Abstract
Cancers are frequently sensitive to restoration of oncogenic lesions to normal physiologic regulation, which can elicit dramatic reversal of their neoplastic properties through the phenomena of oncogene addiction. In some cases, this is associated with the compete elimination of a tumor. However, in other cases the tumor undergoes conversion to differentiated and/or non-self-replicating cells; alternatively, some of the tumor cells are converted to dormant latent tumor cells that can regain the ability to self-renew upon oncogene reactivation. The ability to predict when oncogene inactivation will elicit complete and sustained tumor elimination versus tumor dormancy would have important implications for cancer therapies. One potentially important mechanistic insight into tumor dormancy is that oncogene addiction involves both tumor cell-intrinsic, cell-autonomous mechanisms and host-dependent, tumor cell-non-autonomous programs that both converge upon the regulation of a decision between self-renewal and cellular senescence. Another insight is that the tumor microenvironment, known to be critical during tumor initiation, prevention, and progression, also appears to dictate when oncogene inactivation will elicit the permanent loss of self-renewal through cellular senescence. Thus, oncogene addiction may be best modeled as a consequence of the interplay amongst cell-autonomous and host-dependent programs that converge upon the regulation of self-renewal programs that define when a therapy will result in tumor dormancy.
Keywords: Oncogene Addiction, MYC, Tumor Domancy, Cellular Senescence, Self-Renewal, Transgenic Models
Introduction
Cancers are frequently sensitive to restoration of oncogenic lesions to normal physiologic regulation, which can elicit dramatic reversal of their neoplastic properties through the phenomena of oncogene addiction [1]. In some cases, this is associated with the compete elimination of a tumor. However, in other cases the tumor undergoes conversion to differentiated and/or non-self-replicating cells; alternatively, some of the tumor cells are converted to dormant latent tumor cells that can regain the ability to self-renew upon oncogene reactivation. The ability to predict when oncogene inactivation will elicit complete and sustained tumor elimination versus tumor dormancy would have important implications for cancer therapies. One potentially important mechanistic insight into tumor dormancy is that oncogene addiction involves both tumor cell-intrinsic, cell-autonomous mechanisms and host-dependent, tumor cell-non-autonomous programs that both converge upon the regulation of a decision between self-renewal and cellular senescence [2-5]. Another insight is that the tumor microenvironment, known to be critical during tumor initiation [6, 7], prevention [8], and progression [9], also appears to dictate when oncogene inactivation will elicit the permanent loss of self-renewal through cellular senescence [10-12]. Thus, oncogene addiction may be best modeled as a consequence of the interplay amongst cell-autonomous and host-dependent programs that converge upon the regulation of self-renewal programs that define when a therapy will result in tumor dormancy.
Oncogene Addiction: A Mechanism of Tumor Regression
Oncogene addiction is the phenomenon by which tumor cells, through the consequence of a multitude of genetic and epigenetic changes, remain exquisitely dependent upon a single oncogenic lesion for the persistence of their neoplastic phenotype (Figure 1, 2) [13, 14]. The first suggestion that tumors cells could be addicted to tumor cells came from in vitro observations that tumor-derived cell lines sometimes exhibited proliferative arrest and/or apoptosis upon the suppression of an oncogene or the restoration of expression of a tumor suppressor [15]. These observations first hinted that therapeutic agents targeting the repair or suppression of these mutant gene products could be generally effective for the treatment of cancer.
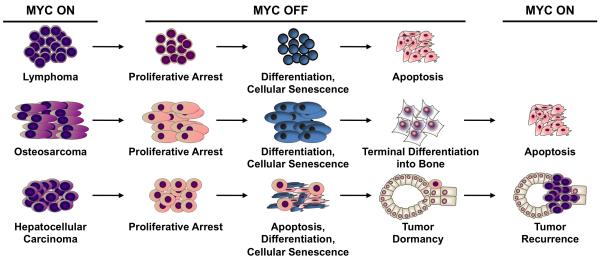
Figure 1. Oncogene addiction elicits tissue-specific effects.
Oncogene inactivation has been observed to have different outcomes depending upon the tissue origin of tumors, including proliferative arrest, differentiation, apoptosis, and/or cellular senescence. The specific consequences of oncogene addiction have a dramatic impact on whether targeted therapies will result in tumor dormancy, as in the case of MYC-induced hepatocellular carcinoma, or tumor elimination, as shown for MYC-induced lymphoma.
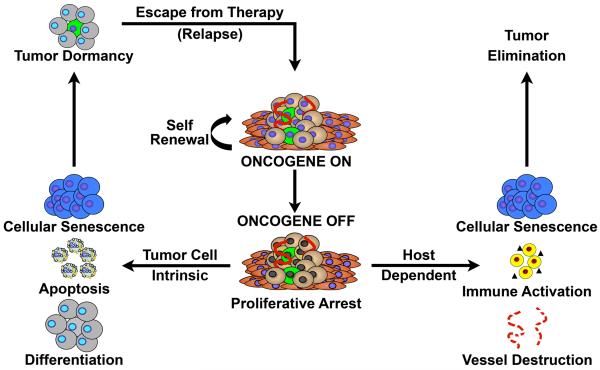
Figure 2. Oncogene addiction comprises both cancer cell-autonomous and non-cell-autonomous mechanisms of tumor regression.
