elias JA, Chupp GL. Effect of variation in CHI3L1 on serum YKL-40 level risk of asthma and lung function. N Engl J Med. 2008;358(April 17):1682–1691. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reese TA, Liang HE, Tager AM, Luster AD, van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447(May 3):92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ober C, Chupp GL. The chitinase and chitinase-like proteins: a review of genetic and functional studies in asthma and immune-mediated diseases. Curr Opin Allergy Clin Immunol. 2009;9(4):401–408. doi: 10.1097/ACI.0b013e3283306533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metz M, Maurer M. Innate immunity and allergy in the skin. Curr Opin Immunol. 2009;21(6):687–693. doi: 10.1016/j.coi.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458(April 16):899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung JJ, Ratnapala LA, Cooke IM, Yanagihara AA. Partial purification and characterization of a hemolysin (CAH1) from Hawaiian box jellyfish (Carybdea alata) venom. Toxicon. 2001;39(7):981–990. doi: 10.1016/s0041-0101(00)00237-3. [DOI] [PubMed] [Google Scholar]
- 22.Prasad P, Yanagihara AA, Small-Howard AL, Turner H, Stokes AJ. Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J Immunol. 2008;181(7):5024–5034. doi: 10.4049/jimmunol.181.7.5024. [DOI] [PubMed] [Google Scholar]
- 23.Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48(7):830–859. doi: 10.1016/j.toxicon.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Burnett JW. Treatment of Atlantic cnidarian envenomations. Toxicon. 2009;54(8):1201–1205. doi: 10.1016/j.toxicon.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Edwards LP, Whitter E, Hessinger DA. Apparent membrane pore-formation by Portuguese Man-of-war (Physalia physalis) Toxicon. 2002;40(9):1299–1305. doi: 10.1016/s0041-0101(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 26.Gollan JA. The dangerous Portuguese Man-of-War. Med J Aust. 1968;1:973. [Google Scholar]
- 27.Edwards EK, Jr, Edwards EK., Sr Immediate anaphylactic and delayed reactions to jellyfish. Contact Dematitis. 2000;43(4):244–245. [PubMed] [Google Scholar]
- 28.Russo AJ, Calton GJ, Burnett JW. The relationship of the possible allergic response to jellyfish envenomation and serum antibody titers. Toxicon. 1983;21(4):475–480. doi: 10.1016/0041-0101(83)90125-3. [DOI] [PubMed] [Google Scholar]
- 29.Burnett JW, Hepper KP, Aurelian L, Calton GJ, Gardepe SF. Recurrent eruptions following unusual solitary coelenterate envenomations. J Am Acad Dermatol. 1987;17(1):86–92. doi: 10.1016/s0190-9622(87)70177-7. [DOI] [PubMed] [Google Scholar]
- 30.Loten C, Stokes B, Worsley D, Seymour JE, Jiang S, Isbister GK. A randomised controlled trial of hot water (45 ° C) immersion versus ice packs for pain relief in bluebottle stings. Med J Aust. 2006;184(7):329–333. doi: 10.5694/j.1326-5377.2006.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimoto CM, Yanagihara AA. Cnidarian (coelenterate) envenomations in Hawai’i improve following heat application. Trans R Soc Trop Med Hyg. 2002;96(3):300–303. doi: 10.1016/s0035-9203(02)90105-7. [DOI] [PubMed] [Google Scholar]
- 32.Segura-Puertas L, Ramos ME, Aramburo C, Heimer de la Cotera EP, Burnett JW. One Linuche mystery solved: All 3 stages of the coronate scyphomedusa Linuche unguiculata cause seabather’s eruption. J Am Acad Dermatol. 2001;44(4):624–628. doi: 10.1067/mjd.2001.112345. [DOI] [PubMed] [Google Scholar]
