Abstract
Expanded populations of CD8+ T lymphocytes lacking CD28 expression are associated with a variety of deleterious clinical outcomes, including early mortality in the elderly, more rapid progression to AIDS, cardiovascular disease, and enhanced tumor cell growth. In cell culture, irreversible loss of CD28 expression correlates with increased production of TNF-α as CD8+ T cells are driven to the nonproliferative end stage of replicative senescence by multiple rounds of Ag-driven cell division. Interestingly, in patients with rheumatoid arthritis, inhibition or neutralization of TNF-α reduces the proportion of T cells lacking CD28 in the disease joints, consistent with studies showing a direct involvement of this cytokine in CD28 gene transcription. Here, we show that modulation of TNF-α levels in long-term cultures of human CD8+ T lymphocytes, by chronic exposure either to a neutralizing Ab or to an inhibitor of the TNF-α receptor-1, increases proliferative potential, delays loss of CD28 expression, retards cytokine profile changes, and enhances telomerase activity. We also show that constitutive caspase-3, one of the downstream effectors of TNF-αR1 binding, increases in parallel with the loss of CD28 in long-term cultures, but this effect is blunted in the presence of the TNF-α inhibitors. Consistent with the in vitro culture data, CD8+CD28− T lymphocytes tested immediately ex vivo also show significantly higher levels of caspase-3 compared with their CD28+ counterparts. These findings help elucidate the complex nature of CD28 gene regulation, and may ultimately lead to novel therapeutic approaches for diseases associated with increased proportions of CD28− T lymphocytes.
The CD28 costimulatory receptor, a membrane glycoprotein, is required for optimal activation of T lymphocytes during an immune response (1). Interaction with the CD80 ligand on APCs transduces downstream survival and proliferation signals, including induction of IL-2 and its receptor, telomerase activation, and stabilization of several cytokine mRNAs (2– 4). At birth, nearly 100% of human T lymphocytes express CD28, but there are multiple clinical situations in which there are increased numbers of T cells lacking CD28 expression. The proportion of CD28+ cells within the CD8 T cell subset decreases with age, and is associated with the in vivo accumulation of CD28− cells in both the CD8+ and CD4+ subsets (5, 6). Furthermore, the accumulation of CD8+CD28− T cells correlates with decreased responses to vaccines in the elderly, reduction in the overall TCR repertoire, and diminished control over infections (7–9). Even in younger persons, similar accumulations of CD28− T cells have been documented in the context of persistent infections, such as HIV/AIDS (10 –13), and autoimmune/inflammatory syndromes, such as rheumatoid arthritis (RA3; Ref. 14) and anklylosing spondylitis (15). Interestingly, a similar permanent, irreversible loss of CD28 expression due to chronic immune activation of human T lymphocytes in long term culture is one of the signature changes of replicative senescence (16).
Several lines of evidence have suggested a link between loss of CD28 expression and the proinflammatory cytokine TNF-α. Clinical observations of patients with RA indicate that the higher levels of circulating TNF-α may contribute to the down-regulation of CD28 expression, because anti-TNF-α therapy is associated with the restoration of the CD28+ T cell populations within the diseased joints (17, 18). Longitudinal analysis of CD8+ T cells that reach replicative senescence in response to multiple rounds of Ag-driven proliferation in cell culture shows that the loss of CD28 expression parallels the progressive increase in TNF-α secretion (19). Interestingly, in transformed human CD4+ T cell lines, CD28 gene silencing was linked to TNF-α-mediated inhibition of the αβ-transcription initiator via disruption of α/β-complex formation (20, 21). CD28 transcription regulation is also associated with the caspase pathway, which is downstream of the TNF-α receptor-1 (22–24). Studies on the Jurkat CD4+ T cell tumor line (23, 25) showed that elevated caspase-3, but not to the threshold level required for apoptosis, resulted in down-regulation of the CD28 promoter, and ultimately the loss of CD28 cell surface expression. However, the possible underlying mechanisms responsible for the down-regulation of CD28 in untransformed, normal CD8+ T lymphocytes are not well understood.
The goal of the current study, therefore, was to analyze the mechanisms responsible for regulating CD28 expression in primary human CD8+ T lymphocytes subjected to chronic activation. We show that inhibition of TNF-α (either with neutralizing Ab, or a receptor inhibitor) delays both the loss of CD28 expression, as well as multiple functional changes associated with human CD8+ T cell replicative senescence. Moreover, we observed that caspase-3 is a likely pathway through which TNF-α mediates the CD28 down-regulation. These findings may lead to novel therapeutic approaches aimed at preserving CD28 expression in clinical situations associated with chronic immune activation.