Oncogene inactivation reverses many of the hallmarks of cancer to lead to tumor regression through reinstatement of cell-intrinsic programs such as proliferative arrest, apoptosis, and differentiation, as well as host-dependent phenomena including immune system activation and destruction of the tumor vasculature. Notably, cellular senescence is both a tumor cell-intrinsic and a host-regulated consequence of oncogene addiction; however, it is likely only in an immune intact host that targeted therapy will result in complete tumor elimination.
Later, the development of transgenic mice that can conditionally express oncogenes enabled the direct in situ interrogation of the role of specific oncogenes in the initiation and maintenance of tumorigenesis. Many mouse models have been generated to explore the tumor-specific consequences of the suppression of oncogenes including MYC, RAS, BRAF, and BCR-ABL (Table 1) [2-4, 16-19]. In these models, the particular consequences of oncogene inactivation include proliferative arrest, apoptosis [2], differentiation [3, 4], and senescence [5], as well as the inhibition of angiogenesis [20, 21]. These observations implied that many cancers are addicted to a single oncogene.
Table 1. Examples of immune system-mediated oncogene addiction.
Murine models of MYC and BCR-ABL inactivation as well as P53 restoration directly implicate immune involvement in implementing the consequences of oncogene addiction. Below, oncogenes whose tumorigenicity relies heavily on immune evasion and/or pro-tumor inflammation highlight the potentially broad generalizability of the concept of immune system-mediated oncogene addiction.
| Oncogene | Tumor Type | Immune Compartment | Immune-Mediated Mechanism | Refs | |
|---|---|---|---|---|---|
| Adaptive Immunity | MYC | T cell acute lymphoblastic lymphoma |
CD4+ T cells | Induction of senescence and suppression of angiogenesis |
[76] |
| BCR-ABL | Pro-B cell acute lymphocytic leukemia |
CD4+ T cells | Induction of senescence and suppression of angiogenesis |
[76] | |
| PML | Acute promyelocytic leukemia; prostate carcinoma |
CD8+ T cells | Influencing MHC class I antigen presentation |
[97, 98] | |
| BRAF | Melanoma | Dendritic cells, CTLs | Inhibition of antigen presentation and induction of IL-10, IL-6 |
[99, 130] | |
| Innate Immunity | MYC | B cell lymphoma; pancreatic islet cell tumor |
Macrophages, Mast cells | Macrophage induction of senescence; mast cell promotion of angiogenesis |
[73, 107] |
| P53 | Hepatocellular carcinoma | Neutrophils, Macrophages, NK cells |
Tumor clearance | [77] | |
| RAS | Cervical cancer; renal cell carcinoma |
Neutrophils | Promotion of angiogenesis and tumor growth due to IL6 and IL8 |
[102, 103] |
Further studies established that the specific consequences of oncogene inactivation are highly dependent upon the tissue or origin from which the cancer was initiated. Even upon brief inactivation of an oncogene, the diversity of these outcomes is evidenced by the induction of a permanent loss of the neoplastic phenotype in osteosarcoma and lymphoma (Figure 1) [2, 3]. In marked contrast, oncogene suppression in hepatocellular carcinoma and breast carcinoma [4, 19] induced regression of tumors, but restoration of oncogene activity restored their neoplastic features, suggesting a state of tumor dormancy (Figure 1). In yet other cases, the inactivation of the oncogene failed to cause significant tumor regression, such as in a murine model of MYC-induced lung adenocarcinoma [22]. The instances in which inactivation of a specific oncogene that initiated tumorigenesis is sufficient to reverse tumorigenesis thus depends upon both cellular and genetic context.
Importantly, the clinical relevance of oncogene addiction was established through the development of several, effective targeted therapeutics [23, 24]. The identification of potent agents such as imatinib for chronic myelogenous leukemia and gastrointestinal stromal tumors [25], trastuzumab for the treatment of breast cancer [26], and vemurafenib for the treatment of melanoma [27], amongst other drugs [28], support the paradigm of exploiting oncogene addiction for the therapy of cancer. Moreover, these successes underscore how elucidating the underlying principles of oncogene addiction may be generally exploited as a strategy to treat a broad spectrum of cancers.
Oncogene addiction had been presumed to be largely a consequence of cell-autonomous mechanisms that occur through processes intrinsic and exclusively dependent upon biological programs including proliferation and apoptosis that are governed within a tumor cell (Figure 2). Several mechanisms have been proposed for oncogene addiction including the notion of abnormal tumor cell genetic circuitry [1], reversibility of tumorigenesis [29], oncogenic shock [30], and synthetic lethality [31]. Even more recently, the host microenvironment has been shown to play a critical role in how oncogenes initiate as well as maintain tumorigenesis (Figure 2) [32-35].
Oncogene Addiction, Tumor Dormancy, Senescence, and Self-Renewal Programs
Even brief inactivation of an oncogene may result in tumor regression [2]. However, in some instances, although oncogene inactivation appears to result in the complete loss of the neoplastic properties of a tumor, the reactivation of the oncogene results in the rapid restoration of neoplastic properties [4]. Tumors that are not fully eliminated may also recur due to resistance to the targeted therapy conferred by mutation(s) in either the targeted gene or a downstream pathway [36-38]. This tumor dormancy, or the persistence of a state of minimal residual disease, therefore represents an immense hurdle to tumor elimination and ultimately patient survival [39].