- 33.Wong DE, Meinking TL, Rosen LB, Taplin D, Hogan DJ, Burnett JW. Seabather’s eruption: Clinical, histologic, and immunologic features. J Am Acad Dermatol. 1994;30(3):399–406. doi: 10.1016/s0190-9622(94)70046-x. [DOI] [PubMed] [Google Scholar]
- 34.Burnett JW, Kumar S, Malecki JM, Szmant AM. The antibody response in seabather’s eruption. Toxicon. 1995;33(1):99–104. doi: 10.1016/0041-0101(94)00145-x. [DOI] [PubMed] [Google Scholar]
- 35.Bentlage B, Cartwright P, Yanagihara A, Lewis C, Richards G, Collins A. Evolution of box jellyfishes (Cnidaria: Cubozoa): a group of highly toxic invertebrates. Proc R Soc B. 2010;277(Feb 7):493–501. doi: 10.1098/rspb.2009.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tibballs J, Williams D, Sutherland SK. The effects of antivenom and verapamil on the haemodynamic actions of Chironex fleckeri (box jellyfish) venom. Anaesth Intens Care. 1998;26(1):40–45. doi: 10.1177/0310057X9802600105. [DOI] [PubMed] [Google Scholar]
- 37.Ramasamy S, Isbister GK, Seymour JE, Hodgson WC. The in vivo cardiovascular effects of box jellyfish Chironex fleckeri venom in rats: efficacy of pre-treatment with antivenom, verapamil and magnesium sulphate. Toxicon. 2004;43(6):685–690. doi: 10.1016/j.toxicon.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 38.Mustafa MR, White E, Hongo K, Othman I, Orchard CH. The mechanism underlying the cardiotoxic effect of the toxin from the jellyfish Chironex fleckeri. Toxicol Appl Pharmacol. 1995;133(2):196–206. doi: 10.1006/taap.1995.1142. [DOI] [PubMed] [Google Scholar]
- 39.Ramasamy S, Isbister GK, Seymour JE, Hodgson WC. The in vitro effects of two chirodropid (Chironex fleckeri and Chiropsalmus sp) venoms: efficacy of box jellyfish antivenom. Toxicon. 2003;41(6):703–711. doi: 10.1016/s0041-0101(03)00046-1. [DOI] [PubMed] [Google Scholar]
- 40.Cuypers E, Yanagihara A, Karlsson E, Tytgat J. Jellyfish and other cnidarian envenomations cause pain by affecting TRPV1 channels. FEBS Lett. 2006;580(24):5728–5732. doi: 10.1016/j.febslet.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brinkman D, Burnell J. Identification, cloning and sequencing of two major venom proteins from the box jellyfishChironex fleckeri. Toxicon. 2007;50(6):850–860. doi: 10.1016/j.toxicon.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Brinkman DL, Burnell JN. Biochemical and molecular characterisation of cubozoan protein toxins. Toxicon. 2009;54(8):1162–1173. doi: 10.1016/j.toxicon.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Brinkman D, Burnell J. Partial purification of cytolytic venom proteins from the box jellyfish, Chironex fleckeri. Toxicon. 2008;51(5):853–863. doi: 10.1016/j.toxicon.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Nevalainen TJ, Peuravuori HJ, Quinn RJ, Llewellyn LE, Benzie JAH, Fenner PJ, Winkel KD. Phospholipase A2 in Cnidaria. Comp Biochem & Physiol B: Biochem & Mol Biol. 2004;139(4):731–735. doi: 10.1016/j.cbpc.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Bailey PM, Bakker AJ, Seymour JE, Wilce JA. A functional comparison of the venom of three Australian jellyfish – Chironex fleckeri, Chiropsalmus sp., and Carybdea xaymacana – on cytosolic Ca2+, haemolysis and Artemia sp.lethality. Toxicon. 2005;45(2):233–242. doi: 10.1016/j.toxicon.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Williamson JA. The Marine Stinger Book. Surf Life Saving Association of Australia; Sydney: 1985. [Google Scholar]
