Abstract
Kava (‘Awa) is a traditional water-based beverage in Pacific island communities, prepared from the ground root and stems of Piper methysticum. Kava use is associated with an ichthyotic dermatitis and delayed type hypersensitivity reactions. In the current study we collated preparative methodologies from cultural practitioners and recreational kava users in various Pacific communities. We standardized culturally-informed aqueous extraction methods and prepared extracts that were subjected to basic physicochemical analysis. Mast cells exposed to these extracts displayed robust intracellular free calcium responses, and concomitant release of pro-inflammatory mediators. In contrast, mast cells were refractory to single or combinatorial stimulation with kavalactones including methysticin, dihydromethysticin and kavain. Moreover, we reproduced a traditional modification of the kava preparation methodology, pre-mixing with the mucilage of Hibiscus taliaceus, and observed its potentiating effect on the activity of aqueous extracts in mast cells. Taken together, these data indicate that water extractable active ingredients may play a role in the physiological and pathophysiological effects of kava, and suggests that mast cell activation may be a mechanistic component of kava-related skin inflammations.
Keywords: Kava, mast cells, kava dermopathy, kavalactones, Piper methysticum
Introduction
Kava (Piper methysticum, Hawaiian ‘Awa) is a member of the pepper family that grows throughout the Pacific Islands (Pepping, 1999). A drink prepared from the ground roots and stems of kava is used recreationally throughout the Pacific and, increasingly, in North America and Europe (Balick, et al., 2002; Brunton, 1988; Rychetnik, et al., 2011; Singh, 1992). In Pacific cultures consumption of the Kava drink was traditionally considered sacred and restricted to chiefs and priests and ceremonial events. Kava's desired physiological effects are as a soporific and relaxant, and its involvement in conflict resolution and decision-making processes in Pacific cultures has been documented (Balick, et al., 2002; Brunton, 1988; Gounder, 2006). In traditional Pacific medicine kava extract has been used as an anesthetic, sedative, relaxant, to relieve headaches, as an anti-inflammatory and to promote urination (Gounder, 2006; Pepping, 1999; Rowe, et al., 2011; Singh, 1992).
The pathophysiological effects of Kava have been widely discussed. Chronic and heavy users of kava report various symptoms, including muscle degradation, reddening of the eyes, and rare incidences of severe hepatotoxicity have been documented (Brown, et al., 2007; Fu, et al., 2008; Kava, 2001; Lude, et al., 2008; Rowe, et al., 2011; Teschke, et al., 2011). Moreover, it is being increasingly recognised that chronic kava users commonly present with a scaling of the dermis similar in form to an icthyotic dermatitis (Fu, et al., 2008; Grace, 2005; Guro-Razuman, et al., 1999; Norton, et al., 1994). This is accompanied by hyper-pigmentation and there is no known mechanism for its development (Matsuda, et al., 2006; Ruze, 1990). Generally, ichthyoses involve defective keratin synthesis, cornification or desquamation (Shwayder, 2004). While mast cells are nest understood to direct skin remodelling in the context of both beneficial and pathological inflammations such as wound healing, various urticarias and eczema (Abraham, et al., 2010; Galli, et al., 2010), some studies have shown that mast cell derived factors can influence keratinocyte function (Harvima, et al., 2008; Kohda, et al., 2002). There are also reports of delayed hypersensitivity allergic skin reactions to kava, which present some contact dermatitis-like symptoms (Jappe, et al., 1998; Schmidt, et al., 2000). Mast cells are key players in delayed hypersensitivity reactions and in this paper we investigate whether mast cells are targets for any of the active components in kava.
The active components of kava have been widely studied. The kavalactones (Bilia, et al., 2004; Fu, et al., 2008; Rowe, et al., 2011) are an extensive group of compounds that have been extracted from kava using conventional organic extraction techniques, and have been shown to recapitulate some of the hepatotoxic effects of kava itself. However, traditional and recreational users of kava do not perform organic extractions, and canonical kavalactones such as methistiycin, dihydromethistiycin and kavain are weakly extractable in aqueous solution (Cote, et al., 2004; Kubatova, et al., 2001; Xuan, et al., 2008). The chronic and heavy use of aqueous kava drinks may provide sufficient dosage for these weakly extractable components to be responsible for the physiological effects, and so in the current study we sought to compare the efficacy of purified kavalactones, singly or in combination, with the traditionally-prepared aqueous extract itself. We proposed to survey cultural practitioners and recreational kava uses from various Pacific communities and develop a collation of methodologies that reflect current practises in Kava preparation. Moreover, we reproduced a traditional modification of the kava preparation methodology reported in Fiji and Pohnpei, pre-mixing with the mucilage of Hisbiscus spp., and assessed its effect on the activity of aqueous extracts in mast cells (Balick, et al., 2002). We tested the premise that mast cells exposed to these extracts or purified kavalactones might display modified intracellular free calcium responses, and altered release of pro-inflammatory mediators. Taken together, our data indicate that water extractable active ingredients may play a role in the physiological and pathophysiological effects of kava, and suggests that mast cell activation may be a mechanistic component of kava-related dermatitis.