One convergent feature of oncogene addiction appears to be the rapid and sustained proliferative arrest of tumor cells associated with the loss of self-renewal (Figure 1, 2) [40-43]. The importance of limitless self-renewal as the essential feature of cancer cells has been appreciated for decades [44]. More recently, it has been dramatically illustrated that only a subpopulation of tumor cells retain this limitless lifespan potential and thus are deemed cancer stem cells (CSCs) [45-50]. The self-renewal of CSCs involves complex regulation of multiple signaling pathways and transcription factors, including MYC [50-53]. Therefore, dramatic regression of tumors following oncogene inactivation is anticipated to involve loss of this self-renewal capacity.
At least in some cases, the loss of self-renewal of cancer cells has been associated with molecular and morphological features that have been described as cellular senescence [41]. Senescence is a cellular program that was first described as a barrier to limitless proliferation of normal cells grown in vitro [54, 55], and subsequently has been shown to be a conserved response to many types of cellular stress including telomere shortening [56, 57], DNA damage [58, 59], chemotherapy treatment [60-62], and oncogene activation [63-66]. Cellular senescence is associated with permanent changes in gene expression, chromatin condensation, induction of cell cycle arrest programs that involve p15(INK4b), p16(INK4a) and/or p53, and is correlated with an increase in acidic beta-galactosidase enzymatic activity [67-71].
Oncogene addiction may elicit cellular senescence through at least four different mechanisms: first, through induction of expression of cell cycle arrest proteins including p15(INK4b), p16(INK4a) and p21(CIP1) [5]; second, through the restoration of autocrine programs that induce cellular senescence, such as TGF-beta (TGF-β) signaling [72, 73]; third, through unopposed MAPK signaling [74, 75]; fourth, via immune mechanisms that appear to be mediated through secreted cytokines such as TSP-1 [76, 77].
Thus, oncogene addiction can be modeled as a consequence of the balance between self-renewal and cellular senescence programs (Figure 2). Cellular senescence is defined by its irreversibility. The ability of oncogene inactivation to elicit cellular senescence, and hence prohibit self-renewal of cancer cells, would be a mechanism to permanently suppress the tumor phenotype. Thus, cellular senescence appears to be a likely mechanism that would dictate tumor dormancy. Hence, whether oncogene inactivation induces tumor elimination or tumor dormancy also appears to depend upon the balance between self-renewal and cellular senescence programs.
Oncogene Addiction, Tumor Dormancy, and the Tumor Microenvironment
Tumor cells evolve in a host with an intact immune system [78]. Co-evolution of incipient tumors cells with host cells is integral to each step of tumorigenesis, including tumor initiation [6, 7], prevention [8], and progression [9]. Tumors appear to undergo immune editing, which is important to both their generation and therapeutic destruction [79, 80]. Thus, tumorigenesis is a consequence of interactions between incipient neoplastic cells and host stromal cells [32] that interact to regulate tumorigenesis [81].
Specific immune effectors and secreted factors have been implicated in the initiation of tumorigenesis [6, 7] as well as tumor growth, survival, and metastasis [81]. Immune effectors, including macrophages, T-cells, and B-cells, have been shown to either have a role in promoting [82-84] or inhibiting [73, 85-87] tumorigenesis. For example, NK (natural killer) cells [88] can inhibit metastasis whereas CD4+ T-cells [89] and macrophages [90] have been shown to promote metastasis. Similarly, in human patients, autoimmune stimulation or inflammation can be associated with increased tumorigenesis [78, 91-93]. Immune-compromised hosts exhibit a magnitudes increased incidence of certain tumors [79]. Consequently, the presence or absence of immune effectors, such as CD4+ T-cells is associated with a favorable [94] or a non-favorable prognosis [95] depending on tissue type, thereby indicating the complexity of the interaction between the host immune system and the evolving tumor. Indeed, immune cells and cytokines are important to the pathogenesis of tumorigenesis.
Oncogene activation can directly influence the immune response [96-100]. The RET oncogene in normal human thymocytes induces an inflammatory response leading to tumor tissue remodeling, angiogenesis and metastasis [101]. RAS up-regulates expression of the cytokines IL-6 [102] and IL-8 [103], which contribute to tumorigenesis. MYC can suppress CD4+ T-cells to maintain the angiogenic tumor microenvironment in multiple tumor models [76, 104]. However, MYC activation of macrophages is also associated with tumor suppression [73]. Hence, oncogene activation and inactivation can have dramatic consequences on both the tumor cells and the host tumor microenvironment (see Table 1).
The host immune system also is important to the efficacy of therapeutics [10-12]. Patients with impaired host immunity have decreased overall and progression-free survival in a variety of solid and hematologic malignancies [105, 106]. In colorectal carcinomas, the type, density, and intratumoral location of the T-cell infiltrate has proven a more robust predictor of patient outcome than the TNM or Duke’s classification [11]. More generally, the host immune status influences the efficacy of conventional chemotherapy and radiation therapies [106].