- 47.Winkel K, Hawdon G, Fenner PJ, Gershwin LA, Collins AG, Tibballs J. Jellyfish Antivenoms: Past, Present and Future. J Toxicol Toxin Rev. 2003;22(1):115–127. [Google Scholar]
- 48.Winkel KD, Tibballs J, Molenaar P, Lambert G, Coles P, Ross-Smith M, Wiltshire C, Fenner PJ, Gershwin LA, Hawdon GM, Wright CE, Angus JA. Cardiovascular actions of the venom from the Irukandji (Carukia barnesi) jellyfish: effects in human, rat and guinea-pig tissues in vitro and in pigs in vivo. Clin Exp Pharmacol Physiol. 2005;32(9):777–788. doi: 10.1111/j.1440-1681.2005.04258.x. [DOI] [PubMed] [Google Scholar]
- 49.Flecker H. Irukandji sting to North Queensland bathers without production of weals but with severe general symptoms. Med J Aust. 1952;2:89–91. doi: 10.5694/j.1326-5377.1952.tb100081.x. [DOI] [PubMed] [Google Scholar]
- 50.Barnes JH. Cause and Effect in Irukandji Stingings. Med J Aust. 1964;1:897–904. doi: 10.5694/j.1326-5377.1964.tb114424.x. [DOI] [PubMed] [Google Scholar]
- 51.Southcott RV. Revision of some Carybdeidae (Scyphozoa: Cubomedusae): including a description of the jellyfish responsible for the “Irukandji syndrome”. Aust J Zool. 1967;15(3):651–671. [Google Scholar]
- 52.Fenner PJ, Williamson JA, Burnett JW, Colquhoun DM, Godfrey S, Gunawardane K, Murtha W. The “Irukandji syndrome” and acute pulmonary oedema. Med J Aust. 1988;149(3):150–156. doi: 10.5694/j.1326-5377.1988.tb120544.x. [DOI] [PubMed] [Google Scholar]
- 53.Gershwin L. Two new species of jellyfishes (Cnidaria: Cubozoa: Carybdeida) from tropical Western Australia, presumed to cause Irukandji Syndrome. Zootaxa. 2005:1–30. [Google Scholar]
- 54.Gershwin L. Malo kingi: A new species of Irukandji jellyfish (Cnidaria : Cubozoa : Carybdeida): possibly lethal to humans, from Queensland, Australia. Zootaxa. 2007:55–68. [Google Scholar]
- 55.Gershwin L. Morbakka fenneri, a new genus and species of Irukandji jellyfish (Cnidaria: Cubozoa) Memoirs of the Queensland Museum – Nature. 2008;54(1):23–33. [Google Scholar]
- 56.Little M, Pereira P, Carrette T, Seymour J. Jellyfish responsible for Irukandji syndrome. QJ M. 2006;99(6):425–427. doi: 10.1093/qjmed/hcl057. [DOI] [PubMed] [Google Scholar]
- 57.Fenner PJ, Hadok JC. Fatal envenomation by jellyfish causing Irukandji syndrome. Med J Aust. 2002;177(7):362–363. doi: 10.5694/j.1326-5377.2002.tb04838.x. [DOI] [PubMed] [Google Scholar]
- 58.Huynh TT, Seymour J, Pereira P, Mulcahy P, Cullen P, Carrette T, Little M. Severity of Irukandji syndrome and nematocyst identification from skin scrapings. Med J Aust. 2003;178(1):38–41. doi: 10.5694/j.1326-5377.2003.tb05041.x. [DOI] [PubMed] [Google Scholar]
- 59.Grady JD, Burnett JW. Irukandji-like syndrome in South Florida divers. Ann Emerg Med. 2003;42(6):763–766. doi: 10.1016/s0196-0644(03)00513-4. [DOI] [PubMed] [Google Scholar]
- 60.Pommier P, Coulange M, De Haro L. Envenimation systémique par méduse en Guadeloupe: Irukandji-lke syndrome? Med Trop. 2005;65(4):367–369. [PubMed] [Google Scholar]
