Abstract
Engineered nanomaterials (ENM) are increasingly being utilized in many consumer products and various medical applications thereby leading to the potentiality of increased human exposures. Assessment of the adverse effects on the immune system is an important component for evaluating the overall health and safety of ENM. Tasked with eliminating pathogens and removing cancerous cells, the immune system is constantly functioning to maintain homeostasis. Small modifications to the immune system which may occur following ENM exposure, could lead to impaired protection or an inappropriate immune response resulting in autoimmunity and damage to the host. This review seeks to survey and evaluate the current literature to better understand the impact of ENM exposure on cells critical to the innate and adaptive immune systems.
Keywords: nanoparticle, toxicity, immunotoxicity, carbon nanotube, TiO2, fullerene, ZnO
Introduction
Engineered nanomaterials (ENM) are defined as manufactured particles that have at least one dimension between 1–100 nanometers. The development of numerous ENM has spawned a new dimension to the rapidly expanding field of nanotechnology. The potential applications of ENM widely range from biomedicine to mechanical engineering. ENM have a variety of unique characteristics beyond size that make them highly suitable for a variety of diverse biomedical applications including different shapes, chemical compositions, surface chemistries and solubilities.
The rapid development of nanotechnology raises concerns regarding the potential impact of ENM on the environment and human health and safety. Incidental exposure to ENM may occur in occupational settings during both manufacturing and processing, while intentional exposure is likely to increase dramatically through use of ENM in consumer products and nanomedicines. It is estimated that there are over 1000 consumer products on the market which contain nanomaterials resulting in increased human exposures through multiple routes (Kim et al., 2011a). Specifically, inclusion of ENM in consumer products could increase nasal, oral, intravenous, dermal, pulmonary and other routes of exposure. The possibility of utilizing ENM based drug delivery systems has gained a lot of attention. The use of ENM as drug delivery systems offers many potential advantages, including the capability of reaching molecular targets and penetrating normally intact physiologic barriers by virtue of their size characteristics (Christopher, 2010, Grimm and Scheinberg, 2011, Liu et al., Misra et al., 2010, Zhang et al., 2010). These advantages however also raise concerns regarding unintended off-target toxicity.
The immune system consists of a variety of effector cells and molecules to protect the host from infectious agents and other foreign substances. In order for ENM to be used safely in medical applications, we must understand their interactions with the immune system. The immune system consists of both innate (nonspecific) and adaptive (specific) immune responses. Innate immune responses occur rapidly to combat a wide range of insults, however, does not lead to lasting immunity. Adaptive immune responses are capable of eliminating infections more efficiently than innate immune responses, however, takes days to develop this specificity. Both innate and adaptive immune responses depend on the activation of key immune cells. The proliferation and differentiation of immune cells is influenced by the presence of different cytokines and growth factors. In the normal healthy condition, the major sources of hematopoietic cytokines and growth factors are bone marrow stromal cells. However in disease conditions, cytokines are produced by activated macrophages and T helper cells causing the induction of additional hematopoietic activity, thus resulting in the rapid expansion of the specific immune cell population to combat disease.
Exposure to ENM unintentionally (e.g., environmental and occupational) or intentionally (e.g., medical applications and cosmetics) may induce immunotoxicity resulting in detrimental effects on immune function. In advertent suppressed immunological function caused by ENM exposures may result in increased incidence and severity of infectious diseases, as well as, some types of cancer (Mitchell et al., 2009). In contrast, inappropriate enhancement of immune function or the generation of hypersensitivity caused by ENM exposures could possibly exacerbate the development of allergic and autoimmune diseases.
This review provides an appraisal of our current knowledge regarding modulation of the immune response following ENM exposure. Furthermore, this review will discuss ENM interactions with specific immune cells that may result in immunotoxicity.
ENM Impact on Immune Function
The immune system consists of both innate and adaptive immune components intended to protect the body from foreign organisms or substances. The innate immune response is not specific to any one foreign substance but consists of general mechanisms to facilitate their removal, while the adaptive immune response is responsible for the recognition and selective elimination of specific foreign substances. Innate and adaptive immune responses do not operate in total independence of each other. Innate immune responses facilitate the participation of adaptive immune responses allowing the body to readily recognize pathogens and damaged self-macromolecules thereby increasing the efficiency of elimination. On the other hand, the adaptive immune system can produce a variety of signaling mediators which can stimulate and increase the effectiveness of the innate immune response. To promote the safety of ENM, interactions of ENM with immune system and potential consequences such as immunosuppression, allergic disease and autoimmunity need to be considered.
Impact of ENM on Innate Immune Responses
The innate immune system plays a critical role in the protection against pathogens, damaged self-macromolecules and cells, and other foreign particles, including ENM (Salvador-Morales et al., 2006). The innate immune response is activated by recognition of non-self antigen via pattern recognition receptors (PRRs) and pathogen-associated molecular patterns (PAMPs). Phagocytosis of ENM by macrophages and other phagocytic cells is a highly effective line of innate defense, resulting in the rapid release of a variety of pro-inflammatory factors, such as IL-1β, IL-6 and TNF-α. The secretion of mature IL-1β from macrophages is dependent on the cleavage of the inactive pro-IL-1β by caspase-1, which is activated within the inflammasome (Davis et al., 2011). The inflammasome is a large multimeric structure that is formed when each component is overexpressed in the cytosol and is activated by a diverse series of pathogens or cellular stress. The activation of the inflammasome by ENM has been an active area of investigation. Yazdi et al. reported that nano-sized titanium dioxide (TiO2) and nano-sized silica dioxide (SiO2) activated the nod-like recepter-3 inflammasome in myeloid cells and results in secretion of IL-1β in primary human keratinocytes (Yazdi et al., 2010). The activation of the inflammasome, however, did not require phagocytosis of nano-TiO2. Yang et al. demonstrated that silver nanoparticles induced the formation of the inflammasome, caspase-1 activation, and the subsequent release of mature IL-1β from human blood monocytes (Yang et al., 2012). In addition, the formation of the inflammasome was dependent on leakage of cathepsins from lysosomes and the efflux of intracellular K+ induced by silver nanoparticle exposure. Moreover, silver nanoparticles have shown synergistic effects on the activated immune response by LPS (Yang et al., 2012). The findings suggest that inflammasome activation is a critical step in ENM effects on the innate immune system which may exacerbate the immune response to subsequent exposure to pathogens.
Formation of reactive oxygen species (ROS) is a critical mechanism by which the innate immune system responds to pathogens. Excessive ROS production, however, may lead to adverse inflammatory responses and toxicity. Indeed, production of ROS has been proposed as a major mechanism by which ENM exposure may impact immune function. An increase in ROS production by ENM is an initiating step which has the capability to trigger an innate immune response through the activation of the inflammasome. Schanen et al. reported that TiO2 nanoparticle exposure induced a 10–20 fold increase in ROS levels in peripheral blood mononuclear cells (PBMC) and human umbilical vein endothelial cells (HUVEC) following a 48 hour treatment in vitro (Schanen et al., 2009). The authors found that the greatest ratio of ROS producers were CD14+ monocytes, indicating that phagocytes are responsible for the majority of ROS generation during an inflammatory responses (Schanen et al., 2009). In addition, the authors observed an increase in IL-1β which suggests inflammasome activation as a response to ROS production following treatment with TiO2 nanoparticle. These findings support the implication that inflammasome activation is linked to nanoparticle exposure which could ultimately promote inflammatory diseases, including autoimmune disease.
Complement activation is a critical component of the innate immune response which needs to be taken into account when studying ENM biological interactions. The complement system is composed of over 40 soluble and cell surface proteins which interact with each other to not only identify, but opsonize foreign pathogens, altered-self and synthetic substances (Rybak-Smith and Sim, 2011). Complement activation can occur though three pathways upon recognition of a target: classical, alternative, and the lectin pathway. Complement activation by any pathway results in turnover of the complement protein C3, the production of inflammatory peptides C3a, C4a, and C5a, as well as formation of C5b-9 complex or membrane attack complex (Rybak-Smith and Sim, 2011, Carroll and Sim, 2011). Cui et al. demonstrated that the mRNA expression of complement factor D (Cfd) was significantly down-regulated in mouse liver following TiO2 nanoparticles exposure (Cui et al., 2012). Cfd, a serine protease is essential for activation of the alternative pathway in the complement system, and plays a key role in the innate immune response by stimulating the elimination of foreign substances. The reduction of Cfd due to TiO2 nanoparticles exposure reduced the activity of C3b factor B which cleaves C3 molecules to generate C3a and C3b and induces the complement system. Insufficient C3 convertase for cleaving additional C3 molecules to generate C3a and C3b may result in activation of other immune components instead of the complement system and may further prevent effective opsonization of microbes. Direct binding of ENM to complement proteins may alter the biodistribution of ENM and facilitate their rapid clearance from systemic circulation via complement receptor binding (Dobrovolskaia et al., 2008). Non-functionalized carbon nanotubes are able to directly bind C1q, the recognition subunit of the C1 complex (Salvador-Morales et al., 2006). In these studies, it was demonstrated that direct binding of C1q to carbon nanotubes activated the complement system via the classical pathway. PEGylated carbon nanotubes actuated complement activation by significantly increasing serum SC5b-9 levels (Hamad et al., 2008). Carbon nanotube mediated elevation of SC5b-9 was based on direct binding of C4 with PEGylated carbon nanotubes subsequently forming C4b2a convertase resulting in complement activation.