Materials and Methods
Cell culture and stimulation
RBL2H3 are a rat basophilic leukemia cell line that recapitulates many aspects of basophil and mucosal mast cell signaling (Passante, et al., 2009) were grown at 37 °C, 5% CO2, in a 95% humidified atmosphere in Dulbecco's Modification of Eagle Medium (Mediatech Inc., Herndon, VA) supplemented with 10% heat-inactivated Fetal Bovine Serum (Mediatech) and 2mM Glutamine. For stimulation via FcεRI, cells were incubated for 16 h with 0.1μg/ml IgE- anti DNP (clone SPE7, Sigma, St Louis, MO). Cells were then washed three times and stimulated with the indicated concentrations of Keyhole Limpet Hemocyanin coupled DNP (KLH-DNP, Sigma).
Beta-hexoseaminidase assay
RBL2H3M1 were plated in cluster plates at 5×104 cells/well. Monolayers were washed and incubated in 200 μl Tyrode's buffer before stimulating as described in the figure legends. After 30 min at 37°C, 25 μl supernatant was removed, clarified by microcentrifugation, and transferred to a 96 well plate containing 100 μl per well p-NAG substrate solution (1 mM p-N-acetyl glucosamine (Sigma) in 0.05 M citrate buffer pH 4.5). After 1,5 h at 37°C reactions were quenched by addition of 100 μl per well 0.2 M glycine, pH 9.0. Beta-hexoseaminidase levels were read as OD at 405 nm. Results are shown as the mean ± standard deviation.
Calcium assay
RBL2H3 were washed and incubated with 1μM Fluo-4 for 30min at 37°C in a standard modified Ringer's solution of the following composition (in mM): NaCl 145, KCl 2.8, CsCl 10, CaCl2 1, MgCl2 2, glucose 10, Hepes·NaOH 10, pH 7.4, 330 mOsm. Cells were transferred to 96-well plates at 50 000 cells/well and stimulated as indicated. Calcium signals were acquired using a Flexstation 3 (Molecular Devices, Sunnydale, USA). Data was analyzed using SoftMax® Pro 5 (Molecular Devices).
Kava preparation
The intent of this study was to approximate the kava preparation methodology used, in practice, in contemporary Pacific island recreational and ceremonial situations. Ground kava root/stem mixtures from Piper methysticum were purchased from commercial sources in Hawaii, Fiji, Vanuatu and Samoa. All ground kava was prepared from 80:20 by weight mixtures of Piper methysticum root:stem. No other ingredients were present in the preparations. Ground kava was produced by sequential grinding to a particle size of 500 microns. Ground kava was stirred in room temperature tap water for 20 min at the indicated w/v concentrations. Paper filters were used to gravity filter the kava suspension, approximating the cloth filtration step reported by survey respondents and available literature.
Hibiscus spp. mucilage preparation
Hisbiscus tiliaceus Hau bark was harvested on Oahu, Hawaii. Hibiscus bark was flattened and the mucilage layer was harvested by gentle scraping. Mucilage was aliquoted and stored at -20°C. Mucilage was mixed with ground kava in a 1:1 volumetric ratio for 5 min at RT before kava extraction was performed in aqueous solution as described above. Voucher specimens of Hibiscus tiliaceus (Hawaii, Maui and Kauai) have been previously deposited in the herbaria of the New York Botanical Garden and the University Museum, Tokyo, Japan (Takayam, 2006).
Analysis
All experiments are n of at least 3. Results are shown as the mean ± standard deviation. Statistical significance was determined based on a two way analysis of variance (Student's t-test). Adjacent to data points in the respective graphs, significant differences were recorded as follows: single asterisk, p < 0.05; double asterisk, p < 0.01; triple asterisk, p < 0.001; no symbol, p > 0.05. All experiments are n of at least 3.
Results
Preparation methodologies for kava in Pacific cultures
Kava is prepared in multiple Pacific Island cultures (Pepping, 1999; Singh, 1992). We prepared a survey instrument that was delivered to cultural practitioners and recreational users of Kava in several Pacific communities. Table 1 summarizes the data from 24 returned surveys. Respondents reported on their personal experience of kava preparation in the Marshall Islands, Vanuatu, Kiribati, the Solomon Islands, Hawaii, Samoa, Fiji, Palau and Guam. Of the respondents 8 self-identified as having a formal role as a cultural practitioner, while the remainder were recreational users of Kava. Commonalities between preparative methodologies are shown in Table 1. In general, users reported that the starting material was ground kava root and stem mixture prepared locally, and in some cases ground by hand. The diluent is tap water, predominantly at RT, and the Kava mixture is stirred briefly before filtration through a readily available material such as cloth, although in Fiji and Pohnpei Hibiscus bark with mucilage is used as a strainer. In Fiji users reported the practice of pre-mixing the Kava powder with the mucilage of the Hau (Hibiscus spp.) tree prior to water extraction. Concentrations (w/v) of Kava extract between 0.5% and 3.0% were reported. Respondents reported consuming numerous cups of Kava per night and that their frequency of use was as high as daily in 29% of users (panel B). Panel C shows that almost half of the respondents reported that the effects of Kava lasted more that 24h, and the longest reported duration of effect was 4-5 days (2 respondents). Finally, respondents were asked if they or anyone they knew presented a scaly skin rash associated with kava consumption. Of the 14 who answered this question, 13 replied in the affirmative (Norton, et al.).
