Abstract
Exposure to high levels of manganese (Mn) results in a neurological condition termed manganism, which is characterized by oxidative stress, abnormal dopamine (DA) signaling, and cell death. Epidemiological evidence suggests correlations with occupational exposure to Mn and the development of the movement disorder Parkinson's disease (PD), yet the molecular determinants common between the diseases are ill-defined. Glutathione S-transferases (GSTs) of the class pi (GSTπ) are phase II detoxification enzymes that conjugate both endogenous and exogenous compounds to glutathione to reduce cellular oxidative stress, and their decreased expression has recently been implicated in PD progression. In this study we demonstrate that a Caenorhabditis elegans GSTπ homologue, GST-1, inhibits Mn-induced DA neuron degeneration. We show that GST-1 is expressed in DA neurons, Mn induces GST-1 gene and protein expression, and GST-1-mediated neuroprotection is dependent on the PD-associated transcription factor Nrf2/SKN-1, as a reduction in SKN-1 gene expression results in a decrease in GST-1 protein expression and an increase in DA neuronal death. Furthermore, decreases in gene expression of the SKN-1 inhibitor WDR-23 or the GSTTπ-binding cell death activator JNK/JNK-1 result in an increase in resistance to the metal. Finally, we show that the Mn-induced DA neuron degeneration is independent of the dopamine transporter DAT, but is largely dependent on the caspases CED-3 and the novel caspase CSP-1. This study identifies a C. elegans Nrf2/SKN-1-dependent GSTπ homologue, cell death effectors of GSTTπ-associated xenobiotic-induced pathology, and provides the first in vivo evidence that a phase II detoxification enzyme may modulate DA neuron vulnerability in manganism.
Keywords: manganism, neurodegeneration, neurotoxicity, caspase, Nrf2, Parkinson's disease
1. Introduction
Parkinson's disease (PD) is the second most prevalent neurodegenerative disease, and results in motor deficits, abnormal dopamine (DA) signaling, and DA neuron degeneration (Martin et al., 2011; Nass and Przedborski, 2008). The majority of PD cases are considered to be idiopathic (IPD), with less than 10% due to a known inherited mutation (Nass and Przedborski, 2008). It is likely that the environment and/or multiple genes contribute significantly to the development of the disorder. Exposure to high levels of manganese (Mn) can result in a neurological disorder termed manganism (Burton and Guilarte, 2009). Individuals with manganism present with motor deficits, dysfunctional DA signaling, and DA neuron degeneration. Epidemiological evidence suggests an association between occupational exposure to Mn and the development of PD (Crossgrove and Zheng, 2004; Jiang et al., 2006; Roth, 2009; Martin, 2011; Racette et al., 2012). Welders and Mn miners are particularly susceptible to developing parkinsonism and PD (Jiang et al., 2006; Racette et al., 2001; Sriram et al., 2010). The molecular basis of the increased propensity of individuals exposed to high levels of Mn to develop manganism and PD is ill-defined.
GSTπ (glutathione S-transferase pi) is a member of the phase II class of detoxification enzymes that are responsible for conjugating a broad range of electrophiles with glutathione to prepare them for cellular excretion (Goto et al., 2009). Decreased GSTπ expression has been identified as a significant risk factor for the development and progression of PD (Kelada et al., 2003; Menegon et al., 1998; Wilk et al., 2006; Shi et al., 2009; Vilar et al., 2007). GSTπ is expressed in the substantia nigra and plays a role in DA neuron sensitivity to the PD-associated neurotoxicants 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) and rotenone (Shi et al., 2009; Smeyne et al., 2007). The protein has been shown to inhibit apoptosis by binding to c-Jun N-terminal kinase (JNK)
(Wang et al., 2001; Burg et al., 2006; Thevenin et al., 2011). The role and regulation of GST π in manganism or Mn-induced cell death has not been previously defined. In rat primary hepatocytes, the expression of GSTπ has recently been shown to be regulated by the PD-associated transcription factor Nrf2, a key redox sensitive regulator of antioxidant enzymes, suggesting that Nrf2 may also play an important role in modulating GSTπ-associated DA neuron vulnerability (Lin et al., 2012).
The nematode Caenorhabditis elegans (C. elegans) is a robust genetic model to dissect the molecular players involved in DA neuron vulnerability (Nass et al., 2008; Nass and Blakely, 2003). The DA neurons contain the full complement of genes involved in DA neurotransmission and signaling in vertebrates. As the animals are transparent, a transgenic strain expressing green fluorescent protein (GFP) in the 8 DA neurons allows for analysis of neuronal integrity in vivo (Nass et al., 2002; Nass and Settivari, 2008). C. elegans are also sensitive to a number of PD-associated toxicants, including 6-OHDA, rotenone, and heavy metals, as well as to the human PD-associated protein α-synuclein, and contain homologues to genes involved in cell death pathways (Lakso et al., 2003; Nass and Settivari, 2008; Vanduyn et al., 2013; Ved et al., 2005; Vistbakka et al., 2012).
The development of C. elegans models for PD and manganism and the recent discovery that the nematode homologue of Nrf2, SKN-1, is expressed in DA neurons, provide an opportunity to identify mechanisms of Mn-associated DA neuron vulnerability (Oliveira et al., 2010; Park et al., 2009; Settivari et al., 2009; Vanduyn et al., 2010). In this study we asked whether the PD-associated GSTp and its molecular modulators contribute to DA neuron vulnerability to Mn. Here we show that the C. elegans SKN-1 regulates DA neuron vulnerability to Mn through the GSTπ homologue GST-1. We also identify upstream and downstream modulators of toxicant-associated cell death, and identify a novel caspase involved in the toxicant-induced DA neurodegeneration.
2. Materials and Methods
2.1 C. elegans strains and maintenance
The following strains were obtained from the Caenorhabditis Genetics Center: Wild-type Bristol N2 and RNA mediated interference (RNAi) sensitive NL2099 rrf-3(pk1426). BY250 (Pdat-1∷GFP) is an integrated, transgenic line expressing GFP from behind the dat-1 promoter, and has been previously described (Lakso et al., 2003; Nass et al., 2002; Settivari et al., 2009). BY200 vtIs1 [Pdat-1∷GFP; rol-6(su1006)], BY215 vtIs1 [P dat-1∷GFP; dat-1(ok157); rol-6(su1006)] and RJ928 (Pdat-1∷GFP; rrf-3(pk1426)) have also been previously described (Nass et al., 2002; Settivari et al., 2009). C. elegans strains were cultured on OP50 or NA22 bacteria on NGM or 8P media, respectively, at 20°C according to standard methods (Hope, 1999; Brenner, 1974).
2.2 RNA extraction and cDNA synthesis
Total RNA was isolated from a synchronized C. elegans population using Trizol reagent largely as previously described (Novillo et al., 2005; Settivari et al., 2009). Briefly, nematode pellets were resuspended in Trizol after treatment with MnCl2 (1 ml/100 μl compact worm pellet). Impurities were separated from nucleic acids using chloroform, and RNA was precipitated with isopropyl alcohol. The RNA pellet was washed with 75% ethanol and dissolved in RNase-free water, and stored at -80°C. RNA concentrations were measured with a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA). One microgram of total RNA was reverse transcribed to cDNA using oligo dT primers (Integrated DNA Technologies, Coralville, IA) and the iScript cDNA synthesis kit (Bio Rad, Hercules, CA) following manufacturer's instructions. The cDNA was purified using Microcon YM30 filters (Millipore, Billerica, MA) and quantified using a NanoDrop ND-1000 spectrophotometer.
2.3 qPCR measurements
Gene specific primers were designed using Primer3 software, and the primers were designed to be exon spanning to avoid amplification of contaminating genomic DNA. Glyceraldehyde-3-dehydrogenase (GAPDH) was selected as the housekeeping gene, as its expression did not change as a result of MnCl2 treatment. The following primers were used to determine changes in gene expression of gst-1 following MnCl2 exposure: gst-1 F – CAAGGACGTTCTTCCAGGAG, gst-1 R - CTGGAACACCATCAAGAGCA GAPDH F - GAAACTGCTTCAACGCATCA, GAPDH R - CCTTGGCGACAAGAAGGTAG. Real-time PCR was performed using 2× SYBR Green PCR master mix and the ABI Prism 7500 sequence detection system (Applied Biosystems, Grand Island, NY). Gene expression studies were performed in triplicate and the formation of a single PCR product was confirmed using dissociation curves. Negative controls with the primers consisted of all of the components of PCR mix except cDNA. Relative fold change in gene expression for each gene was calculated using normalized CT values (the cycle number at which the fluorescence passes the threshold).