In mouse models, the immune system can be directly interrogated mechanistically to define its role in therapeutic response [11]. For example, in mouse models of hepatocellular carcinoma, pancreatic tumor, and B-cell lymphoma innate immune components such as mast cells [107] and macrophages [73] have been implicated as barriers to tumor growth and facilitators of tumor regression. In models of colon and breast adenocarcinomas, chemotherapeutic agents and radiation therapies have been shown to elicit immunogenic apoptosis of cancer cells [108].
Multiple mechanisms of the immune contribution to the therapeutic response have been suggested, including both innate and adaptive immune effectors as well as specific cytokines [10-12]. Recently, it has been proposed that restoration in tumor cells of the “find me” and “eat me” immune stimulatory signals could potentially be used therapeutically to treat cancer [108, 109]. Hence, the promotion of both the adaptive and innate arms of host immunity may be highly useful towards the complete elimination of tumor cells [108, 109].
Immune Effectors and Tumor Dormancy versus Elimination
Specific cellular and cytokine-mediated immune effectors may define the consequences of oncogene inactivation. Experimentally, CD4+ T-cells appear to be essential to the mechanism of tumor regression upon oncogene inactivation in mouse models of MYC- or BCR-ABL-induced hematopoietic tumorigenesis (Figure 3, Table 1) [76]. Oncogene inactivation in MYC-induced tumors in CD4+ T-cell immunodeficient mice resulted in significantly delayed kinetics of tumor regression and failed to completely eradicate tumor cells, leaving up to 1000-fold more minimal residual disease (MRD) than in wildtype hosts [76]. Other effectors are also recruited to the tumor site suggesting their possible contribution [110].
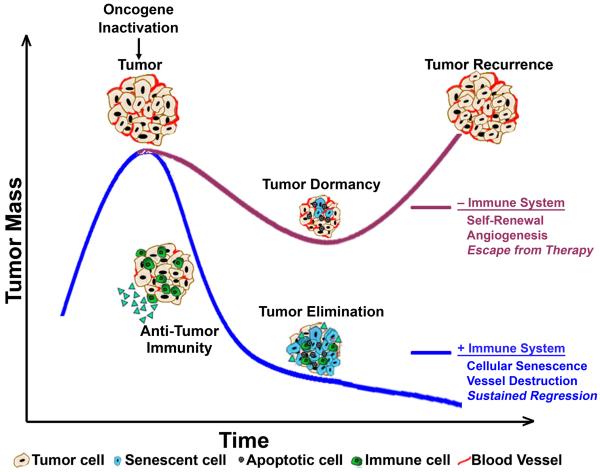
Figure 3. Tumor dormancy versus tumor elimination is regulated by an intact host immune system.
Oncogene inactivation results in tumor regression regardless of host immune status due to proliferative arrest, apoptosis, and/or differentiation. In an intact host, the immune system is subsequently activated to facilitate elimination of the residual tumor cells via induction of cellular senescence and destruction of the tumor vasculature. However, in the absence of an immune system, tumors establish a state of dormancy, due to lack of inhibition of self-renewal and angiogenesis, followed by eventual escape from therapy and tumor recurrence.
CD4+ T-cells contribute to sustained tumor regression at least by two mechanisms: enforcing both the induction of cellular senescence and the suppression of angiogenesis (Figure 3, Table 1) [76]. Of import, both of these processes previously have been characterized as hallmarks of oncogene addiction (Figure 2, 3). CD4+ T-cells may mediate their influence on the tumor and tumor microenvironment directly or indirectly through the expression of many cytokines [111-114].
Thrombospondin-1 (TSP-1) was found to be a critical mediator of CD4+ T-cell-mediated sustained tumor regression upon MYC inactivation (Figure 3). TSP-1 may play a role in contributing to remodeling of the tumor microenvironment upon oncogene inactivation [76, 115]. TSP-1 is a potent anti-angiogenic and immune-modulatory cytokine that can induce apoptosis of endothelial cells and regulate T-cell chemotaxis [116]. TSP-1 also may mediate its effects through the regulation of TGF-β [117]. TGF-β can play a tumor-suppressive role in the tumor microenvironment [118, 119]. In particular, TGF-β can contribute to both the restraint of tumor onset as well as oncogene addiction through the regulation of cellular senescence upon MYC activation and inactivation [72, 73].
Additional cytokines and effectors that may be involved inCD4+ T-cell-mediated oncogene addiction. Cytokines that appear to play a role include: eotaxin-1, IL-5, IFN-γ and TNF-α, as well as the down-regulation of “pro-tumor” cytokines such as VEGF, IL-1β, and MCP-1 upon MYC inactivation [76]. Whether any of these cytokines contribute more generally to the phenomenon of oncogene addiction remains to be seen.
CD4+ T-cells coordinate multiple components of both the innate and adaptive immune system [120], suggesting the contribution of other immune effectors is likely. Indeed, in oncogene-induced hepatocellular carcinoma, pancreatic tumor, and B-cell lymphoma, innate immune cell types such as mast cells [107] and macrophages [73] have been implicated as barriers to tumor growth and facilitators of tumor regression.
Notably, the restoration of the p53 tumor suppressor has been shown previously to induce tumor senescence, elicit chemokine expression, and induce the activation and recruitment of innate immune cells that contribute to tumor clearance [77]. Thus, the restoration of normal cellular function of a single tumor suppressor or oncogene can elicit oncogene addiction through changes in the tumor microenvironment dependent upon various host immune effectors.