Table 1. Survey of Kava preparers in Pacific Island cultures.
Data from 24 respondents from the indicated Pacific islands are collated in this table. Anonymous surveys were prepared and delivered between January 1 and July 1, 2011. A. Overview of preparative methodologies and reported consumption in specific locations surveyed. B. Reported frequency of Kava ingestion among survey respondents. C. Reported duration of the physiological effects of Kava ingestion among survey respondents.
| Respondents (24): | Male | 20 | Female | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Ages: | <30 | 5 | 31-50 | 8 | 51-70 | 7 | >70 | 2 |
|
| ||||||||
| Kava usage: | Cultural practitioner | 8 | Recreational | 16 | ||||
|
| ||||||||
| Location | Formulation | Diluent (temperature) | Filtration | Mucilage | w/v preparation | Consumption/person | ||
| Marshall Islands | root/stem | tap water (RT) | cloth/coconut sheath | nu | 1.0-2.5% | 1.5-3.0 liters | ||
| Vanuatu | root/stem | tap water (RT) | cloth | no | 0.1-3.0% | 2.0-4.0 liters | ||
| Kiribati | root/stem | tap water (boiled) | cloth | no | 1.0-5.0% | 3.0-5.0 liters | ||
| Solomon Islands | root/stem | tap water (RT) | cloth/metal filter | no | 0.5-1.0% | 2.0-3.0 liters | ||
| Hawaii | root/stem | tap water (RT) | cloth | no | 0.5-1.0% | 3.0-5.0 liters | ||
| American Samoa | root/stem | tap water (RT) | cloth | no | 0.5-1.0% | 2.0-3.0 liters | ||
| Fiji | root/stem/leaves | tap water (RT) | cloth/Hau bast | yes | 0.5-1.0% | 2.0-4.0 liters | ||
| Palau | root/stem | tap water (RT) | cloth | no | 0.5-1.0% | 2.0-4.0 liters | ||
| Guam | root/stem | tap water (RT) | cloth | no | 0.5-1.0% | 3.0-5.0 liters | ||
|
| ||||||||
| ||||||||
We used the data obtained in our survey, as well as a survey of available literature, to construct a Kava preparation process that reflected cultural practices in the laboratory. We selected commercially pounded kava root/stem preparations as our source materials. Our data suggest that the broad parameters for Kava preparation across Pacific cultures are the production of a 0.5-3.0% solution in room temperature-tepid (24-27°C) tap water, with a mixing period of 10-20 minutes with agitation, followed by filtration through cloth or equivalent. The major variation on this that we sought to also evaluate was the inclusion of an equal volume of Hibiscus spp. mucilage in the pre-filtration mixture, as practiced in Fiji and Pohnpei. Aqueous Kava extract prepared using these methodologies is slightly acidic (approximately pH 6.8).
Traditionally prepared aqueous Kava extracts elevate intracellular free calcium in mast cells
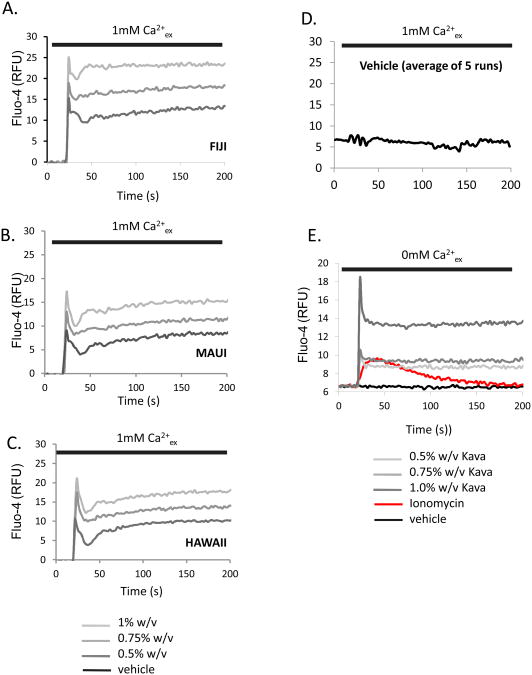
We sought to test the hypothesis that traditionally prepared aqueous Kava extracts may regulate the pro-inflammatory function of mast cells. Since the elevation of intracellular free calcium is a critical step in mast cell activation, we evaluated the effect of the extracts of Hawaiian, Fijian, and Mauian kava extracts, prepared as described, on calcium levels. The data in Figure 1A, B and C show that all aqueous extracts elicit robust calcium responses in RBL2H3 mast cells in a dose-dependent fashion. Vehicle controls were unresponsive (Figure 1D). Figure 1E demonstrates that the traditionally prepared aqueous kava extracts elicit calcium responses when cells are in a nominally calcium free external milieu, indicating that the downstream signaling mechanism of the active components is capable of causing calcium release from intracellular stores.
Figure 1. Aqueous extracts of kava cause elevation in intracellular free calcium levels in mast cells.