Besides binding to complement proteins, ENM may interact with other surrounding proteins, such as serum albumin, to form a protein corona (Walkey and Chan, 2012). The protein corona may affect how ENM are internalized by immune cells and cleared from the body, which will likely further modulate the toxicity of ENM. Dutta et al. demonstrated that albumin-coated SWCNTs reduced LPS-mediated Cox2-induction from RAW 264.7 macrophages under serum-free conditions (Dutta et al., 2007). Sometimes the protein corona may not fully saturate the surface area of ENM, and instead bind at discreet sites, leaving much of ENM surface unobstructed, which could also affect the toxicity of ENM (Rybak-Smith and Sim, 2011). On the other hand, proteins coated on the surface of ENM may undergo conformation changes, resulting in the exposure of new epitopes which could be presented as antigen by antigen presenting cells to initiate adaptive immune response (Nel et al., 2009).
In occupational settings, ENM may become associated with other contaminates such as microbial agents, LPS, and other inhalable pollutants which could further perturb innate immune response compared to ENM alone (Kim et al., 2011b). For example, LPS contamination exacerbates multi-walled carbon nanotubes (MWCNT)-induced lung fibrosis in mice by amplifying production of platelet-derived growth factor-AA in macrophages and epithelial cells, while also upregulating PDGF-Rα in pulmonary fibroblasts (Cesta et al., 2010).
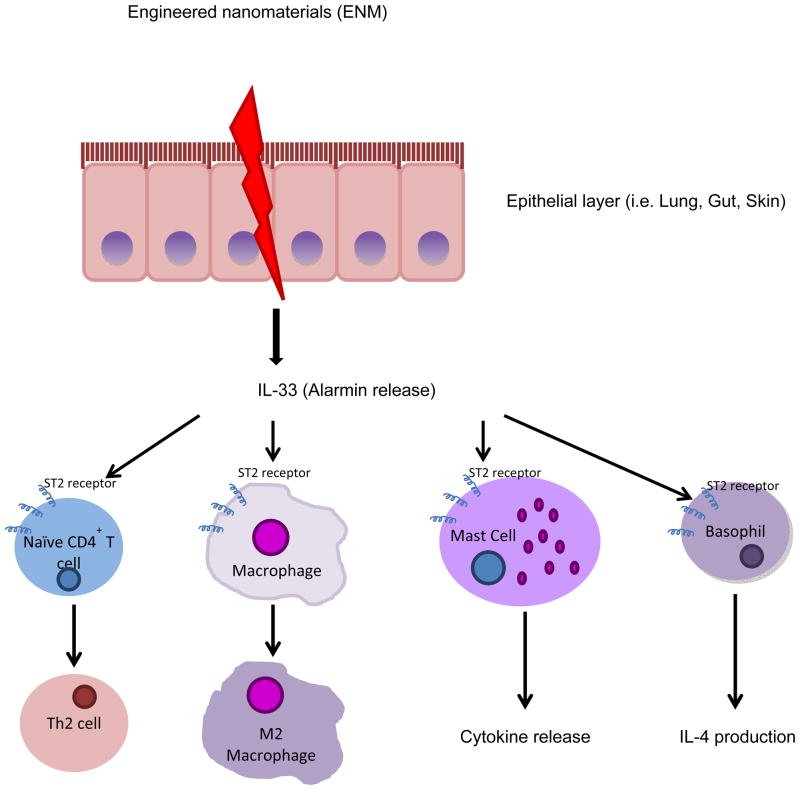
Innate immune responses can also be initiated by ‘alarmins’ which are the released or produced in response to infection or tissue injury. Alarmins are released very rapidly from necrotic cells or innate immune cells (i.e. macrophages) in response to infection (Gougeon et al., 2012). Recent studies in our lab have established a critical role for thealarmin, IL-33, in directing immune responses to ENM. IL-33, a novel member of the IL-1 family of cytokines, is a potent activator of mast cells and promoter of Th2 immunity. The interaction of IL-33 with its membrane-bound receptor, ST2, induces maturation and cytokine production in mast cells (Miller, 2011). In addition, soluble ST2 can bind IL-33 directly and act as a decoy receptor (Hayakawa et al., 2007). Pulmonary exposure to MWCNT results in significant IL-33 production by epithelial cells leading to mast cell activation and subsequent neutrophilic inflammation and impairment of lung function (Wang et al., 2011, Katwa et al., 2012). These findings highlight IL-33, and possibly other alarmins, as key initiators in the immune response to ENM that direct both innate and adaptive immunity (Figure 1).
Figure 1. Proposed mechanism by which alarmins initiate immune responses to ENM.
Alarmins, such as IL-33, are released rapidly from epithelial cells or innate immune cells (i.e. macrophages) in response to engineered nanomaterial exposure. IL-33 binds to its ligand, ST2, which is expressed on the cell surface of T cells, macrophages, mast cells, and basophils. The interaction of IL-33 with the ST2 receptor results in differentiation of naïve T cells to Th2 cells, alternative activation of macrophages (M2 phenotype), mast cell activation, as well as IL-4 production from basophils.
Impact of ENM on Adaptive Immune Responses
The adaptive immune system is responsible for specificity and memory against invading foreign pathogens. Antigen presentation is a key component in activating and expanding T and B cells that recognize specific pathogenic epitopes. For naïve T helper and naïve B cell activation, antigen presentation via MHC class II is critical. This powerful system has been exploited through vaccines to provide protection against pathogens without having to be exposed to the devastating version of these pathogens. Vaccine development with the aid of ENMs is a rapidly expanding area of study due to the potential role of ENMs as adjuvants and/or delivery vehicles and also provides examples of how ENM could adversely interact with the immune system.
Adjuvants are crucial for vaccine efficacy and are added to antigens to enhance the immune response. For an adjuvant to be effective, it must recruit antigen presenting cells, activate these antigen presenting cells, persist for an extended period of time and be delivered by the most relevant route for the foreign pathogen. Nanoparticles offer new opportunities for the enhancement of vaccine adjuvants in all of these categories. Nanoparticles are attractive platforms for adjuvant development due to the possible addition of ligands specific for receptors on immune cells that enhance targeting as well as activation of antigen presenting cells. In addition, some nanoparticles form a core that allows preloading of antigens such as peptides for MHC I and MHC II presentation to T and B cells. For example, polylactic-coglycolic acid (PGLA) nanoparticle cores can be loaded with peptides for MHC I & II (Cruz et al., 2012). In this example, the PGLA cores were decorated with human DC-SIGN ligands using conventional carbohydrates such as the HIV protein gp120 or monoclonal antibodies to target dendritic cells. In these studies, the authors demonstrated that the monoclonal antibodies against DC-SIGN receptors were more efficient for MCH class II delivery and the addition of toll-like receptor (TLR) ligands significantly upregulated co-stimulatory molecules such as CD80/86 (Cruz et al., 2012). In addition, TLR ligands have also been added to nanoparticles to stimulate innate immune responses (Kasturi et al., 2011).
Despite these promising aspects of using ENM for vaccine development and similarly for targeted tumor therapy, there is concern that the same properties, which make ENM useful, may also lead to adverse adaptive immune effects. For example, nanoparticles have been shown to exacerbate disease conditions associated with adaptive immunity such as allergic asthma. Hussain et al. demonstrated that TiO2 and gold nanoparticles increase airway hyper-responsiveness and total lung cells counts in a toluene diisocyanate model of murine asthma (Hussain et al., 2011). Additionally, Ryman-Rasmussen et al. showed MWCNT increases lung fibrosis in mice challenged with ovalbumin 14 days post co-administration (Ryman-Rasmussen et al., 2009). MWCNT and ovalbumin delivered together also resulted in increased IL-5 (mRNA), PDGF-AA (protein), and neutrophils in BAL fluid at 1 day post-inhalation. These studies suggest that ENMs can amplify adaptive immune responses which create conditions know to exacerbate Th2 diseases such as allergic asthma.
In addition to activating the adaptive immune system, nanoparticles have also been shown to down regulate this system. Mitchell et al. demonstrated that mice exposed to 1 mg/m3 MWCNT in whole body inhalation chambers displayed suppressed immune function with decreased T cell dependent antibody response (Mitchell et al., 2009). This suppressed immune function involved activation of cyclooxygenase enzymes in the spleen in response to a signal from the mouse lungs. Impairment of T cell dependent antibody response is cause for concern, as activation of naïve B cells could be compromised thereby leading to immunosuppression and a possible increase in susceptibility to invading pathogens.
Immunotoxicity of ENM through Different Immune Cells
The immune system consists of a complex network of cells which are responsible for acquired immunity and the immunologic attributes of diversity, specificity, memory, and self/non-self recognition. Cells involved in immune system function include but are not limited to phagocytes, lymphoid cells, and mast cells. To better understanding the toxicity induced by ENM, interactions of ENM with cells of immune system need to be assessed.
Phagocytes
When the physical and chemical barriers that prevent foreign substances from entering the body are overcome or evaded, the first cells that respond are phagocytes. Phagocytes are able to ingest and digest foreign substances and endogenous cellular debris resulting in the production of a variety of cytokines, chemokines, ROS and powerful degradative enzymes. The role of phagocytes is not absolute but often ancillary. There are three types of professional phagocytes in the immune system: macrophages/monocytes, granulocytes and dendritic cells. These professional phagocytes express a multitude of receptors, including Fcγ receptors, complement receptors, scavenger receptors, and TLR on their surfaces which can detect signals from damaged tissues, non-self antigens, and antibody opsonized materials (Murray and Wynn, 2011). These receptors enhance the ability of phagocytes to internalize ENM. Studies have been shown that the efficient internalization of ENM by phagocytes is size dependent (Kunzmann et al., 2011, Manolova et al., 2008), however, due to unique size of ENMs the exact mechanism(s) of uptake are not entirely understood. In addition to size, the shape of ENM may impact internalization by phagocytes. For example, when a phagocyte engulfsfiber shaped ENM, such as carbon nanotubes, frustrated phagocytosis can occur resulting in the production of superoxide anions and pro-inflammatory mediators such as TNF-α (Brown et al., 2007).