2.4 Toxicant exposures
Synchronized L1 stage worms were obtained by hypochlorite treatment of gravid adults followed by incubation of the embryos in M9 buffer for 18 h, and washed at least three times in dH2O using standard protocols (Nass et al., 2002; Nass and Hamza, 2007; Settivari et al., 2009). For Mn treatment, L1 stage worms (10 worms/μl) were incubated with dH2O ± 50 mM manganese chloride (MnCl2, Fisher Scientific, Fair Lawn, NJ), as previously described for 30 min at room temperature (∼22°C) with gentle mixing every 10 min (Nass and Hamza, 2007; Settivari et al., 2009). Following treatment, the worms were placed onto NGM/OP50 plates and allowed to recover for 72 h at 20°C. After exposure and recovery, 50-60 worms were immobilized on 2% agarose pads with 2% sodium azide and were scored for DA neurodegeneration under a fluorescence microscope (Leica MZ 16FA, Switzerland). Worms were scored positive for DA neuron degeneration when GFP in any part of the four cephalic dendrites (CEPs, which run from the nerve ring to tip of the nose) was absent (Nass et al., 2002; Settivari et al., 2009). Each of the experiments was performed at least in triplicate, and the results are reported as mean ± S.E.
2.5 Antibodies and Western blot analysis
Antibodies to amino acids 85-184 from the putative C. elegans protein GST-1 (WP:CE00302) were generated using Genomic Antibody Technology at Strategic Diagnostics Inc. (SDI, Newark, DE). Rabbit polyclonal antibodies were further purified at SDI. GAPDH (ab36840 Abcam, Cambridge, MA) was used as a loading control. To prepare protein for Western blot analysis, synchronized L1 stage worms were exposed to MnCl2 for 30 min, washed three times with water and allowed to recover on NGM plates at 20°C for 24 h. Following toxicant exposures and recovery, worms were washed from media plates and pelleted. 150 μl of buffer (20 mM HEPES, pH 7.5, 250 mM sucrose, 1 mM EDTA, 1 mM EGTA, 10 mM KCl, 1.5 mM MgCl2, 1 mM DTT, 0.1 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml pepstatin, 2 μg/ml aprotinin) was added to 300-400 μl of pelleted worms and the tubes were frozen at -20°C until protein purification. Nematode samples stored at -20°C were thawed and homogenized on ice using 50-60 strokes with a 2 ml glass homogenizer. The lysate was centrifuged at 400×g at 4°C for 4 min, the supernatant was collected in a sterile tube, and protein concentration was determined using the Bradford assay with bovine gamma globulin as the standard (Bradford, 1976). The samples were diluted in NuPAGE LDS buffer (Invitrogen, Carlsbad, CA), heated at 95°C for 20 min, and total cell lysates (50 μg protein) were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to PVDF membranes (Bio-Rad, Hercules, CA). Membranes were blocked with 5% non-fat dry milk dissolved in TBST (tris-buffered saline, 0.1% Tween-20) for 2 h at RT, followed by incubation with the appropriate primary antibody dilution (anti-GST-1 at 1:10,000; anti-GAPDH at 1:10,000) at 4°C overnight. The membranes were washed 3 times at RT for 15 min, and incubated with HRP-conjugated secondary anti-rabbit IgG (611-1302 Rockland, Gilbertsville, PA). The membrane was developed using enhanced chemiluminescence (ECL) (Amersham Biosciences, Pittsburgh, PA), captured using Bio-Rad ChemiDoc XRS, and total-protein intensities were measured using QuantityOne software (Bio-Rad, Hercules, CA).
2.6 Immunohistochemistry
Primary C. elegans cultures were prepared as previously described, but with slight modifications (Bianchi and Driscoll, 2006, Settivari et al., 2009). Gravid adult worms were lysed with the synchronization solution, and the egg pellet was washed using egg buffer (118 mM NaCl, 48 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 25 mM HEPES). The eggs were separated from the debris using a 60% sucrose solution. The eggshell was digested using 4 mg/ml chitinase (Sigma, St. Louis, MO), embryonic cells were dissociated using syringe aspiration, and cells were resuspended in L-15 medium (containing 10% FBS and 1% pen/strep) and grown on polylysine-coated slides at 20°C. Following growth for 72 h, the cells were fixed in 4% paraformaldehyde, permeabilized in 0.5% Triton X-100, and blocked using 2% BSA and 20% normal goat serum. The cells were then incubated with GST-1 primary antibodies (1:5,000) at 4°C overnight (14 h), followed by incubation with a Texas Red conjugated goat anti-rabbit secondary antibody (Invitrogen, Eugene, OR) (1:5,000) at RT for 1 h. Images were captured using a Zeiss LSM 510 confocal microscope (Zeiss, Jena, Germany).
2.7 RNA interference
The RNAi sensitive strain RJ928 has been described previously (Settivari et al., 2009; Vanduyn et al., 2010). RNAi studies were performed on NGM plates containing 1 mM isopropyl β-d-thiogalactoside (IPTG) and 100 μg/ml ampicillin and seeded with HT115 (DE3), an RNase III-deficient E. coli strain carrying the L4440 vector with the gene fragment (gst-1, skn-1, wdr-23, jnk-1, ced-3, or csp-1) (OpenBiosystems, Thermo Scientific, Waltham, MA) or empty vector (Addgene, Cambridge, MA) (Timmons and Fire, 1998). Synchronized L1 stage RJ928 worms were transferred onto RNAi plates and the feeding protocol was followed with modifications (Kamath and Ahringer, 2003). 50 to 100 second generation gravid adults grown on RNAi bacteria were transferred to fresh RNAi media plates and allowed to lay eggs for 5 h. Adults were then removed from the plate. L1s hatched from the eggs (3rd generation) were exposed to Mn as described above. For skn-1, RNAi is embryonic lethal, therefore first generation L1s were exposed to the toxicants (Vanduyn et al., 2010). The animals were allowed to recover on the RNAi plates for 72 h, and evaluated as previously described (Nass et al., 2002; Settivari et al., 2009;Vanduyn et al., 2010).
2.8 Statistical analysis
Data in all graphs is presented as the mean ± SEM. GraphPad Prism software (GraphPad Software, San Diego, CA) was used for statistical analysis. A t-test was used to compare the difference between two groups. One-way ANOVA followed by a Bonferroni post hoc test was used for the comparison of multiple groups. For experiments involving RNAi and toxicant exposure, two-way ANOVA analysis with a Bonferroni post hoc test was performed. Differences were considered significant when p < 0.05, or lower where indicated.
3. Results
3.1 Manganese induces GST-1 gene and protein expression
Manganese is an environmental toxicant that has been positively correlated with the development of IPD (Hudnell, 1999; Gorell et al., 1999; Racette et al., 2001; Roth, 2009; Tanner et al., 2011; Martin, 2011). Epidemiological and neuropathological studies implicate differential expression of the inducible phase II detoxification enzyme GSTπ as a possible contributor to the development of IPD (Shi et al., 2009; Vilar et al., 2007). A BLAST search with human GSTπ (accession number: AAH10915.1) and the C. elegans genome indicates that the nematode GST-1 is the most homologous protein. A sequence alignment of the results using ClustalW2 indicates that the C. elegans GST-1 is highly conserved with its human counterpart (58% similar, 39% identical), and contains the 11 residues that are involved in the formation of the glutathione binding site as well as the 8 conserved C-terminal amino acids that are characteristic of the pi class GSTs (data not shown). The alignment suggests that GST-1 may be a functional GSTπ homologue, and its expression may be induced upon xenobiotic exposure.