Aqueous kava extracts at 0.5, 0.75 and 1.0% (w/v) were added to Fluo-4 loaded mast cells in a standard sodium Ringer's solution containing 1mM CaCl2 (A,B,C) or made nominally calcium free (0mM CaCl2 with 1mM EGTA, E). Averaged response to vehicle is shown in D. Baselines were established for 25s before addition of kava extract. For comparison, response to 0.5μM ionomycin is shown in red. RFU, Relative Fluorescence Units.
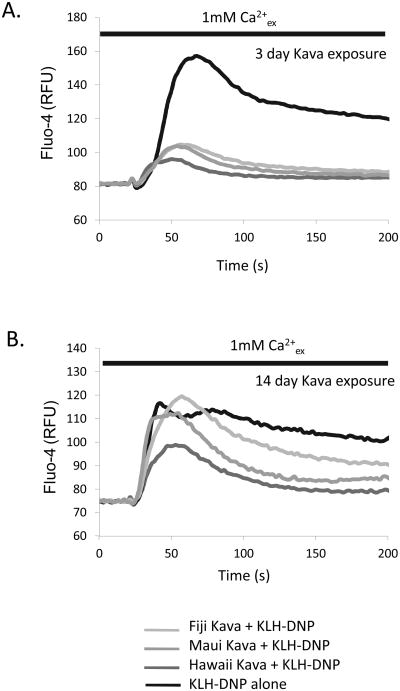
Chronic exposure to active components may be a feature of heavy and sustained kava usage. We asked if sustained kava extract exposure caused any effects on responsiveness of mast cells to stimulation. Figure 2A and B show that cells cultured in 1.0% kava extracts over a period of 3-14 days show suppressed calcium responses to antigen receptor ligation. These data indicate that prolonged exposure to kava components may alter the outcome of mast cell responses to subsequent challenge.
Figure 2. Chronic exposure to kava affects intracellular free calcium responses in mast cells.
RBL2H3 mast cells were cultured in the presence or absence of the indicated kava extracts (1% w/v final concentration) for periods up to 14 days. Cells were sampled at 3 and 14 days and loaded with Fluo-4. Cells were stimulated with IgE/KLH-DNP in a standard sodium Ringer's solution containing 1mM CaCl2.
Traditionally prepared aqueous Kava extracts cause degranulation in mast cells
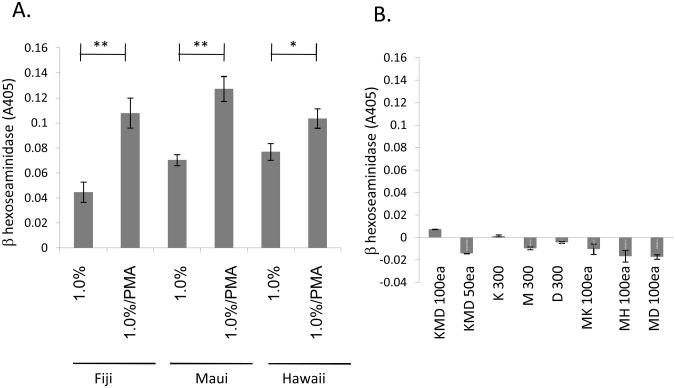
Mast cell degranulation is a central step in the generation of acute and chronic inflammation (Abraham, et al., 2010; Galli, et al., 2010). Secretion of mast cell mediators from pre-formed cytoplasmic granules has a well-established role in the generation of urticarias, and other dermal inflammations. We assessed the effect of traditionally-prepared aqueous kava extracts in an assay of mast cell degranulation. Figure 3A shows that these extracts cause secretory responses when added in isolation in a beta-hexoseaminidase release assay. There is extensive literature showing that PMA (through activation of serine/threonine kinase pathways) synergizes with calcium signals (for example provided by the ionophore ionomycin). Similarly, we observe that the addition of PMA to the calcium-mobilizing Kava extracts causes an increase in the observed level of mast cell degranulation. (Figure 3A).
Figure 3. Aqueous extracts of kava cause mast cell degranulation.
A. RBL2H3 mast cells were stimulated with the indicated concentration of kava extract in the absence or presence of 500nM PMA. Release of beta-hexoseaminidase was assayed after 30 min as described in Methods. Background release due to vehicle was subtracted from each reading. B. RBL2H3 mast cells were stimulated with the indicated concentrations (in μM) of Kavain (K), Methysticin (M) or dihydromethysticin (D). Release of beta-hexoseaminidase was assayed after 30 min as described in Methods. Background release due to vehicle (or PMA alone) was subtracted from each reading.
The kavalactones methysticin (M), dihydromethysticin (D) and kavain (K) are recognized as prominent active ingredients in kava, extractable readily in organic solvents and to a lesser degree in aqueous solution (Cote, et al., 2004; Kubatova, et al., 2001; Xuan, et al., 2008). We compared the effects of these purified kavalactones, applied singly and in combination, to the effect of the traditionally-prepared aqueous kava extracts described above. Figure 3B shows that when applied singly, these three compounds do not affect degranulation at concentrations of up to 0.3 mM. Moreover, when applied in combination (Figure 3B) these kavalactones are without effect on mast cell degranulation.