Macrophage
Macrophages are important immune effector cells which play a crucial role in host defense. Macrophages are prodigious phagocytic cells that engulf and remove foreign particles, pathogens, and cell debris generated from apoptotic and necrotic cells. Macrophages are located in tissues throughout the entire body and are able to recruit additional macrophages from the circulation to inflamed tissues in response to an infection or following injury. Macrophages can be divided into different subpopulations, such as Langerhans cells, alveolar macrophages, osteoclasts and splenic macrophages, depending on their anatomical location and functional phenotype (Gordon and Taylor, 2005). In general, macrophage activation occurs following ENM exposure and includes production of ROS, activation of the inflammasome, as well as secretion of pro-inflammatory cytokines. For example, amorphous nanostructured silica has been shown to induce cytotoxicity, ROS production, lipid peroxidation, nitric oxide synthesis and production of TNF-α in murine alveolar macrophages (MH-S cell line) (Gazzano et al., 2012). Lee et al. demonstrated that MWCNTs could induce COX-2 production in mouse RAW264.7 macrophages through a MAPK-dependent mechanism, while iNOS production by MWCNTs is a MAPK-independent mechanism (Lee et al., 2012).
Phagocytosis of ENM by macrophages is found to be size dependent (Tsai et al., 2011). Carlson et al. evaluated size-dependent cellular interaction of biologically active silver nanoparticles with alveolar macrophages (Carlson et al., 2008). Silver nanoparticles exhibit a unique size-dependent toxicity in macrophages based on the data from MTT and LDH assays. Exposure of macrophages to 15 nm silver nanomaterials results in cytotoxicity and an increase in ROS production in a dose-dependent manner over 24 hour. In addition, Tsai et al. demonstrated that gold nanoparticles engulfed by RAW264.7 macrophages inhibit proinflammatory cytokine production and attenuate TLR9 signaling in a size-dependent manner (Tsai et al., 2012).
Lung macrophages
One of the major routes for environmental or occupational exposure is via inhalation, therefore interactions of ENM with macrophages located in the lung which include alveolar and tissue resident macrophages are of importance. Alveolar macrophages are the first cells of the immune system to encounter inhaled pathogens and particles within the lung. Alveolar macrophages are excellent phagocytes capable of rapidly clearing large numbers of foreign particles from the host lung and also play an important role in regulating immune responses in the lung (Braciale et al., 2012). The importance of alveolar macrophages in the uptake and clearance of inhaled ENM is demonstrated by a study conducted in rats showing that over 90% of inhaled gold nanoparticles appeared to be internalized by alveolar macrophages within the first 24 hours (Takenaka et al., 2012). Similar results of efficient macrophage phagocytosis have been demonstrated in vitro. For example, an in vitro study with nanosilver particles revealed that nonselective uptake by macrophages occurred within minutes (Haase et al., 2011). This rapid uptake by alveolar macrophages suggests that they may play a central role in mediating the pulmonary effects following inhalation of ENM.
A major role of alveolar macrophages is to migrate from the lung to the draining lymph nodes (DLN) where antigen is presented through MHC II to prime and activate T cells (Kirby et al., 2009). However, cationic-charged nanomaterials can be easily taken up by macrophages, and result in acute cytotoxicity which prevents translocation to lymph nodes and causes severe local lung injury (Choi et al., 2010).
Upon inhalation, nanoparticles have been shown to interact with pulmonary surfactant components which enhances ENM phagocytosis by macrophages (Kapralov et al., 2012). Following pharyngeal aspiration of SWCNT, phosphotidylcholine represented 74% of the total surfactant phospholipids adsorbed on the SWCNT, while 14% of the SWCNT phospholipids were phosphatidylglycerol and surfactant proteins A, B, and D. The presence of adsorbed surfactant phospholipids significantly enhanced the uptake of SWCNTs by murine RAW264.7 macrophages when compared with uncoated SWCNT. The authors speculated that the association of SWCNTs with the lipid and protein components of the surfactant markedly enhanced the efficiency of their phagocytosis by macrophages and may determine the recognition of them in vivo thereby impacting their biodistribution and biodegradation (Kapralov et al., 2012). In addition, another study has shown that magnetic nanoparticles with high binding to surfactant protein A have a greater potential to interact with an alveolar macrophage cell line resulting in enhanced internalization and clearance (Ruge et al., 2011). This suggests that recognition of ENM by alveolar macrophages is largely dependent on the constituents of the protein corona that forms on the surface of ENM. Taken together, these data suggest that coating of ENM with proteins or lipids relevant for the lung (i.e. SPA) likely enhances ENM interaction with alveolar macrophages through recognition, tethering, and engulfment thereby influencing the subsequent biodistribution and other immunological effects of the ENM.
Macrophages are also present in the pleural space of the lung and play a critical role in host defense and immune regulation (Jantz and Antony, 2008). Pleural macrophages, as a phagocyte, play an important role in the removal of inhaled foreign particles retained in the pleural space. An in vitro study with direct exposure to carbon nanotube (CNT) samples containing long fibers resulted in significant increases in cytokine release including IL-1β, IL-6 and IL-8 from human macrophage cell line (THP-1) due to frustrated phagocytosis (Murphy et al., 2012). The authors also speculated that pro-inflammatory cytokines released by activated pleural macrophages may stimulate the adjacent pleural mesothelial cells possible leading to the induction of mesothelioma.
Dendritic cells
Dendritic cells (DCs) are a heterogeneous population of cells serving as a professional antigen presenting cell in most peripheral tissues where antigens normally first encounter the immune system. DCs are able to uptake exogenous antigens through phagocytosis and present them on major histocompatibility complex (MHC) class I or II for recognition by CD8+ or CD4+ T cells, respectively. DCs have been shown to possess strong phagocytic capacity of gold nanoparticles which accumulate in endocytic compartments of the DCs (Christian et al., 2010). This suggests that such accumulation of gold nanoparticles inside the endocytic compartments, which is dedicated to antigen processing, might influence antigen presentation. In fact, it was demonstrated that gold nanoparticles and antigen, when presented simultaneously by DCs, inhibited the specific immune response (Christian et al., 2010). However, the opposite may be true depending on the ENM. Uptake of ENM and presentation of antigen from nanovectors is a promising approach for enhancing immune responses to vaccinations (Elamanchili et al., 2004). For example, mRNA-loaded nanoparticles, which are biodegradable core-shell structured nanoparticles with a poly core enveloped by a phospholipid bilayer shell, enhanced uptake by dendritic cells and led to mRNA delivery to the cytosol with low cytotoxicity (Su et al., 2011).
Phagocytosis of antigen or ENM by DCs is an actin-dependent, receptor mediated process (Blander and Medzhitov, 2006). These processes may or may not require recognition of the ENM by a specific receptor. In vitro, it has been shown that monocyte-derived DCs readily take up quantum dots (QD) through a clathrin-mediated mechanism and scavenger receptors that is regulated by F-actin and phospholipase C (Zhang et al., 2011). However, the process of ENM uptake may be modulated by PRR and DC maturation. DC maturation induced by LPS actuated an increase in QD uptake compared with DCs without LPS stimulation. However, QD exposures cause a decrease in CD80/CD86 expression on the surface of DCs after LPS stimulation. These results indicated that QD can interfere with LPS-induced DC maturation and activation. In addition, QD are capable of inhibiting antigen presentation, since CD80/CD86 facilitate antigen presentation to T lymphocytes. Beside phagocytosis, DCs can also internalize macromolecules and particles though macropinocytosis. Migdal et al. reported that hybrid titanium dioxide (TiO2)/para-amino benzoic acid nanoparticles (TiO2/PABA NPs) were internalized in DCs through macropinocytosis and not by a receptor-mediated mechanism (Migdal et al., 2010). However, despite efficient internalization of hybrid TiO2/PABA NPs, DC activation, including ROS production and release of pro-inflammatory cytokines was not observed. The route of ENM uptake by DC therefore likely impacts cellular responses due to the activation of intracellular signaling cascades through transmembrane receptor engagement.
Activation of DCs induces morphological transformation with development of long dendrites and increased expression of MHC-II, CD80 and CD86 which facilitate presentation of antigen to T lymphocytes. Meanwhile, activated DCs are able to activate naïve T cells though production of large amounts of IL-12 and interaction of these surface molecules with CD40 ligand and CD28 on the surface of T cells. A recent study has shown that DC exposure to zinc oxide (ZnO) nanoparticles induced maturation and activation by upregulating CD80 and CD86 expression and releasing pro-inflammatory cytokines IL-6 and TNF-α (Heng et al., 2011). TiO2- and silicon dioxide (SiO2)-nanoparticles also have been shown to upregulate MHC-II, CD80, and CD86 expression on DC (Winter et al., 2011). Taken together, these observations suggest that certain NP may promote DC maturation and activation thereby leading to activation of lymphocytes.
Lymphocytes
ENMs may enter the body by breaching the epithelial or endothelial barriers followed by clearance via monocytes/macrophages or the mononuclear phagocytic system (MPS) and the complement system leading to recruitment of lymphocytes from the circulation. This leads to the typical signs of inflammation at specific local sites. Lymphocytes are key players for the initiation of most responses of the adaptive immune system and can be broadly subdivided into three populations, T cells, B cells, and natural killer (NK) cells, based on their function and cell-membrane components.