Materials and Methods
Cell cultures
Human peripheral blood samples were acquired after informed consent and in accordance with the University of California Los Angeles Institutional Review Board. After centrifugation, the PBMC layer was carefully removed and washed twice in complete RPMI 1640 (10% FBS, 10 mM HEPES, 2 mM glutamine, 50 IU/ml penicillin/streptomycin) The PBMC were then used to initiate long-term T cell cultures, as described previously (26). Briefly, PBMC were combined (1:1) with irradiated (8000 Rads) allogeneic EBV-transformed B lymphoblastoid cells, which serve as APCs in complete RPMI 1640 supplemented with rIL-2 (20 U/ml). Cultures were restimulated with the APCs every 21–28 days. Before the second stimulation, CD8+ T cells were isolated by CD3 negative selection using the PanT cell isolation kit (Mitenyi Biotec), followed by removal of the CD4+ T cells using CD4 Microbeads (Miltenyi Biotec). Purity of the CD8+ T cells was verified by flow cytometry. Every 3– 4 days, viable cell concentration was determined by trypan blue exclusion, and when the concentration reached ≥8 × 105/ml, cells were subcultivated to a density of 5 × 105 cells/ml. Population doublings (PDs) were determined according to the formula PDs = log2 (final cell concentration/initial cell concentration).
TNF-α inhibition
Cultures, established as described above, were exposed to a neutralizing Ab for TNF-α (R&D Systems), or an inhibitor of the TNF-α receptor-1 (R1 inhibitor, Peg sTNF-R1, Amgen) every 3– 4 days. Control cultures were not exposed to either agent. Based on initial titration experiments (data not shown), a concentration of 40 ng/ml Ab and 10 μg/ml of the R1 inhibitor was found to be optimal in terms of cell viability and maximum CD28 expression.
Flow cytometry
Surface expression of CD4, CD28, CD8, and CD3 was examined by immunostaining and flow cytometry. Cells were isolated once the cultures reached quiescence (21–30 days after previous stimulation) and were immediately stained and analyzed on the same day. Cells were incubated with FITC-conjugated anti-CD4, PE-conjugated anti-CD28, PerCp-conjugated anti-CD8, and APC-conjugated anti-CD3 (BD Biosciences) mAbs at 4°C for 20 min, washed, and fixed in PBS containing 1% paraformaldehyde. Parallel samples were incubated with Ig isotype controls (BD Biosciences). All samples were analyzed on a flow cytometer (BD Biosciences). Fluorescence data from at least 25,000 cells were collected. Analysis of data was performed using Cell Quest Pro (BD Biosciences).
Telomerase activity measurements
Telomerase activity was determined by the telomeric repeat amplification protocol (TRAP), using the reagents, protocol, and calculation details provided in the TRAPeze kit (Millipore, cat. no. S7710), as in our previous studies (4, 27). The amplified TRAP reaction products were separated on an 8% polyacrylamide gel, and the resulting bands were probed and analyzed using Packard InstantImager software (Packard Instrument). Telomerase activity for all samples was calculated for 10,000 cell-equivalents, according to the TRAPeze kit formula for “Total Product Generated.”
IL-6 production
Culture supernatants were harvested 96 h poststimulation and analyzed for IL-6 in an ELISA Ready-Set-Go (eBioscience). All measurements were performed in triplicate wells and in accordance to manufacturer’s recommendation.
Real-time quantitative PCR
RNA was isolated using the Qiagen RNAeasy kit (Qiagen), and quantified using the QuantiT Ribogreen RNA Assay Kit (Molecular Probes). cDNAs were synthesized with the iScript cDNA sythesis kit (Bio-Rad) using 500 ng RNA. Quantitative real-time PCR assays were performed using the iQ SYBER Green SuperMix and IQCycler (Bio-Rad). GAPDH was used as an internal control. The sequences were designed with the aid of Beacon Designer software and synthesized at Integrated DNA Technologies. CD28, 5′-TCCTTACCTAGACAATGAGAAGAGCAATC, 3′-CCACCAACC ACCACCAGCAC; IL-2, 5′-TCACCAGGATGCTCACATTTAAGTTT TAC, 3′-TTCCTCCAGAGGTTTGAGTTCTTCTTC; GAPDH, 5′-GGT CATGAGTCCTTCCACGATACCA, 3′-CCTCAAGATCATCAGCAAT GCCTCCT. Samples were run in triplicate in a 96-well plate at the following settings: 95°C for 15 s, 61°C for 30 s, and 72°C for 30 s using single fluorescence measurement.
Separation of cell subpopulations
Purified CD3+ T cells were obtained by negative selection using the PanT cell isolation kit (Mitenyi Biotec) followed by a CD8+ T cell negative selection (using CD4 Microbeads, Miltenyi Biotec). For some experiments, CD8+ T cells were further divided into either CD28− or CD28− subsets, using the CD28 Microbead Kit (Miltenyi Biotec). Purity of the various cell populations was verified by flow cytometry.
Caspase-3 assay
The Apo-Alert assay kit (Calbiochem) was used to detect caspase-3 activity. Briefly, 2 × 106 cells were removed immediately before each restimulation, centrifuged, and the pellet was lysed using the lysis buffer provided in the kit. Each lysate was incubated with 2× reaction buffer containing 10 mM DTT and incubated for 1 h at 37°C. Caspase-3 protease activity was detected by spectrophotometry (405 nm).
Statistical analysis
Mean values and SD were calculated for each time point. Significance was established by using a two-tail Student’s t test, and a p value < 0.05 is considered significant.