The effect of aqueous Kava extracts on mast cell calcium responses cannot be recapitulated by purified kavalactones singly or in combination
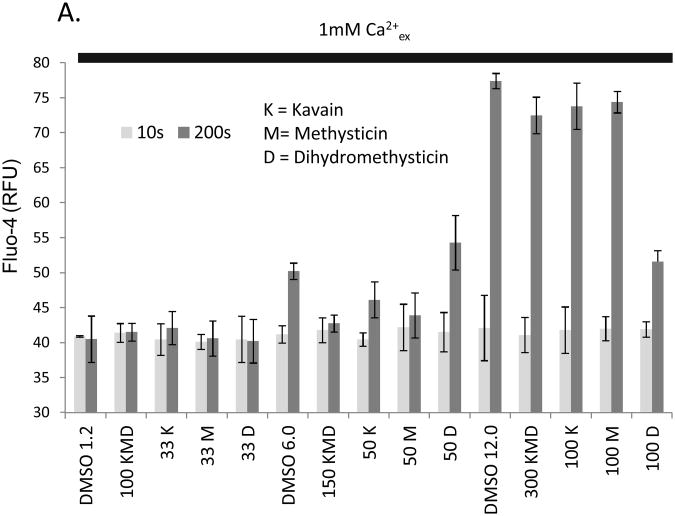
We asked whether the purified kavalactones methysticin (M), dihydromethysticin (D) and kavain (K) were capable of causing elevations in intracellular free calcium. Figure 4 shows that these compounds do not affect calcium levels in mast cells in a manner that cannot be attributed to vehicle (DMSO) effects. Taken together, these data suggest that application of these purified kavalactones cannot recapitulate the effect of aqueous kava extracts on mast cells.
Figure 4. Purified kavalactones do not elevate intracellular free calcium in mast cells.
Kavalactones at the indicated concentrations (μM) were added to Fluo-4 loaded mast cells in a standard sodium Ringer's solution containing 1mM CaCl2. Baselines were established for 25s before addition of kava extract. Light grey bars show RFU at 10s prior to compound addition (basal intensity) and dark grey bars show RFU at 200s (final attained intensity of calcium response). Vehicle controls comprised equivalent volumes of DMSO (indicated in microliters) to those used in stimuli.
Effect of pre-mixing with the mucilage of Hibiscus spp. bark on the potency of traditionally prepared aqueous kava extracts
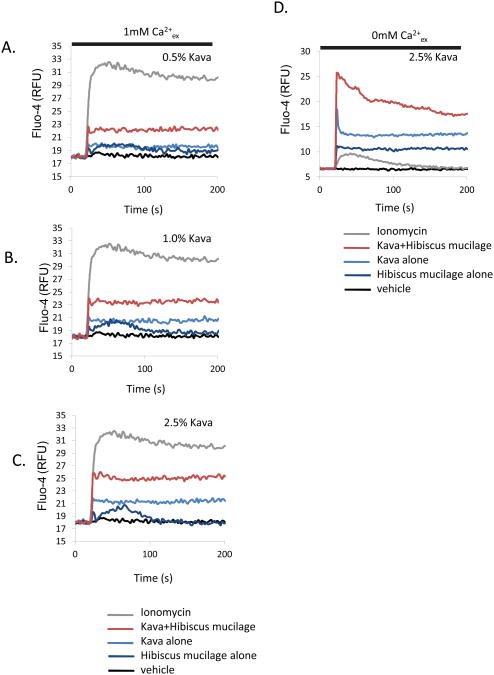
In some Pacific cultures a pre-mixing step is included in the preparation of kava for drinking. In Fiji and on the Micronesian island of Pohnpei, for example, traditional preparation of kava involves the kneading of the root/stem powder with approximately equal volume of Hibiscus spp. mucilage. This process is followed by filtration through either a fine cloth or, more traditionally, the moistened outer bark fibers of the hibiscus tree itself. We approximated this process in the laboratory (see Methods) and assessed the effect upon the potency and efficacy of subsequent aqueous extracts. Figure 5 shows a comparison of calcium responses, in kava prepared with and without the Hibiscus spp. mucilage pre-mixing step. Here, pre-mixing with the mucilage causes an apparent increase in the potency of applied kava extracts, in terms of enhanced calcium release (Figure 5A, B, C) and influx (Figure 5 D).
Figure 5. Pre-mixing with Hibiscus spp. mucilage increases the potency of aqueous kava extracts on mast cell calcium responses.
Kava powder was premixed with the mucilage of Hibiscus tiliaceus as described in Methods. Aqueous kava extracts at 0.5, 0.75. 1.0% and 2.5% (w/v) (with and without hibiscus mucilage pre-mix) were added to Fluo-4 loaded mast cells in a standard sodium Ringer's solution containing 1mM CaCl2 (A,B,C) or made nominally calcium free (0mM CaCl2 with 1mM EGTA, D, E, F). Baselines were established for 25s before addition of kava extract.