Both cellular and cytokine-associated immune mechanisms are essential components of oncogene addiction. They define the kinetics, extent, and durability of tumor elimination (Figure 3). In the absence of an immune system, upon oncogene inactivation tumor cells persist, in a dormant state, whereas in the presence of a fully intact immune system there is complete elimination of tumor cells.
Therapeutic Implications: Tumor Dormancy versus Elimination
For maximal clinical efficacy, ideally a therapeutic for cancer would either completely eliminate a tumor or induce a permanent state of dormancy. Since both tumor cell-intrinsic and host-dependent programs appear to be required to elicit oncogene addiction, it would seem that in designing a therapeutic that is most efficacious it would be critical to consider both the tumor and the host. Therapies that target programs in cancer cells but suppress the immune system, or those that stimulate the immune system but have no effect on the biology of a tumor cell, may not be as effective as therapies that modulate both processes in concert. In particular, therapies that target the tumor but suppress the immune system could blunt their overall efficacy. Many existing anti-cancer therapies cause immunosuppression and lymphodepletion that may undermine their efficacy [10].
To best identify anti-cancer therapies, it would be critical to perform pre-clinical evaluation in host model systems that have an intact immune system and recapitulate a tumor microenvironment. In vitro or animal models in which a host is immuno-compromised would not correctly identify the best therapeutic agents precisely because the kinetics of tumor cell elimination, the degree of tumor elimination, the ablation of minimal residual disease (MRD), and the duration of a clinical response could all be dictated by mechanisms related to the host.
The ability to identify whether a therapy will induce dormancy versus elimination would be critical to evaluating potential therapeutics. The regulation of self-renewal versus cellular senescence appears to be the key determinant of the fate of a tumor. The ability to interrogate self-renewal may be intrinsic to evaluating and predicting therapeutic activity. The direct targeting of self-renewal/cellular senescence programs through the inactivation of particular oncogenes or other gene products may therefore be a particularly effective strategy for treating cancer. This critical decision in cell fate appears to be tightly coupled to interactions between tumor cells, host cells, and cytokines and appear to define whether a tumor expands, regresses, or becomes dormant (Figure 3) [39]. Hence, therapeutics that target self-renewal and/or activate cellular senescence could be very effective, including, for example, the induction of p53 or the modulation of genes that regulate the cell cycle machinery [41]. Therapeutic strategies that modulate the tumor microenvironment also may be useful adjuncts, including drugs that target angiogenesis [121]. A combination of approaches is likely to be most effective for tumor elimination.
Finally, the appreciation that immune mechanisms can dictate the balance between self-renewal and senescence suggests that the therapeutic manipulation of host immune system and secreted cytokines may be an important treatment strategy. Specific host immune effectors and chemokines profoundly influence the consequences of therapeutic oncogene inactivation, radiation therapy, and chemotherapy [11]. The integration of targeted- and immune-therapy may be the most efficacious strategy in treating cancer [122].
Modeling and Predicting Tumor Dormancy versus Elimination
Through mechanistic understanding of oncogene addiction it should be possible to predict therapeutic efficacy. Oncogene addiction involves both tumor cell-intrinsic and host-dependent programs that regulate self-renewal and cellular senescence. Thus, it should be possible to generate models that predict oncogene addiction, incorporating these cellular outcomes [30, 123]. One such possible approach would be to presume that cancer cells behave stochastically and can exist in three states: proliferating, apoptotic, or quiescent/dormant. Then, the acquisition of even very simple measurements of proliferation and apoptosis combined with assessments of tumor size could be used to mathematically predict oncogene addiction [123].
Such modeling has revealed some possible insights into the mechanism of oncogene addiction and tumor regression following oncogene inactivation [123]. A simple differential decay between pro-survival and pro-death signals is sufficient to explain the majority of what occurs upon oncogene inactivation. A decay of both pro-survival and pro-death signals follows targeted oncogene inactivation. Although, the final level of the pro-death signal is comparable to the pro-survival signal, it is precisely because the death signals induced by the oncogene are extinguished more slowly after oncogene inactivation than the survival signals that tumors regress. These results support the oncogenic shock hypothesis, first suggested by both Settleman and Kaelin [30, 31].
Mathematical modeling of the response to targeted therapy indicates that simple measurements of tumors before and after initiation of a therapeutic may be useful to predict therapeutic outcome [124]. A variety of different computational approaches could be used and this could potentially be very useful in enabling the more rapid identification of therapeutics as well as the more rapid discontinuation of therapies that are not effective (Figure 4). This approach would exploit existing as well as emerging imaging techniques to rapidly and reliably assess tumor cell proliferation and apoptosis ex vivo [125-129].
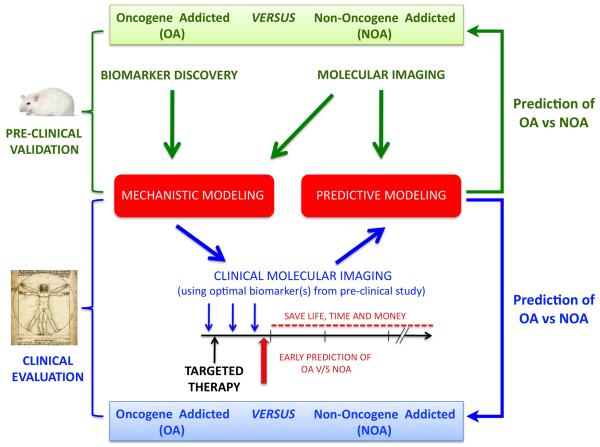
Figure 4. Modeling and predicting when oncogene inactivation will result in tumor dormancy versus tumor elimination.