T cells
T cells play a crucial role in adaptive and memory immune responses as well as in the pathogenesis of many diseases. T cells increase the phagocytic activity of macrophages and stimulate the production of antibodies by B cells. In addition, T cells are responsible for the generation of immunological memory. During the final stage of maturation, most T cells proceed along two different developmental pathways, which generate functionally distinct CD4+ and CD8+ subpopulations. CD4+ T cells generally function as T helper (Th) cells and are MHC class II restricted, whereas CD8+T cells generally function as T cytotoxic (Tc) cells and are MHC class I restricted. Duan et al. reported that the intragastrical administration of nanoparticle anatase TiO2 significantly decreased the proliferation of both CD4+ and CD8+ T cells, as well as the ratio of CD4+ to CD8+ cells in the liver of ICR mice, which led to an inhibition of the immune response in those mice (Duan et al., 2010). In contrast, work in our laboratory has revealed that, intravenous administration of MWCNT leads to significant increases in both CD4+ and CD8+ T cells in the spleen of C57BL/6 mice (unpublished data). The increase in T cells may be responsible for enhancing the immune response in C57BL/6 mice. This discrepancy might be due to different characteristics of nanoparticles and administration routes. Similar to our results, Park et al. reported that mice intraperitoneally exposed with high dose of silica nanoparticles had an increase in the number of splenocytes at 24 hours after treatment (Park and Park, 2009). The distribution of T cells in the spleen was increased by>100% of control following exposure. Due to a decrease in B cells in the spleen, the authors speculated that silica nanoparticles initiated cellular immunity rather than humoral immunity.
Antigen-presenting cells can present peptide to naïve CD4+ T cells by their MHC class II molecule and deliver co-stimulatory signals, thereby driving T cell activation. The activated CD4+ T cells undergo extensive cell division and differentiation, and give rise to distinct subsets of effector T cells, including T helper1 (Th1), Th2, and Th17. Th1 and Th2 cells are characterized by their production of interferon-γ and IL-4, respectively, whereas Th17 cells produce IL-17. Limited studies have shown the effects of ENM on the differentiation of CD4+ T cells. Hirai et al. demonstrated that silica nanoparticles synergistic increased total IgE production and a systemic Th2 response induced by Dermatophagoides pteronyssinus(Hirai et al., 2012). Gras et al. reported that the carbosilane dendrimer 2G-NN16 suppressed Th17 response genes and proteins including IL-17A, IL-17F, IL-22, and IL-23, in CD4+ T lymphocytes in vitro as well as inhibition of IL17A production and increased C. albicans kidney burden in vivo (Gras et al., 2012). The impact of ENM on Th-skewing largely depends on the characteristic of the nanomaterials. Exposure route may also lead to different outcomes as for Th polarization induced by the ENM.
In healthy, normal adults, around 1010 T cells leave the circulation to enter the different tissues and then return to the circulation every 30 min. Kennedy et al. demonstrated that gold nanoparticles (AuNP) can be taken up by activated human T cells without impairing their viability or cellular function (Kennedy et al., 2011). The AuNP-T cells can specifically migrate to subcutaneous large cell lymphoma sites in immune deficient SCID mice. This technique maximized AuNP accumulation at the tumor site and enhanced the potential photothermal cancer therapy. Due to the high mobility of T cells, the possible application of an ENM-T cell delivery system could further be extended to imaging and drug delivery applications. Nembrini et al. showed that effector memory CD8+ T cells recruited to the mouse lung following pulmonary immunization with nanoparticles-ova and CpG killed infected cells during influenza infection (Nembrini et al., 2011). This suggests that mice vaccinated with nanoparticles showed a decrease in disease morbidity due to CD8+ T-cell protection.
Many reports have been released on the relationship between oxidative stress and T cell activation from ENM exposure. Eom et al. investigated the potential toxic effects of silver nanoparticles on immortalized human T lymphocytes, Jurkat T cells, by measuring oxidative stress, cell cycling, and DNA damage in vitro (Eom and Choi, 2010). This study reported that cell viability revealed higher sensitivity of Jurkat T cells to Ag nanoparticles than Ag ion exposure. ROS production was increased in the Jurkat T cells exposed to both Ag nanoparticles and Ag ions compared to unexposed cells, however, the production of ROS was significantly increased in the cells 24 hours after treatment with Ag nanoparticles compared to those exposed to Ag ions. This oxidative stress was mediated by p38 MAPK through the Nrf-2 and NF-kB signaling pathways. Furthermore, Jurkat T cell exposure to Ag nanoparticles led to an increase in the G2/M and S phases and a decrease in the G1 phase as well as DNA damage suggesting AgNP may be cytotoxic to T cells. Whole blood CD4+ T cells have also been shown to significantly decrease when TiO2 was given intragastrically for 30 and 90 days (Duan et al., 2010, Sang et al., 2012). These studies suggest ENM can adversely impact T cell homeostasis by increasing the rate of cellular division while also increasing ROS which could be damaging DNA faster than repair mechanisms can compensate. These findings suggest that chronic ENM exposure could possibly lead to lymphopenia, as well as, impaired adaptive immunity.
B cells
B cells are antibody producing cells that contribute to the adaptive immune response by providing humoral protection. B cells generate specific antibodies through random rearrangements of segments of their DNA. However, some DNA rearrangements result in self reactive B cells which are eliminated in the bone marrow. The remaining B cells in the bone marrow, designated transitional B cells, exit the bone marrow and migrate to spleen, where they transit from type 1 to type 2 and develop into mature B cells (Yeramilli and Knight, 2011). Moon et al. observed a drop in the number of the CD21+ IgM+ transitional type 2, the mature IgD+IgM+, and the recirculating IgD+IgM− B lymphocytes from the spleen when TiO2 nanoparticles were intraperitoneally administered once a day for 7 days (Moon et al., 2011). A similar drop in whole blood B cell numbers has also been observed when TiO2 is delivered intraperitoneally for 30 days (Duan et al., 2010). Additionally, B cells in whole blood and serum IgM levels continually decreased when TiO2 was delivered intragastrically every day for 90 days (Sang et al., 2012). B cells can be activated via two pathways, thymus dependent and thymus independent.
Thymus dependent activation requires cross linking of membrane antibody (IgM) by the antigen and from CD40 binding on Th2 cells plus the presence of Th2 cytokines such as IL-4, IL-5, and IL-10. Thymus independent activation occurs when antigen crosslinks IgM in addition to highly repetitive epitopes found on bacteria such as LPS which provide a second signal through receptors such as TLR4. Intraperitoneal administration of TiO2 nanoparticles not only leads to a drop in splenic B cell numbers but also decreases the proliferative capacity of B220+ B cells when challenged with LPS (Moon et al., 2011). The decrease in proliferative capacity of B cells when challenged with LPS suggests ENMs have the capacity to interfere with thymus independent activation. This impairment could ultimately compromise immunity against pathogenic bacteria.
Once a B cell becomes activated it can begin antibody class switching. Class switching involves changing the Fc portion of the antibody for different effector functions. IgG is typically favored for opsinization (antibody enhanced phagocytosis), IgA is secreted into mucous membranes, while IgE is meant to combat parasitic infections, but also triggers allergic diseases by stimulating mast cells and basophils. Park et al. has demonstrated increases in B cell numbers in the spleen and whole blood when platinum nanoparticles or TiO2 nanoparticles were delivered in a single intratracheal instillation (Park et al., 2010, Park et al., 2009). Both types of metal nanoparticles resulted in increased serum IgE levels. TiO2 nanoparticles resulted in increased serum Th2 cytokines IL-4, IL-5 and IL-10 (Park and Park, 2009)which likely stimulated B cells to produce IgE. These data support a humoral allergic response when these metal nanoparticles are delivered in an acute manner to the pulmonary system. ENM have the ability to significantly alter B cell function. The possible impairment or enhancement of B cell activation by ENM is cause for future studies and assessment to prevent development of pathogenic antibodies that could lead to autoimmunity, allergic disease or impaired host defense.
Natural Killer cells
Natural killer (NK) cells are lymphocytes that provide innate immunity against intracellular pathogens as well as protection against development tumor cells. Moon et al. demonstrated that TiO2 nanoparticles delivered intraperitoneally once a day for 7 days resulted in reduced numbers of resident NK cells in spleens of mice (Moon et al., 2011). Additionally, in the same study, TiO2 nanoparticles were delivered intraperitoneally once a day for 4 weeks and the mice were subsequently implanted with B16F10 mouse melanoma cells. Mice receiving TiO2 nanoparticles and B16F10 tumors showed a significant increase in tumor size over the course of 12 days compared to control animals due to a reduction in the number of NK cells (Moon et al., 2011).
NK cells receive activating signals as well as inhibitory signals. It is the balance of these signals that determines the fate of the target cell. Activating signals include many surface ligands induced by cellular stress while inhibitory receptors include MHC class I receptors, NKP-P1A, as well as many others (Vivier et al., 2008). Therefore, an increase in stress induced ligands combined with a decrease in MHC class I expression will result in target cell death as is often the case with tumor cells. When TiO2 nanoparticles are delivered via a single intratracheal installation to the lung, a rise in NK cell numbers in bronchoalveolar fluid is observed at 8 days post delivery and remains elevated over baseline through 90 days post delivery (Gustafsson et al., 2011). However, expression of the NKR-P1A receptor on the NK cells displayed a transient increase from day 2–16. Since the NKR-P1A is an inhibitor receptor, upregulation of this receptor could decrease the effector function of NK cells (Gustafsson et al., 2011).