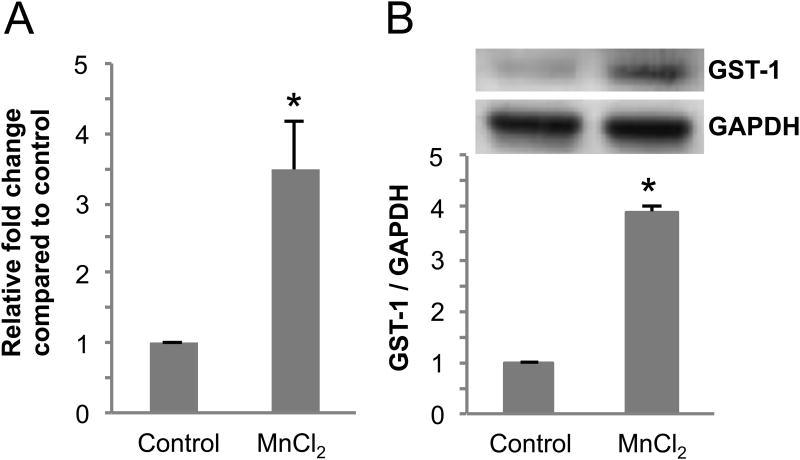
Mn exposures have been used to model manganism and PD in vertebrates, C. elegans, and cell culture (Settivari et al., 2009; Shi et al., 2009, Sanchez-Betancourt et al., 2012; Ved et al., 2005). In order to determine if GST-1 gene expression is induced upon exposure to the toxicants, we utilized RT-PCR to determine levels of gene expression in controls and in worms exposed to sub-lethal concentrations of Mn as previously described (Nass and Hamza, 2007; Settivari et al., 2009). Young nematodes exposed to MnCl2 for 30 min show a 3-fold increase in GST-1 mRNA levels (Fig. 1A). These results suggest that GST-1 protein levels may also be induced following exposure to Mn. In order to determine if Mn can induce GST-1 protein expression, we generated antibodies to the putative GST. The antigenic sequence is unique to GST-1 and spans from amino acid 85 to 184. As can be seen in Fig. 1B, exposure to Mn results in a 3-4 fold increase in GST-1 protein levels. These results indicate that GST-1 protein levels are sensitive to Mn, and suggest that the enzyme may play a role in modulating DA neuron sensitivity to the metal.
Fig 1. Exposure to Mn induces GST-1 gene and protein expression.
(A) Synchronized L1 stage WT C. elegans were exposed to 50 mM MnCl2 for 30 mins, mRNA was extracted and reverse transcribed to cDNA. Relative gene expression changes of gst-1 were quantitated using real-time PCR. The fold change in gene expression relative to GAPDH was calculated following the ΔΔCt method. Shown are mean ± S.E. of three individual replicates. p value was calculated using t-test analysis. Asterisk indicates p ≤ 0.04 between control and toxicant-exposed group ΔCt values. (B) Synchronized L1 stage WT nematodes were exposed to 50 mM MnCl2 for 30 mins and allowed to recover for 24 h on NGM plates at 20°C. Following recovery/exposure, the worms were collected, homogenized and protein was quantified following standard a Bradford assay. For Western blot analysis, protein samples were separated by electrophoresis, transferred to a membrane, and probed with anti-GST-1 or GAPDH primary antibodies. Shown are mean ± SE of at least three individual replicates. p values were calculated using t-test analysis. Asterisks indicate p ≤ 0.03 between controls and Mn-exposed groups.
3.2 GST-1 is expressed in DA neurons
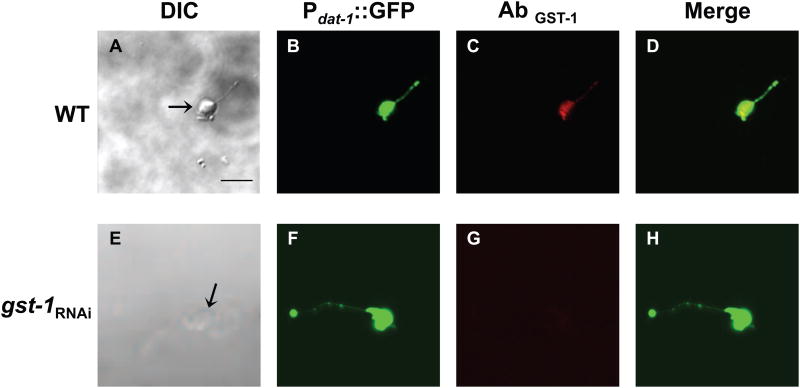
Vertebrate studies indicate that GSTπ is expressed in DA neurons and can affect DA neuron sensitivity to toxicants (Smeyne et al., 2007). To determine whether GST-1 is expressed in C. elegans DA neurons, we generated primary cultures from RJ928 animals fed RNAi bacteria containing the empty vector (WT) or RNAi bacteria targeting gst-1 to decrease its expression (gst-1RNAi) (Settivari et al., 2009). The DA neurons are easily identified under a fluorescent dissecting scope, as GFP is expressed at high levels in the neurons both in vivo and in vitro (Settivari et al., 2009; Vanduyn et al., 2010). The morphology of the DA neurons in animals in which GST-1 expression is reduced appears identical to WT, suggesting that GST-1 does not play a significant role in maintaining DA neuron integrity (data not shown). We used affinity-purified anti-GST-1 to evaluate the GST-1 expression levels. GST-1 immunoreactivity is observed in all DA neurons, as well as in other cell types (Fig. 2A-D; data not shown). There is no apparent staining in DA primary neurons or other cells exposed to gst-1 RNAi, suggesting that the RNAi significantly reduces GST-1 protein expression (Fig. 2E-H). These results indicate that GST-1 is expressed in DA neurons, and suggest that the antibody is likely specific for this GST.
Fig 2. GST-1 is expressed in DA neurons.
Primary C. elegans cultures expressing GFP in the dopamine neurons were generated with WT (A-D) or gst-1 knockdown animals (E-H). Primary cultures were incubated with GST-1 primary antibody followed by incubation with Texas Red conjugated goat-anti-rabbit secondary antibody. DIC images (A) and (E) of WT and gst-1RNAi cultures, respectively. DA neurons from WT and gst-1RNAi animals expressing GFP driven by the dat-1 promoter (B) and (F), respectively. GST-1 is expressed in DA neurons in WT animals (C), but not in gst-1RNAi (G). (D) Overlay of (B-C) and (H) overlay of (F-G). Images were observed under a Zeiss confocal microscope (Zeiss LSM 510). Scale bar represents 5 μm.
3.3 GST-1 inhibits toxicant-induced DA neuron degeneration
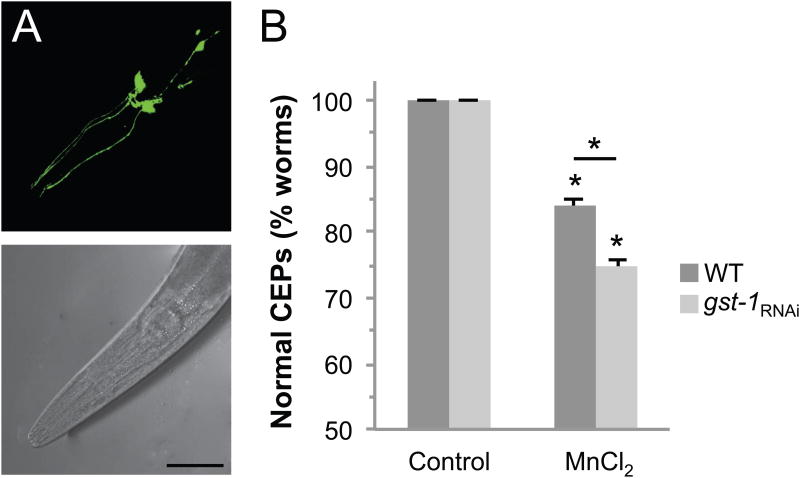
Compounds that generate reactive oxygen species (ROS) and confer oxidative stress have been shown to contribute to DA neuron vulnerability (Bove et al., 2005; Sherer et al., 2003). GSTπ has been shown to protect against oxidative stress (Goto et al., 2009). We have previously demonstrated that a brief exposure of C. elegans to Mn can increase oxidative stress and lead to DA neuronal death (Settivari et al., 2009). In order to determine if GST-1 may inhibit Mn-associated DA neuropathology, we exposed WT or gst-1RNAi transgenic worms expressing GFP in the eight DA neurons to the toxicant (Nass et al., 2002; Settivari et al., 2009). An acute low concentration exposure to Mn results in approximately 15% of the WT animals displaying significant DA neurodegeneration (Fig. 3A, B). The Mn-induced DA neurodegeneration that we observe is similar to our prior studies in which we characterized Mn- and 6-OHDA-induced DA neurodegeneration by loss of dendritic GFP and loss of neuronal integrity by electron microscopy (Nass et al., 2002; Settivari et al., 2009). As described earlier, only the CEP processes that cannot be visually followed from the cell body to the tip of the nose are considered to have DA neuron degeneration (Nass et al., 2002). Following genetic knockdown of GST-1, we found that Mn exposure resulted in degeneration in an additional 10% of nematodes (Fig. 3C). These results show that Mn exposures cause DA neuronal death, and that expression of GST-1 inhibits the toxicant-associated pathology.
