Abstract
Immunoprevention refers to a strategy of preventing pathogen-associated and spontaneous cancers through the use of vaccines, antibodies, and immune modulators. Immune modulators function by enhancing the endogenous ability of the immune system to monitor for malignancy, so-called “immunosurveillance.” There is growing evidence that many of the most promising cancer chemoprevention agents including aspirin, COX-2 inhibitors, aromatase inhibitors, and bisphosphonates mediate their effects, in part, by enhancing immunosurveillance and reversing the immune evasive mechanisms that premalignant lesions employ. In the following review, we introduce critical components of the human immune surveillance system—dendritic cells, T cells, and immune suppressive cells—and discuss the emerging data suggesting that common chemoprevention agents may modulate the function of these immunologic cells.
Keywords: Cancer, prevention, chemoprevention, immunomodulation, immune surveillance
Introduction
Cancer prevention strategies include (1) risk reduction through elimination of environmental factors (asbestos, tobacco, and alcohol); (2) chemoprevention in high-risk populations with agents such as cyclo-oxygenase-2 (COX-2) inhibitors and aromatase inhibitors (AIs); and (3) immunoprevention with vaccines, antibodies, and immune modulators. The most successful application of immunoprevention to date has been vaccination against the infectious causative agents of hepatocellular carcinoma (HCC) and cervical cancer, two of the most common cancers worldwide. In prevention of HCC, a nationwide hepatitis B vaccination program in Taiwan was shown to reduce the average annual incidence of HCC in children over several years from 0.7 per 100,000 children (1981-1986) to 0.57 (1986-1990) and 0.36 (1990-1994) with a decrease in corresponding rates of mortality (1). In the prevention of cervical and other gynecologic cancers related to human papillomavirus (HPV), two international, double blind, placebo controlled randomized trials (FUTURE I/II) evaluated the efficacy of the quadrivalent HPV vaccine (serotypes 6, 11, 16, and 18). In lesions caused by virus corresponding to the specific serotypes included in the quadrivalent vaccine, efficacy at preventing cervical intraepithelial neoplasia grade I was 96% and for vulvar and vaginal intraepithelial neoplasia reached 100% (2). Vaccines designed to stop infection associated cancers have been one of the most successful prevention strategies to date.
Most cancers, however, have not been shown to be caused by infectious agents, instead arising from genetic alterations, and the immune system may inhibit tumor growth even in this setting. Thomas and Burnet developed the “tumor surveillance” theory in the 1950s in which they hypothesized that the immune system protects against nascent cancers by destroying abnormal cells before evolution to invasive malignancy. Burnet predicted that “if there were tumor immunity, it would be invisible,” anticipating the difficulty of providing evidence in humans to support immune surveillance (3). Evidence for the role of the immune system in modulating the growth of common cancers now exists. First, many cancer patients across a variety of tumor types spontaneously develop significant levels of antibodies and/or T cells specific for antigens expressed on their tumors, which, in some cases, are associated with prognosis including the occasional spontaneous regression (4). Second, the composition of tumor infiltrating lymphocytes (TILs) has been shown to have prognostic implications in a variety of different malignancies (5). Indeed, for colon cancer an immune scoring system based on enumerating CD8+ T cells and memory T cells can predict prognosis with greater accuracy than standard TNM staging (6). Third, immunodeficiency has been associated with cancer risk. Patients with impaired immunity, e.g. HIV infection or the use of antirejection drugs for transplantation, have a higher risk of both virally associated and non-virally induced tumors, suggesting a cancer protective effect via an intact immune system (7).
Standard cytotoxic chemotherapy has long been thought to work primarily by selectively causing death of rapidly proliferating tumor cells. Recently, however, many chemotherapeutic agents have been shown to stimulate tumor-specific immune responses by inducing immunogenic cell death or stimulating/activating immune effector cells which contribute to drug efficacy (8). Within the nascent field of cancer immunoprevention, similar data is emerging that many of the most promising chemoprevention agents under study may exert their effects, in part, by enhancing immune surveillance. As with cytotoxic drugs, chemoprevention agents have been shown to increase antigen processing by potent antigen presenting cells (APC), stimulate the proliferation and anti-tumor capabilities of CD8+ T cells, and inhibit the function or decrease the number of immune suppressive cells. In the following review, we discuss relevant elements of cancer destructive immunity and explain how chemoprevention agents may have immunomodulatory effects.
Cancer chemoprevention agents may enhance the immunologic activity of antigen presenting cells
Dendritic cells (DCs) are the most effective of the APCs in presenting immunogenic proteins to T cells. DCs sample antigens in peripheral tissues and process them into small peptides as they mature and migrate to lymphoid organs. After antigen uptake, APCs must receive suitable activation or stimulatory signals that result in sufficient maturation so that they differentiate to promote immunity rather than tolerance, as most immunogenic cancer associated proteins are self-antigens. Once activated, APCs present processed peptides to naïve T cells, stimulating a cellular immune response composed of CD4+ T helper cells (Th) and cytotoxic effector CD8+ T cells that are critical for destruction of pre-invasive and invasive lesions (Fig. 1A, B) (9).
Figure 1. Cancer chemoprevention agents enhance the immunologic activity of antigen presenting cells.
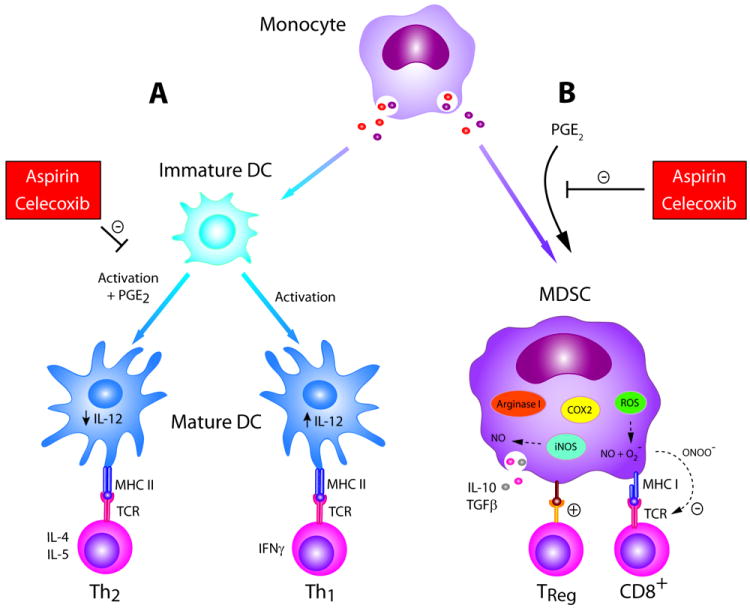
(A) PGE2 can inhibit the ability of maturing DCs to produce IL-12 during priming, biasing the resulting adaptive immune response toward a Th2 profile. COX-2 inhibitors such as aspirin and celecoxib can reverse this effect, allowing generation of Th1; (B) PGE2 facilitates differentiation of monocytes into immunosuppressive MDSCs, which function to inhibit the adaptive immune response and promote Treg populations through depletion of environmental arginine, expression of nitric oxide synthase, production of reactive oxygen species, and elaboration of Th2 cytokines IL-10 and TGF-B. COX-2 inhibitors aspirin and celecoxib can reverse this effect.