Results
Inhibition of TNF-α increases the proliferative potential of CD8+ T lymphocytes
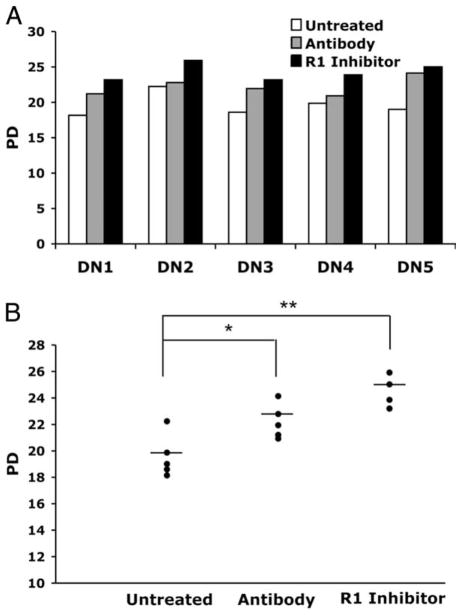
Human CD8+ T lymphocytes that are subjected to repeated rounds of Ag-driven proliferation in cell culture reach the end stage of replicative senescence, which is associated with irreversible cell cycle arrest and increased production of TNF-α (28). Moreover, the proportion of CD28+ cells correlates with the remaining proliferative potential of the culture (29), consistent with proliferative differences between CD28+ and CD28− T cells tested immediately ex vivo (30). Based on studies showing that TNF-α inhibits the activity of the CD28 minimal promoter in CD4+ T lymphocytes (6), we hypothesized that inhibition of TNF-α might modulate the process of CD8+ T cell replicative senescence. In preliminary experiments (data not shown) we observed that the increase of CD28 expression occurred in a dose-dependant manner in cultures exposed to varying concentrations of both Ab (5 ng/ml – 100 ng/ml) and the R1 inhibitor (0.1 μg/ml – 20 μg/ml) as compared with control (untreated) cultures. We selected the 40 ng/ml and 10 μg/ml concentrations of Ab and R1 inhibitor, respectively, for further analysis, based on the maximal effects on CD28 cell surface expression and cell viability. We compared the long-term proliferative dynamics of CD8+ T lymphocyte cultures established from five individual healthy donors in the presence or absence of Ab or the R1 Inhibitor. For all donors tested, inhibition of TNF-α signaling resulted in a significant increase in the total number of PDs the CD8+ T cells achieved before reaching replicative senescence (Fig. 1A). Overall, the effect of the R1 inhibitor on the proliferative potential was slightly more dramatic than that of the neutralizing Ab. Whereas the control (untreated) cultures underwent a mean total PDs of 19.6, the R1 inhibitor- and Ab-treated cultures reached 24.2 (p < 0.001), and 22.2 (p < 0.05), respectively (Fig. 1B).
FIGURE 1.
Inhibition of TNF-α increases the proliferative potential of CD8 T lymphocytes. Long-term CD8+ T lymphocyte cultures from PBMC of healthy donors (n = 5) were established. At each subcultivation, Ab to TNF-α (40 ng/ml), TNF-α R1-inhibitor (10 μg/ml), or medium was added, and PD followed until replicative senescence was reached. A, Comparison of total PD in treated and untreated cultures for each donor. B, Mean number of total PD for untreated and treated cultures. DN, Donor.
Loss of CD28 expression is delayed by inhibition of TNF-α
Treatment of RA patients with medications that inhibit TNF-α is associated with a reduction in the proportion of CD4+ T cells lacking CD28 expression in the affected joints (17). Based on these in vivo observations, we sought to investigate the dynamics of inhibiting TNF-α on the CD28 expression in the CD8+ T cell subset in long-term cultures that are subjected to repeated rounds of antigenic stimulation. Flow cytometry results for a representative culture (Fig. 2A) illustrate that chronic exposure to either the Ab or to the receptor inhibitor resulted in increased proportions of cells expressing CD28 at both early (6 PDs) and late (18 PDs) time points in the long-term culture. Indeed, at every time point tested, inhibition of TNF-α was associated with an increased proportion of CD28+ T cells (data not shown). Analysis of CD28 expression in cultures from five individual donors show a highly significant effect (p < 0.01) for the receptor inhibitor at both early and late culture time-points, and a significant effect of the Ab (p < 0.05) at the late time-point (Fig. 2B). No additive effect on CD28 expression was observed in cultures that were treated with the combination of Ab and receptor inhibitor (data not shown). Because TNF-α has been shown to function as a growth factor (31), it was important to confirm that the increased proportion of CD28+ cells in cultures that lacked TNF-α, due to presence of the Ab or the receptor inhibitor (Fig. 2), was not due to possible differential growth effects of TNF-α on CD28+ and CD28− T cells. To formally exclude this possibility, we analyzed the proliferative activity of purified populations of CD8+CD28−, CD8+CD28+, and total CD8+ T cells over a 14 day culture period (Fig. 2C). Our results show that exposure to TNF-α did not result in increased proliferation of CD28− T cells. In fact, the CD8+CD28− subset underwent fewer PDs than the CD28+ subset, irrespective of whether or not TNF-α was present in the culture. Because TNF-α has been shown to down-regulate both CD28 protein and mRNA levels (18, 32), we compared CD28 mRNA by quantitative real-time PCR in the various cultures. We found that the increased surface expression of CD28 was associated with increased levels of CD28 mRNA at both 6 and 18 PD (Fig. 2D).