NK cell mediated target cell death occurs by the release of enzymes from granules that initiate apoptosis through the activation of caspases. It is important for NK cells to initiate apoptosis as lysis would release virus particles which would only amplify the infection. NK cells are inherently capable of inducing apoptosis in target cells; however, in the presence of interferon this capability is significantly enhanced. It should be noted that although NK cells are derived from the lymphoid lineage in hematopoiesis, they are considered part of the innate immune system and require no prior exposure to a pathogen to function. Moon et al. reported that specifically spleen resident CD11b− NK cells were significantly reduced by TiO2 nanoparticle exposure while CD11b+ spleen resident NK cell numbers were unaffected. This suggests TiO2 nanoparticles may interfere with development of NK cell hematopoiesis, since NK cells acquire CD11b expression during maturation and acquisition of effector capabilities (Chiossone et al., 2009). Another study in which TiO2 nanoparticles were delivered intragastrically for 90 days revealed a decrease in whole blood NK cell numbers in mice (Sang et al., 2012). The decrease in number of NK cells resulted in severe histopathological changes in spleen morphology consistent with splenocyte apoptosis. Taken together, these data suggest exposure to ENM may have the capacity to impair NK cell anti-tumor properties and therefore should be examined carefully in future studies of ENM toxicity.
Granulocytic cells
Basophils
Basophils are the rarest granulocyte population accounting for less than 0.5% of leukocytes in circulation. Basophils play a fundamental role in the induction and maintenance of Th2 cytokine-dependent immunity and inflammation by producing abundant quantities of secreted mediators including cytokines and chemokines. Basophils are commonly associated with IgE-mediated allergic diseases such as allergic asthma. Besides allergic disease, basophils contribute to host protection and are involved in aberrant inflammatory responses as well as autoimmune disease. Very few studies have examined basophil activation in relation to ENM exposure despite their importance in systemic allergic reactions and their potential to negatively impact intravenous delivery of nanomedicines. In fact, the few studies that have examined the interaction of basophils and ENM have examined the ability of novel ENM to inhibit basophil activation for the treatment of allergic disease. For example, Ryan et al. reported that fullerenes (C60) are able to inhibit peripheral blood basophil activation in vitro which prevents allergic responses and anaphylaxis in vivo (Ryan et al., 2007). In support of these findings, it was reported that C70-tetraglycolic acid and C70-tetrainositol, two fullerene derivatives, significantly inhibited degranulation and cytokine production from basophils (Norton et al., 2010). Due to the importance of basophils in mediating allergic disease, further studies are needed to examine the potential role of basophils in the health and safety of ENMs.
Neutrophils
Neutrophils are highly phagocytic short-lived leukocytes capable of quickly migrating to sites of inflammation. Neutrophils reside primarily in circulation and are the most abundant leukocyte in circulation. By tethering and rolling, neutrophils can quickly diapedese through capillary walls. Once out of circulation, neutrophils migrate to sites of inflammation through chemotaxis where phagocytosis is enhanced by multiple PRRs, including antibody receptors, complement receptors, toll like receptors, and scavenger receptors. Phagocytosis through PRRs on the surface of neutrophils leads to increased production of free radicals such as NO and superoxide to aid in the digestion and elimination of foreign threats. Neutrophils have primarily been examined as markers of inflammation resulting from ENM exposure. Indeed, recent findings from an NIH sponsored consortium identified neutrophils as one of the most reliable markers of inflammation resulting from TiO2 and MWCNT pulmonary exposure (Bonner et al., In submission).
Numerous studies have shown ENMs have the capacity to recruit neutrophils. Iron oxide nanoparticles have been shown to significantly increase neutrophils in the BAL fluid when delivered intratracheally (Ban et al., 2012). The authors discovered nano-sized iron oxide produced higher BAL inflammatory cell numbers with a greater neutrophil percentage compared to micro-sized iron oxide. Gustafsson et al. reported a dose-dependent increase in neutrophils recovered from bronchoalveolar lavage following oropharyngeal instillation of TiO2 nanoparticles (Gustafsson et al., 2011). Additionally neutrophils continued to increase in the bronchoalveolar lavage fluid, reaching a maximum at 16 days post installation. Zinc oxide (ZnO) nanoparticles have been shown to lead to an increase in the percentage of neutrophils in BAL fluid when delivered intratracheally (Sayes et al., 2007). Chronic delivery of ENM has also been shown to negatively impact neutrophil numbers. Neutrophils numbers in the blood decreased when TiO2 nanoparticles were delivered intragastrically for 90 days (Sang et al., 2012). It is plausible that neutrophils were being recruited from circulation to the gastric compartment due to the intragastrically delivery of TiO2 nanoparticles. However impairment of hematopoietic development in the bone marrow could also be an explanation.
Neutrophil recruitment depends on inflammatory cytokines and chemokines. Understanding the source of these inflammatory mediators that recruit neutrophils is paramount to understanding ENM toxicity. Cytokine production could result from cells such as macrophages experiencing frustrated phagocytosis of ENM. There is also evidence ENM can have cytotoxic properties leading to necrosis of cells. Internalization of ZnO nanoparticles by lung epitheal cells resulted in an increase in cytotoxic markers such as lactate dehydrogenase (Sayes et al., 2007). Necrosis of cells can lead to increased inflammatory cytokines which could then serve to recruit neutrophils. Future ENM toxicity studies will undoubtedly involve assaying for inflammation and neutrophils may well be a reliable marker.
Mast Cells
Mast cells are derived from hematopoietic progenitors in the bone marrow and become differentiated after migrating to peripheral tissues, such as the skin, mucosa and airways (Gilfillan and Tkaczyk, 2006). These locations expose mast cells to the external environment and facilitate them to act as sentinel cells in host defense (Brown et al., 2008). Mast cells are highly heterogeneous due to local tissue environments in which they reside or due to encountering unique pathogen challenges (Abraham and St. John, 2010). Mast cells can respond to environmental stimulation by rapid degranulation and release of their preformed mediators, such as histamine, to elicit protective immune responses and also by synthesizing de novo mediators to promote adaptive immune responses. It is important to study mast cells in the context of ENMs due to their anatomic location as well as their ability to orchestrate innate and adaptive immune responses. A recent study has shown that TiO2 nanoparticles could potentially exacerbate allergic inflammatory responses by activating mast cells through membrane L-type Ca2+ channels in vitro (Chen et al., 2012). Influx of extracellular Ca2+ triggers histamine secretion from mast cells. The authors speculated that nanoparticle exposure may significantly augment and intensify mast cell degranulation with or without IgE sensitization. Therefore, mast cell associated inflammatory diseases including arthritis, atherosclerosis and coronary diseases may worsen following nanoparticle exposure (Chen et al., 2012). Cerium oxide (CeO2) nanoparticles can induce mast cell activation in C57BL/6 mice leading to the production of pro-inflammatory mediators, including IL-6, MIP-1α, and osteopontin in the lung as well as TGF-β in the heart (Wingard et al., 2011). The release of mediators from mast cells leads to pulmonary inflammation by recruiting inflammatory cells, as well as subsequent vascular responses and myocardial I/R injury in response to CeO2 nanoparticle exposure. However, mice deficient in mast cells did not show any inflammatory response in the pulmonary and vascular systems (Wingard et al., 2011). These findings support the activation and degranulation of mast cells as an important mechanism which may mediate ENM-induced inflammation and injury.
Mast cells play an important role in allergic responses. Mast cells can be activated through the high-affinity IgE receptor, FcεRI, for mediator release. Cross-linking IgE on FcεRI actuates the release of various preformed mediators, including histamine, and initiated early phase allergic responses. The newly formed mediators can also be released by activation of mast cells via IgE-dependent pathways to maintain late phase responses. The fullerene C70-tetraglycolic acid significantly inhibited degranulation and cytokine production from human mast cells, while C70-tetrainositol blocked only cytokine production in mast cells (Norton et al., 2010). The inhibition of mast cell activation was due to the blocking of FcεRI-induced intracellular calcium release and ROS responses. Furthermore, the inhibition of calcium store release and ROS production in response to FcεRI aggregation has been found to be caused by fullerene inhibiting phosphorylation of several signaling intermediates including extracellular signal regulated kinase 1/2, p38-mitogen-activated protein kinase, linker of activated T cells, Rho family GTPase, serine threonine protein kinase, phosphoinositide 3-kinase, as well as v-src sarcoma viral oncogene homolog (Norton et al., 2010).
Mast cells can also be activated by a variety of stimuli, such as IL-33, in addition to cross-linking of the IgE-receptor. In our lab, we have demonstrated that exposure to MWCNTs by oropharyngeal aspiration in C57BL/6 mice induced a significant increase in production of IL-33 (Wang et al., 2011). We speculated that the increased levels of IL-33, released from damaged lung epithelial cells, subsequently activated mast cells resulting in adverse pulmonary and cardiovascular responses in mice (Shannahan et al., 2012). These MWCNT induced toxicities appear to be mast cell dependent as MWCNT failed to induce these toxicities in mast cell-deficient mice. However, mast cell-deficient mice reconstituted with mast cells from wild-type but not ST2−/− mice restored the ability to respond to pulmonary MWCNT exposure (Katwa et al., 2012).
Taken together, unintentional activation of mast cell by ENM exposure through different pathways could result in adverse immune responses. However, the precise underlying mechanisms by which ENM affect mast cells are not fully clarified. A better understanding of ENM interaction with mast cells should help to prevent possible immunotoxicity, provide effective treatment for ENM induced toxicity, as well as to optimize ENM for a desired application.