Aspirin and the COX-2 inhibitors are well studied chemoprevention agents. Aspirin and the COX-2 inhibitor celecoxib have both been shown to reduce the risk of colorectal cancer (10-14). Additionally, systematic reviews of the results of aspirin in cardiovascular studies have suggested that low-dose aspirin reduces overall cancer incidence and mortality (15-17). NSAIDs may limit carcinogenesis by preventing prostaglandin E2 (PGE2)-mediated inhibition of DCs. PGE2 is a product of COX enzymes and, normally, mediates physiologic functions such as maintenance of gastric mucosal integrity and renal blood flow when constitutively expressed. However, components of the tumor microenvironment can also produce PGE2 through COX-2 expression during oncogenesis. PGE2 alters the balance and function of DCs in different ways dependent on their maturation state at the time of exposure to the prostaglandin. Early in development, PGE2 has been shown to suppress differentiation of human monocytes into functional Th1-inducing APC (Fig. 1A), instead redirecting monocytes to become immunosuppressive MDSCs (Fig. 1B). Th1 are critical in mediating tumor regression (9). When monocyte-derived immature DCs are matured with IL-1β and tumor necrosis factor alpha (TNF-α) in the presence of PGE2 in vitro, resultant DCs are phenotypically identical but have reduced capacity to produce IL-12 when stimulated with CD40L and IFN-g (Fig. 1A). IL-12 is necessary for efficient generation of Th1 and cytotoxic T cells (Type I cells). While naïve Th cells primed with PGE2-DCs have similar expansion kinetics as compared to controls, the cells also have an enhanced ability to produce Th2-type cytokines, IL-4 and IL-5, and a reduced ability to produce the Th1 cytokine IFN-g. Th2 cells can contribute to tumor growth by dampening the generation of cytotoxic T cells and support cancer proliferation. The effects of PGE2 on DCs may be most important early in oncogenesis, as cervical, breast, head and neck, and colorectal precursor lesions have been shown to overexpress COX-2 (18-21). In a cervical model, COX-2 expression was inversely correlated with density of DCs and ability to stimulate T cells (20). NSAIDs and COX-2 inhibitors may be particularly suited to cancer prevention by protecting the integrity of DCs in mediating immunosurveillance by allowing selective induction and proliferation of Type I T cells (Fig. 1).
Cancer chemoprevention agents may stimulate the adaptive immune system
APCs must induce protective T cell responses through antigen recognition (9). This adaptive T cell response is largely composed of Th1 CD4+ T cells and CD8+ T cells. Th1 cells are critical for propagation of the acute tissue destructive inflammatory response, secreting cytokines such as IFN-g, TNF-α, and IL-2 that support CD8+ T cells and tumor destruction. This is in contrast to Th2 cells that secrete cytokines such as IL-4, IL-5, and IL-10, which limit CD8+ T cell proliferation and promote tumor growth (Fig. 2A). CD8+ T cells are activated after binding antigen presented by MHC class I molecules on APCs and some tumor cells and can deliver cytokines and cytotoxic enzymes that result in tumor cell lysis (9). Lesions that escape cell-mediated death do so by subverting this arm of the immune system, shifting the tumor environment to a Th2 type and inhibiting proliferation of CD8+ T cells. Cancer escapes immune surveillance in a myriad of ways including down regulation of MHC class I molecules, rendering them invisible to CD8+ T cells; production of factors that inhibit CD8+ T cell survival and expansion; and production of cytokines and chemokines that attract immune suppressive cells (22).
Figure 2. Cancer chemoprevention agents stimulate the adaptive immune system.
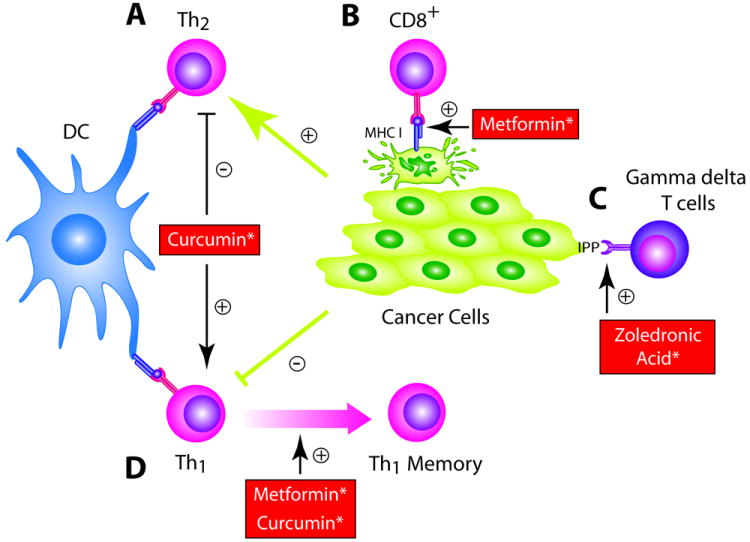
(A) Curcumin administration can enhance the Th1 and decrease Th2 immune response; (B) Metformin and curcumin increase effector CD8+ T cell populations and resulting memory cells, both of which are critical for an effective adaptive immune response; (C) Metformin may increase MHC-I expression on tumor cells, increasing visibility to effector CD8+ T cells; (D)Bexarotene inhibits apoptosis in T cells by increasing expression of BCL2; (E) ZA and other bisphosphonates increase phosphoantigens in PBMCs and on cancer cells themselves, resulting in activation of anti-tumor gamma delta T cells. * Only preclinical data exists to support concept at this time.