FIGURE 2.
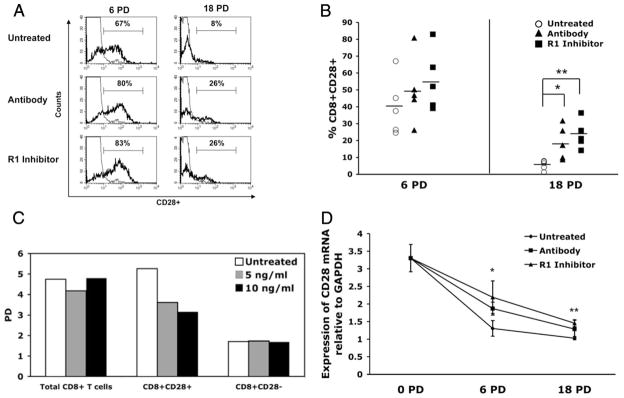
Loss of CD28 expression is delayed by inhibition of TNF-α. Cultures were established as in Fig. 1, and CD28 expression determined by flow cytometry once the cultures had reached quiescence following each stimulation (~21–30 days poststimulation). A, Flow histogram plot for a representative donor, showing CD28 expression at 6 and 18 PD. B, Mean (±SD) percentage of the CD8+ T cells that expressed CD28 at early (6 PD) and late (18 PD) time points. C, Comparison of CD8+CD28+, CD8−CD28−, and total CD8+ T cells stimulated with PHA (6 μg/ml) of a representative donor in the absence or presence of TNF-α (5 ng/ml and 10 ng/ml). PDs were calculated as described in the Materials and Methods section. D, CD28 transcriptional expression (n = 5) normalized against GAPDH. *, p < 0.05; **, p < 0.01.
TNF-α inhibition delays the onset of the senescent phenotype
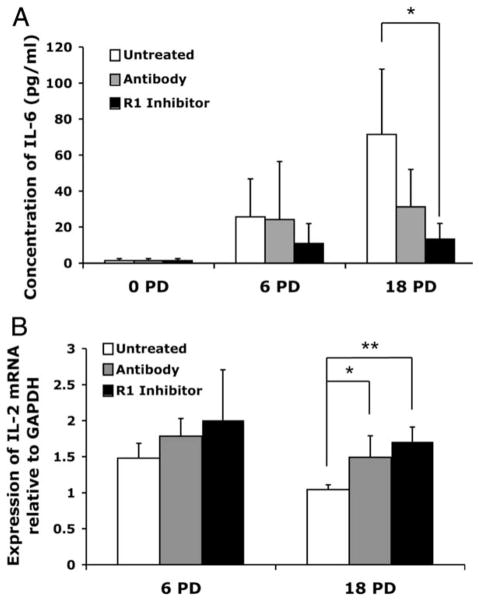
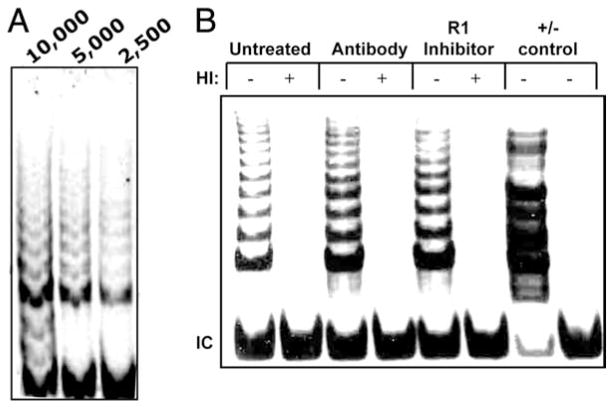
CD8+ T lymphocyte replicative senescence is associated with a variety of functional characteristics in addition to the proliferative arrest. These include altered cytokine secretion patterns (33–35), and inability to up-regulate telomerase activity (4). ELISA evaluation of the culture supernatants showed that the IL-6 production increased with progressive PDs in the control cultures, confirming previous studies (36), but this effect was blunted in cultures in which TNF-α activity/binding was inhibited (Fig. 3A). The effects of TNF-α inhibition on IL-2 production were determined by analyzing mRNA rather than protein in the supernatants, because the culture medium already contained high levels of exogenous IL-2. A mild increase in IL-2 mRNA was already evident in the early culture period, but at 18 PDs, the effect was more dramatic (Fig. 3B). There was a significant increase in IL-2 mRNA in the cultures that were treated with anti-TNF-α Ab (p < 0.01) or the TNF-α R1 inhibitor (p < 0.0001). This increased message is consistent with the significantly greater proportion of CD28+ T cells in the culture (Fig. 2C), because IL-2 production is one of the downstream effects of CD28 signal transduction (37, 38). Finally, telomerase activity was evaluated, because CD28 signaling is required for optimal telomerase up-regulation and loss of telomerase activity with chronic stimulation parallels the loss of CD28 expression (4). Moreover, the significance of enhanced telomerase activity is underscored by the observation that maintenance of high telomerase activity is associated with increased anti-viral function in CD8+ T cells (27, 39). We had previously shown that at various stages of the long-term culture, if we purified the CD28+ T cells from the mixture of CD28+ and CD28− T cells within the culture, the CD28+ T cells had significantly greater telomerase activity than the mixed population of cells. In addition, when the CD28 ligand on APCs was blocked, using Abs to B7.1 and B7.2, telomerase activity was significantly reduced (4). To further investigate the relationship between CD28 and telomerase activity, we analyzed control and treated cultures on day 4 following the second stimulation, using the TRAP assay. Preliminary titration of the cell lysates was performed (Fig. 4A), and using this information, the effect of the TNF-α modulation was determined on 5000 cell-equivalents from cultures established for all five donors. As shown in a representative TRAP gel image (Fig. 4B), inhibition of TNF-α was associated with increased telomerase activity. A summary of the telomerase activity changes seen in cultures where TNF-α was inhibited is shown in Table I.