Discussion
In the present report we collated survey information on preparative methodologies for a soporific drink made from an aqueous extract of the kava plant, Piper methysticum. Survey data was collected from the Marshall Islands, Vanuatu, Kiribati, the Solomon Islands, Hawaii, Samoa, Fiji, Palau and Guam. The rationale in this study was to reflect in our experiments the actual source materials that are used in practice in the field, rather than to develop a standardized extraction process in the laboratory from raw Kava plant. This latter type of (usually organic solvent-based) extraction has been extensively documented elsewhere (Bilia, et al., 2004; Cote, et al., 2004; Kubatova, et al., 2001; Xuan, et al., 2008). We then reproduced these traditional techniques in the laboratory and assessed the efficacy of traditionally-prepared aqueous extracts of kava on cultured mast cells. Our data show that mast cells are sensitive to active components of the traditionally-prepared extracts. Specifically, there is a component of these extracts which can activate the release of intracellular free calcium and subsequent calcium influx from the extracellular milieu. These extracts are sufficient to cause mast cell degranulation, a key step in the development of inflammation (Abraham, et al., 2010; Galli, et al., 2010). Extracts from Hawaii and Fiji are presented in this paper, but essentially identical data were obtained using Samoan and Vanuatuan kava (data not shown).
Survey respondents and numerous published papers report an icthyotic dermopathy that manifests among ‘heavy, chronic’ users of kava throughout the Pacific (Gounder, 2006; Grace, 2005; Guro-Razuman, et al., 1999; Norton, et al., 1994). Clear prevalence data on this pathology are not available and its etiology has been poorly studied. The idea of a relationship between the kava-associated dermopathy and a niacin deficiency related to kava consumption has been proposed but is not supported by published evidence (Fu, et al., 2008; Ruze, 1990; Teschke, et al., 2011). Moreover, there are reports of a delayed type hypersensitivity to kava, in which mast cells would be expected to play a central role (Jappe, et al., 1998; Schmidt, et al., 2000) . Our current data suggest that mast cells are indeed sensitive to kava components. There is an obvious caveat to our study that these are in vitro experiments and it is unknown whether kava ingestion would lead to dermal mast cell exposure in vivo, although mucosal mast cell exposure in the GI tract would seem likely. If tissue concentrations of kava components were indeed demonstrated to be sufficient to activate dermal mast cells, then the chronic presentation of a dermal inflammatory response initiated by mast cell secretory components such as matrix active chymase, tryptase as well as fibroblast growth factors and angiogenic factors could be hypothesized. Mast cells also release factors that are active at keratinocytes, such as IL-4 and histamine. Interestingly, kava dermopathy is also accompanied by hyperpigmentation, possibly reflecting the overlap between mast cell activation pathways and those found in secretory melanocytes (Matsuda, et al., 2006; Norton, et al., 1994). Future experiments will focus upon the isolation of active components from aqueous kava extracts and their evaluation in vitro and in vivo for mast cell activation properties. An in vivo model of kava associated skin pathologies would enable the further test of our hypothesis using mast cell–stabilizing drugs and mast cell deficient mice.
The kavalactones have been the main focus of studies of the active components of kava. However, while these compounds are readily extractable in typical natural products discovery protocols using organic solvents published comparison data suggests that they are only poorly represented in the aqueous extracts that are actually ingested by humans (Cote, et al., 2004; Kubatova, et al., 2001; Xuan, et al., 2008). Our data suggest that the three purified kavalactones here are not capable, singly or in combination, and at high doses, of causing the mast cell responses to aqueous kava extracts that we observe. While this may be a dose or chronic exposure issue, we cannot exclude that these, or other kavalactones, are at some level capable of activating mast cells. For example, stem peelings contain pipermethystine, which has some cellular toxicity. Our evaluation of pipermethystine showed that its effects are somewhat similar to these other kavalactones assayed here (data not shown). Alternatively, there may be another class of compounds entirely or property of the aqueous extract that is responsible for the mast cell-activity. Xuan et al have noted that gluathione and compounds including cinnamic acid bornyl ester, several flavanones, trimethylnapththol, 5-methyl-1-phenylhexen-3-yn-5-ol, 8,11-octadecadienoic acid-methyl ester, pinostrobin and a chalcone are present in aqueous kava extracts (Xuan, et al., 2008). Of these, various flavanones and pinostrobin have been shown to be inactive, inhibitory or stimulatory at mast cells (Middleton, et al., 2000). Cinnamic acid has been shown to elicit immediate contact dermatitis, a mast cell-mediated process, and to mobilize calcium in pancreatic beta cells (Smith, et al., 2000). Cinnamic acid esters activate TRPA1, a mast cell calcium channel (Sadofsky, et al., 2011; Stokes, et al., 2006).
Differences in potency between the extracts of kava sourced from various Pacific Islands should be noted but may represent different harvesting and grinding methodologies that are beyond the scope of study in the current paper. However, we did examine the effect of pre-mixing ground kava powder with the mucilage extract of Hibiscus spp. The effect of this pre-mixing step, which approximated traditional practices in Fiji and Pohnpei, was to cause a potentiation in the intensity of calcium responses to aqueous Kava extracts. The Hisbiscus spp. mucilage extract has been proposed, anecdotally, to increase potency of kava but the chemistry of its effect has not been investigated. It may be acting as an emulsifying agent or may participate more actively in the chemistry of extraction. Hisbiscus mucilage has been investigated as a hydrophilic excipient (Jani, et al., 2008). Other similar excipients in this class have been shown to improve extraction efficacy of natural product-derived compounds and to modulate absorption dynamics (Pescitelli, et al., 2010). Taken together it seems likely that the mucilage is acting as an excipient with properties of binding, emulsification and stabilization. Medicinal chemistry and GCMS/HPLC-MS deconvolution of mucilage-mixed and untreated kava extracts will enable better understanding of whether the mucilage plays an active role in changing the chemistry of the kava extraction.
