Abstract
Background
Delirium or acute brain dysfunction is extremely prevalent in medical intensive care unit (ICU) patients, but limited data exist regarding its prevalence and risk factors among surgical (SICU) and trauma ICU (TICU) patients. The purpose of this study was to determine the prevalence and risk factors for delirium in surgical and trauma ICU patients.
Methods
SICU and TICU patients requiring mechanical ventilation (MV) >24 hours were prospectively evaluated for delirium using the Richmond Agitation Sedation Scale (RASS) and the Confusion Assessment Method for the ICU (CAM-ICU). Those with baseline dementia, intracranial injury, or ischemic/hemorrhagic strokes that would confound the evaluation of delirium were excluded. Markov models were used to determine predictors for daily transition to delirium.
Results
One-hundred patients (46 SICU and 54 TICU) were enrolled. Prevalence of delirium was 73% in the SICU and 67% in the TICU. Multivariable analyses identified midazolam [OR 2.75 (CI 1.43–5.26, p = 0.002)] exposure as the strongest independent risk factor for transitioning to delirium. Opiate exposure showed an inconsistent message such that fentanyl was a risk factor for delirium in the SICU (p = 0.007) but not in the TICU (p = 0.936), while morphine exposure was associated with a lower risk of delirium (SICU, p = 0.069; TICU p = 0.024).
Conclusions
Approximately 7 out of 10 SICU and TICU patients experience delirium. In keeping with other recent data on benzodiazepines, exposure to midazolam is an independent and potentially modifiable risk factor for the transitioning to delirium.
Keywords: trauma, surgery, cognitive impairment, delirium, mechanical ventilation, critical care, sedatives and analgesic
INTRODUCTION
Delirium is a global disturbance of consciousness characterized by fluctuating mental status, inattention, and disorganized thinking. Although health care providers have long recognized components of this syndrome, delirium has been historically dismissed as an expected complication of the hospitalized elderly patient, and its impact on outcomes thought to be negligible. Recent studies using standardized and validated tools have shown that delirium is significantly under-diagnosed,1, 4 not limited to the elderly patient, and more importantly is a predictor of a 3-fold higher mortality over 6 months1 and higher cost of care.2 Additionally patients with delirium may experience significant cognitive impairment long after discharge.3
The development of tools such as the Confusion Assessment Method for the ICU (CAM-ICU)4 and the Intensive Care Delirium Screening Checklist5 have allowed for the rapid diagnosis of delirium in patients (even while mechanically ventilated) by non-psychiatric physicians and other healthcare personnel. In light of this, the Society of Critical Care Medicine (SCCM) has proposed guidelines6 for more routine and diligent monitoring of delirium, using the CAM-ICU. To date, however, there have been no prospective studies performed in surgical and trauma patients, using standardized tools to identify the true prevalence in this population of patients. In addition, the risk factors for developing delirium among mechanically ventilated surgical and trauma ICU patients have yet to be defined. The objective of this prospective, observational trial was to study the prevalence of delirium in surgical and trauma ICU patients and identify potentially modifiable risk factors.
METHODS
Patients
The institutional review board approved this investigation with a waiver of consent, due to the non-interventional nature of the study. We enrolled patients from January 01, 2005 through March 15, 2005. Enrollment criteria included (1) all patients 18 years or older, (2) requiring mechanical ventilation (MV) for greater than 24 hours and (3) admitted to the SICU or TICU at Vanderbilt University Medical Center (VUMC). Patients were excluded who had significant baseline neurological diseases or intracranial neurotrauma that would confound the evaluation of delirium, inability to understand English, significant hearing loss and moribund patients not expected to survive > 24 hours.
Patient setting
VUMC is a 631-bed academic hospital that includes a level I trauma center, with a 14 bed TICU, staffed by surgical intensivists. The trauma center admits approximately 3,000 acutely injured patients annually with over 900 being admitted to the TICU. The SICU, managed by a multidisciplinary intensivist team, is a 21 bed critical care unit that serves all surgical services (excluding cardiac) including general, vascular, hepatobiliary, urology, plastics, otolaryngology, thoracic, transplant, and obstetrics-gynecology.