FIGURE 3.
Effect of TNF-α inhibition alters senescence-associated cytokine patterns. Cytokine profiles were evaluated at 6 and 18 PD in the treated and untreated cultures. A, Concentration of IL-6 (pg/ml) in culture supernatants for untreated and treated cultures from each blood donor (n =5) was evaluated by ELISA. B, IL-2 mRNA was analyzed by real time quantitative PCR, and results expressed relative to GAPDH. *, p < 0.05; **, p < 0.01.
FIGURE 4.
TNF-α inhibition is associated with increased telomerase activity. On day 4 after the second stimulation, telomerase activity present in lysates from 10,000 cells was analyzed using the TRAP assay, as described in Materials and Methods. A, Representative gel showing that three dilutions (10,000; 5,000; and 2,500 cell equivalents) all fall in the linear range of telomerase activity. B, TRAP assay gel from a representative culture, using 5000 cell-equivalents per lane. HI, Heat inactivated; IC, internal control.
Table I.
Telomerase activity is increased in cultures treated with Ab to TNF-α or R1 inhibitor
| Donor | Fold Increasea
|
|
|---|---|---|
| Ab | R1 Inhibitor | |
| 1 | 1.48 | 1.62 |
| 2 | 1.47 | 1.78 |
| 3 | N/A | 1.42 |
| 4 | 1.25 | 1.20 |
| 5 | 1.46 | 1.41 |
| Mean ± SD | 1.41 ± 0.113 | 1.49 ± 0.221 |
| p value | 0.00033 | 0.0022 |
Telomerase activity for each donor was determined as the fold increase over their respective control (untreated) at 5000 cell-equivalents. The mean fold increase is given and p value determined compared to control.
Reduced caspase-3 levels in senescent cultures and in ex vivo CD28- T lymphocytes
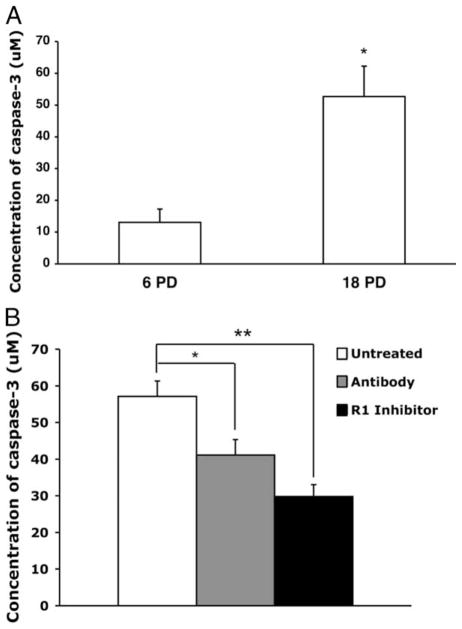
Recent studies have shown that caspase-3, at levels well below those required to induce apoptosis, down-regulates CD28 expression in the Jurkat CD4+ T cell tumor line. The effect is mediated via interaction with the gene promoter, and the addition of caspase-3 inhibitors was shown to restore CD28 promoter activity (23). Because the caspase cascade is one of the downstream effects of TNF-α receptor 1-mediated signaling (22, 24), we hypothesized that the CD28 expression changes induced by modulation of TNF-α in long-term cultures of CD8+ T lymphocytes (Fig. 2) might be associated with altered caspase levels. We first evaluated the untreated cultures, where we observed a significant increase in caspase-3 in the late vs early passage cells (Fig. 5A). This increase was significantly diminished in the treated cultures, even as early as six PDs. (Fig. 5B). The effect was even more dramatic in the late passage cultures (Fig. 5B). Interestingly, no changes in caspase-8 levels at different culture time points were observed (data not shown). Based on the inverse correlation between the proportion of CD28+ T cells in the culture and caspase-3 levels (data not shown), we evaluated whether CD8+ T cells present in vivo would also show an association between caspase-3 and CD28 expression. CD8+ T lymphocytes were isolated from PBMC and the cells were further separated into CD8+CD28+ and CD8+CD28− subsets, each of which was evaluated for caspase-3. Consistent with our results from long-term in vitro cultures, CD8+CD28− T cells tested immediately ex vivo showed significantly greater caspase-3 activity than CD8+ CD28+ T cells from the same donor (Fig. 6). This difference was observed in experiments on cells from all 10 individuals tested, independent of whether they were healthy (n = 5) or HIV+ (n = 5).
FIGURE 5.