After antigenic stimulation, CD8+ T cells undergo expansion of antigen-specific effector populations followed by persistence of long-lived central and effector TM cells. The number of tumor infiltrating central and effector TM cells is inversely correlated with tumor invasion including vascular emboli, lymphatic invasion, and perineural invasion (23). In addition, the presence of TM cells is associated with reduced risk of tumor recurrence, suggesting that these cells are important for secondary prevention (23, 24). The anti-diabetic drug metformin has been suggested as a chemoprevention agent that may enhance the generation of the TM cell compartment (Fig. 2B). Preclinical and epidemiologic studies have suggested a cancer prevention role of metformin with a recent meta-analysis showing a 30% overall reduction in cancer incidence in diabetics on metformin compared to other diabetics (25-27). Metformin significantly decreased both aberrant crypt foci and proliferating cell nuclear antigen index over a single month compared to controls in a small, randomized pilot study (28). Metformin may enhance TM cell numbers via modulation of fatty acid metabolism. Preclinical experiments with mice deficient in tumor necrosis factor (TNF) receptor associated factor 6 (TRAF-6) demonstrated that, while mice mounted normal effector CD8+ T cell responses to infections, they had compromised CD8+ TM cell generation. Microarray data revealed altered expression of genes that regulate fatty acid metabolism with defective AMP-activated kinase (AMPK) activation and mitochondrial fatty acid oxidation. Metformin, which has been shown to promote AMPK activation, when administered restored the ability to generate TM cells (Fig. 2B) (29). Furthermore, metformin promoted survival of CD8+ T cells in wild type mice, resulting in enhanced generation of TM cells (Fig. 2B).
Effective CD8+ T cell immunosurveillance is dependent on MHC I-mediated antigen presentation. Immunohistochemical staining across a spectrum of premalignant and malignant lesions has demonstrated an association between malignant transformation of cells and HLA class I antigen defects, suggesting that loss of Class I expression is an early, critical step in selection and outgrowth of malignant lesions (30, 31). Strategies that increase MHC I expression on transformed cells may restore immunosurveillance and prevent the development of overt malignancy (32). In a preclinical study, metformin increased MHC I expression on cancer cells (Fig. 2C). Most cancer cells shift from oxidative phosphorylation to glycolysis to generate energy. In vitro studies with leukemic cells demonstrated culture conditions that forced respiration also had the effect of upregulating MHC I transcription and protein levels at the cell surface, suggesting a link between the bioenergetic signature of cancer cells and their visibility to the immune system (33). In the SKBR3 breast cancer cell line, metformin enhanced oxidative phosphorylation resulting in a 25-fold increase in cell surface associated MHC-I protein levels (Fig. 2C) (34). Since MHC-I down regulation has been well documented in premalignant lesions, metformin could serve to increase the immunogenicity of pre-invasive disease. The RXR agonist bexarotene also has the potential to enhance T cell numbers and function. Bexarotene has been extensively studied in pre-clinical models and has been shown to be a potent anti-proliferative agent (35). Indeed, clinical trials assessing the potential for bexarotene as a chemoprevention agent are ongoing (36). Bexarotene has also been shown to upregulate the expression of high affinity IL-2 receptor on the surface of immune cells, when cultured together in vitro, potentially allowing an enhanced proliferation when exposed to an environment rich in IL-2 (37). In addition, the use of bexarotene may increase the lifespan of T cells as the agent has been shown to increase BCL2 expression and inhibit the development of apoptosis in T cells (Fig. 2D) (38).
Curcumin is a potential cancer chemoprevention agent currently being tested in clinical trials that may promote a Th1 environment that increases the number of CD4+ and CD8+ T cells. Curcumin inhibits targets important in oncogenesis including COX-2, tumor growth factor beta (TGF-β), and indoleamine 2,3-dixoygenase (IDO), which suppresses the adaptive T cell immune response (39). Preclinical studies have demonstrated chemoprevention effects of curcumin across multiple tumor types (39). Results of a phase IIa trial revealed that curcumin treatment significantly reduced aberrant crypt foci (40). In the ApcMin/+ mouse, curcumin administration reduced colonic tumor formation by approximately 70%. Immunohistochemistry of the mucosal lymphoid population of curcumin treated mice revealed a 30% increase in CD4+ T cells compared to controls, although these cells were not further characterized (41). In a mouse model of mammary carcinoma, curcumin inhibited tumor growth by (1) reversing tumor-induced depletion of CD4+ and CD8+ T cells (Fig. 2B,C) and potentiating CD8+ T cell cytotoxicity (Fig. 2C); (2) restoring memory T cell (TM) populations to levels comparable to controls (Fig. 2B); and (3) shifting the cytokine signature from Th2 to Th1 (Fig. 2A) (42).
Gamma-delta T cells are T lymphocytes with attributes of both the innate and adaptive immune system and account for 1-10% of all peripheral blood T cells. Gamma delta T cells exhibit many qualities of the innate immune system such as the capability of being activated by non-self ligands and phosphoantigens generated by the isoprenoid pathway used by microorganisms or mevalonate pathway in infected or transformed cells. Once activated, gamma delta T cells can expand, exhibit cytotoxicity in both a MHC-dependent and independent fashion, and release Th1 cytokines that further support the adaptive immune system (43). In mouse models of prostate and carcinogen-induced cutaneous malignancy, mice lacking gamma delta T cells developed higher disease burdens and progression of premalignant lesions to overt malignancy than controls and adoptive transfer of gamma delta cells could abrogate this effect (44, 45). In a longitudinal case-control study of renal transplant patients, 18 patients who developed cancer 2-6 years after transplantation were compared to a control group of 45 transplant recipients with similar demographics. Interestingly, patients who developed cancer had significantly fewer gamma delta T cells (<4%) measured in blood at 6, 12, and 18 months before their diagnosis of cancer compared to control patients (46). These findings have increased interest in the potential utility of gamma delta T cells in cancer prevention.