Study procedures
Baseline demographics as well as information pertaining to known baseline risk factors for delirium identified from review of the literature in post surgical ward and medical ICU patients and from focus group meetings were collected at time of enrollment. These included pre-existing neurological disease, depression, hearing and visual impairment, history of alcohol, nicotine or substance abuse, acquired immunodeficiency syndrome, and chronic immunosuppressant medication use.
Level of arousal was measured by using the Richmond Agitation Sedation Scale (RASS),7,8 which is a 10 point scale ranging from + 4 to −5, with a RASS score of 0 denoting a calm and alert patient. Positives RASS scores denote positive or aggressive symptomology ranging from +1 (mild restlessness) to +4 (dangerous agitation). The negative RASS scores, differentiates between response to verbal commands (RASS score −1 to −3) and physical stimulus (RASS score −4 and −5). Patients were evaluated once daily for delirium using the CAM-ICU4,9 for a maximum of 10 days or until ICU discharge. This delirium assessment instrument is valid4,9 and extremely reliable in the hands of health care providers, taking an average of 30–60 seconds for the bedside nurses.10,11 The CAM-ICU comprises four features which assess the following: acute change or fluctuation in mental status (Feature 1), inattention (Feature 2), disorganized thinking (Feature 3) or an altered level of consciousness (Feature 4). Each day the patient was categorized as being either in a coma, normal or delirious based on previously published standardized definitions.4 Coma was defined as a RASS score of −4 or −5, in which case the CAM-ICU is not assessed due to lack of any response to any verbal stimulation. Normal was defined as RASS scores −3 and above and CAM-ICU negative. To be diagnosed as delirious, one needed to have a RASS score of −3 or higher, with an acute change or fluctuation in mental status (Feature 1), accompanied by inattention (Feature 2) and either disorganized thinking (Feature 3) or an altered level of consciousness (Feature 4). Prevalent delirium was defined as a positive CAM-ICU assessment during the first non-comatose mental status evaluation. We chose to evaluate and label our delirium assessment as prevalent delirium instead of incident delirium (the first positive CAM-ICU assessment following a period of normal mental status) because it was difficult to obtain a reliable assessment of a patients’ pre-enrollment delirium status prior to ICU admission especially in the trauma patients. We believe most of our patients were not delirious prior to their ICU admission, and that the new or incident delirium rates would be the same as the prevalent delirium rates. Prior to initiation of the study the authors agreed that the delirium evaluations would be performed after morning rounds by the primary and critical care teams and following the assessment of the patient by the bedside nurse as to not interfere with direct patient care.
Daily laboratory data, as ordered by the treating ICU team, were also collected to gather information related to metabolic disturbances that may contribute to delirium. Sedatives and analgesic medications were prescribed by physicians according to an institutional protocol adapted from the guidelines of the Society of Critical Care Medicine (SCCM),6 which can be viewed on our educational web site (www.icudelirium.org). The medications were titrated by the bedside nurses to achieve a target sedation level determined by the treating team using the Richmond Agitation Sedation Scale (RASS)7,8 and for pain using the behavioral pain scale.12 Exposure to sedative and analgesic medications, anticholinergics, antipsychotics, general anesthesia, histamine blockers, antiarrhythmics, antidepressants as well as steroids and non-steroidal anti-inflammatory drugs was also collected daily. Hypoxemia, another risk factor for delirium, was captured by collecting the lowest oximetric saturations daily in the enrolled patients.