Reduced accumulation of caspase-3 in CD8+ T cell cultures subjected to TNF-α inhibition. Constitutive caspase-3 activity was measured using the Apo-Alert colorimetric assay kit (Chemicon International) in CD8+ T cells during quiescence. A, Early (6 PD) vs late (18 PD) time points in untreated cultures. B, At the late (18 PD) time point, the concentration of caspase-3 is shown for all condition in all donors (n = 5). *, p < 0.05; **, p < 0.01.
FIGURE 6.
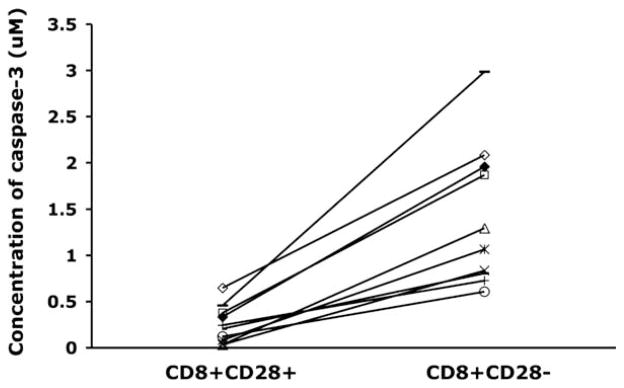
Higher levels of caspase-3 in CD8+CD28− vs CD8+CD28− T cells tested immediately ex vivo. CD8+ T cells were separated into CD28+ or CD28− subpopulations by magnetic bead selection, and purity of each subpopulation was confirmed by flow cytometry. Caspase-3 activity was measured as in Fig. 5, using the Apo-Alert colorimetric assay (n = 10). Results for the CD28+ and CD28− subpopulations from each donor are connected by a line. The difference in caspase-3 activity was highly significant (p < 0.001).
Discussion
The present study shows that inhibition of TNF-α alters the dynamics of replicative senescence in human CD8+ T lymphocytes, and in particular, the loss of CD28 expression. Our data also suggest that the CD28 regulatory pathway mediated by TNF-α may involve caspase-3. The expression of CD28, a key costimulatory molecule required for proper cellular immune responses, is irreversibly down-regulated in CD8+ T lymphocytes in a wide variety of clinical situations. Increased proportions of clonally expanded populations of CD8+CD28− cells are a consistent biological feature of human aging, and are predictors of reduced immune function in the elderly (20). Similar accumulations are seen in chronic HIV-1 infection, with CD28− T lymphocytes occupying more than 50% of the peripheral blood CD8+ T cell pool (5, 6, 13, 16). Cells with this phenotype are also associated with various forms of cancer, and their abundance varies according to the degree of chronic immune activation (7, 9, 40 – 44). Therefore, understanding the dynamics of CD28 regulation and the possible cause of its permanent, irreversibly suppressed expression is critical to translational studies on a variety of diseases.
We observed that chronic exposure, over a period of several months, to either a neutralizing Ab or an R1 inhibitor resulted in a significant delay in the loss of CD28 surface expression, extending the studies showing similar results during short-term (~3 wk) experiments (18). Our data show that the effect was dose-dependent, and it involved increased levels of TNF-α mRNA. Inhibition of TNF-α also had an effect on the proliferative potential of CD8+ T cells. Although all the cultures eventually did reach the end stage of replicative senescence (26, 45), inhibition of TNF-α was associated with a significantly greater number of PDs before senescence. The nearly 5-fold increase in PDs achieved in culture in the presence of the receptor inhibitor (Fig. 1B) would translate into an ~30-fold expansion of CD8+ T cells, which, assuming all the cells were functional, could have a significant impact on the control of infection or cancer in vivo.
We also investigated the effect of TNF-α inhibition on telomerase activity, because CD8+ T cells lose the ability to up-regulate telomerase activity with chronic activation (4, 46). Moreover, optimal telomerase up-regulation is dependent on CD28 signaling, as shown by the significant reduction in T cell telomerase activity in the presence of Abs to the B7 ligands on APC (4). The present study extended those results by showing that the delayed loss of CD28 induced by inhibition of TNF-α coincided with the increased telomerase activity. The increased telomerase activity may explain the greater number of PDs in the treated cultures, because maintenance of telomerase activity in virus-specific CD8+ T cells, either via gene transduction or by small molecule telomerase activators, also results in increased proliferative potential (27).
In addition to its involvement in telomerase up-regulation, CD28 signal transduction is also essential for the production of cytokines and stabilization of several cytokine mRNAs (1, 3). T cells that reach senescence in long-term culture show significant reduction in the production of IL-2, but our data show that this decrease is abrogated in cultures in which the loss of CD28 expression is delayed. The cultures in which TNF-α was inhibited showed both increased levels of CD28+ T cells, as well as increased levels of IL-2 transcript. Conversely, the production of IL-6, a proinflammatory cytokine, which, like TNF-α, increases in cultures that reach replicative senescence (19), as well as during aging in vivo (34), was reduced in cultures treated with the Ab or receptor inhibitor. Whether this reduced level of IL-6 secretion was the direct outcome of higher CD28 expression, which is known to stabilize several cytokine mRNAs (2), or through inhibition of TNF-α, has yet to be determined. Nonetheless, our data show that delaying the loss of CD28 expression in CD8+ T lymphocytes via manipulation of TNF-α signaling retards a variety of changes associated with replicative senescence.
