Abstract
Currently, Δ9-tetrahydrocannabinol (THC) is the analyte quantified for oral fluid cannabinoid monitoring. The potential for false-positive oral fluid cannabinoid results from passive exposure to THC-laden cannabis smoke raises concerns for this promising new monitoring technology. Oral fluid 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH) is proposed as a marker of cannabis intake since it is not present in cannabis smoke and was not measureable in oral fluid collected from subjects passively exposed to cannabis. THCCOOH concentrations are in the picogram per milliliter range in oral fluid and pose considerable analytical challenges. A liquid chromatography–tandem mass spectrometry (LCMSMS) method was developed and validated for quantifying THCCOOH in 1 mL Quantisal-collected oral fluid. After solid phase extraction, chromatography was performed on a Kinetex C18 column with a gradient of 0.01 % acetic acid in water and 0.01 % acetic acid in methanol with a 0.5-mL/min flow rate. THCCOOH was monitored in negative mode electrospray ionization and multiple reaction monitoring mass spectrometry. The THCCOOH linear range was 12–1,020 pg/mL (R2>0.995). Mean extraction efficiencies and matrix effects evaluated at low and high quality control (QC) concentrations were 40.8–65.1 and −2.4–11.5 %, respectively (n=10). Analytical recoveries (bias) and total imprecision at low, mid, and high QCs were 85.0–113.3 and 6.6–8.4 % coefficient of variation, respectively (n=20). This is the first oral fluid THCCOOH LCMSMS triple quadrupole method not requiring derivatization to achieve a <15 pg/mL limit of quantification. The assay is applicable for the workplace, driving under the influence of drugs, drug treatment, and pain management testing.
Keywords: Cannabinoids, Carboxy THC, Oral fluid, Metabolites, Analytical method, LCMSMS
Introduction
Oral fluid testing is a useful monitoring tool for driving under the influence of cannabis and workplace, drug treatment, and parolee programs [1, 2]. Currently, as a result of growing interest, the Substance Abuse and Mental Health Services Administration (SAMHSA) is considering oral fluid testing guidelines for federally mandated workplace drug testing. Although SAMHSA guidelines are not yet approved, oral fluid testing in the US nonregulated sector is rapidly growing, and oral fluid testing programs are firmly in place in Australia and Europe [1, 3].
Oral fluid can be collected under direct observation without requiring same-sex observers and reduces opportunities for sample adulteration compared to urine collection [2]. Risks to analysts from infectious disease exposure is lower than for blood, and the presence of the parent drug in oral fluid might provide better correlation with pharmacodynamic effects than urine testing [2]. Numerous commercially available oral fluid collection devices are capable of accurately collecting standardized oral fluid volumes. Most devices include a collection pad and proprietary buffer for recovering and stabilizing drugs during storage prior to analysis.
Oral fluid poses analytical challenges with small specimen volumes and drug concentrations that are much lower than for urine [4]. This is confounded when there are multiple drug classes present requiring multiple drug confirmation analyses [4]. Furthermore, most collection device buffers include surfactants that could cause matrix effect challenges for liquid chromatography–mass spectrometry analysis [2, 5].
Cannabis is the most commonly abused drug of abuse [6] and is often present in drug treatment, pain management, and forensic and workplace cases. Δ9-Tetrahydrocannabinol (THC) is the primary psychoactive component in cannabis and is metabolized via cytochrome P450 to several metabolites, most prominently 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH) [7, 8]. The conditions under which exposure to cannabis smoke can produce false-positive oral fluid THC results and for how long are still unclear. Parent THC predominates in oral fluid following controlled smoked cannabis administration [9-11] and was found in oral fluid during passive exposure studies [12-14], but no THCCOOH was found in oral fluid collected from nonsmokers 0.3–22 h after 3 h exposure to smoke from multiple cannabis smokers in a Groningen café [14]. THCCOOH exceeded 7.5 pg/mL for up to 29 days in chronic, daily cannabis smokers during sustained, monitored abstinence providing a longer detection window than THC [15]. Therefore, monitoring THCCOOH has been proposed to minimize false-positive oral fluid results possibly caused by passive cannabis exposure while providing effective detection of cannabis smoking [14]. THCCOOH also documents oral THC administration with THCCOOH detected in oral fluid for at least 10.5 h after 15 mg oral THC, while THC was undetectable in most participants [16]; THC was only detected in 20.7 % of specimens and THCCOOH was present in 98.2 % of specimens during 37 around-the-clock oral THC administrations [17].
Monitoring picogram per milliliter THCCOOH concentrations in oral fluid presents an analytical sensitivity challenge requiring two-dimensional gas chromatography negative chemical ionization mass spectrometry (2D-GCMS) [18, 19], gas chromatography—tandem mass spectrometry (GCMSMS) [20], or liquid chromatography–tandem mass spectrometry analysis (LCMSMS) [21-23]. Lee et al. and Coulter et al. employed chemical derivatization with dansyl chloride or triphenylphosphine and 2-picolylamine, respectively, prior to LCMSMS, achieving oral fluid limits of quantification (LOQ) of 5 and 10 pg/mL, respectively [21, 22]. He et al. employed drydown, reconstitution, and ultrafiltration (DRUF) prior to online trapping and microflow liquid chromatography with high-resolution Orbitrap MS achieving a THCCOOH LOQ of 7.5 pg/mL [23]. Our aim was to develop and validate a simple, rapid, and robust method via traditional liquid chromatography–triple quadrupole mass spectrometry without derivatization that was capable of high-throughput picogram per milliliter THCCOOH quantification in oral fluid. This assay is applicable for workplace, drug treatment, pain management, and forensic testing.