Definitions of clinical injury severity and critical illness scoring systems
Several clinical injury severity and critical illness scoring systems were used. The Charlson Comorbidity Index (calculated using the Deyo method)13,14 represents the sum of a weighted index that takes into account the number and seriousness of pre-existing co-morbidities. The Acute Physiology and Chronic Health Evaluation (APACHE) II15 is a severity of illness scoring system, and these data were calculated using the most abnormal parameters during the first 24 hours following admission to the intensive care unit. APACHE II scores range from 0 (best) to 71 (worst). The Sequential Organ Failure Assessment (SOFA)16,17 is an organ failure scoring system that was calculated using the most abnormal parameters during the first 24 hours following admission to the intensive care unit. SOFA scores range from 0 (best) to 24 (worst). The Injury Severity Score (ISS) is an anatomical scoring system that provides an overall score for patients with multiple injuries. Each injury is assigned an Abbreviated Injury Scale (AIS) score and is allocated to one of six body regions (head, face, chest, abdomen, extremities, including pelvis, and external). Only the highest AIS score in each body region is used. The 3 most severely injured body regions have their score squared and added together to produce the ISS score. The Revised Trauma Score (RTS) is to be used to rapidly assess the trauma patient at the scene. It assigns points for respiratory rate, systolic blood pressure and the Glasgow coma score. The Trauma Related Injury Severity Score (TRISS) was designed to be used to compare outcomes from different treatment centers, and calculates expected survival based on patient characteristics including the ISS, RTS, age as well as blunt versus penetrating trauma.
Statistical Analysis
Patients’ baseline demographic and clinical variables were presented using medians and interquartile range (IQRs) for continuous variables, and proportions for categorical variables. The aim of the analysis was to assess the effect of daily use of sedative and analgesic drug administration on daily transitions to delirium independent of pre-determined, clinically relevant covariates. As one transition model, first-order Markov chains were used to estimate the probability of transitioning from any previous cognitive status to the next (assuming such transition depends only on the previous status).18 In this study, our Markov chain models included the following 6 (3 by 2) transitions: from normal, delirious or comatose during the previous 24 hours to either normal or delirious status in the following 24 hours. These transition probabilities were estimated within a regression framework called “Markov regression” – i.e., a model that included the patients' cognitive status measured 24 hours prior along with other clinically relevant covariates. Generalized Estimating Equation (GEE) methods19 were used to account for correlations within patients over time. Analyses were performed separately per surgical patients, trauma patients, and all patients combined. Drugs examined included analgesics (morphine and fentanyl), sedatives (lorazepam, propofol and midazolam), anesthetics, antipsychotics and histamine (H2) blockers, which were included separately in the Markov regression model in order to prevent over-fitting. Clinically relevant covariates at baseline included patient’s age, body mass index, Charlson co morbidities index, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and presence of sepsis. To further prevent over-fitting, especially for subgroup analyses by ICU type, the 5 clinically relevant covariates, were combined into two summary components using principle component analysis, which reduce dimension of correlated to preserve the power of the analysis. These two principle components were included in Markov regression model along with daily use (yes/no) of each psychoactive drug and the 24-hour prior cognitive status expressed by two dummy variables (delirium and coma versus normal status). For all analyses, R-software version 2.1.1 (www.r-project.org) and SAS version 9.0 (SAS Institute, Cary NC) were used. Two-sided 5% significance level was used for all statistical inferences.
RESULTS
One hundred and forty two consecutive patients from the surgical and trauma ICU were evaluated during the study period for eligibility in the study. Forty were excluded due to significant neurological injury and two patients were moribund and not expected to survive for greater than 24 hours, leaving a total of 100 patients who were enrolled into the study. Three patients remained comatose for the entire duration of the study and subsequently died; these 3 were not included in the delirium analyses. Demographic data on the remaining 97 patients are presented in Table 1. Ventilator days, ICU and hospital lengths of stay and mortality data for the entire group as well as for each individual ICU are presented in Table 1 as well.
Table 1.