A combinatorial, iterative approach can be utilized to model the consequences of oncogene addiction, thereby allowing for prediction of response to targeted therapy in patients. Continuing progress in molecular imaging and biomarker discovery will be crucial for the validation of mathematical models of oncogene addiction in primary animal models of cancer. In parallel, the advent of new molecular imaging tools in the clinic will allow for incorporation of discoveries in these pre-clinical models into assessment of human cancer patient response to therapy.
Even simples models may be able to predict oncogene addiction with measurement of proliferation and apoptosis alone [123]. However, the inclusion of additional parameters such as immune cell infiltration, onset of cellular senescence, loss of self-renewal, and suppression of angiogenesis would likely improve the modeling. New molecular imaging approaches as well as proteomic technologies may enable the measurement of such parameters (Figure 4). Then, the application of both mechanistic and predicting modeling may further enable the goal of predicting when targeted inactivation of a gene product or combination of products would elicit tumor elimination or tumor dormancy.
Acknowledgements
The authors would like to acknowledge current members of the Felsher laboratory for critical discussion and previous members who have contributed to characterizing various models of oncogene addiction.
References
- 1.Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297(5578):63–4. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 2.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4(2):199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 3.Jain M, et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297(5578):102–4. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- 4.Shachaf CM, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431(7012):1112–7. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 5.Wu CH, et al. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc Natl Acad Sci U S A. 2007;104(32):13028–33. doi: 10.1073/pnas.0701953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7(5):411–23. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Dougan M, et al. A dual role for the immune response in a mouse model of inflammation-associated lung cancer. J Clin Invest. 2011;121(6):2436–46. doi: 10.1172/JCI44796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–11. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 9.Ruffell B, et al. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21(1):3–10. doi: 10.1016/j.cytogfr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8(3):151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 11.Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 12.Dave SS, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351(21):2159–69. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 13.Felsher DW. Cancer revoked: oncogenes as therapeutic targets. Nat Rev Cancer. 2003;3(5):375–80. doi: 10.1038/nrc1070. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68(9):3077–80. doi: 10.1158/0008-5472.CAN-07-3293. discussion 3080. [DOI] [PubMed] [Google Scholar]
- 15.Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007;21(24):3214–31. doi: 10.1101/gad.1609907. [DOI] [PubMed] [Google Scholar]
- 16.Huettner CS, et al. Reversibility of acute B-cell leukaemia induced by BCR-ABL1. Nat Genet. 2000;24(1):57–60. doi: 10.1038/71691. [DOI] [PubMed] [Google Scholar]
- 17.Chin L, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400(6743):468–72. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- 18.Hoeflich KP, et al. Oncogenic BRAF is required for tumor growth and maintenance in melanoma models. Cancer Res. 2006;66(2):999–1006. doi: 10.1158/0008-5472.CAN-05-2720. [DOI] [PubMed] [Google Scholar]
- 19.Boxer RB, et al. Lack of sustained regression of c-MYC-induced mammary adenocarcinomas following brief or prolonged MYC inactivation. Cancer Cell. 2004;6(6):577–86. doi: 10.1016/j.ccr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Giuriato S, et al. Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch. Proc Natl Acad Sci U S A. 2006;103(44):16266–71. doi: 10.1073/pnas.0608017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shchors K, et al. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1beta. Genes Dev. 2006;20(18):2527–38. doi: 10.1101/gad.1455706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran PT, et al. Combined Inactivation of MYC and K-Ras oncogenes reverses tumorigenesis in lung adenocarcinomas and lymphomas. PLoS One. 2008;3(5):e2125. doi: 10.1371/journal.pone.0002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hait WN, Hambley TW. Targeted cancer therapeutics. Cancer Res. 2009;69(4):1263–7. doi: 10.1158/0008-5472.CAN-08-3836. discussion 1267. [DOI] [PubMed] [Google Scholar]
- 24.Sawyers C. Targeted cancer therapy. Nature. 2004;432(7015):294–7. doi: 10.1038/nature03095. [DOI] [PubMed] [Google Scholar]
- 25.Druker BJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 26.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 27.Chapman PB, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cataldo VD, et al. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med. 2011;364(10):947–55. doi: 10.1056/NEJMct0807960. [DOI] [PubMed] [Google Scholar]
- 29.Felsher DW. Reversibility of oncogene-induced cancer. Curr Opin Genet Dev. 2004;14(1):37–42. doi: 10.1016/j.gde.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Sharma SV, Settleman J. Oncogenic shock: turning an activated kinase against the tumor cell. Cell Cycle. 2006;5(24):2878–80. doi: 10.4161/cc.5.24.3598. [DOI] [PubMed] [Google Scholar]
- 31.Kaelin WG., Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5(9):689–98. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 32.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7(2):139–47. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 33.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Coussens LM, et al. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–90. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1(1):46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi PS, et al. Lymphomas that recur after MYC suppression continue to exhibit oncogene addiction. Proc Natl Acad Sci U S A. 2011;108(42):17432–7. doi: 10.1073/pnas.1107303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorre ME, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293(5531):876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 38.Pao W, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS medicine. 2005;2(1):e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7(11):834–46. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felsher DW. MYC Inactivation Elicits Oncogene Addiction through Both Tumor Cell-Intrinsic and Host-Dependent Mechanisms. Genes & cancer. 2010;1(6):597–604. doi: 10.1177/1947601910377798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nardella C, et al. Pro-senescence therapy for cancer treatment. Nat Rev Cancer. 2011;11(7):503–11. doi: 10.1038/nrc3057. [DOI] [PubMed] [Google Scholar]