Our study also identified a possible mechanism by which TNF-α inhibits CD28 expression. Previous research has shown that in the Jurkat CD4+ T cell tumor line, constitutive caspase-3 at a level well below the threshold required for the programmed cell death cascade can down-regulate CD28 gene expression via interaction with its promoter (23, 32). Interestingly, CD28+ and CD28− T cells show differences in caspase-3 activity during apoptosis (47). Consistent with these reports linking caspase-3 based and CD28 expression, we observed that the treated cultures, which showed increased CD28 expression, also had lower levels of constitutive caspase-3 compared with the control cultures. The physiological relevance of this observation was underscored by our analysis of T lymphocytes tested immediately ex vivo, where the same inverse relationship between CD28 expression and caspase-3 was observed. Specifically, for all 10 donors tested, CD8+CD28− T cells had significantly higher levels of caspase-3 compared with CD8+CD28+ T cells from the same individual. This observation is consistent with the fact that the TNF-α receptor-1 is an upstream signal for the caspase cascade. Interestingly, we found no difference in the level of caspase-8, which precedes caspase-3 in the apoptosis-induced caspase cascade, suggesting that the apoptosis and CD28 pathways involving caspase-3 may be divergent.
In summary, our results further elucidate the dynamics of CD28 modulation and other changes associated with chronic immune activation. Our data show that inhibition of TNF-α in long-term human CD8+ T cell cultures, either via neutralization of the protein or by inhibiting binding to its receptor, delays the loss of CD28 cell surface expression, and also retards the process of replicative senescence. These effects are associated with coordinate regulation of constitutive caspase-3 and CD28 expression. Our findings may help expedite the development of novel therapeutic approaches to prevent the generation of senescent CD8 T cells in vivo in such clinical situations as aging, cancer, and HIV/AIDS.
Footnotes
This research was supported by National Institutes of Health Grants AG023720 and AI060362 (to R.B.E.).
Abbreviations used in this paper: RA, rheumatoid arthritis; PD, population doubling; R1 inhibitor, an inhibitor of the TNF-α receptor-1; TRAP, telomeric repeat amplification protocol.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 3.Powell JD, Ragheb JA, Kitagawa-Sakakida S, Schwartz RH. Molecular regulation of interleukin-2 expression by CD28 costimulation and anergy. Immunol Rev. 1998;165:287–300. doi: 10.1111/j.1600-065x.1998.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 4.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 5.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallejo AN, Nestel AR, Schirmer M, Weyand CM, Goronzy JJ. Aging-related deficiency of CD28 expression in CD4+ T cells is associated with the loss of gene-specific nuclear factor binding activity. J Biol Chem. 1998;273:8119–8129. doi: 10.1074/jbc.273.14.8119. [DOI] [PubMed] [Google Scholar]
- 7.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Bock G, Schonitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8+CD28− T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 9.Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 10.Lewis DE, Yang L, Luo W, Wang X, Rodgers JR. HIV-specific cytotoxic T lymphocyte precursors exist in a CD28-CD8+ T cell subset and increase with loss of CD4 T cells. AIDS. 1999;13:1029–1033. doi: 10.1097/00002030-199906180-00005. [DOI] [PubMed] [Google Scholar]
- 11.Neil GA, Summers RW, Cheyne BA, Carpenter C, Huang WL, Waldschmidt TJ. Analysis of T-lymphocyte subpopulations in inflammatory bowel diseases by three-color flow cytometry. Dig Dis Sci. 1994;39:1900–1908. doi: 10.1007/BF02088123. [DOI] [PubMed] [Google Scholar]
- 12.Moosig F, Csernok E, Wang G, Gross WL. Costimulatory molecules in Wegener’s granulomatosis (WG): lack of expression of CD28 and preferential up-regulation of its ligands B7-1 (CD80) and B7-2 (CD86) on T cells. Clin Exp Immunol. 1998;114:113–118. doi: 10.1046/j.1365-2249.1998.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutra WO, Martins-Filho OA, Cancado JR, Pinto-Dias JC, Brener Z, Gazzinelli G, Carvalho JF, Colley DG. Chagasic patients lack CD28 expression on many of their circulating T lymphocytes. Scand J Immunol. 1996;43:88–93. doi: 10.1046/j.1365-3083.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7− CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Effros RB. From Hayflick to Walford: the role of T cell replicative senescence in human aging. Exp Gerontol. 2004;39:885–890. doi: 10.1016/j.exger.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–124. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Bryl E, Vallejo AN, Matteson EL, Witkowski JM, Weyand CM, Goronzy JJ. Modulation of CD28 expression with anti-tumor necrosis factor α therapy in rheumatoid arthritis. Arthritis Rheum. 2005;52:2996–3003. doi: 10.1002/art.21353. [DOI] [PubMed] [Google Scholar]