Baseline Characteristics of the Patients
| Variable | Combined (N=97) |
Surgical ICU (N=45) |
Trauma ICU (N=52) |
|---|---|---|---|
| Demographic data | |||
| Age | 48 [36–60] | 55 [46–67] | 41 [25–50] |
| Sex (Male) | 52% (50) | 38% (17) | 63% (33) |
| Race (White) | 88% (84) | 93% (42) | 84% (42) |
| Charlson Comorbidity Index | 0 [0–2] | 1 [0–3] | 0 [0–0] |
| APACHE II | 24 [18–28] | 25 [18–29] | 23 [19–27] |
| SOFA | 8 [7–10] | 9 [7–10] | 8 [7–9] |
| Injury Severity Score (ISS) | NA | NA | 20 [16–29] |
| Revised Trauma Score (RTS) | NA | NA | 6 [4–7] |
| Trauma and Injury Severity | NA | NA | 0.9 [0.6–1.0] |
| Score (TRISS) | |||
| Primary admission diagnoses | |||
| Hemorrhage | 14% (14) | 18% (8) | 12% (6) |
| Airway or facial trauma | 21% (20) | 11% (5) | 29% (15) |
| Chest trauma | 8% (8) | 0% (0) | 15% (8) |
| Colonic or gastric trauma | 5% (5) | 0% (0) | 10% (5) |
| Gastric surgery | 8% (8) | 16% (7) | 2% (1) |
| Hepatobiliary-pancreatic surgery | 4% (4) | 7% (3) | 2% (1) |
| Neurosurgical trauma | 7% (7) | 2% (1) | 12% (6) |
| Orthopedic trauma | 7% (7) | 2% (1) | 12% (6) |
| Septic shock or ARDS | 9% (9) | 20% (9) | 0% (0) |
| Other primary diagnosis | 15% (15) | 24% (11) | 8% (4) |
| Outcomes | |||
| Days on ventilator | 2 [1–6] | 4 [1–13] | 2 [1–3] |
| Length of hospital stay | 11 [7–20] | 13 [8–23] | 9.5 [5–19.2] |
| Length of ICU stay | 5 [3–10] | 8 [4–19] | 5 [3–8] |
| Hospital survival | 86% (83) | 78% (35) | 92% (48) |
Median with interquartile range when applicable
We found the prevalence of delirium to be 70% in the combined surgical and trauma ICU patients with 73% of surgical and 67% of trauma patients having delirium. Surgical patients had a median (IQR) duration of delirium of 3 (0–4) days, while that for the trauma ICU patients was 1(0 to 4) days..
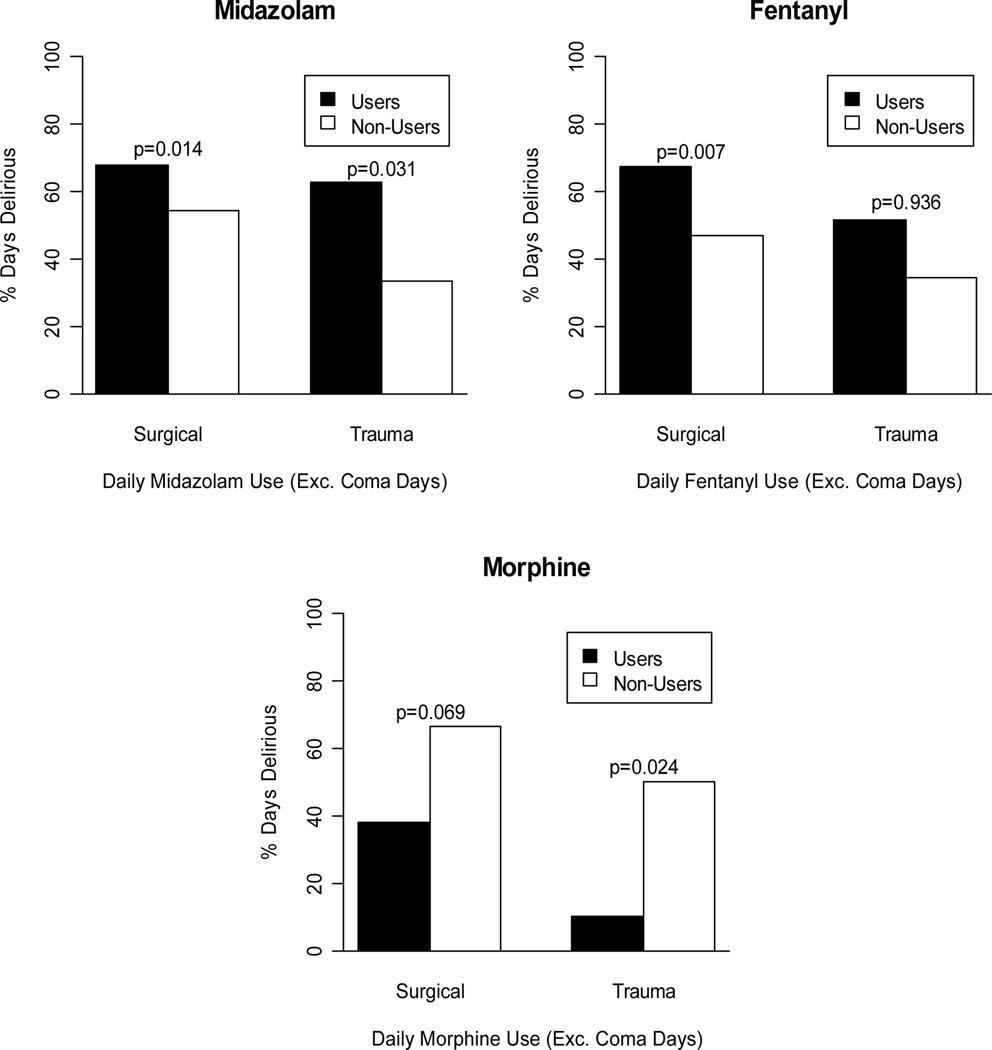
In the multivariable analyses, adjusting for previous cognitive status and clinically relevant covariates at baseline, midazolam exposure [Odds ratio (OR) 2.75 (CI 1.43–5.26, p = 0.002)] was the strongest independent predictor of transitioning to delirium (Table 2). Narcotic exposure showed an inconsistent message such that fentanyl was a risk factor for delirium in the SICU (p = 0.007) but not in the TICU (p = 0.936) patients and morphine was potentially beneficial against delirium (SICU, p = 0.069; TICU p = 0.024). The association