In summary, cultural practices for kava extraction informed our study and provided methodological information, the resulting aqueous extracts have distinct properties from purified kavalactones and the organic extracts typically prepared in natural products chemistry studies. Components of aqueous extracts of kava are highly active in mast cells, promoting calcium release, influx and the secretion or pro-inflammatory mediators. These data provide the basis for future studies of mast cells as a causative component of kava-induced skin inflammation. In addition, mast cell active compounds in kava extracts remain to be identified.
Acknowledgments
This work was funded by the NSF EPSCOR EPS-0903833 (to H.T.) and The Hawaii Community Foundation (Victoria S. and Bradley L. Geist Foundation) award 45408 to H.T. The authors acknowledge with gratitude the contributions of undergraduate students Tautasi Falanai, Noelani Tu'u, Franscis Galavao, Erick Paul, Jack Kuh, Daniel Randall and Micah Yoshinaga, Timothy Ng; Dr. Thomas C. Wesselkamper (Chaminade University), Mr. Dako Nating (Republic of the Marshall Islands) and Dr. Irene Taafaki (University of the South Pacific), Januaria Balajadia and William Greineisen.
Footnotes
The authors disclose no conflicts of interest.
Literature Cited
- Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balick MJ, Lee R. Traditional use of sakau (kava) in Pohnpei: lessons for integrative medicine. Altern Ther Health Med. 2002;8:96–98. [PubMed] [Google Scholar]
- Bilia AR, Scalise L, Bergonzi MC, Vincieri FF. Analysis of kavalactones from Piper methysticum (kava-kava) J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:203–214. doi: 10.1016/j.jchromb.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Brown AC, Onopa J, Holck P, Kaufusi P, Kabasawa D, Craig WJ, Dragull K, Levine AM, Baker JD. Traditional kava beverage consumption and liver function tests in a predominantly Tongan population in Hawaii. Clin Toxicol (Phila) 2007;45:549–556. doi: 10.1080/15563650701365875. [DOI] [PubMed] [Google Scholar]
- Brunton R. Kava: Use and Abuse in Australia and the South Pacific. No 5. Sydney: National Drug and Alcohol Research Centre Monograph; 1988. A harmless substance? Anthropological aspects of kava in the South Pacific. [Google Scholar]
- Cote CS, Kor C, Cohen J, Auclair K. Composition and biological activity of traditional and commercial kava extracts. Biochem Biophys Res Commun. 2004;322:147–152. doi: 10.1016/j.bbrc.2004.07.093. [DOI] [PubMed] [Google Scholar]
- Fu PP, Xia Q, Guo L, Yu H, Chan PC. Toxicity of kava kava. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26:89–112. doi: 10.1080/10590500801907407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol. 2010;40:1843–1851. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounder R. Kava consumption and its health effects. Pac Health Dialog. 2006;13:131–135. [PubMed] [Google Scholar]
- Grace R. Kava-induced urticaria. J Am Acad Dermatol. 2005;53:906. doi: 10.1016/j.jaad.2005.04.068. [DOI] [PubMed] [Google Scholar]
- Guro-Razuman S, Anand P, Hu Q, Mir R. Dermatomyositis-like illness following kava-kava ingestion. J Clin Rheumatol. 1999;5:342–345. doi: 10.1097/00124743-199912000-00008. [DOI] [PubMed] [Google Scholar]
- Harvima IT, Nilsson G, Suttle MM, Naukkarinen A. Is there a role for mast cells in psoriasis? Arch Dermatol Res. 2008;300:461–478. doi: 10.1007/s00403-008-0874-x. [DOI] [PubMed] [Google Scholar]
- Jani GK, Shah DP. Evaluation of mucilage of Hibiscus rosasinensis Linn as rate controlling matrix for sustained release of diclofenac. Drug Dev Ind Pharm. 2008;34:807–816. doi: 10.1080/03639040801925768. [DOI] [PubMed] [Google Scholar]
- Jappe U, Franke I, Reinhold D, Gollnick HP. Sebotropic drug reaction resulting from kava-kava extract therapy: a new entity? J Am Acad Dermatol. 1998;38:104–106. doi: 10.1016/s0190-9622(98)70547-x. [DOI] [PubMed] [Google Scholar]
- Kava R. The adverse effects of kava. Pac Health Dialog. 2001;8:115–118. [PubMed] [Google Scholar]
- Kohda F, Koga T, Uchi H, Urabe K, Furue M. Histamine-induced IL-6 and IL-8 production are differentially modulated by IFN-gamma and IL-4 in human keratinocytes. J Dermatol Sci. 2002;28:34–41. doi: 10.1016/s0923-1811(01)00147-5. [DOI] [PubMed] [Google Scholar]