Conclusion
After surveying the current available literature, it is clear that certain ENM, as well as, their route of administration seem to enhance immune responses, while other ENM and exposure routes seem to damage or phenotypically inhibit cells of the immune system (Table 1). Human exposures to ENM will continue to increase in both occupational and environmental settings as the field of nanotechnology continues its rapid expansion and growth. Unintentional emissions or exposures to ENM raise concerns regarding their potential effects on human health, especially in populations already suffering from immune disorders. By understanding the mechanisms of ENM interaction with the immune system, we can better anticipate adverse reactions and identify populations at risk. When specific cells of the immune system are compromised by ENM exposure, the immune system can become ineffective against subsequent challenges by pathogens or other toxicants. Additionally, when cytokine profiles are altered by ENM exposure, it can initiate or exacerbate conditions such as asthma, lymphoma, arthritis, and other autoimmune diseases. By modifying the physicochemical properties of ENMs and minimizing exposures, possible adverse health effects related to the immune system can likely be mitigated.
Table 1.
Studies regarding possible mechanisms responsible for immunotoxicity after exposure to selected engineered nanomaterials
| Engineered nanomaterial | Administration routes | Target immune cells | Immune effects |
|---|---|---|---|
| TiO2 | Oropharyngeal instillation | Neutrophils | Increase in numbers of neutrophils in BAL (Gustafsson et al., 2011) |
| Intragastrical | Neutrophils | Decrease in numbers of neutrophils in the blood (Sang et al., 2012) | |
| In vitro | Dendritic cells | Upregulate MHC-II, CD80, and CD86 expression on DC (Winter et al., 2011) | |
| Intragastrical | T cells | Decreased the proliferation of both CD4+ and CD8+ T cells, as well as the ratio of CD4+ to CD8+ cells in the liver (Duan et al., 2010) | |
| Intraperitoneal | B cells | Delayed B-lymphocyte development (Moon et al., 2011) | |
| Intratracheal installation | B cells | Increase in numbers of B cells in the spleen and whole blood as well as increased production of IgE in BALF (Park and Park, 2009) | |
| Intraperitoneal | NK cells | Decrease in numbers of spleen resident NK cells, specifically CD11b− NK cells (Moon et al., 2011) | |
| Intratracheal installation | NK cells | Increase in numbers of NK cells in BAL as well as a transient increased expression of the NKR-P1A receptor on the NK cells (Gustafsson et al., 2011) | |
| Intragastrical | NK cells | Decrease in whole blood NK cell numbers (Sang et al., 2012) | |
| In vitro | Mast cells | Activating mast cells to release histamine through membrane L-type Ca2+ channels (Chen et al., 2012) | |
| Carbon Nanotubes | In vitro | Macrophages | MWCNTs induce COX-2 production through a MAPK-dependent mechanism and iNOS production through a MAPK-independent mechanism (Lee et al., 2012) |
| In vitro | Macrophages | Coating of SWCNTs with SP-D enhanced the efficiency of their phagocytosis by murine RAW264.7 macrophages (Kapralov et al., 2012) | |
| In vitro | Macrophages | Increases in cytokine release including IL-1β, IL-6 and IL-8 from human macrophage cell line (Murphy et al., 2012) | |
| Intravenous | T cells | Increases in both CD4+ and CD8+ T cells in the spleen of C57BL/6 mice (unpublished data) | |
| Oropharyngeal aspiration | Mast cells | IL-33, released from damaged lung epithelial cells by MWCNT exposure, subsequently activated mast cells resulting in adverse cardiopulmonary responses (Wang et al., 2011) (Katwa et al., 2012) | |
| Fullerenes | In vitro | Basophils | Inhibit IgE dependent activation of peripheral basophil activation (Ryan et al., 2007) (Norton et al., 2010) |
| Intraperitoneal | Mast cells | A significant reduction in the mast cell-dependent anaphylactic-induced drop in core body temperature as well as behavioral responses that accompany anaphylactic shock (Norton et al., 2010) | |
| Silica | In vitro | Macrophages | Silica NP induce cytotoxicity, ROS production, lipid peroxidation, nitric oxide synthesis and production of TNF-α (Gazzano et al., 2012) |
| Intraperitoneal | T cells | Silica NP influence the distribution of T cells in the spleens (Park and Park, 2009) | |
| Intradermal | T cells | Silica NP synergistic increased a systemic Th2 response induced by Dermatophagoides pteronyssinus (Hirai et al., 2012) | |
| Intraperitoneal | B cells | Silica NP lead to a decrease in B cells in spleens (Park and Park, 2009) | |
| ZnO | In vitro | Dendritic cells | ZnO induced maturation and activation by upregulating CD80 and CD86 expression and releasing pro-inflammatory cytokines IL-6 and TNF-α (Heng et al., 2011) |
| Intratracheal | Neutrophils | ZnO lead to an increase in the percentage of neutrophils in BAL fuild (Sayes et al., 2007) |
Acknowledgments
This work was supported by NIH RO1 ES019311 (JMB). The authors would like to thank Dr. Jonathan Shannahan for extensively reviewing this article.
Footnotes
Declaration of Interest
The authors report no declarations of interest.
References
- ABRAHAM SN, ST JOHN AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAN M, LANGONNÉ I, HUGUET N, GOUTET M. Effect of submicron and nano-iron oxide particles on pulmonary immunity in mice. Toxicol Lett. 2012;210:267–275. doi: 10.1016/j.toxlet.2012.02.004. [DOI] [PubMed] [Google Scholar]
- BLANDER JM, MEDZHITOV R. On regulation of phagosome maturation and antigen presentation. Nat Immunol. 2006;7:1029–1035. doi: 10.1038/ni1006-1029. [DOI] [PubMed] [Google Scholar]
- BONNER J, SILVA R, TAYLOR A, BROWN J, HILDERBRAND S, CASTRANOVA V, PORTER D, ELDER A, OBERDORSTER G, HARKEMA J, BRAMBLE L, KAVANAGH T, BOTTA D, NEL A, PINKERTON K. Interlaboratory Evaluation of Rodent Pulmonary Responses to Engineered Nanomaterials. In submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRACIALE TJ, SUN J, KIM TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN DM, KINLOCH IA, BANGERT U, WINDLE AH, WALTER DM, WALKER GS, SCOTCHFORD CA, DONALDSON K, STONE V. An in vitro study of the potential of carbon nanotubes and nanofibres to induce inflammatory mediators and frustrated phagocytosis. Carbon. 2007;45:1743–1756. [Google Scholar]
- BROWN JM, WILSON TM, METCALFE DD. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clinical & Experimental Allergy. 2008;38:4–18. doi: 10.1111/j.1365-2222.2007.02886.x. [DOI] [PubMed] [Google Scholar]
- CARLSON C, HUSSAIN SM, SCHRAND AM, BRAYDICH-STOLLE K, HESS L, JONES KL, SCHLAGER RL, JJ Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. The Journal of Physical Chemistry B. 2008;112:13608–13619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- CARROLL MV, SIM RB. Complement in health and disease. Advanced Drug Delivery Reviews. 2011;63:965–975. doi: 10.1016/j.addr.2011.06.005. [DOI] [PubMed] [Google Scholar]
- CESTA MF, RYMAN-RASMUSSEN JP, WALLACE DG, MASINDE T, HURLBURT G, TAYLOR AJ, BONNER JC. Bacterial Lipopolysaccharide Enhances PDGF Signaling and Pulmonary Fibrosis in Rats Exposed to Carbon Nanotubes. American Journal of Respiratory Cell and Molecular Biology. 2010;43:142–151. doi: 10.1165/rcmb.2009-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN E, GARNICA M, WANG YC, MINTZ A, CHEN CS, CHIN WC. A mixture of anatase and rutile TiO2 nanoparticles induces histamine secretion in mast cells. Part Fibre Toxicol. 2012;9:2. doi: 10.1186/1743-8977-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIOSSONE L, CHAIX J, FUSERI N, ROTH C, VIVIER E, WALZER T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- CHOI HS, ASHITATE Y, LEE JH, KIM SH, MATSUI A, INSIN N, BAWENDI MG, SEMMLER-BEHNKE M, FRANGIONI JV, TSUDA A. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotech. 2010;28:1300–1303. doi: 10.1038/nbt.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIAN V, HEIDI F, RACHEL C, MARIE-BERNADETTE V, PATRICE M. Analysis of the toxicity of gold nano particles on the immune system: effect on dendritic cell functions. Journal of Nanoparticle Research. 2010;12:55–60. doi: 10.1007/s11051-009-9692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTOPHER C. The immune effects of naturally occurring and synthetic nanoparticles. Journal of Autoimmunity. 2010;34:J234–J246. doi: 10.1016/j.jaut.2009.11.009. [DOI] [PubMed] [Google Scholar]