Nitrogen-bisphosphonates (N-BP) such as zoledronic acid (ZA) are being studied as chemoprevention agents that may stimulate the proliferation of gamma delta T cells (Fig. 2E) (47). N-BPs are primarily used for osteoporosis therapy and to reduce skeletal-related events in patients with bone metastases in solid tumors (48). Preclinical evidence for a cancer preventative effect was seen with the bisphosphonate ibadronate, which reduced colorectal dysplasia induced in an experimental mouse model of ulcerative colitis (49). Multiple observational studies have suggested that bisphosphonates are associated with reductions in the incidence of both breast and colon cancer (50, 51). N-BPs stimulate gamma delta T cells indirectly by increasing concentrations of isopentenyl pyrophosphate (IPP), a precursor in the mevalonate pathway, in peripheral blood mononuclear cells, which subsequently activates gamma delta T cell receptors (TCRs). There is also evidence that N-BPs can increase IPP in tumor cells themselves, resulting in a chemotactic and stimulatory signal for gamma delta T cells (Fig. 2E) (52). Activated gamma delta T cells release Th1 cytokines such as TNF-a, IL-6, and IFN-g that are important in immune surveillance (53, 54). Both preclinical data and phase I studies have shown N-BPs can activate tumoricidal gamma delta T cells in a broad range of tumors including breast, prostate, and renal cell carcinoma (55-58).
Cancer chemoprevention agents may inhibit the function of immune suppressor cells
Foxp3 regulatory T cells (Tregs) constitute 5% of all peripheral CD4+ T cells in healthy adults and are important in the regulation of immune responses to both self and foreign antigens and maintenance of immune homeostasis. Tumor-infiltrating Tregs have been shown to correlate with poor prognosis across a spectrum of different cancers. Studies have shown that Tregs suppress both proliferation and activity of effector T cells. In cancer prevention, the strongest support for an important role of Tregs comes from preclinical rodent studies with carcinogen-induced tumors. In studies with methylcholanthrene (MCA)-induced fibrosarcomas, depletion of 50-70% the total number of Tregs with specific antibodies prevented fibrosarcoma development compared to control mice, suggesting that Tregs interfere with immune surveillance (59, 60). Modulation of Tregs could be a means of cancer prevention.
PGE2 increases the inhibitory potential of Tregs and there is evidence that COX-2 inhibitors reverse this effect (Fig. 3A). In the cancer prevention setting, a mouse model of azoxymethane-induced colon cancer was studied to assess effects of PGE2 reduction. Mice underwent genetic deletion of mPGES-1, an inducible terminal synthase that produces PGE2, resulting in 40% reduction of premalignant aberrant crypt foci in the distant colon; 85% suppression of tumor number; and a 90% reduction in tumor load. Evaluation of colon histology of mPGES-1 knockout mice revealed presence of macroscopically inflamed mesenteric lymph nodes with markedly elevated CD4+ and CD8+ lymphocytes and 55% reduction of CD4+ Foxp3+ cells (61). Further support comes from the cancer literature where multiple studies have demonstrated that PGE2 enhances the ability of Tregs to suppress effector T cell proliferation and that COX-2 inhibition abrogated this effect (62, 63). Curcumin has also been shown to have similar effects, preventing cancer-induced Treg expansion and reducing Th2 cytokine release (Fig. 3B) (42). These findings suggest that both COX-2 inhibition and curcumin may decrease Treg function and contribute to enhanced immune surveillance (Fig. 3A,B).
Figure 3. Cancer chemoprevention agents inhibit the function of immune suppressor cells.
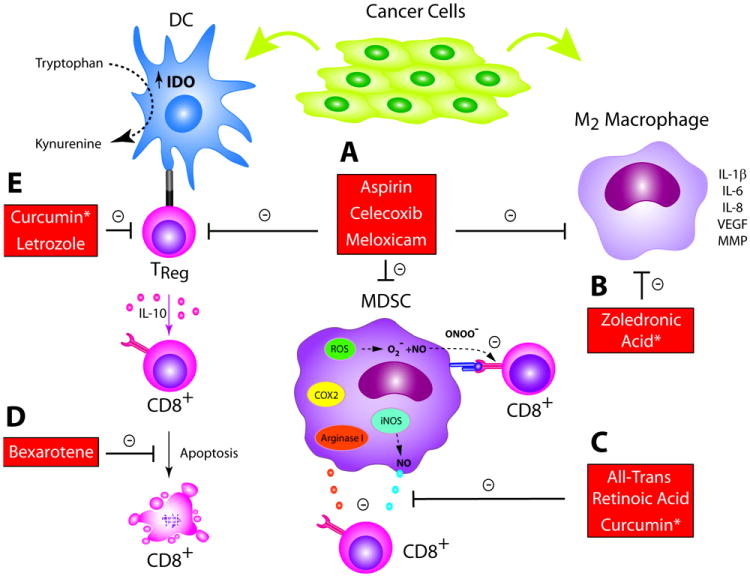
(A) PGE2 can increase immunosuppressive Treg, MDSC, and M2 macrophage populations. COX inhibitors aspirin, celecoxib, and meloxicam can reverse this effect; (B) Both the AI letrozole and curcumin have been shown to reduce Treg populations; (C) The retinoid ATRA can differentiate MDSCs into immature DCs, which may account for its ability to enhance proliferation of both effector and memory CD8+ T cells. Curcumin can inhibit MDSCs and differentiate them toward a M1-like phenotype; (D)ZA decreases populations of M2 macrophages and may re-polarize them to the anti-tumor M1 phenotype. * Only preclinical data exists to support concept at this time.
Aromatase inhibitors (AI) are breast cancer chemoprevention agents associated with up to a 65% relative reduction in annual incidence of invasive breast cancer (64). AIs may mediate this effect in part by decreasing Treg populations (Fig. 3B) (47). AIs function by decreasing the peripheral conversion of androgenic precursors into estrogen. Estrogen has been shown to promote a Th2 cytokine profile and expand Tregs, raising the possibility that AIs could shift this balance to Th1 and resolution of aberrant lesions. Consistent with this observation, in a preclinical mouse study of inflammatory arthritis, AI-treatment resulted in a lower percentage of splenic and lymph node Tregs and increased Th1-cytokine release in response to lymphocyte stimulation compared to untreated mice (Fig. 3B) (65). In a randomized phase II trial, patients with locally advanced ER+ breast cancer received the AI letrozole or letrozole plus cyclophosphamide. There was a significant reduction in Treg number for all patients after treatment with a nonsignificant trend toward the letrozole and cyclophosphamide arm and Treg number at residual histology showed a significant, inverse relationship with response (Fig. 3B) (66).