of sedatives and analgesic medication with delirium by ICU type is shown in Figure 1. This illustrates the proportion of time that patients were delirious in the surgical and trauma ICU while receiving midazolam, fentanyl or morphine (users) in comparison to those that were not exposed to the medications (non-users). Exposure to anesthetics was associated with differential effect on transitioning to delirium, with it appearing protective for the trauma group (p = 0.020) but not for the SICU (p= 0.734). For a limited sample size, no significant interaction was detected for midazolam (p for interaction = 0.89), fentanyl (p = 0.13) or morphine (p= 0.40) by ICU type. Additionally there were no significant interactions between the antipsychotic drugs and midazolam [OR 0.47 (0.09–2.42), p=0.37], or fentanyl [OR 0.77 (0.26–2.24), p=0.63]. Baseline demographics, admission diagnosis and severity of illness indicators did not predict transition to delirium.
Table 2.
Multivariable Analysis of Sedative and Analgesic Medications as Risk Factors for Transitioning to Delirium
| Medication | Odds Ratio (95% CI) * | p-value † |
|---|---|---|
| Surgical and trauma patients (n=97) | ||
| Anesthetics | 0.52 (0.23, 1.16) | 0.108 |
| H2 blockers | 1.45 (0.80, 2.62) | 0.217 |
| Lorazepam | 0.45 (0.16, 1.27) | 0.131 |
| Midazolam | 2.75 (1.44, 5.26) | 0.002 |
| Fentanyl | 1.88 (0.99, 3.55) | 0.053 |
| Morphine | 0.36 (0.16, 0.82) | 0.015 |
| Surgical Patients (n=4 5) | ||
| Anesthetics | 1.23 (0.37, 4.04) | 0.735 |
| H2 blockers | 1.71 (0.74, 3.95) | 0.212 |
| Lorazepam | 0.46 (0.10, 2.05) | 0.307 |
| Midazolam | 3.22 (1.27, 8.20) | 0.014 |
| Fentanyl | 3.99 (1.47, 10.85) | 0.007 |
| Morphine | 0.37 (0.13, 1.08) | 0.069 |
| Trauma Patients (n= 52) | ||
| Anesthetics | 0.18 (0.04, 0.77) | 0.020 |
| H2 blockers | 1.25 (0.52, 3.04) | 0.618 |
| Lorazepam | 0.51 (0.12, 2.17) | 0.360 |
| Midazolam | 2.45 (1.09, 5.52) | 0.031 |
| Fentanyl | 1.03 (0.47, 2.25) | 0.936 |
| Morphine | 0.22 (0.06, 0.82) | 0.024 |
Odds ratios in this table can be interpreted as indicating the odds of transitioning to delirium for patients who received any dose of the given medication in the previous 24 hours, adjusted for the following baseline variables: age, body mass index, Charlson comorbidity index, APACHE II severity of illness score, and diagnosis of sepsis, septic shock or ARDS.