Fig 3. GST-1 inhibits dopamine neuron degeneration.
Third generation L1 stage RJ928 animals were exposed to 50 mM MnCl2 for 30 mins and the dopamine neurons were visualized after a 72 h recovery on control or gst-1 RNAi plates at 20°C. DA neurons were considered degenerated when a break in or complete loss of GFP expression was observed in the CEPs (dendritic processes ending at the tip of the nose). An unexposed (normal) nematode has 4 complete CEP processes (A). Mn induces DA neuron degeneration (B) (arrows indicate the location of missing process), scale bar is 50 μm. Quantification of DA neuron degeneration is expressed as the percentage of worms with normal CEPs after exposure to MnCl2 in WT and gst-1RNAi animals (C). Shown are mean values ± SE of at least three individual replicates. Two-way ANOVA analysis was used and an asterisks indicates p ≤ 0.001. For both genotypes, Mn exposure significantly decreased the % normal CEPs, and the difference between WT and gst-1RNAi Mn exposed groups is significant.
3.4 SKN-1 modulates GST-1 protein expression
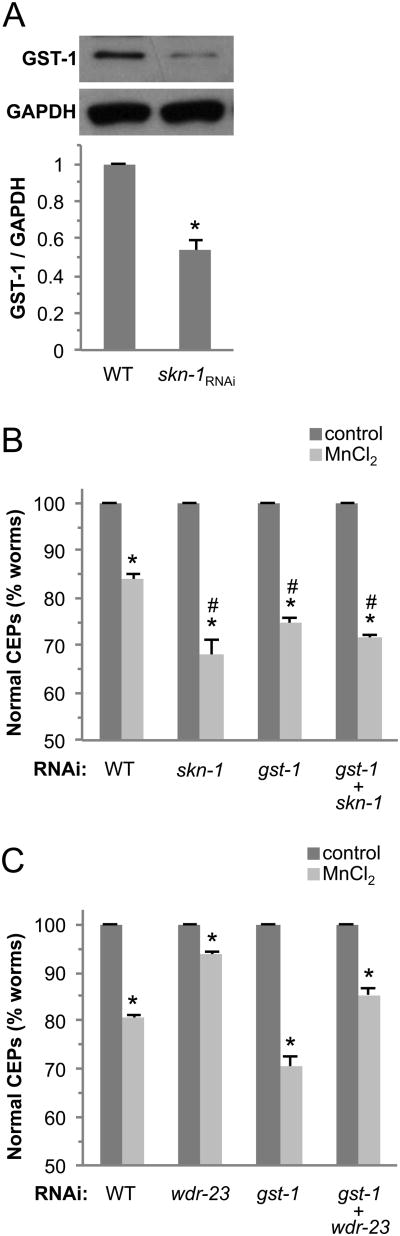
SKN-1, the homologue to the vertebrate PD-associated transcription factor Nrf2, can regulate antioxidant and detoxification enzymes including PD-associated proteins and GSTs. We have recently shown that SKN-1 is expressed in C. elegans DA neurons (Vanduyn et al., 2010). Analysis of the sequence 5′ of the GST-1 start codon suggests that there is a SKN-1 binding site approximately 230 bases upstream from the ATG in the promoter region that may be involved in GST-1 regulation (data not shown). In order to determine if GST-1 protein expression may be dependent on SKN-1, we knocked down SKN-1 gene expression by RNAi and examined GST-1 protein levels by Western blot analysis. As can be seen in Fig. 4A, a reduction in SKN-1 gene expression resulted in approximately 2-fold reduction of GST-1 protein levels. These results indicate that SKN-1 can regulate GST-1, and suggest that the transcription factor could play a role in GST-1-associated neuroprotection following toxicant exposure.
Fig 4. GST-1 requires SKN-1 for inhibition of toxicant-associated DA neurodegeneration.
(A) GST- 1 expression is partially dependent on SKN-1. Synchronized RJ928 animals were placed on RNAi plates spread with skn-1 RNAi bacteria. After 48 h, the worms were collected, homogenized and total protein was quantified following the standard Bradford assay. Protein samples were separated by electrophoresis, transferred to a membrane, and probed with anti-GST-1 or GAPDH primary antibodies. Band intensity was quantified relative to GAPDH expression and normalized to WT. p value was calculated with a t-test and the asterisk indicates p ≤ 0.0001 between WT and skn-1RNAi. skn-1RNAi results in increased DA neuron sensitivity to Mn (B), and wdr-23RNAi decreases sensitivity (C). L1 stage RJ928 worms were exposed to 50 mM MnCl2 for 30 mins with a 72 h recovery (B and C) on RNAi plates as indicated, and DA neurons were visualized for degeneration. Shown are mean values ± SE of at least three individual replicates. p values were calculated using two-way ANOVA analysis. Asterisks indicate p ≤ 0.01 between control and toxicant exposed groups within each genotype. Number sign indicates p ≤ 0.01 between toxicant-exposed WT and gene knockdown groups (B). In C, comparisons between all toxicant-exposed groups are statistically different with p ≤ 0.01.
3.5 SKN-1 inhibits Mn-induced DA neuron degeneration and contributes to GST-1-associated neuroprotection
We have previously shown that the PD-associated transcription factor SKN-1 can inhibit MeHg-associated DA neuron degeneration (Vanduyn et al., 2010). It has previously been demonstrated that SKN-1 lowers the incidence of C. elegans death in presence of high Mn concentrations, but to date SKN-1 has not been shown to play a role in Mn-induced DA neuroprotection (Benedetto et al., 2010). We therefore asked whether SKN-1 may inhibit Mn-associated DA neuronal death following toxicant exposure. As can be seen in Fig. 4B, genetic knockdown of SKN-1 alone significantly increases DA neuronal death following exposure to Mn, indicating that the loss of SKN-1 increases DA neuron vulnerability to this metal. Since GST-1 protein expression is partially dependent on SKN-1, we asked whether SKN-1 and GST-1 could function in the same molecular pathway to inhibit toxicant-associated DA neuron degeneration. The double knockdown of skn-1/gst-1 demonstrates similar levels of DA neuronal death, approximately 30%, as the RNAi of each of the single genes following Mn exposure (Fig. 4B). These results are consistent with SKN-1 and GST-1 functioning via a similar molecular pathway. The WD40 repeat protein WDR-23 negatively regulates SKN-1-associated transcription, and can also play a significant role in the response of cells to stress (Choe et al., 2009). To determine if WDR-23 may contribute to the vulnerability of DA neurons to Mn, we knocked down WDR-23 gene expression using RNAi. Consistent with WDR-23 negatively regulating SKN-1, we find a significant increase in DA neuron viability following WDR-23 genetic knockdown and Mn exposure (Fig. 4C). Furthermore, there is very little difference in DA neuron degeneration between WT and wdr-23/gst-1 double knockdown, suggesting that the neuroprotection conferred by WDR-23 may also largely function through a SKN-1-associated pathway. Taken together, these results indicate that DA neuron vulnerability to Mn is dependent on SKN-1 and GST-1 expression that may function in overlapping molecular pathways.