MDSCs are regulatory cells that suppress tumor immune surveillance. In healthy individuals, these immature myeloid cells generally differentiate into mature cells of the myeloid lineage. In cancer patients, MDSCs accumulate and can contribute to oncogenesis through inhibition of Type I immunity (67). MDSCs can inhibit IL-2 production by activated intratumoral T cells as well as activation and proliferation of CD4+ and CD8+ T cells. In addition, MDSC have the capacity to stimulate Treg recruitment and proliferation. MDSCs act via the depletion of environmental arginine (Arg), an essential amino acid for T cell function. MDSC stimulate the secretion of inducible nitric oxide synthase (iNOS) and the reactive oxygen species (ROS), which promote mutagenesis and inhibit T cells (Fig. 3C) (67). Studies modulating MDSC through inhibition of function or selective depletion have resulted in prevention of carcinogen-mediated neoplasia and restoration of immune surveillance (68, 69).
PGE2 influences differentiation of monocytes, promoting the MDSC phenotype, and COX-2 inhibition may prevent this effect (Fig. 1B). In preclinical studies, PGE2 shifted development of monocytes from immature DCs to MDSCs when added to a standard preparative regimen of GM-CSF and IL-4 (Fig. 1B) (70). In addition, PGE2 exposure promoted COX-2 expression in MDSCs, suggesting that PGE2 initiates a COX2-mediated positive feedback loop, perpetuating the immunosuppressive signal (71). PGE2 has also been shown to be responsible for chemotaxis of MDSCs to tumor sites through chemokine induction and COX-2 inhibition reversed this effect (72). In a carcinogen induced mouse model of intestinal cancer, the COX-2 inhibitor celecoxib administration delayed tumor development and reduced number of tumors at autopsy compared to controls. Coinciding with this, there was a significant reduction in tumor infiltrating MDSCs and splenic MDSCs and a decrease in NOS and Arg mRNA levels from splenic cells (73). In a mouse model of glioma prevention, treatment with aspirin or celecoxib reduced systemic PGE2 production, MDSC number, and consequently significantly delayed glioma development (74).
Retinoids are promising chemoprevention agents that may function by redirecting development of MDSCs to immature DCs (Fig. 3C). Initial studies suggested that retinoids were important in myeloid development, as vitamin A deficient mice had significant myeloid expansion in bone marrow, spleen, and peripheral blood, and this effect was reversed with introduction of vitamin A (75). In prevention, 13-cis-retinoic acid decreased leukoplakia lesion size in 67% of patients compared to 10% in placebo and reversed dysplasia in 54% of patients compared to 10% in the placebo group (76). In vitro studies using MDSCs isolated from patients with a variety of solid tumors demonstrated that all-trans retinoic acid (ATRA) could reverse their suppressive effects on CD8+ T cells (Fig. 3C). ATRA was shown to mediate this effect by re-differentiating MDSCs into immature DCs (77-79). In a therapeutic vaccine trial, a recombinant HPV protein vaccine inhibited HPV-related tumor growth by 85% when combined with ATRA compared to 42% with the vaccine alone and this coincided with a significant decrease in the number of MDSCs, an increase in mature DCs, and enhanced HPV-specific CD8+ T cell response (80). In addition to reducing MDSCs, there is evidence that ATRA can enhance observed proliferation of effector CD8+ T cells by increasing IL-2 release and can augment TM cells when given in combination with a viral vaccine (81). These studies suggest retinoids can reduce suppressive MDSCs and, as a consequence, enhance proliferation and effector function of CD8+ T cells (Fig. 3C). Curcumin has been shown to have similar effects, reducing percentages of MDSCs in peripheral tissues of mice and may actually repolarize them toward a Type 1 (M1) macrophage phenotype (Fig. 3C) (82).
Tumor associated macrophages (TAMs) are recruited early in dysplastic or premalignant lesions and contribute significantly to oncogenesis (83-86). M1 macrophages are tumoricidal while type 2 (M2) macrophages support tumorigenesis and immunosuppression. M2 macrophages express a host of tumorigenic factors including vascular endothelial growth factor (VEGF), COX-2, epidermal growth factor receptor (EGFR) and matrix metalloproteinases (MMP), which support angiogenesis, tissue repair, and remodeling in tumors (Fig. 3D). M2 macrophages also induce immunosuppression through elaboration of cytokines such as IL-10 and TGF-β and inhibit T cell proliferation through expression of Arg and IDO (Fig. 3D) (87). The presence of M2 macrophages correlates with poor prognosis in a number of different tumor types (88). There is evidence that TAMs are recruited early in preneoplasia (89). In a transgenic mouse of mammary cancer (PyMT mice), mice progress through four stages from benign hyperplasia to overt malignancy with metastases. There was a significant correlation between low density of macrophages in primary tumors and a delay in vascular development and malignant transition. Macrophage depletion resulted in delayed progression of premalignant lesions (90).
In addition to their other anti-tumor effects, COX-2 inhibitors inhibit M2 macrophage accumulation (Fig. 3A). PGE2 overexpression increases M2 macrophage density in models of dysplastic and premalignant gastric, esophageal, and colon lesions, which is associated with progression to overt malignancy (83-85). In a rodent model of prevention, surgically induced duodenal reflux resulted in inflammation-induced dysplasia that progressed to squamous cell carcinoma in the forestomach. In control mice, 90% developed dysplasia and 38% of mice developed SCC at week 60 compared to 20% and 0%, respectively, in mice given the COX-2 inhibitor meloxicam. COX-2 was predominantly detected in infiltrating macrophages, suggesting that these cells mediated inflammation-induced COX-2 expression and oncogenesis (91). In another study, ApcMin/+ mice developed premalignant polyps heavily infiltrated by M2 macrophages with a Th2 cytokine profile. Celecoxib administration for 8 weeks (1) reduced the size and number of polyps compared to controls; (2) shifted the TAM phenotype from M2 to M1 macrophage predominant infiltrate; and (3) increased the Th1 signature in the environment (Fig. 3A) (92).