- 42.Felsher DW. Reversing cancer from inside and out: oncogene addiction, cellular senescence, and the angiogenic switch. Lymphat Res Biol. 2008;6(3-4):149–54. doi: 10.1089/lrb.2008.63403. [DOI] [PubMed] [Google Scholar]
- 43.Felsher DW. Tumor dormancy and oncogene addiction. APMIS. 2008;116(7-8):629–37. doi: 10.1111/j.1600-0463.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 44.Bishop JM. Molecular themes in oncogenesis. Cell. 1991;64(2):235–48. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- 45.Reya T, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 46.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112(13):4793–807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 47.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends in cell biology. 2005;15(9):494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Sell S. Stem cell origin of cancer and differentiation therapy. Critical reviews in oncology/hematology. 2004;51(1):1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen LV, et al. Cancer stem cells: an evolving concept. Nature reviews. Cancer. 2012;12(2):133–43. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 50.Das B, et al. The Idea and Evidence for the Tumor Stemness Switch. In: Rajasekhar V, Vemuri M, editors. Regulatory Networks in Stem Cells. Humana Press; New York: 2009. pp. 473–487. [Google Scholar]
- 51.Zheng H, et al. Pten and p53 converge on c-Myc to control differentiation, self-renewal, and transformation of normal and neoplastic stem cells in glioblastoma. Cold Spring Harbor symposia on quantitative biology. 2008;73:427–37. doi: 10.1101/sqb.2008.73.047. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, et al. c-Myc is required for maintenance of glioma cancer stem cells. PLoS One. 2008;3(11):e3769. doi: 10.1371/journal.pone.0003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marquardt JU, et al. Human hepatic cancer stem cells are characterized by common stemness traits and diverse oncogenic pathways. Hepatology. 2011;54(3):1031–42. doi: 10.1002/hep.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Experimental cell research. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 55.Hayflick L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Experimental cell research. 1965;37:614–36. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 56.Lundblad V, Szostak JW. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57(4):633–43. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- 57.Yu GL, et al. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990;344(6262):126–32. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- 58.Chen Q, Ames BN. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(10):4130–4. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Leonardo A, et al. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes & development. 1994;8(21):2540–51. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 60.Schmitt CA, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109(3):335–46. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 61.Chang BD, et al. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer research. 1999;59(15):3761–7. [PubMed] [Google Scholar]
- 62.Michishita E, et al. 5-Bromodeoxyuridine induces senescence-like phenomena in mammalian cells regardless of cell type or species. Journal of biochemistry. 1999;126(6):1052–9. doi: 10.1093/oxfordjournals.jbchem.a022549. [DOI] [PubMed] [Google Scholar]
- 63.O’Brien W, Stenman G, Sager R. Suppression of tumor growth by senescence in virally transformed human fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(22):8659–63. doi: 10.1073/pnas.83.22.8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serrano M, et al. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 65.Zhu J, et al. Senescence of human fibroblasts induced by oncogenic Raf. Genes & development. 1998;12(19):2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Braig M, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436(7051):660–5. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 67.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(20):9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Narita M, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113(6):703–16. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 69.Collado M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436(7051):642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 70.Chan HM, et al. The p400 E1A-associated protein is a novel component of the p53 --> p21 senescence pathway. Genes & development. 2005;19(2):196–201. doi: 10.1101/gad.1280205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Z, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436(7051):725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Riggelen J, et al. The interaction between Myc and Miz1 is required to antagonize TGFbeta-dependent autocrine signaling during lymphoma formation and maintenance. Genes Dev. 2010;24(12):1281–94. doi: 10.1101/gad.585710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reimann M, et al. Tumor Stroma-Derived TGF-beta Limits Myc-Driven Lymphomagenesis via Suv39h1-Dependent Senescence. Cancer Cell. 2010;17(3):262–272. doi: 10.1016/j.ccr.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 74.Zhuang D, et al. C-MYC overexpression is required for continuous suppression of oncogene-induced senescence in melanoma cells. Oncogene. 2008;27(52):6623–34. doi: 10.1038/onc.2008.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin AW, et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes & development. 1998;12(19):3008–19. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rakhra K, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18(5):485–98. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445(7128):656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 79.Dunn GP, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 80.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 81.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 82.Andreu P, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17(2):121–34. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Girardi M, et al. Characterizing the protective component of the alphabeta T cell response to transplantable squamous cell carcinoma. J Invest Dermatol. 2004;122(3):699–706. doi: 10.1111/j.0022-202X.2004.22342.x. [DOI] [PubMed] [Google Scholar]