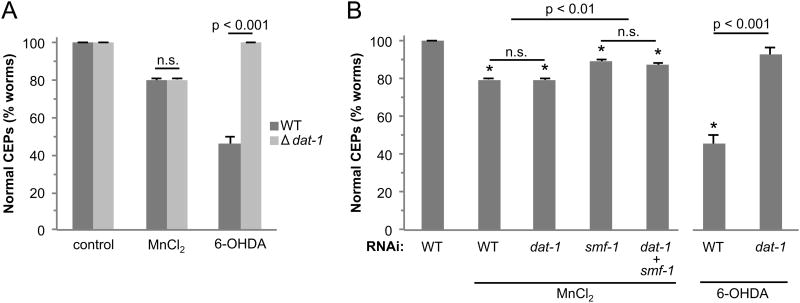
3.6 Mn-induced DA neurodegeneration is not dependent on DAT-1
Mn-induced neurotoxicity is largely dependent on the transport of Mn into cells through the divalent metal transporter, DMT-1 (Liu and Culotta, 1999; Culotta et al., 2005; Supek et al., 1996). The Mn-induced DA neurotoxicity in vertebrates has been demonstrated to be independent of the expression of the dopamine transporter DAT (Hirata et al., 2008; Roth et al., 2013). Studies in C. elegans also indicate that the primary mechanism of Mn-induced DA neurotoxicity is dependent on the C. elegans orthologue of DMT-1, SMF-1 (Settivari et al., 2009), although a recent nematode study suggests Mn-induced DA neurodegeneration is dependent on the nematode orthologue of DAT, DAT-1 (Benedetto et al., 2010). In order to determine if DAT-1 contributes to Mn-induced DA neuron degeneration in the nematode, we exposed WT animals and animals containing a deletion of dat-1 to 50 mM Mn, and examined DA neuron integrity 72 hours later. We also exposed both animals to the DA neurotoxin 6-OHDA as a control, as DA neurons lacking DAT-1 are resistant to 6-OHDA induced DA neurodegeneration (Nass et al., 2002; Nass and Blakely, 2003). As can be seen in Fig. 5A, there is no difference in the extent of Mn-induced DA neurodegeneration between WT and dat-1 knockout animals. Importantly, approximately 60% of WT animals exposed to 6-OHDA display DA neuron degeneration, while none of the dat-1 knockout animals display neurodegeneration, consistent with the complete loss of DAT-1. These results indicate that dat-1 knockout DA neurons are not more resistant to Mn-induced DA neuron degeneration relative to WT animals. In order to further elucidate whether DAT may play a role in Mn-induced DA neuron pathology, we utilized RNAi to knockdown dat-1 and/or smf-1 expression in animals sensitive to RNAi. As can be seen in Fig. 5B, there is no difference in Mn-induced DA neurodegeneration between WT and dat-1RNAi animals, or between animals in which the primary DA neuron Mn transporter, SMF-1, is knocked down and smf-1/dat-1 double knockdown. Furthermore as reported earlier, the loss of SMF-1 results in a significant reduction of Mn-induced DA neuronal death, indicating that Mn-induced DA neuron degeneration is dependent on SMF-1 expression (Settivari et al., 2009). Exposure to 6-OHDA as a control in the dat-1 RNAi experiment suggests that the vast majority of DAT-1 expression is reduced. These studies strongly indicate that Mn-induced DA neuron degeneration is independent of DAT-1 expression.
Fig 5. Mn-induced cell death is not dependent on dat-1.
WT (BY200) and dat-1 knockout (BY215) synchronized L1 worms (A) or third generation RJ928 nematodes grown on dat-1 or smf-1 RNAi or the combination (B) were exposed to 50 mM MnCl2 or 1 mM 6-OHDA for 30 mins and allowed to recover on NGM plates (A) or plates with RNAi bacteria (B) for 72 h. DA neurons expressing GFP were visualized under a fluorescent microscope following the exposure or recovery (> 50 worms/condition). Shown are mean ± SE of at least three individual replicates. Two-way ANOVA analysis indicates a significant difference between WT and dat-1 genetic knockout animals exposed to 6-OHDA (A). Oneway ANOVA was performed in (B). Asterisks indicate p ≤ 0.01 for all Mn-exposed groups compared to untreated. Within the Mn-treated groups, WT and dat-1RNAi are significantly different from smf-1RNAi and dat-1/smf-1RNAi. A t-test was used to compare 6-OHDA-treated WT animals vs dat-1RNAi.
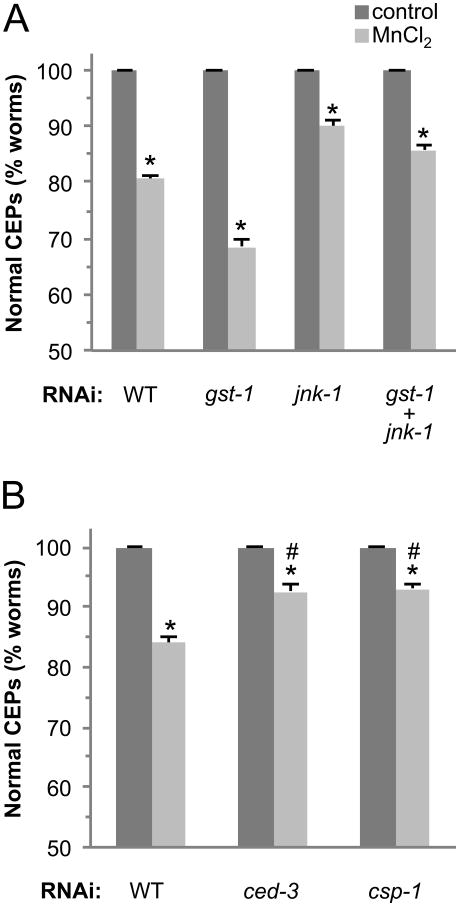
3.7 JNK-1 contributes to toxicant-associated DA neuron degeneration
Under non-stress conditions in vertebrates, GSTπ inhibits the activation of JNK by binding JNK in a protein complex with c-Jun (Wang et al., 2001). Under oxidative stress, GSTπ dissociates from JNK allowing JNK to participate in downstream events that include apoptotic signaling and cell death (Ruscoe et al., 2001). To determine if the C. elegans JNK homologue, JNK-1, may play a role in cell death following Mn exposure, we knocked down jnk-1 gene expression using RNAi and exposed the animals to Mn as described above. As can be seen in Fig. 6A, genetic knockdown of jnk-1 results in significant inhibition of DA neuronal death following exposure to Mn, consistent with JNK's role in apoptotic signaling. The double knockdown of gst-1 and jnk-1 shows a significant yet small difference in the percentage of animals demonstrating DA neuron pathology relative to the jnk-1RNAi exposed animals, suggesting that JNK-1 and GST-1 may largely function in the same signaling pathway. Taken together, these results indicate that JNK-1 contributes to Mn-induced DA neuron pathology, and suggest that the toxicants may be precipitating cell death through apoptosis.
Fig 6. Mn-induced cell death is dependent on apoptosis.
Third generation RJ928 nematodes grown on gst-1 or jnk-1 RNAi or the combination (A) or ced-3 or csp-1 RNAi (B) were exposed to 50 mM MnCl2 for 30 mins and allowed to recover on plates with RNAi bacteria for 72 h. DA neurons expressing GFP were visualized under a fluorescent microscope following exposure and recovery (> 50 worms/condition). Shown are mean ± SE of at least three individual replicates. p values were calculated using two-way ANOVA. Asterisks indicate p ≤ 0.01 between control and toxicant-exposed groups. Comparisons between all Mn-treated groups are significant with p ≤ 0.001 (A) or p ≤ 0.01 (B).
3.8 Mn-induced DA neuron degeneration is dependent on the caspases CED-3 and CSP-1
The mechanisms of cell death in PD and manganism remain elusive. Apoptosis has been implicated in both disorders, although the role of classical apoptosis is controversial and not well defined, as recent studies suggest that several types of programmed cell death may contribute to DA neuron degeneration (Venderova and Park, 2012). In order to determine if the canonical programmed cell death/apoptotic pathway in C. elegans contributes to Mn-induced DA neuron toxicity, we examined DA neurodegeneration in ced-3 deficient backgrounds (Nass et al., 2002). Animals in which CED-3 expression is reduced have 50% less DA neuron degeneration following Mn exposure relative to WT (Fig. 6B). These results suggest that Mn activates the classical apoptotic pathway to confer DA neuron degeneration, and that another cell death pathway may also be contributing to the toxicant-associated DA neurodegeneration.