Bisphosphonates have been shown to have inhibitory effects on TAMs as well, which is not unexpected given the shared lineage between macrophages and osteoclasts (Fig. 3D). Macrophages endocytose bisphosphonates and release them into the cytosol where they can induce apoptosis by inhibiting the mevalonate pathway (93). Bisphosphosphonates decrease release of protumorigenic factors such as MMP-9 by activated macrophages and decrease other proangiogenic factors like VEGF associated with activated macrophages (Fig. 3D) (93). In the transgenic BALB-neuT mouse, mice develop spontaneous mammary tumors in a stepwise manner similar to human breast cancer. In one study, mice received either control drug or cycles of ZA mimicking standard dosing schedules. Mice treated with ZA had significant extension of median tumor-free survival, delayed growth kinetics, and overall survival compared to control. Tumor stroma of control mice exhibited heavy infiltration of VEGF-expressing macrophages, while ZA-treated mice had a marked reduction. Interestingly, macrophages from control mice exhibited a Th2 cytokine pattern, while macrophages from ZA-treated mice were strongly positive for IFN-g, suggesting that in addition to reducing total macrophage numbers, ZA repolarized M2 macrophages to M1 type (94). In a murine model expressing HPV-16 genes (HPV/E2 mouse) of cervical carcinogenesis, mice develop cervical intraepithelial neoplasia (CIN) lesions that progress to invasive SCCs. In a trial comparing effect of ZA on mice with CIN-3 lesions to controls, at 5 months, controls had SCC incidence of 85%, while ZA-treated mice had an incidence of 30%, suggesting a strong preventative effect. In addition to inducing apoptosis of tumor cells themselves, ZA reduced infiltrating macrophages by 10% and MMP-9 expression by 73% in remaining macrophages relative to control again suggesting a repolarizing effect of the drug (Fig. 3D) (95).
Conclusion
The immune system is a powerful sentinel against cancer with several types of cells surveying the environment and eliminating pre-invasive lesions before the development of overt malignancy. For immunocompetent individuals, a hallmark of cancer involves evolution of the mechanisms for the tumor to evade the immune system (96). In the field of immunoprevention, an emerging strategy involves enhancing immune surveillance via pharmacologic means. Tumor vaccines and antibodies hold promise in terms of bolstering the adaptive immune system for tumor prevention. However, many of the most promising chemoprevention agents may exert their effects, in part, by enhancing function of both DCs and effector T cells and decreasing the functional impact of immunosuppressive cells. As the immune effects of chemoprevention agents are further delineated, the exciting possibility of using them in combination to elicit effective tumor immune surveillance will be within reach.
Acknowledgments
Financial support: This work was supported for EM by T32 5T32CA009515-27 and for MLD by NCI N01-CN-53300/WA#10, N01-CN-53300, and DOD W81XWH-11-1-0760.
Abbreviation
- AI
aromatase inhibitors
- AMPK
adenosine monophosphate kinase-activated kinase
- APC
antigen presenting cells
- Arg
arginine
- ATRA
all-trans retinoic acid
- CIN
cervical intraepithelial neoplasia
- COX-2
cyclo-oxygenase 2
- DC
dendritic cells
- EGFR
epidermal growth factor receptor
- FOXP3
forkhead box protein P3
- FPP
farnesyl disphosphate synthase
- HCC
hepatocellular carcinoma
- HLA
human leukocyte antigen
- HPV
human papillomavirus
- IDO
indoleamine 2,3-dixoygenase
- IFN-γ
interferon gamma
- IL
interleukin
- IPP
isopentenyl pyrophosphate
- MCA
methylcholanthrene
- MDSC
myeloid-derived suppressor cell
- MMP
matrix metalloprotein
- mPGES-1
terminal prostaglandin synthase 1
- N-BP
nitrogen-bisphosphonate
- NOS
nitrogen oxide synthetease
- NSAIDs
non-steroidal anti-inflammatory drugs
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- RXR
Rexinoid receptor
- SCC
squamous cell carcinoma
- SERMS
selective estrogen receptor modulators
- SIL
squamous intraepithelial lesions
- TAM
tumor-associated macrophage
- TCR
T cell receptor
- TGF-β
tumor growth factor β
- Th
T-helper
- TILs
tumor infiltrating lymphocyte
- TM
memory T cells
- TNF-α
tumor necrosis factor α
- TRAF
tumor necrosis factor receptor associated factor 6
- Treg
regulatory T cells
- VEGF
vascular endothelial growth factor
- ZA
zoledronic acid
Footnotes
Disclosure of Potential Conflicts of Interest: There are no conflicts of interest.