- CRUZ LJ, TACKEN PJ, POTS JM, TORENSMA R, BUSCHOW SI, FIGDOR CG. Comparison of antibodies and carbohydrates to target vaccines to human dendritic cells via DC-SIGN. Biomaterials. 2012;33:4229–39. doi: 10.1016/j.biomaterials.2012.02.036. [DOI] [PubMed] [Google Scholar]
- CUI Y, LIU H, ZE Y, ZENGLI Z, HU Y, CHENG Z, CHENG J, HU R, GAO G, WANG L, TANG M, HONG F. Gene Expression in Liver Injury Caused by Long-term Exposure to Titanium Dioxide Nanoparticles in Mice. Toxicological Sciences. 2012;128:171–185. doi: 10.1093/toxsci/kfs153. [DOI] [PubMed] [Google Scholar]
- DAVIS BK, WEN H, TING JPY. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annual Review of Immunology. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBROVOLSKAIA MA, AGGARWAL P, HALL JB, MCNEIL SE. Preclinical Studies To Understand Nanoparticle Interaction with the Immune System and Its Potential Effects on Nanoparticle Biodistribution. Molecular Pharmaceutics. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUAN Y, LIU J, MA L, LI N, LIU H, WANG J, ZHENG L, LIU C, WANG X, ZHAO X, YAN J, WANG S, WANG H, ZHANG X, HONG F. Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice. Biomaterials. 2010;31:894–899. doi: 10.1016/j.biomaterials.2009.10.003. [DOI] [PubMed] [Google Scholar]
- DUTTA D, SUNDARAM SK, TEEGUARDEN JG, RILEY BJ, FIFIELD LS, JACOBS JM, ADDLEMAN SR, KAYSEN GA, MOUDGIL BM, WEBER TJ. Adsorbed Proteins Influence the Biological Activity and Molecular Targeting of Nanomaterials. Toxicological Sciences. 2007;100:303–315. doi: 10.1093/toxsci/kfm217. [DOI] [PubMed] [Google Scholar]
- ELAMANCHILI P, DIWAN M, CAO M, SAMUEL J. Characterization of poly (d, l-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004;22:2406–2412. doi: 10.1016/j.vaccine.2003.12.032. [DOI] [PubMed] [Google Scholar]
- EOM HJ, CHOI J. p38 MAPK Activation, DNA Damage, Cell Cycle Arrest and Apoptosis As Mechanisms of Toxicity of Silver Nanoparticles in Jurkat T Cells. Environmental Science & Technology. 2010;44:8337–8342. doi: 10.1021/es1020668. [DOI] [PubMed] [Google Scholar]
- GAZZANO E, GHIAZZA M, POLIMENI M, BOLIS V, FENOGLIO I, ATTANASIO A, MAZZUCCO G, FUBINI B, GHIGO D. Physico-chemical determinants in the cellular responses to nanostructured amorphous silicas. Toxicological Sciences. 2012 doi: 10.1093/toxsci/kfs128. [DOI] [PubMed] [Google Scholar]
- GILFILLAN AM, TKACZYK C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- GORDON S, TAYLOR PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- GOUGEON ML, MELKI MT, SAIDI H. HMGB1, an alarmin promoting HIV dissemination and latency in dendritic cells. Cell Death Differ. 2012;19:96–106. doi: 10.1038/cdd.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAS R, RELLOSO M, GARCÍA MI, JAVIER DE LA MATA F, GÓMEZ R, LÓPEZ-FERNÁNDEZ LA, MUÑOZ-FERNÁNDEZ MA. The inhibition of Th17 immune response in vitro and in vivo by the carbosilane dendrimer 2G-NN16. Biomaterials. 2012;33:4002–4009. doi: 10.1016/j.biomaterials.2012.02.018. [DOI] [PubMed] [Google Scholar]
- GRIMM J, SCHEINBERG DA. Will nanotechnology influence targeted cancer therapy? Semin Radiat Oncol. 2011;21:80–7. doi: 10.1016/j.semradonc.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSSON Å, LINDSTEDT E, ELFSMARK LS, BUCHT A. Lung exposure of titanium dioxide nanoparticles induces innate immune activation and long-lasting lymphocyte response in the Dark Agouti rat. Journal of Immunotoxicology. 2011;8:111–121. doi: 10.3109/1547691X.2010.546382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAASE A, ARLINGHAUS HF, TENTSCHERT J, JUNGNICKEL H, GRAF P, MANTION A, DRAUDE F, GALLA S, PLENDL J, GOETZ ME, MASIC A, MEIER W, THUNEMANN AF, TAUBERT A, LUCH A. Application of Laser Postionization Secondary Neutral Mass Spectrometry/Time-of-Flight Secondary Ion Mass Spectrometry in Nanotoxicology: Visualization of Nanosilver in Human Macrophages and Cellular Responses. ACS Nano. 2011;5:3059–3068. doi: 10.1021/nn200163w. [DOI] [PubMed] [Google Scholar]
- HAYAKAWA H, HAYAKAWA M, KUME A, TOMINAGA SI. Soluble ST2 Blocks Interleukin-33 Signaling in Allergic Airway Inflammation. Journal of Biological Chemistry. 2007;282:26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- HENG B, ZHAO X, TAN E, KHAMIS N, ASSODANI A, XIONG S, RUEDL C, NG K, LOO J. Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles. Arch Toxicol. 2011;85:1517–1528. doi: 10.1007/s00204-011-0722-1. [DOI] [PubMed] [Google Scholar]
- HIRAI T, YOSHIKAWA T, NABESHI H, YOSHIDA T, TOCHIGI S, ICHIHASHI KI, UJI M, AKASE T, NAGANO K, ABE Y, KAMADA H, ITOH N, TSUNODA SI, YOSHIOKA Y, TSUTSUMI Y. Amorphous silica nanoparticles size-dependently aggravate atopic dermatitis-like skin lesions following an intradermal injection. Part Fibre Toxicol. 2012;9:3. doi: 10.1186/1743-8977-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUSSAIN S, VANOIRBEEK JAJ, LUYTS K, DE VOOGHT V, VERBEKEN E, THOMASSEN LCJ, MARTENS JA, DINSDALE D, BOLAND S, MARANO F, NEMERY B, HOET PHM. Lung exposure to nanoparticles modulates an asthmatic response in a mouse model. European Respiratory Journal. 2011;37:299–309. doi: 10.1183/09031936.00168509. [DOI] [PubMed] [Google Scholar]
- JANTZ MA, ANTONY VB. Pathophysiology of the pleura. Respiration. 2008;75:121–33. doi: 10.1159/000113629. [DOI] [PubMed] [Google Scholar]
- KAPRALOV AA, FENG WH, AMOSCATO AA, YANAMALA N, BALASUBRAMANIAN K, WINNICA DE, KISIN ER, KOTCHEY GP, GOU P, SPARVERO LJ, RAY P, MALLAMPALLI RK, KLEIN-SEETHARAMAN J, FADEEL B, STAR A, SHVEDOVA AA, KAGAN VE. Adsorption of Surfactant Lipids by Single-Walled Carbon Nanotubes in Mouse Lung upon Pharyngeal Aspiration. ACS Nano. 2012 doi: 10.1021/nn300626q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASTURI SP, SKOUNTZOU I, ALBRECHT RA, KOUTSONANOS D, HUA T, NAKAYA HI, RAVINDRAN R, STEWART S, ALAM M, KWISSA M, VILLINGER F, MURTHY N, STEEL J, JACOB J, HOGAN RJ, GARCIA-SASTRE A, COMPANS R, PULENDRAN B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATWA P, WANG X, URANKAR RN, PODILA R, HILDERBRAND SC, FICK RB, RAO AM, KE PC, WINGARD CJ, BROWN JM. A Carbon Nanotube Toxicity Paradigm Driven by Mast Cells and the IL-33/ST2 Axis. Small. 2012 doi: 10.1002/smll.201200873. n/an/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY L, BEAR A, YOUNG J, LEWINSKI N, KIM J, FOSTER A, DREZEK R. T cells enhance gold nanoparticle delivery to tumors in vivo. Nanoscale Research Letters. 2011;6:283. doi: 10.1186/1556-276X-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM JA, ABERG C, SALVATI A, DAWSON KA. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nat Nanotechnol. 2011a;7:62–8. doi: 10.1038/nnano.2011.191. [DOI] [PubMed] [Google Scholar]
- KIM JS, ADAMCAKOVA-DODD A, O’SHAUGHNESSY P, GRASSIAN V, THORNE P. Effects of copper nanoparticle exposure on host defense in a murine pulmonary infection model. Part Fibre Toxicol. 2011b;8:29. doi: 10.1186/1743-8977-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY AC, COLES MC, KAYE PM. Alveolar Macrophages Transport Pathogens to Lung Draining Lymph Nodes. The Journal of Immunology. 2009;183:1983–1989. doi: 10.4049/jimmunol.0901089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNZMANN A, ANDERSSON B, VOGT C, FELIU N, YE F, GABRIELSSON S, TOPRAK MS, BUERKI-THURNHERR T, LAURENT S, VAHTER M, KRUG H, MUHAMMED M, SCHEYNIUS A, FADEEL B. Efficient internalization of silica-coated iron oxide nanoparticles of different sizes by primary human macrophages and dendritic cells. Toxicology and Applied Pharmacology. 2011;253:81–93. doi: 10.1016/j.taap.2011.03.011. [DOI] [PubMed] [Google Scholar]
- LEE JK, SAYERS B, CHUN KS, LAO HC, SHIPLEY-PHILLIPS J, BONNER J, LANGENBACH R. Multi-walled carbon nanotubes induce COX-2 and iNOS expression via MAP Kinase-dependent and - independent mechanisms in mouse RAW264.7 macrophages. Part Fibre Toxicol. 2012;9:14. doi: 10.1186/1743-8977-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Z, ROBINSON JT, TABAKMAN SM, YANG K, DAI H. Carbon materials for drug delivery & cancer therapy. Materials Today. 14:316–323. [Google Scholar]
- MANOLOVA V, FLACE A, BAUER M, SCHWARZ K, SAUDAN P, BACHMANN MF. Nanoparticles target distinct dendritic cell populations according to their size. European Journal of Immunology. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- MIGDAL C, RAHAL R, RUBOD A, CALLEJON S, COLOMB E, ATRUX-TALLAU N, HAFTEK M, VINCENT C, SERRES M, DANIELE S. Internalisation of hybrid titanium dioxide/para-amino benzoic acid nanoparticles in human dendritic cells did not induce toxicity and changes in their functions. Toxicol Lett. 2010;199:34–42. doi: 10.1016/j.toxlet.2010.07.017. [DOI] [PubMed] [Google Scholar]