C. elegans contains several other putative caspase genes that may be involved in the activation of ced-3, or in an independent cell death pathway (Shaham, 1998). Three caspase-like genes, csp-1, csp-2, and csp-3, have been shown to encode a total of at least 7 RNA transcripts, although only the CSP-1 splice variant CSP-1B has been shown to be an active protease (Shaham, 1998). Recently CSP-1 has been shown to be a pro-apototic caspase involved in programmed cell death in embryogenesis (Denning et al., 2013). In order to determine if CSP-1 may contribute to Mn-associated DA neuron degeneration, we evaluated the DA neuronal death following genetic knockdown of csp-1 and exposure to Mn. Mn-induced DA neuron death is inhibited by approximately 50% in csp-1 genetic knockdown animals (Fig. 6B). Taken together, these results indicate that both caspase CED-3 and CSP-1 play a role in Mn-associated DA neuron vulnerability.
4. Discussion
Mn-induced neurotoxicity resembles a number of aspects of IPD, including tremors, bradykinesia, and rigidity (Burton and Guilarte, 2009). As in IPD, high Mn exposures result in mitochondrial dysfunction, increases in ROS levels and oxidative stress, and DA neurodegeneration. Epidemiological evidence also suggests that there may be a correlation between Mn exposure and the development of PD (Gorelle, et al., 1999; Verina et al., 2013). The similarity between manganism, and PD suggests that there may be common modalities in the underlying pathophysiology, although the molecular determinants involved in both disorders have largely remained elusive. (Sherer et al., 2003, Tanner et al., 2011). A contributing factor to the sensitivity of DA neurons to PD-associated neurotoxicants may be the expression or activity of GSTπ. GSTπ enzymes conjugate endogenous and exogenous electrophiles, including pesticides, with glutathione to reduce ROS, oxidative stress, and cellular toxicity. GSTπ polymorphisms have been shown to be a risk factor for the development of PD, and the PD-associated risk can be exacerbated by exposure to herbicides (Menegon et al., 1998; Wilk et al., 2006; Sherer et al., 2003; Tanner et al., 2011). In this study, we show that the C. elegans GSTπ homologue GST-1 is expressed in DA neurons, Mn exposures induce GST-1 gene and protein expression, and the loss of SKN-1 or GST-1 gene expression results in an increase in DA neurodegeneration. This study also shows for the first time that Mn-induced DA neuron degeneration is associated with GSTπ gene expression, and further supports a possible common genetic linkage between manganism and IPD.
In vertebrates, the basic leucine zipper class transcription factor Nrf2 regulates the expression of antioxidant genes, and is negatively regulated by Keap1 (kelch-like ECH-associated protein 1) (Nguyen et al., 2009). Under non-oxidative stress conditions, Keap1 physically interacts with Nrf2 and promotes Nrf2 ubiquitination that targets the transcription factor for proteosomal degradation (Itoh et al., 1999; Kobayashi et al., 2004; Nguyen et al., 2009). Under conditions of oxidative stress, the interaction of Keap1 with Nrf2 is decreased, resulting in decreased ubiquitination and an increase in Nrf2 binding to antioxidant response elements (AREs) in the promoters of cytoprotective genes. Nrf2 regulates a number of phase II detoxification enzymes including GSTs, and Nrf2 deficient vertebrate cells are sensitive to PD-associated toxicants (Kobayashi et al., 2009; Lee et al., 2003; Toyama et al., 2007). SKN-1 is the C. elegans homologue to Nrf2, and although there does not appear to be a nematode homologue to Keap1 based on genetic sequence, the WD40 repeat containing protein WDR-23 appears to be a functional homologue, as it physically interacts with and negatively regulates SKN-1, likely through a ubiquitination-associated pathway, and plays a role in stress resistance and longevity (Choe et al., 2009). We have recently shown that SKN-1 is expressed in the C. elegans DA neurons and can modulate expression of at least two other GSTs (Vanduyn et al., 2010). Chromatin immunoprecipitation binding studies indicate that SKN-1 binds in the putative promoter of GST-1, consistent with the binding site predicted by the consensus sequence (An and Blackwell, 2003). Our current studies show that constitutive expression of GST-1 is partially dependent on SKN-1 expression, and the loss of SKN-1 expression results in increase sensitivity of DA neurons to Mn. This study shows for the first time that Mn-associated
DA neuron pathology is associated with SKN-1/Nrf-2 expression. Consistent with the role of SKN-1 in DA neuroprotection, the loss of the SKN-1 inhibitor WDR-23 renders the neurons significantly more resistant to Mn. Furthermore, genetic repression of both GST-1 and WDR-23 renders the DA neurons in the double knockdown similarly vulnerable to exposures as WT, suggesting that the role of WDR-23 in the stress response may be at least partially through a GST-1-associated pathway. The small but significant increase in resistance relative to WT following Mn exposure in the double knockdown suggests that WDR-23 may contribute to the DA neuroprotection also through a GST-1-independent pathway, possibly through a reduction in target protein ubiquitination (Kobayashi et al., 2009; Nguyen et al., 2009).
Our studies show that Mn-induced DA neurodegeneration is not dependent on DAT-1. This is in contrast to Aschner and colleagues, whose studies indicate that DAT-1 is required for Mn-induced DA neuronal death. The reasons for the different results from the studies are not clear. It is possible that the genetic background of the dat-1 knockout strain used in Benedetto et al. 2010 was different from the WT strain; the full genotype of the dat-1 knockout strain used in their experiments was not reported (Benedetto et al., 2010). Also, the genotype of the WT strain (BY200) used in their study was reported incorrectly (see Nass et al., PNAS, 2002, for details on the genotype), therefore it is not clear which WT strain may have been used in discerning the role of DAT in Mn-induced toxicity. In the current study, we compare Mn-induced DA neurodegeneration in a WT strain (BY200) and a strain with a deletion in dat-1 (BY215) from identical genetic backgrounds (each contain the selection marker rol-6), and find that DAT-1 does not contribute to Mn-induced DA neuron vulnerability (Nass et al., 2002). We also show that a genetic knockdown of dat-1 does not alter Mn-induced DA neuron sensitivity relative to WT animals in a similar genetic background. Taken together, these results are consistent with vertebrate studies that demonstrate that DAT does not contribute to Mn-induced DA neuron toxicity.
JNKs are a class of MAPKs that are activated by endogenous or environmental stressors and play a role in proapoptotic signaling (Kanda and Miura, 2004; Liu and Lin, 2005; Lu et al., 2007). GSTπ enzymes have been shown to negatively regulate kinase pathways by directly binding to the C-terminus of JNK and inhibiting JNK activation and the subsequent apoptosis (Wang et al., 2001; Thevenin et al., 2011). Our findings are consistent with JNK playing a role in apoptosis, as genetic knockdown of JNK results in increase in DA neuron viability following Mn exposure. Conversely, genetic knockdown of gst-1 alone results in an increase in DA neuronal death, consistent with a loss of JNK inhibition and an increase in apoptosis.
The two types of vertebrate caspases involved in apoptosis are initiator caspases and effector caspases. Initiator caspases (caspase-8, caspase-9, and caspase-10) cleave inactive pro-forms of effector caspases (caspase-3, caspase-6, and caspase-7), resulting in activation of the effector caspase and initiation of the apoptotic cascade leading to cell death (Bratton and Salvesen, 2010; Venderova and Park, 2012). In C. elegans, one caspase has been shown to function as a positive regulator of apoptosis in vivo. CED-3 is the core cell-death effector (homologous to caspase-3) that acts both in programmed cell death and as recently shown, in neuronal regeneration (Pinan-Lucarre et al., 2012; Yuan et al., 1993). Our studies show that CED-3 contributes to Mn-induced DA neuronal death. The nematode also contains three proteins that have some homology to vertebrate caspases CSP-1-3 (Shaham, 1998). Considering that CSP-2 has a highly divergent consensus caspase sequence and CSP-3 lacks a middle caspase domain, these proteins are unlikely to function as active caspases. In addition, both proteins have been shown to be negative regulators of CED-3 (Brady and Duckett, 2009; Geng et al., 2008; Geng et al., 2009). CSP-1 is highly conserved with vertebrate caspase proproteins, and in vitro studies show that activated CSP-1 can cleave the CED-3 protein, and activated CED-3 and CSP-1 have different substrate specificities (Shaham, 1998). Furthermore, CSP-1 has recently been shown to promote programmed cell death during C. elegans embryogenesis (Denning et al., 2013). Here we provide the first evidence that CSP-1 contributes to neuronal vulnerability to a toxicant. CSP-1 may act upstream of CED-3 as an initiator caspase in vivo, or cleave other substrates downstream to initiate apoptosis and cell death (Shaham, 1998). Considering that apoptosis plays a significant role in PD-associated DA neurodegeneration, yet the precise molecular pathways in the neuropathology are ill-defined, the identification of CSP-1's role in Mn-associated DA neuron pathology may provide new insights into the molecular basis of DA neuron vulnerability.