References
- 1.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al. The New England journal of medicine. Vol. 336. Taiwan Childhood Hepatoma Study Group; 1997. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children; pp. 1855–9. [DOI] [PubMed] [Google Scholar]
- 2.Group FIIS. Dillner J, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. Bmj. 2010;341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darnell RB, Posner JB. Observing the invisible: successful tumor immunity in humans. Nature immunology. 2003;4:201. doi: 10.1038/ni0303-201. [DOI] [PubMed] [Google Scholar]
- 4.Dougan M, Dranoff G. Immune therapy for cancer. Annual review of immunology. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 5.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature reviews Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 6.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:610–8. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 7.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annual review of immunology. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 8.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nature reviews Immunology. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 9.Disis ML. Immune regulation of cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4531–8. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. The New England journal of medicine. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 11.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. The lancet oncology. 2009;10:501–7. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 12.Force USPST. Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2007;146:361–4. [PubMed] [Google Scholar]
- 13.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. The New England journal of medicine. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 14.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nature reviews Clinical oncology. 2012;9:259–67. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 15.Mills EJ, Wu P, Alberton M, Kanters S, Lanas A, Lester R. Low-dose aspirin and cancer mortality: a meta-analysis of randomized trials. The American journal of medicine. 2012;125:560–7. doi: 10.1016/j.amjmed.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–12. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 18.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–8. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 19.Half E, Tang XM, Gwyn K, Sahin A, Wathen K, Sinicrope FA. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer research. 2002;62:1676–81. [PubMed] [Google Scholar]
- 20.Herfs M, Herman L, Hubert P, Minner F, Arafa M, Roncarati P, et al. High expression of PGE2 enzymatic pathways in cervical (pre)neoplastic lesions and functional consequences for antigen-presenting cells. Cancer immunology, immunotherapy : CII. 2009;58:603–14. doi: 10.1007/s00262-008-0584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saba NF, Choi M, Muller S, Shin HJ, Tighiouart M, Papadimitrakopoulou VA, et al. Role of cyclooxygenase-2 in tumor progression and survival of head and neck squamous cell carcinoma. Cancer prevention research. 2009;2:823–9. doi: 10.1158/1940-6207.CAPR-09-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biragyn A, Longo DL. Neoplastic “Black Ops”: cancer’s subversive tactics in overcoming host defenses. Seminars in cancer biology. 2012;22:50–9. doi: 10.1016/j.semcancer.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. The New England journal of medicine. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 24.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 25.Bhalla K, Hwang BJ, Dewi RE, Twaddel W, Goloubeva OG, Wong KK, et al. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer prevention research. 2012;5:544–52. doi: 10.1158/1940-6207.CAPR-11-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer prevention research. 2010;3:1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 27.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer prevention research. 2010;3:1066–76. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer prevention research. 2010;3:1077–83. doi: 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- 29.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–7. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer immunology, immunotherapy : CII. 2007;56:227–36. doi: 10.1007/s00262-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cromme FV, Meijer CJ, Snijders PJ, Uyterlinde A, Kenemans P, Helmerhorst T, et al. Analysis of MHC class I and II expression in relation to presence of HPV genotypes in premalignant and malignant cervical lesions. British journal of cancer. 1993;67:1372–80. doi: 10.1038/bjc.1993.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lampen MH, van Hall T. Strategies to counteract MHC-I defects in tumors. Current opinion in immunology. 2011;23:293–8. doi: 10.1016/j.coi.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Charni S, de Bettignies G, Rathore MG, Aguilo JI, van den Elsen PJ, Haouzi D, et al. Oxidative phosphorylation induces de novo expression of the MHC class I in tumor cells through the ERK5 pathway. Journal of immunology. 2010;185:3498–503. doi: 10.4049/jimmunol.1001250. [DOI] [PubMed] [Google Scholar]
- 34.Oliveras-Ferraros C, Cufi S, Vazquez-Martin A, Menendez OJ, Bosch-Barrera J, Martin-Castillo B, et al. Metformin rescues cell surface major histocompatibility complex class I (MHC-I) deficiency caused by oncogenic transformation. Cell cycle. 2012;11:865–70. doi: 10.4161/cc.11.5.19252. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Zhang Y, Hill J, Kim HT, Shen Q, Bissonnette RP, et al. The rexinoid, bexarotene, prevents the development of premalignant lesions in MMTV-erbB2 mice. British journal of cancer. 2008;98:1380–8. doi: 10.1038/sj.bjc.6604320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strecker TE, Shen Q, Zhang Y, Hill JL, Li Y, Wang C, et al. Effect of lapatinib on the development of estrogen receptor-negative mammary tumors in mice. Journal of the National Cancer Institute. 2009;101:107–13. doi: 10.1093/jnci/djn436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorgun G, Foss F. Immunomodulatory effects of RXR rexinoids: modulation of high-affinity IL-2R expression enhances susceptibility to denileukin diftitox. Blood. 2002;100:1399–403. doi: 10.1182/blood-2002-01-0300. [DOI] [PubMed] [Google Scholar]
- 38.Rasooly R, Schuster GU, Gregg JP, Xiao JH, Chandraratna RA, Stephensen CB. Retinoid × receptor agonists increase bcl2a1 expression and decrease apoptosis of naive T lymphocytes. Journal of immunology. 2005;175:7916–29. doi: 10.4049/jimmunol.175.12.7916. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer research. 2003;23:363–98. [PubMed] [Google Scholar]
- 40.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer prevention research. 2011;4:354–64. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Churchill M, Chadburn A, Bilinski RT, Bertagnolli MM. Inhibition of intestinal tumors by curcumin is associated with changes in the intestinal immune cell profile. The Journal of surgical research. 2000;89:169–75. doi: 10.1006/jsre.2000.5826. [DOI] [PubMed] [Google Scholar]
- 42.Bhattacharyya S, Md Sakib Hossain D, Mohanty S, Sankar Sen G, Chattopadhyay S, Banerjee S, et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cellular & molecular immunology. 2010;7:306–15. doi: 10.1038/cmi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hannani D, Ma Y, Yamazaki T, Dechanet-Merville J, Kroemer G, Zitvogel L. Harnessing gammadelta T cells in anticancer immunotherapy. Trends in immunology. 2012;33:199–206. doi: 10.1016/j.it.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–9. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z, Eltoum IE, Guo B, Beck BH, Cloud GA, Lopez RD. Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. Journal of immunology. 2008;180:6044–53. doi: 10.4049/jimmunol.180.9.6044. [DOI] [PubMed] [Google Scholar]
- 46.Couzi L, Levaillant Y, Jamai A, Pitard V, Lassalle R, Martin K, et al. Cytomegalovirus-induced gammadelta T cells associate with reduced cancer risk after kidney transplantation. Journal of the American Society of Nephrology : JASN. 2010;21:181–8. doi: 10.1681/ASN.2008101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuzick J. Aromatase inhibitors for breast cancer prevention. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:1636–43. doi: 10.1200/JCO.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 48.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. The Journal of experimental medicine. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sassa S, Okabe H, Nemoto N, Kikuchi H, Kudo H, Sakamoto S. Ibadronate may prevent colorectal carcinogenesis in mice with ulcerative colitis. Anticancer research. 2009;29:4615–9. [PubMed] [Google Scholar]