- Kubatova A, Miller DJ, Hawthorne SB. Comparison of subcritical water and organic solvents for extracting kava lactones from kava root. J Chromatogr A. 2001;923:187–194. doi: 10.1016/s0021-9673(01)00979-7. [DOI] [PubMed] [Google Scholar]
- Lude S, Torok M, Dieterle S, Jaggi R, Buter KB, Krahenbuhl S. Hepatocellular toxicity of kava leaf and root extracts. Phytomedicine. 2008;15:120–131. doi: 10.1016/j.phymed.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Hirata N, Kawaguchi Y, Naruto S, Takata T, Oyama M, Iinuma M, Kubo M. Melanogenesis stimulation in murine B16 melanoma cells by Kava (Piper methysticum) rhizome extract and kavalactones. Biol Pharm Bull. 2006;29:834–837. doi: 10.1248/bpb.29.834. [DOI] [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Norton SA, Ruze P. Kava dermopathy. J Am Acad Dermatol. 1994;31:89–97. doi: 10.1016/s0190-9622(94)70142-3. [DOI] [PubMed] [Google Scholar]
- Passante E, Frankish N. The RBL-2H3 cell line: its provenance and suitability as a model for the mast cell. Inflamm Res. 2009;58:737–745. doi: 10.1007/s00011-009-0074-y. [DOI] [PubMed] [Google Scholar]
- Pepping J. Kava: Piper methysticum. Am J Health Syst Pharm. 1999;56:957–958. 960. doi: 10.1093/ajhp/56.10.957. [DOI] [PubMed] [Google Scholar]
- Pescitelli G, Bilia AR, Bergonzi MC, Vincieri FF, Di Bari L. Cyclodextrins as carriers for kavalactones in aqueous media: spectroscopic characterization of (S)-7,8-dihydrokavain and beta-cyclodextrin inclusion complex. J Pharm Biomed Anal. 2010;52:479–483. doi: 10.1016/j.jpba.2010.01.037. [DOI] [PubMed] [Google Scholar]
- Rowe A, Zhang LY, Ramzan I. Toxicokinetics of kava. Adv Pharmacol Sci. 2011;2011:326724. doi: 10.1155/2011/326724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruze P. Kava-induced dermopathy: a niacin deficiency? Lancet. 1990;335:1442–1445. doi: 10.1016/0140-6736(90)91458-m. [DOI] [PubMed] [Google Scholar]
- Rychetnik L, Madronio CM. The health and social effects of drinking water-based infusions of kava: a review of the evidence. Drug Alcohol Rev. 2011;30:74–83. doi: 10.1111/j.1465-3362.2010.00184.x. [DOI] [PubMed] [Google Scholar]
- Sadofsky LR, Boa AN, Maher SA, Birrell MA, Belvisi MG, Morice AH. TRPA1 is activated by direct addition of cysteine residues to the N-hydroxysuccinyl esters of acrylic and cinnamic acids. Pharmacol Res. 2011;63:30–36. doi: 10.1016/j.phrs.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Boehncke WH. Delayed-type hypersensitivity reaction to kava-kava extract. Contact Dermatitis. 2000;42:363–364. [PubMed] [Google Scholar]
- Shwayder T. Disorders of keratinization: diagnosis and management. Am J Clin Dermatol. 2004;5:17–29. doi: 10.2165/00128071-200405010-00004. [DOI] [PubMed] [Google Scholar]
- Singh YN. Kava: an overview. J Ethnopharmacol. 1992;37:13–45. doi: 10.1016/0378-8741(92)90003-a. [DOI] [PubMed] [Google Scholar]
- Smith CK, Moore CA, Elahi EN, Smart AT, Hotchkiss SA. Human skin absorption and metabolism of the contact allergens, cinnamic aldehyde, and cinnamic alcohol. Toxicol Appl Pharmacol. 2000;168:189–199. doi: 10.1006/taap.2000.9025. [DOI] [PubMed] [Google Scholar]
- Stokes A, Wakano C, Koblan-Huberson M, Adra CN, Fleig A, Turner H. TRPA1 is a substrate for de-ubiquitination by the tumor suppressor CYLD. Cell Signal. 2006;18:1584–1594. doi: 10.1016/j.cellsig.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Takayam K, Kajita T, Murata J, Tateshi Y. Phylogeography and genetic structure of Hibiscus tiliaceus — speciation of a pantropical plant with sea-drifted seeds. Molecular Ecology. 2006;15:2871–2881. doi: 10.1111/j.1365-294X.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- Teschke R, Sarris J, Schweitzer I. Kava hepatotoxicity in traditional and modern use: The presumed Pacific kava paradox hypothesis revisited. Br J Clin Pharmacol. 2011;73:170–174. doi: 10.1111/j.1365-2125.2011.04070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan TD, Fukuta M, Wei AC, Elzaawely AA, Khanh TD, Tawata S. Efficacy of extracting solvents to chemical components of kava (Piper methysticum) roots. J Nat Med. 2008;62:188–194. doi: 10.1007/s11418-007-0203-2. [DOI] [PubMed] [Google Scholar]