Highlights.
GST-pi expression in C. elegans DA neurons inhibits Mn-associated DA neuron pathology
A PD-associated transcription factor and molecular modulators affect DA neuron vulnerability to Mn
Dopamine transporter does not play a role in Mn-associated DA neurodegeneration
Identifies apoptosis-associated caspases involved in Mn-induced DA neurodegeneration
Acknowledgments
Some of the strains were provided by the Caenorhabditis Genetics Center, which is supported by the National Institutes of Health's National Center for Research Resources. This study was partially supported by grants R011ES014459 and ES015559 from the National Institute of Environmental Health Sciences (RN) and an EPA STAR graduate fellowship (NV).
Abbreviations used
- DA
dopamine
- ECL
enhanced chemiluminescence
- GFP
green fluorescent protein
- PAGE
polyacrylamide gel electrophoresis
- PD
Parkinson's disease
- ROS
reactive oxygen species
- SN
substantia nigra
- TH
tyrosine hydroxylase
- WT
wild type
Footnotes
Conflict of Interest Statement: The authors state that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–93. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto A, Au C, Avila DS, Milatovic D, Aschner M. Extracellular dopamine potentiates mn-induced oxidative stress, lifespan reduction, and dopaminergic neurodegeneration in a BLI-3-dependent manner in Caenorhabditis elegans. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi L, Driscoll M. Culture of embryonic C. elegans cells for electrophysiological and pharmacological analyses. WormBook. 2006:1–15. doi: 10.1895/wormbook.1.122.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson's disease. NeuroRx. 2005;2:484–94. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brady GF, Duckett CS. A caspase homolog keeps CED-3 in check. Trends Biochem Sci. 2009;34:104–7. doi: 10.1016/j.tibs.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton SB, Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci. 2010;123:3209–14. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg D, Riepsaame J, Pont C, Mulder G, van de Water B. Peptide-bond modified glutathione conjugate analogs modulate GSTpi function in GSH-conjugation, drug sensitivity and JNK signaling. Biochem Pharmacol. 2006;71:268–77. doi: 10.1016/j.bcp.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Burton NC, Guilarte TR. Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates. Environ Health Perspect. 2009;117:325–32. doi: 10.1289/ehp.0800035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta VC, Yang M, Hall MD. Manganese transport and trafficking: lessons learned from Saccharomyces cerevisiae. Eukaryot Cell. 2005;4:1159–65. doi: 10.1128/EC.4.7.1159-1165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KP, Przybysz AJ, Strange K. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol. 2009;29:2704–15. doi: 10.1128/MCB.01811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossgrove J, Zheng W. Manganese toxicity upon overexposure. NMR Biomed. 2004;17:544–53. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning DP, Hatch V, Horvitz HR. Both the Caspase CSP-1 and a Caspase-Independent Pathway Promote Programmed Cell Death in Parallel to the Canonical Pathway for Apoptosis in Caenorhabditis elegans. PLoS Genet. 2013;9:e1003341. doi: 10.1371/journal.pgen.1003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Shi Y, Nakagawa A, Yoshina S, Mitani S, Shi Y, et al. Inhibition of CED-3 zymogen activation and apoptosis in Caenorhabditis elegans by caspase homolog CSP-3. Nat Struct Mol Biol. 2008;15:1094–101. doi: 10.1038/nsmb.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Zhou QH, Kage-Nakadai E, Shi Y, Yan N, Mitani S, et al. Caenorhabditis elegans caspase homolog CSP-2 inhibits CED-3 autoactivation and apoptosis in germ cells. Cell Death Differ. 2009;16:1385–94. doi: 10.1038/cdd.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, et al. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson's disease. Neurotoxicology. 1999;20:239–47. [PubMed] [Google Scholar]
- Goto S, Kawakatsu M, Izumi S, Urata Y, Kageyama K, Ihara Y, et al. Glutathione S-transferase pi localizes in mitochondria and protects against oxidative stress. Free Radic Biol Med. 2009;46:1392–403. doi: 10.1016/j.freeradbiomed.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Suzuno H, Tsuruta T, Oh-hashi K, Kiuchi K. The role of dopamine transporter in selective toxicity of manganese and rotenone. Toxicology. 2008;244:249–56. doi: 10.1016/j.tox.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Hope I. elegans: A Practical Approach. New York: Oxford University Press; 1999. [Google Scholar]
- Hudnell HK. Effects from environmental Mn exposures: a review of the evidence from non-occupational exposure studies. Neurotoxicology. 1999;20:379–97. [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YM, Mo XA, Du FQ, Fu X, Zhu XY, Gao HY, et al. Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J Occup Environ Med. 2006;48:644–9. doi: 10.1097/01.jom.0000204114.01893.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 2003;30:313–21. doi: 10.1016/s1046-2023(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Kanda H, Miura M. Regulatory roles of JNK in programmed cell death. J Biochem. 2004;136:1–6. doi: 10.1093/jb/mvh098. [DOI] [PubMed] [Google Scholar]
- Kelada SN, Stapleton PL, Farin FM, Bammler TK, Eaton DL, Smith-Weller T, et al. Glutathione S-transferase M1, T1, and P1 polymorphisms and Parkinson's disease. Neurosci Lett. 2003;337:5–8. doi: 10.1016/s0304-3940(02)01286-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–9. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, et al. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Vartiainen S, Moilanen AM, Sirvio J, Thomas JH, Nass R, et al. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. Journal of Neurochemistry. 2003;86:165–72. doi: 10.1046/j.1471-4159.2003.01809.x. [DOI] [PubMed] [Google Scholar]
- Lee JM, Shih AY, Murphy TH, Johnson JA. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278:37948–56. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- Liu XF, Culotta VC. Post-translation control of Nramp metal transport in yeast. Role of metal ions and the BSD2 gene. J Biol Chem. 1999;274:4863–8. doi: 10.1074/jbc.274.8.4863. [DOI] [PubMed] [Google Scholar]
- Lin AH, Chen HW, Liu CT, Tsai CW, Lii CK. Activation of Nrf2 is required for up-regulation of the pi class of glutathione S-transferase in rat primary hepatocytes with L-methionine starvation. J Agric Food Chem. 2012;60:6537–45. doi: 10.1021/jf301567m. [DOI] [PubMed] [Google Scholar]
- Lu GD, Shen HM, Chung MC, Ong CN. Critical role of oxidative stress and sustained JNK activation in aloe-emodin-mediated apoptotic cell death in human hepatoma cells. Carcinogenesis. 2007;28:1937–45. doi: 10.1093/carcin/bgm143. [DOI] [PubMed] [Google Scholar]
- Martin I, Dawson VL, Dawson TM. Recent advances in the genetics of Parkinson's disease. Annu Rev Genomics Hum Genet. 2011;12:301–25. doi: 10.1146/annurev-genom-082410-101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR. Fuming over Parkinson disease: are welders at risk? Neurology. 2011;76:1286–7. doi: 10.1212/WNL.0b013e31821528ab. [DOI] [PubMed] [Google Scholar]
- Menegon A, Board PG, Blackburn AC, Mellick GD, Le Couteur DG. Parkinson's disease, pesticides, and glutathione transferase polymorphisms. Lancet. 1998;352:1344–6. doi: 10.1016/s0140-6736(98)03453-9. [DOI] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM, 3rd, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3264–9. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Hamza I. The nematode Caenorhabditis elegans as a model to explore toxicology in vivo: Solid and axenic growth culture conditions and compound exposure parameters. Current Protocols in Toxicology. 2007:1.9.1–9.18. doi: 10.1002/0471140856.tx0109s31. [DOI] [PubMed] [Google Scholar]