- 50.Rennert G, Pinchev M, Rennert HS. Use of bisphosphonates and risk of postmenopausal breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3577–81. doi: 10.1200/JCO.2010.28.1113. [DOI] [PubMed] [Google Scholar]
- 51.Rennert G, Pinchev M, Rennert HS, Gruber SB. Use of bisphosphonates and reduced risk of colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:1146–50. doi: 10.1200/JCO.2010.33.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benzaid I, Monkkonen H, Stresing V, Bonnelye E, Green J, Monkkonen J, et al. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo. Cancer research. 2011;71:4562–72. doi: 10.1158/0008-5472.CAN-10-3862. [DOI] [PubMed] [Google Scholar]
- 53.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 54.Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. The New England journal of medicine. 1999;340:737–8. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 55.Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, et al. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–1. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 56.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer research. 2007;67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marten A, Lilienfeld-Toal M, Buchler MW, Schmidt J. Zoledronic acid has direct antiproliferative and antimetastatic effect on pancreatic carcinoma cells and acts as an antigen for delta2 gamma/delta T cells. Journal of immunotherapy. 2007;30:370–7. doi: 10.1097/CJI.0b013e31802bff16. [DOI] [PubMed] [Google Scholar]
- 58.Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clinical and experimental immunology. 2010;161:290–7. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Betts G, Twohig J, Van den Broek M, Sierro S, Godkin A, Gallimore A. The impact of regulatory T cells on carcinogen-induced sarcogenesis. British journal of cancer. 2007;96:1849–54. doi: 10.1038/sj.bjc.6603824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teng MW, Ngiow SF, von Scheidt B, McLaughlin N, Sparwasser T, Smyth MJ. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer research. 2010;70:7800–9. doi: 10.1158/0008-5472.CAN-10-1681. [DOI] [PubMed] [Google Scholar]
- 61.Nakanishi Y, Nakatsuji M, Seno H, Ishizu S, Akitake-Kawano R, Kanda K, et al. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis. 2011;32:1333–9. doi: 10.1093/carcin/bgr128. [DOI] [PubMed] [Google Scholar]
- 62.Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc’h N, Zeng G, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. Journal of immunology. 2005;175:1483–90. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 63.Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. Journal of immunology. 2006;177:246–54. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- 64.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. The New England journal of medicine. 2011;364:2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Zhang Q, Jin S, Feng M, Kang X, Zhao S, et al. Immoderate inhibition of estrogen by anastrozole enhances the severity of experimental polyarthritis. Experimental gerontology. 2009;44:398–405. doi: 10.1016/j.exger.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Generali D, Bates G, Berruti A, Brizzi MP, Campo L, Bonardi S, et al. Immunomodulation of FOXP3+ regulatory T cells by the aromatase inhibitor letrozole in breast cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:1046–51. doi: 10.1158/1078-0432.CCR-08-1507. [DOI] [PubMed] [Google Scholar]
- 67.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. Journal of immunology. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morales JK, Kmieciak M, Graham L, Feldmesser M, Bear HD, Manjili MH. Adoptive transfer of HER2/neu-specific T cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer immunology, immunotherapy : CII. 2009;58:941–53. doi: 10.1007/s00262-008-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. The Journal of experimental medicine. 2003;198:1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer research. 2007;67:4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 71.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118:5498–505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer research. 2011;71:7463–70. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. International immunopharmacology. 2007;7:140–51. doi: 10.1016/j.intimp.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 74.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer research. 2011;71:2664–74. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuwata T, Wang IM, Tamura T, Ponnamperuma RM, Levine R, Holmes KL, et al. Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood. 2000;95:3349–56. [PubMed] [Google Scholar]
- 76.Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. The New England journal of medicine. 1986;315:1501–5. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 77.Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:8270–8. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 78.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer research. 2006;66:9299–307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer research. 2007;67:11021–8. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- 80.Song X, Ye D, Liu B, Cui J, Zhao X, Yi L, et al. Combination of all-trans retinoic acid and a human papillomavirus therapeutic vaccine suppresses the number and function of immature myeloid cells and enhances antitumor immunity. Cancer science. 2009;100:334–40. doi: 10.1111/j.1349-7006.2008.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tan X, Sande JL, Pufnock JS, Blattman JN, Greenberg PD. Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. Journal of virology. 2011;85:8316–27. doi: 10.1128/JVI.00781-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G, et al. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer prevention research. 2012;5:205–15. doi: 10.1158/1940-6207.CAPR-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Femia AP, Dolara P, Luceri C, Salvadori M, Caderni G. Mucin-depleted foci show strong activation of inflammatory markers in 1,2-dimethylhydrazine-induced carcinogenesis and are promoted by the inflammatory agent sodium dextran sulfate. International journal of cancer Journal international du cancer. 2009;125:541–7. doi: 10.1002/ijc.24417. [DOI] [PubMed] [Google Scholar]
- 84.Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131:1086–95. doi: 10.1053/j.gastro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 85.Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. The EMBO journal. 2004;23:1669–78. doi: 10.1038/sj.emboj.7600170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmid MC, Avraamides CJ, Foubert P, Shaked Y, Kang SW, Kerbel RS, et al. Combined blockade of integrin-alpha4beta1 plus cytokines SDF-1alpha or IL-1beta potently inhibits tumor inflammation and growth. Cancer research. 2011;71:6965–75. doi: 10.1158/0008-5472.CAN-11-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mancino A, Lawrence T. Nuclear factor-kappaB and tumor-associated macrophages. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:784–9. doi: 10.1158/1078-0432.CCR-09-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer research. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 89.Kawsar HI, Weinberg A, Hirsch SA, Venizelos A, Howell S, Jiang B, et al. Overexpression of human beta-defensin-3 in oral dysplasia: potential role in macrophage trafficking. Oral oncology. 2009;45:696–702. doi: 10.1016/j.oraloncology.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 90.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer research. 2006;66:11238–46. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 91.Oba M, Miwa K, Fujimura T, Harada S, Sasaki S, Oyama K, et al. A selective cyclooxygenase-2 inhibitor prevents inflammation-related squamous cell carcinogenesis of the forestomach via duodenogastric reflux in rats. Cancer. 2009;115:454–64. doi: 10.1002/cncr.23990. [DOI] [PubMed] [Google Scholar]
- 92.Nakanishi M, Menoret A, Tanaka T, Miyamoto S, Montrose DC, Vella AT, et al. Selective PGE(2) suppression inhibits colon carcinogenesis and modifies local mucosal immunity. Cancer prevention research. 2011;4:1198–208. doi: 10.1158/1940-6207.CAPR-11-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. Journal of translational medicine. 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C, et al. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. Journal of cellular and molecular medicine. 2010;14:2803–15. doi: 10.1111/j.1582-4934.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. The Journal of clinical investigation. 2004;114:623–33. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]