- 18.Bryl E, Vallejo AN, Weyand CM, Goronzy JJ. Down-regulation of CD28 expression by TNF-α. J Immunol. 2001;167:3231–3238. doi: 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- 19.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 20.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 21.Lewis DE, Merched-Sauvage M, Goronzy JJ, Weyand CM, Vallejo AN. Tumor necrosis factor-α and CD80 modulate CD28 expression through a similar mechanism of T-cell receptor-independent inhibition of transcription. J Biol Chem. 2004;279:29130–29138. doi: 10.1074/jbc.M402194200. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S. Tumor necrosis factor-α-induced apoptosis in T cells from aged humans: a role of TNFR-I and downstream signaling molecules. Exp Gerontol. 2002;37:293–299. doi: 10.1016/s0531-5565(01)00195-4. [DOI] [PubMed] [Google Scholar]
- 23.Ma S, Ochi H, Cui L, He W. FasL-induced downregulation of CD28 expression on Jurkat cells in vitro is associated with activation of caspases. Cell Biol Int. 2003;27:959–964. doi: 10.1016/s1065-6995(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 24.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 25.Ma S, Ochi H, Cui L, Zhang J, He W. Hydrogen peroxide induced down-regulation of CD28 expression of Jurkat cells is associated with a change of site α-specific nuclear factor binding activity and the activation of caspase-3. Exp Gerontol. 2003;38:1109–1118. doi: 10.1016/s0531-5565(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 26.Perillo NL, Walford RL, Newman MA, Effros RB. Human T lymphocytes possess a limited in vitro life span. Exp Gerontol. 1989;24:177–187. doi: 10.1016/0531-5565(89)90009-0. [DOI] [PubMed] [Google Scholar]
- 27.Dagarag M, Evazyan T, Rao N, Effros RB. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: enhanced antiviral functions accompany the increased proliferative potential and telomere length stabilization. J Immunol. 2004;173:6303–6311. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]
- 28.Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-α and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- 29.Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, Kronenberg M, Cohen D, Schachter F. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29:601–609. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 30.Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, Harley CB, Villeponteau B, West MD, Giorgi JV. Shortened telomeres in the expanded CD28−CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10:F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 32.Walker LS, McLeod JD, Boulougouris G, Patel YI, Hall ND, Sansom DM. Down-regulation of CD28 via Fas (CD95): influence of CD28 on T-cell apoptosis. Immunology. 1998;94:41–47. doi: 10.1046/j.1365-2567.1998.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Effros RB. Replicative senescence of CD8 T cells: effect on human ageing. Exp Gerontol. 2004;39:517–524. doi: 10.1016/j.exger.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 35.Tarazona R, DelaRosa O, Alonso C, Ostos B, Espejo J, Pena J, Solana R. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121:77–88. doi: 10.1016/s0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 36.Effros RB, Svoboda K, Walford RL. Influence of age and caloric restriction on macrophage IL-6 and TNF production. Lymphokine Cytokine Res. 1991;10:347–351. [PubMed] [Google Scholar]
- 37.Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 38.Verweij CL, Geerts M, Aarden LA. Activation of interleukin-2 gene transcription via the T-cell surface molecule CD28 is mediated through an NF-κB-like response element. J Biol Chem. 1991;266:14179–14182. [PubMed] [Google Scholar]
- 39.Fauce SR, Jamieson BD, Chin AC, Mitsuyasu RT, Parish ST, Ng HL, Kitchen CM, Yang OO, Harley CB, Effros RB. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J Immunol. 2008;181:7400–7406. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sze DM, Giesajtis G, Brown RD, Raitakari M, Gibson J, Ho J, Baxter AG, Fazekas de St Groth B, Basten A, Joshua DE. Clonal cytotoxic T cells are expanded in myeloma and reside in the CD8+CD57+CD28− compartment. Blood. 2001;98:2817–2827. doi: 10.1182/blood.v98.9.2817. [DOI] [PubMed] [Google Scholar]
- 41.Turka LA, Ledbetter JA, Lee K, June CH, Thompson CB. CD28 is an inducible T cell surface antigen that transduces a proliferative signal in CD3+ mature thymocytes. J Immunol. 1990;144:1646–1653. [PubMed] [Google Scholar]
- 42.Vingerhoets JH, Vanham GL, Kestens LL, Penne GG, Colebunders RL, Vandenbruaene MJ, Goeman J, Gigase PL, De Boer M, Ceuppens JL. Increased cytolytic T lymphocyte activity and decreased B7 responsiveness are associated with CD28 down-regulation on CD8+ T cells from HIV-infected subjects. Clin Exp Immunol. 1995;100:425–433. doi: 10.1111/j.1365-2249.1995.tb03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28− T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52:599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hakansson A, Hakansson L, Gustafsson B, Krysander L, Rettrup B, Ruiter D, Bernsen MR. Biochemotherapy of metastatic malignant melanoma: on down-regulation of CD28. Cancer Immunol Immunother. 2002;51:499–504. doi: 10.1007/s00262-002-0304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 46.Effros RB. Telomerase induction in T cells: a cure for aging and disease? Exp Gerontol. 2007;42:416–420. doi: 10.1016/j.exger.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spaulding C, Guo W, Effros RB. Resistance to apoptosis in human CD8+ T cells that reach replicative senescence after multiple rounds of antigen-specific proliferation. Exp Gerontol. 1999;34:633–644. doi: 10.1016/s0531-5565(99)00033-9. [DOI] [PubMed] [Google Scholar]