- Nass R, Merchant KM, Ryan T. Caenohabditis elegans in Parkinson's disease drug discovery: addressing an unmet medical need. Mol Interv. 2008;8:284–93. doi: 10.1124/mi.8.6.6. [DOI] [PubMed] [Google Scholar]
- Nass R, Przedborski S. Parkinson's disease: Molecular and Therapeutic Insights from Model Systems. 1st. Elsevier Academic Press; 2008. p. 652. [Google Scholar]
- Nass R, Blakely RD. The Caenorhabditis elegans dopaminergic system: opportunities for insights into dopamine transport and neurodegeneration. Annual Review of Pharmacology & Toxicology. 2003;43:521–44. doi: 10.1146/annurev.pharmtox.43.100901.135934. [DOI] [PubMed] [Google Scholar]
- Nass R, Settivari RS. Caenorhabditis elegans Models of Parkinson's Disease: A Robust Genetic System to Identify and Characterize Endogenous and Environmental Components involved in Dopamine Neuron Degeneration. In: Nass R, Przedborski S, editors. Parkinson's Disease: Molecular and Therapeutic Insights from Model Systems. Elsevier Academic Press; 2008. pp. 347–60. [Google Scholar]
- Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–5. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novillo A, Won SJ, Li C, Callard IP. Changes in Nuclear Receptor and Vitellogenin Gene Expression in Response to Steroids and heavy metal in Caenorhabditis elegans. Integrative and Comparative Biology. 2005;45:61–71. doi: 10.1093/icb/45.1.61. [DOI] [PubMed] [Google Scholar]
- Oliveira RP, Porter Abate J, Dilks K, Landis J, Ashraf J, Murphy CT, Blackwell TK. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1NNrf. Aging Cell. 2009;8:524–41. doi: 10.1111/j.1474-9726.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Tedesco PM, Johnson TE. Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell. 2009;8:258–69. doi: 10.1111/j.1474-9726.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinan-Lucarre B, Gabel CV, Reina CP, Hulme SE, Shevkoplyas SS, Slone RD, et al. The core apoptotic executioner proteins CED-3 and CED-4 promote initiation of neuronal regeneration in Caenorhabditis elegans. PLoS Biol. 2012;10:e1001331. doi: 10.1371/journal.pbio.1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- Racette BA, Aschner M, Guilarte TR, Dydak U, Criswell SR, Zheng W. Pathophysiology of manganese-associated neurotoxicity. Neurotoxicology. 2012;33:697–702. doi: 10.1016/j.neuro.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JA. Are there common biochemical and molecular mechanisms controlling manganism and parkinsonism. Neuromolecular Med. 2009;11:281–96. doi: 10.1007/s12017-009-8088-8. [DOI] [PubMed] [Google Scholar]
- Roth JA, Li Z, Sridhar S, Khoshbouei H. The effect of manganese on dopamine toxicity and dopamine transporter (DAT) in control and DAT transfected HEK cells. Neurotoxicology. 2013;35:121–8. doi: 10.1016/j.neuro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscoe JE, Rosario LA, Wang T, Gate L, Arifoglu P, Wolf CR, et al. Pharmacologic or genetic manipulation of glutathione S-transferase P1-1 (GSTpi) influences cell proliferation pathways. J Pharmacol Exp Ther. 2001;298:339–45. [PubMed] [Google Scholar]
- Sanchez-Betancourt J, Anaya-Martinez V, Gutierrez-Valdez AL, Ordonez-Librado JL, Montiel-Flores E, Espinosa-Villanueva J, et al. Manganese mixture inhalation is a reliable Parkinson disease model in rats. Neurotoxicology. 2012;33:1346–55. doi: 10.1016/j.neuro.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Settivari R, Levora J, Nass R. The divalent metal transporter homologues SMF-1/2 mediate dopamine neuron sensitivity in caenorhabditis elegans models of manganism and parkinson disease. J Biol Chem. 2009;284:35758–68. doi: 10.1074/jbc.M109.051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham S. Identification of multiple Caenorhabditis elegans caspases and their potential roles in proteolytic cascades. J Biol Chem. 1998;273:35109–17. doi: 10.1074/jbc.273.52.35109. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, et al. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci. 2003;23:10756–64. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Bradner J, Bammler TK, Eaton DL, Zhang J, Ye Z, et al. Identification of glutathione S-transferase pi as a protein involved in Parkinson disease progression. Am J Pathol. 2009;175:54–65. doi: 10.2353/ajpath.2009.081019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne M, Boyd J, Raviie Shepherd K, Jiao Y, Pond BB, Hatler M, et al. GSTpi expression mediates dopaminergic neuron sensitivity in experimental parkinsonism. Proc Natl Acad Sci U S A. 2007;104:1977–82. doi: 10.1073/pnas.0610978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Lin GX, Jefferson AM, Roberts JR, Wirth O, Hayashi Y, et al. Mitochondrial dysfunction and loss of Parkinson's disease-linked proteins contribute to neurotoxicity of manganese-containing welding fumes. FASEB J. 2010;24:4989–5002. doi: 10.1096/fj.10-163964. [DOI] [PubMed] [Google Scholar]
- Supek F, Supekova L, Nelson H, Nelson N. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc Natl Acad Sci U S A. 1996;93:5105–10. doi: 10.1073/pnas.93.10.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, et al. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect. 2011;119:866–72. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenin AF, Zony CL, Bahnson BJ, Colman RF. GST pi modulates JNK activity through a direct interaction with JNK substrate, ATF2. Protein Sci. 2011;20:834–48. doi: 10.1002/pro.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Toyama T, Sumi D, Shinkai Y, Yasutake A, Taguchi K, Tong KI, et al. Cytoprotective role of Nrf2/Keap1 system in methylmercury toxicity. Biochem Biophys Res Commun. 2007;363:645–50. doi: 10.1016/j.bbrc.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Vanduyn N, Settivari R, Levora J, Zhou S, Unrine J, Nass R. The metal transporter SMF-3/DMT-1 mediates aluminum-induced dopamine neuron degeneration. J Neurochem. 2013;124:147–57. doi: 10.1111/jnc.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanduyn N, Settivari R, Wong G, Nass R. SKN-1/Nrf2 inhibits dopamine neuron degeneration in a Caenorhabditis elegans model of methylmercury toxicity. Toxicol Sci. 2010;118:613–24. doi: 10.1093/toxsci/kfq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ved R, Saha S, Westlund B, Perier C, Burnam L, Sluder A, et al. Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J Biol Chem. 2005;280:42655–68. doi: 10.1074/jbc.M505910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venderova K, Park DS. Programmed cell death in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verina T, Schneider JS, Guilarte TR. Manganese exposure induced a-synuclein aggregation in the frontal cortex of non-human primates. Toxicol Lett. 2013:177–83. doi: 10.1016/j.toxlet.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar R, Coelho H, Rodrigues E, Gama MJ, Rivera I, Taioli E, et al. Association of A313 G polymorphism (GSTP1*B) in the glutathione-S-transferase P1 gene with sporadic Parkinson's disease. Eur J Neurol. 2007;14:156–61. doi: 10.1111/j.1468-1331.2006.01590.x. [DOI] [PubMed] [Google Scholar]
- Vistbakka J, Vanduyn N, Wong G, Nass R. C. elegans as a Genetic Model System to Identify Parkinson's Disease-associated Therapeutic Targets. CNS Neurol Disord Drug Targets. 2012 doi: 10.2174/1871527311211080004. [DOI] [PubMed] [Google Scholar]
- Wang T, Arifoglu P, Ronai Z, Tew KD. Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J Biol Chem. 2001;276:20999–1003. doi: 10.1074/jbc.M101355200. [DOI] [PubMed] [Google Scholar]
- Wilk JB, Tobin JE, Suchowersky O, Shill HA, Klein C, Wooten GF, et al. Herbicide exposure modifies GSTP1 haplotype association to Parkinson onset age: the GenePD Study. Neurology. 2006;67:2206–10. doi: 10.1212/01.wnl.0000249149.22407.d1. [DOI] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–52. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]