- 84.Lin EY, et al. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193(6):727–40. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hung K, et al. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188(12):2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martin-Manso G, et al. Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells. Cancer Res. 2008;68(17):7090–9. doi: 10.1158/0008-5472.CAN-08-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou P, et al. Mature B cells are critical to T-cell-mediated tumor immunity induced by an agonist anti-GITR monoclonal antibody. J Immunother. 2010;33(8):789–97. doi: 10.1097/CJI.0b013e3181ee6ba9. [DOI] [PubMed] [Google Scholar]
- 88.Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–56. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 89.DeNardo DG, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 93.Ekbom A, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323(18):1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 94.Wakabayashi O, et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94(11):1003–9. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang JP, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50(5):980–9. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 96.Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 97.Zhang H, et al. Concordant down-regulation of proto-oncogene PML and major histocompatibility antigen HLA class I expression in high-grade prostate cancer. Cancer Immun. 2003;3:2. [PubMed] [Google Scholar]
- 98.Zheng P, et al. Proto-oncogene PML controls genes devoted to MHC class I antigen presentation. Nature. 1998;396(6709):373–6. doi: 10.1038/24628. [DOI] [PubMed] [Google Scholar]
- 99.Sumimoto H, et al. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203(7):1651–6. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boni A, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70(13):5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 101.Borrello MG, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci U S A. 2005;102(41):14825–30. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21(14):1714–9. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6(5):447–58. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 104.Sodir NM, et al. Endogenous Myc maintains the tumor microenvironment. Genes & development. 2011;25(9):907–16. doi: 10.1101/gad.2038411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boshoff C, Weiss R. AIDS-related malignancies. Nat Rev Cancer. 2002;2(5):373–82. doi: 10.1038/nrc797. [DOI] [PubMed] [Google Scholar]
- 106.Ray-Coquard I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–91. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Soucek L, et al. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13(10):1211–8. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 108.Shiao SL, Coussens LM. The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia. 2010;15(4):411–21. doi: 10.1007/s10911-010-9194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 110.Restifo NP. Can antitumor immunity help to explain “oncogene addiction”? Cancer Cell. 2010;18(5):403–5. doi: 10.1016/j.ccr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Acosta JC, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133(6):1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 112.Beatty G, Paterson Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J Immunol. 2001;166(4):2276–82. doi: 10.4049/jimmunol.166.4.2276. [DOI] [PubMed] [Google Scholar]
- 113.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 114.Muller-Hermelink N, et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13(6):507–18. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Li SS, et al. T lymphocyte expression of thrombospondin-1 and adhesion to extracellular matrix components. Eur J Immunol. 2002;32(4):1069–79. doi: 10.1002/1521-4141(200204)32:4<1069::AID-IMMU1069>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 116.Li SS, et al. Endogenous thrombospondin-1 is a cell-surface ligand for regulation of integrin-dependent T-lymphocyte adhesion. Blood. 2006;108(9):3112–20. doi: 10.1182/blood-2006-04-016832. [DOI] [PubMed] [Google Scholar]
- 117.Young GD, Murphy-Ullrich JE. The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-beta complex. J Biol Chem. 2004;279(46):47633–42. doi: 10.1074/jbc.M404918200. [DOI] [PubMed] [Google Scholar]
- 118.Becker C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21(4):491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 119.Tang B, et al. Transforming growth factor-beta can suppress tumorigenesis through effects on the putative cancer stem or early progenitor cell and committed progeny in a breast cancer xenograft model. Cancer Res. 2007;67(18):8643–52. doi: 10.1158/0008-5472.CAN-07-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr Opin Immunol. 2009;21(2):200–8. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gonzalez-Angulo AM, Hortobagyi GN, Ellis LM. Targeted therapies: peaking beneath the surface of recent bevacizumab trials. Nat Rev Clin Oncol. 2011;8(6):319–20. doi: 10.1038/nrclinonc.2011.66. [DOI] [PubMed] [Google Scholar]
- 122.Wrzesinski C, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 2010;33(1):1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tran PT, et al. Survival and death signals can predict tumor response to therapy after oncogene inactivation. Science translational medicine. 2011;3(103):103ra99. doi: 10.1126/scitranslmed.3002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Califano A. Striking a balance between feasible and realistic biological models. Science translational medicine. 2011;3(103):103ps39. doi: 10.1126/scitranslmed.3003079. [DOI] [PubMed] [Google Scholar]
- 125.Willmann JK, et al. Molecular imaging in drug development. Nature reviews. Drug discovery. 2008;7(7):591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 126.Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clinical radiology. 2010;65(7):500–16. doi: 10.1016/j.crad.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes & development. 2003;17(5):545–80. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 128.Nguyen QD, Aboagye EO. Imaging the life and death of tumors in living subjects: Preclinical PET imaging of proliferation and apoptosis. Integrative biology: quantitative biosciences from nano to macro. 2010;2(10):483–95. doi: 10.1039/c0ib00066c. [DOI] [PubMed] [Google Scholar]
- 129.Michalski MH, Chen X. Molecular imaging in cancer treatment. European journal of nuclear medicine and molecular imaging. 2011;38(2):358–77. doi: 10.1007/s00259-010-1569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boni A, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 70(13):5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]