p ≤ 0.05 considered statistically significant
Figure 1.
Histogram illustrating the proportion of time that patients were delirious in the surgical and trauma ICU while receiving midazolam, fentanyl or morphine (users) in comparison to those that were not exposed to the medications (non-users). Patients receiving midazolam spent a greater proportion of time with delirium than the non users in the surgical and trauma ICU. Users of fentanyl in the SICU spent a greater proportion of time with delirium but not those in the TICU, while patients receiving morphine spent a lower proportion of time with delirium than the non users in both the ICUs.
DISCUSSION
Sedative and analgesic medications are administered nearly universally to patients on mechanical ventilation in accordance with widely recognized Society of Critical Care Medicine’s clinical practice guidelines6 in order to reduce pain and anxiety. This study has several important findings. First, we diagnosed delirium in 7 out of 10 surgical/trauma patients. This high prevalence was not known as this organ dysfunction has not been studied with tools validated for mechanically ventilated patients in this population. Second, and perhaps the chief finding, is that one of the most widely used sedative is independently associated with the development of delirium. Specifically, midazolam is associated with a 2–3 fold higher odds of transitioning into delirium during each subsequent 24-hour period even after adjusting for relevant covariates. This is consistent with recent data from our MICU cohort study in which we assessed the temporal association between administration of sedative and analgesic medications and transitioning to delirium and found that lorazepam was an independent risk factor for transitioning to delirium.20 The current study further supports the notion that it is not the individual drug (lorazepam or midazolam), but the benzodiazepine class of drugs that are risk factors for transitioning to delirium.
Despite the high prevalence of delirium in surgical and trauma ICU patients seen in our study, delirium is often missed because health care teams are more attuned to noticing the patient with hyperactive delirium, with positive symptomology like agitation, than the hypoactive delirium, manifesting negative symptomology like inattention and depressed level of consciousness. Peterson et al.21 recently showed that pure hyperactive delirium occurred in only 1.6 % of 614 medical ICU patients while the majority of patients had either hypoactive (43.5%) or a mixed delirium (54.9%). With the development of tools such as the CAM22 and CAM-ICU4, non-psychiatric physicians and other healthcare personnel, can now reliably diagnose delirium, including the hypoactive subtype, even while mechanically ventilated.