- MILLER A. Role of IL-33 in inflammation and disease. Journal of Inflammation. 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISRA R, ACHARYA S, SAHOO SK. Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov Today. 2010;15:842–50. doi: 10.1016/j.drudis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- MITCHELL LA, LAUER FT, BURCHIEL SW, MCDONALD JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat Nano. 2009;4:451–456. doi: 10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOON EY, YI GH, KANG JS, LIM JS, KIM HM, PYO S. An increase in mouse tumor growth by an in vivo immuno modulating effect of titanium dioxide nanoparticles. Journal of Immunotoxicology. 2011;8:56–67. doi: 10.3109/1547691X.2010.543995. [DOI] [PubMed] [Google Scholar]
- MURPHY F, SCHINWALD A, POLAND C, DONALDSON K. The mechanism of pleural inflammation by long carbon nanotubes: interaction of long fibres with macrophages stimulates them to amplify pro-inflammatory responses in mesothelial cells. Part Fibre Toxicol. 2012;9:8. doi: 10.1186/1743-8977-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY PJ, WYNN TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEL AE, MADLER L, VELEGOL D, XIA T, HOEK EMV, SOMASUNDARAN P, KLAESSIG F, CASTRANOVA V, THOMPSON M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- NEMBRINI C, STANO A, DANE KY, BALLESTER M, VAN DER VLIES AJ, MARSLAND BJ, SWARTZ MA, HUBBELL JA. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proceedings of the National Academy of Sciences. 2011;108:E989–E997. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTON SK, DELLINGER A, ZHOU Z, LENK R, MACFARLAND D, VONAKIS B, CONRAD D, KEPLEY CL. A New Class of Human Mast Cell and Peripheral Blood Basophil Stabilizers that Differentially Control Allergic Mediator Release. Clinical and Translational Science. 2010;3:158–169. doi: 10.1111/j.1752-8062.2010.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK EJ, KIM H, KIM Y, PARK K. Intratracheal instillation of platinum nanoparticles may induce inflammatory responses in mice. Archives of Pharmacal Research. 2010;33:727–735. doi: 10.1007/s12272-010-0512-y. [DOI] [PubMed] [Google Scholar]
- PARK EJ, PARK K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol Lett. 2009;184:18–25. doi: 10.1016/j.toxlet.2008.10.012. [DOI] [PubMed] [Google Scholar]
- PARK EJ, YOON J, CHOI K, YI J, PARK K. Induction of chronic inflammation in mice treated with titanium dioxide nanoparticles by intratracheal instillation. Toxicology. 2009;260:37–46. doi: 10.1016/j.tox.2009.03.005. [DOI] [PubMed] [Google Scholar]
- RUGE CA, KIRCH J, CAÑADAS O, SCHNEIDER M, PEREZ-GIL J, SCHAEFER UF, CASALS C, LEHR CM. Uptake of nanoparticles by alveolar macrophages is triggered by surfactant protein A. Nanomedicine: Nanotechnology, Biology and Medicine. 2011;7:690–693. doi: 10.1016/j.nano.2011.07.009. [DOI] [PubMed] [Google Scholar]
- RYAN JJ, BATEMAN HR, STOVER A, GOMEZ G, NORTON SK, ZHAO W, SCHWARTZ LB, LENK R, KEPLEY CL. Fullerene Nanomaterials Inhibit the Allergic Response. The Journal of Immunology. 2007;179:665–672. doi: 10.4049/jimmunol.179.1.665. [DOI] [PubMed] [Google Scholar]
- RYBAK-SMITH MJ, SIM RB. Complement activation by carbon nanotubes. Advanced Drug Delivery Reviews. 2011;63:1031–1041. doi: 10.1016/j.addr.2011.05.012. [DOI] [PubMed] [Google Scholar]
- RYMAN-RASMUSSEN JP, TEWKSBURY EW, MOSS OR, CESTA MF, WONG BA, BONNER JC. Inhaled Multiwalled Carbon Nanotubes Potentiate Airway Fibrosis in Murine Allergic Asthma. American Journal of Respiratory Cell and Molecular Biology. 2009;40:349–358. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVADOR-MORALES C, FLAHAUT E, SIM E, SLOAN J, GREEN H, ML, SIM RB. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol. 2006;43:193–201. doi: 10.1016/j.molimm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- SANG X, ZHENG L, SUN Q, LI N, CUI Y, HU R, GAO G, CHENG Z, CHENG J, GUI S, LIU H, ZHANG Z, HONG F. The chronic spleen injury of mice following long-term exposure to titanium dioxide nanoparticles. Journal of Biomedical Materials Research Part A. 2012;100A:894–902. doi: 10.1002/jbm.a.34024. [DOI] [PubMed] [Google Scholar]
- SAYES CM, REED KL, WARHEIT DB. Assessing Toxicity of Fine and Nanoparticles: Comparing In Vitro Measurements to In Vivo Pulmonary Toxicity Profiles. Toxicological Sciences. 2007;97:163–180. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- SCHANEN BC, KARAKOTI AS, SEAL S, DRAKE DR, III, WARREN WL, SELF WT. Exposure to Titanium Dioxide Nanomaterials Provokes Inflammation of an in Vitro Human Immune Construct. ACS Nano. 2009;3:2523–2532. doi: 10.1021/nn900403h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANNAHAN JH, KODAVANTI UP, BROWN JM. Manufactured and airborne nanoparticle cardiopulmonary interactions: a review of mechanisms and the possible contribution of mast cells. Inhal Toxicol. 2012;24:320–339. doi: 10.3109/08958378.2012.668229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SU X, FRICKE J, KAVANAGH DG, IRVINE DJ. In Vitro and in Vivo mRNA Delivery Using Lipid-Enveloped pH-Responsive Polymer Nanoparticles. Molecular Pharmaceutics. 2011;8:774–787. doi: 10.1021/mp100390w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKENAKA S, MOLLER W, SEMMLER-BEHNKE M, KARG E, WENK A, SCHMID O, STOEGER T, JENNEN L, AICHLER M, WALCH A, POKHREL S, MADLER L, EICKELBERG O, KREYLING WG. Efficient internalization and intracellular translocation of inhaled gold nanoparticles in rat alveolar macrophages. Nanomedicine (Lond) 2012 doi: 10.2217/nnm.11.152. [DOI] [PubMed] [Google Scholar]
- TSAI C-Y, LU S-L, HU C-W, YEH C-S, LEE G-B, LEI H-Y. Size-Dependent Attenuation of TLR9 Signaling by Gold Nanoparticles in Macrophages. The Journal of Immunology. 2011 doi: 10.4049/jimmunol.1100344. [DOI] [PubMed] [Google Scholar]
- TSAI CY, LU SL, HU CW, YEH CS, LEE GB, LEI HY. Size-Dependent Attenuation of TLR9 Signaling by Gold Nanoparticles in Macrophages. The Journal of Immunology. 2012;188:68–76. doi: 10.4049/jimmunol.1100344. [DOI] [PubMed] [Google Scholar]
- VIVIER E, TOMASELLO E, BARATIN M, WALZER T, UGOLINI S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- WALKEY CD, CHAN WCW. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chemical Society Reviews. 2012;41:2780–2799. doi: 10.1039/c1cs15233e. [DOI] [PubMed] [Google Scholar]
- WANG X, KATWA P, PODILA R, CHEN P, KE PC, RAO A, WALTERS D, WINGARD C, BROWN J. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Particle and Fibre Toxicology. 2011;8:24. doi: 10.1186/1743-8977-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINGARD CJ, WALTERS DM, CATHEY BL, HILDERBRAND SC, KATWA P, LIN S, KE PC, PODILA R, RAO A, LUST RM, BROWN JM. Mast cells contribute to altered vascular reactivity and ischemia-reperfusion injury following cerium oxide nanoparticle instillation. Nanotoxicology. 2011;5:531–545. doi: 10.3109/17435390.2010.530004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTER M, BEER HD, HORNUNG V, KRÄMER U, SCHINS RPF, FÖRSTER I. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology. 2011;5:326–340. doi: 10.3109/17435390.2010.506957. [DOI] [PubMed] [Google Scholar]
- YANG EJ, KIM S, KIM JS, CHOI IH. Inflammasome formation and IL-1β release by human blood monocytes in response to silver nanoparticles. Biomaterials. 2012;33:6858–6867. doi: 10.1016/j.biomaterials.2012.06.016. [DOI] [PubMed] [Google Scholar]
- YAZDI AS, GUARDA G, RITEAU N, DREXLER SK, TARDIVEL A, COUILLIN I, TSCHOPP J. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1α and IL-1β. Proceedings of the National Academy of Sciences. 2010;107:19449–19454. doi: 10.1073/pnas.1008155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YERAMILLI VA, KNIGHT KL. Somatically Diversified and Proliferating Transitional B Cells: Implications for Peripheral B Cell Homeostasis. The Journal of Immunology. 2011;186:6437–6444. doi: 10.4049/jimmunol.1003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG LW, BAUMER W, MONTEIRO-RIVIERE NA. Cellular uptake mechanisms and toxicity of quantum dots in dendritic cells. Nanomedicine (Lond) 2011;6:777–91. doi: 10.2217/nnm.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Y, BAI Y, YAN B. Functionalized carbon nanotubes for potential medicinal applications. Drug Discov Today. 2010;15:428–35. doi: 10.1016/j.drudis.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]