- 61.de Pender AM, Winkel KD, Ligthelm RJ. A probable case of Irukandji syndrome in Thailand. J Travel Med. 2006;13(4):240–243. doi: 10.1111/j.1708-8305.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- 62.Ramasamy S, Isbister GK, Seymour JE, Hodgson WC. The in vivo cardiovascular effects of the Irukandji jellyfish (Carukia barnesi) nematocyst venom and a tentacle extract in rats. Toxicol Lett. 2005;155(1):135–141. doi: 10.1016/j.toxlet.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Winter KL, Isbister GK, Schneider JJ, Konstantakopoulos N, Seymour JE, Hodgson WC. An examination of the cardiovascular effects of an ‘Irukandji’ jellyfish, Alatina nr mordens. Toxicol Lett. 2008;179(3):118–123. doi: 10.1016/j.toxlet.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Magalhães MM, Pereira MES, Amaral CFS, Rezende NA, Campolina D, Buc\aretchi F, Gazzinelli RT, Cunha-Melo JR. Serum levels of cytokines in patients envenomed by Tityus serrulatus scorpion sting. Toxicon. 1999;37(8):1155–1164. doi: 10.1016/s0041-0101(98)00251-7. [DOI] [PubMed] [Google Scholar]
- 65.Abdoon NA, Fatani AJ. Correlation between blood pressure, cytokines and nitric oxide in conscious rabbits injected with Leiurus quinquestriatus quinquestriatus scorpion venom. Toxicon. 2009;54(4):471–480. doi: 10.1016/j.toxicon.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Corkeron MA. Magnesium infusion to treat Irukandji syndrome. Med J Aust. 2003;178(8):411. doi: 10.5694/j.1326-5377.2003.tb05263.x. [DOI] [PubMed] [Google Scholar]
- 67.Corkeron M, Pereira P, Makrocanis C. Early experience with magnesium administration in Irukandji syndrome. Anaesth Intens Care. 2004;32(5):666–669. doi: 10.1177/0310057X0403200510. [DOI] [PubMed] [Google Scholar]
- 68.Tibballs J, Hawdon G, Winkel K. Mechanism of cardiac failure in Irukandji syndrome and first aid treatment for stings. Anaesth Intens Care. 2001;29(5):552. [PubMed] [Google Scholar]
- 69.Togias AG, Burnett JW, Kagey-Sobotka A, Lichtenstein LM. Anaphylaxis after contact with a jellyfish. J Allergy Clin Immunol. 1985;75(6):672–675. doi: 10.1016/0091-6749(85)90092-2. [DOI] [PubMed] [Google Scholar]
- 70.Månsson T, Randle HW, Mandojana RM, Calton GJ, Burnett JW. Recurrent cutaneous jellyfish eruptions without envenomation. Acta Derm Venereol. 1985;65:72–75. [PubMed] [Google Scholar]
- 71.Ohtaki N, Oka K, Sugimoto A, Akizawa T, Yasuhara T, Azuma H. Cutaneous reactions caused by experimental exposure to jellyfish, Carybdea rastoni. J Dermatol. 1990;17(2):108–114. doi: 10.1111/j.1346-8138.1990.tb03716.x. [DOI] [PubMed] [Google Scholar]
- 72.Tamanaha RH, Izumi AK. Persistent cutaneous hypersensitivity reaction after a Hawaiian box jellyfish sting (Carybdea alata) J Am Acad Dermatol. 1996;35(6):991–993. doi: 10.1016/s0190-9622(96)90130-9. [DOI] [PubMed] [Google Scholar]
- 73.Sendovski U, Goffman M, Goldshlak L. Severe delayed cutaneous reaction due to Mediterranean jellyfish (Rhopilema nomadica) envenomation. Contact Dermatitis. 2005;52(5):282–283. doi: 10.1111/j.0105-1873.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- 74.Ulrich H, Landthaler M, Vogt T. Granulomatous jellyfish dermatitis. J Dtsch Dermatol Ges. 2007;5(6):493–495. doi: 10.1111/j.1610-0387.2007.06335.x. [DOI] [PubMed] [Google Scholar]
- 75.Di Costanzo L, Balato N, Zagaria O, Balato A. Successful management of a delayed and persistent cutaneous reaction to jellyfish with pimecrolimus. J Dermatol Treatment. 2009;20(3):179–180. doi: 10.1080/09546630802562443. [DOI] [PubMed] [Google Scholar]
- 76.Pang KA, Schwartz MS. Guillain-Barré syndrome following jellyfish stings (Pelagia noctiluca) J Neurol Neurosurg Psych. 1993;56(10):1133. doi: 10.1136/jnnp.56.10.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huxley JTH. Huxley’s Diary of the Voyage of HMS. Rattlesnake; London: 1935. p. 63. [Google Scholar]