Our study is the first prospective study in surgical and trauma patients that shows the temporal association of administration of benzodiazepines such as midazolam and the development of delirium. This has tremendous implications for clinicians since benzodiazepines form the cornerstone of sedative regimens to relieve anxiety in the ICU,6,23,24 though significant regional and international variations exist. Midazolam, reflecting the choice of the benzodiazepine most commonly used in our surgical and trauma ICU, was the most consistent and significant predictor of transitioning into delirium in our cohort. This is in light of the fact that benzodiazepines are identified as the “drug of choice” for treating delirium in critically ill patients in a recent text25 and by 16% of respondents in a recent international survey.26 Unfortunately, the mechanism or prognostic implications of delirium caused by the different psychoactive medications is not known (and not answered by the current study). Benzodiazepines and propofol have high affinity for the gamma-amino butyric acid (GABA)-receptor in the central nervous system,27 leading to alterations in the levels of numerous neurotransmitters believed to be deliriogenic. Novel sedative agents which are GABA-receptor sparing may help reduce some of the cognitive dysfunction seen in ICU patients, and a randomized trial is presently underway.28 While it should be emphasized that sedative and analgesic medications have a very important role in patient comfort, healthcare professionals must also strive to achieve the right balance of sedative and analgesic administration through greater focus on reducing unnecessary use. Instituting daily interruption of sedatives and analgesics and protocolizing their delivery have both been shown to improve patients’ outcomes.29–31
Amongst opiates, fentanyl had high and significant odds ratio for transitions to delirium in the SICU but not in the TICU patients, while morphine appeared to have a protective effect by preventing the transition to delirium. This protective effect of morphine needs to be considered in the context that the number of patients who were given morphine were considerably lower than those who received fentanyl. Sedative and analgesic practices vary in ICUs around the world,24 with some physicians opting to use opiates for the “double effect” of analgesia and sedation, thereby reducing the need for benzodiazepines or propofol. We feel this use of fentanyl for achieving deeper levels of sedation and thus being administered at higher doses continuously, rather than as smaller bolus doses like morphine, may explain why fentanyl and not morphine was a risk factor for delirium in our study. Marcantonio et al.32 performed a nested case-control study within a prospective cohort of post operative patient patients who developed delirium, and found an association between benzodiazepines and meperidine use and the occurrence of delirium. Similarly Dubois et al.33 have shown that opiates (morphine and meperidine) administered either IV or via an epidural catheter may be associated with the development of delirium in medical/surgical ICU patients. Morrison et al.34 have shown that pain control with morphine is associated with reduction in delirium, while the use of meperidine increases the incidence of delirium.
Several limitations of this investigation warrant consideration. We used drug exposure rather than drug dosage or serum levels of the medications. Few studies have attempted to use in vivo drug levels, and we are incorporating both these elements in our future studies. We were also not able to look at lorazepam separately, because of an extremely small number of patients on that drug. Our model incorporated numerous covariates that were deemed relevant a priori, though this list was not all-inclusive. It is possible that other unmeasured covariates such as sleep deprivation could have altered the results. In our study we evaluated for delirium once daily and measured sedative and analgesic exposure in the intervening 24 hours to study the effect of these medications in the transition or change in cognitive status. However, within the 24-hour period it is possible that the sedative and analgesic medications were given as a consequence of delirium. In order to completely remove this bias, the only solution is to assess patient’s cognitive status more frequently (every 2–4 hours) and measure sedative and analgesic exposure between these periods, thus accurately assessing temporal association between delirium and sedatives. Since that is extremely difficult to do due to resource and time constraints, the next best alternative is to use Markov modeling which at least provides the temporal association within 24 hour periods and our methods are still much more rigorous than previously published non-ICU databases addressing this topic. Further, this study was also not designed or powered to determine the role of delirium in leading to adverse outcomes such as length of stay, cost, or mortality. This remains unknown in the surgical patient population, though the strong relationship between delirium and these outcomes has been repeatedly demonstrated in many other studies of critically ill patients in medical, geriatric, orthopedic, and other patient populations.1–3, 35–37 Lastly, we were only able to conduct a cursory investigation into the role of anticholinergic medications and their interactions with sedatives and analgesics as risk factors for transition to delirium. Though not statistically significant, we did not have adequate power to make firm conclusions.
CONCLUSIONS
In the current study, we found delirium to be present in 70% of surgical and trauma patients. We used Markov regression modeling and showed that there was an independent and temporal association between receiving sedative and analgesic medications and transitioning to delirium (most notable for midazolam) after adjusting for important covariates. These data suggest that surgical and trauma ICU patients are at a very high risk of developing this form of organ dysfunction that may become even higher depending on treatment choices. The sedative and analgesic medications that are routinely employed have both therapeutic and hazardous ramifications for our patients and must be dosed very carefully. Considering that delirium is a predictor of death, prolonged cognitive impairment, and higher cost of care in previously conducted cohort studies, prospective interventional studies should be conducted to determine whether alternative management strategies (or specific choices of sedative/analgesic agents) are associated with reductions in delirium and other short and long-term clinical outcomes.
Acknowledgments
Grant Support:
Dr. Pandharipande is the recipient of the Vanderbilt Physician Scientist Development Award and the ASCCA-FAER Research Grant.
Dr. Ely is the Associate Director of Research for the VA Tennessee Valley Geriatric Research and Education Clinical Center (GRECC). He is a recipient of the VA MERIT Award from CSRND and a RO-1 from the National Institute of Aging (#AG0727201-A1).
The funding agencies had no role in the design or conduct of the study, data collection, management, analysis or interpretation of the data. In addition, they had no role in the preparation, review or approval of the manuscript.
No other financial support was provided to conduct this investigation.
References
- 1.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 2.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 3.Jackson JC, Gordon SM, Hart RP, et al. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 4.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron N, Dubois MJ, Dumont M, et al. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 6.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 8.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 9.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Pun BT, Gordon SM, Peterson JF, et al. Large-scale implementation of sedation and delirium monitoring in the intensive care unit: A report from two medical centers. Crit Care Med. 2005;33:1199–1205. doi: 10.1097/01.ccm.0000166867.78320.ac. [DOI] [PubMed] [Google Scholar]
- 11.Ely EW, Truman B, Manzi DJ, et al. Consciousness monitoring in ventilated patients: bispectral EEG monitors arousal not delirium. Intensive Care Med. 2004;30:1537–1543. doi: 10.1007/s00134-004-2298-1. [DOI] [PubMed] [Google Scholar]
- 12.Payne JP, Bru O, Bosson JL, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001;29:2258–2263. doi: 10.1097/00003246-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Mendonca Ad, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford: Clarendon Press; 1994. [Google Scholar]
- 19.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 20.Pandharipande PP, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Peterson JF, Pun BT, Dittus RS, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 22.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 23.Ostermann ME, Keenan SP, Seiferling RA, et al. Sedation in the intensive care unit. JAMA. 2000;283:1451–1459. doi: 10.1001/jama.283.11.1451. [DOI] [PubMed] [Google Scholar]
- 24.Soliman HM, Melot C, Vincent JL. Sedative and analgesic practice in the intensive care unit: the results of a European survey. Br J Anaesth. 2001;87:186–192. doi: 10.1093/bja/87.2.186. [DOI] [PubMed] [Google Scholar]
- 25.Wijdicks EFM. Neurologic Complications of Critical Illness. New York: Oxford University Press; 2002. [Google Scholar]
- 26.Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med. 2004;32:106–112. doi: 10.1097/01.CCM.0000098033.94737.84. [DOI] [PubMed] [Google Scholar]
- 27.Mihic S, Harris R. GABA and the GABAA receptor. Alcohol Health and Research World. 1997;21:127. [PMC free article] [PubMed] [Google Scholar]
- 28.Maze M, Scarfini C, Cavaliere F. New agents for sedation in the intensive care unit. Critical Care Clinics. 2001;17:881–897. doi: 10.1016/s0749-0704(05)70185-8. [DOI] [PubMed] [Google Scholar]
- 29.Kollef MH, Levy NT, Ahrens TS, et al. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 30.Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27:2609–2615. doi: 10.1097/00003246-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Kress JP, Pohlman AS, O'Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 32.Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272:1518–1522. [PubMed] [Google Scholar]
- 33.Dubois MJ, Bergeron N, Dumont M, et al. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 34.Morrrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. Journal of Gerontology: Medical Sciences. 2003;58A:76–81. doi: 10.1093/gerona/58.1.m76. [DOI] [PubMed] [Google Scholar]
- 35.Inouye SK, Rushing JT, Foreman MD, et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13:234–242. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32:2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 37.McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162:457–463. doi: 10.1001/archinte.162.4.457. [DOI] [PubMed] [Google Scholar]