Methods
Reagents and supplies
THCCOOH and THCCOOH-d9 were purchased from Cerilliant (Round Rock, TX, USA). Acetonitrile, hexane, and ethyl acetate were obtained from Sigma-Aldrich (St. Louis, MO, USA). Methanol and acetic acid were acquired from Fisher Scientific (Fair Lawn, NJ). Water was purified in house with an ELGA Purelab Ultra Analytic purifier (Siemens Water Technologies, Lowell, MA, USA). All solvents were HPLC grade or better. Strata X-C columns (3 mL/30 mg, Phenomenex Inc, Torrance, CA, USA) were utilized for preparing samples. Specimens were extracted on a Cerex System 48 positive pressure manifold (SPEware Corp, Baldwin Park, CA, USA). Analytical chromatography was performed on a Kinetex C18 column (50×2.1 mm; 2.6 μm particle size) combined with a KrudKatcher Ultra frit purchased from Phenomenex. Quantisal™ oral fluid collection devices were from Immunalysis Corp. (Pomona, CA, USA). Oral synthetic THC, Marinol®, was from Unimed Pharmaceuticals (Marietta, GA, USA).
Instrumentation
Tandem mass spectrometry was performed on an ABSciex 5500 QTrap® triple quadrupole/linear ion trap mass spectrometer with a TurboIonSpray source (ABSciex, Foster City, CA, USA). The high-performance liquid chromatography (HPLC) system consisted of a DGU-20A3 degasser, LC-20ADxr pumps, SIL-20ACxr autosampler, and a CTO-20 column oven (Shimadzu Corp, Columbia, MD, USA). Data were acquired and analyzed with Analyst software version 1.6.1.
Calibrators, quality control, and internal standards
Blank oral fluid for preparation of calibrators and quality controls was collected anonymously via expectoration from volunteers in our laboratory and was evaluated with the methodology detailed in this manuscript to ensure absence of detectable THCCOOH prior to fortification with working stock solutions to prepare calibrators and quality control samples.
Primary stock solution containing THCCOOH at 10 μg/mL was prepared in methanol. Dilutions of the stock solution created calibrators at 12, 30, 90, 360, 720, and 1,020 pg/mL when fortifying 25 μL standard solution into 250 μL of blank oral fluid. All sample concentrations are expressed as picogram per milliliter neat oral fluid throughout this manuscript.
Quality control (QC) samples were prepared with different lot numbers of reference standard solutions than calibrators. 360, 2250 and 9000 pg/mL QC solutions were prepared in methanol. QC samples were prepared by adding working solutions to 0.25 mL blank oral fluid to yield 36, 225, and 900 pg/mL THCCOOH.
Primary stock solutions of THCCOOH-d9 were diluted in methanol, producing a mixed internal standard working solution of 900 pg/mL. Fifty microliters internal standard working solution was added to 250 μL oral fluid yielding 180 pg/mL THCCOOH-d9. All primary and working solutions were stored at −20 °C in amber glass vials.
Solid phase extraction
Two hundred fifty microliters of blank oral fluid and 750 μL Quantisal buffer were aliquoted into a glass 13×100-mm test tube prior to fortification with native calibrator or control stock solution and internal standard. Glacial acetic acid, 0.5 mL, was added to each specimen before vortexing. Solid phase extraction (SPE) columns were conditioned with 3 mL methanol, water, and 0.1 % hydrochloric acid in water prior to application of prepared samples. Columns were washed with 2 mL water and 2 mL 0.1 % hydrochloric acid in water/acetonitrile (70:30 v/v). Columns were dried at 207 kPA for 10 min prior to eluting analytes into clean glass centrifuge tubes with 2 mL of hexane/ethyl acetate/glacial acetic acid (78:20:2 v/v/v). Eluates were dried completely under nitrogen at 40 °C in a Zymark TurboVap. Residues were reconstituted in 150 μL mobile phase A/B (50:50 v/v), vortexed briefly before centrifugation at 2,000×g, 4 °C for 5 min, and transferred to autosampler vials containing 200 μL glass inserts. Fifty microliters was injected onto the LCMSMS instrument.
LCMSMS
Chromatographic separation was performed on a Kinetex C18 column equipped with a KrudKatcher Ultra frit. Gradient elution was performed with (A) 0.01 % acetic acid in water and (B) 0.01 % acetic acid in methanol at a flow rate of 0.5 mL/min. The initial gradient conditions were 20 % B, held for 1 min, then increased to 60 % B at 1.5 min and increased to 98 % B over 2 min. Ninety-eight percent of B was maintained for 3.5 min, at which time the column was reequilibrated to 20 % B over 0.1 min and held for 1.9 min (total runtime, 9 min). Flow rate was increased to 1.0 mL/min at 3.7 to 7.2 min to increase column rinsing efficiency. HPLC eluent was diverted to waste for the first 2.0 min and the final 4 min of analysis. The column oven and autosampler were maintained at 40 and 4 °C, respectively. Mass spectrometric data were collected via negative mode electrospray ionization (ESI). MS/MS parameter settings (Table 1) were optimized via direct infusion of individual analytes (10 ng/mL in methanol) at 10 μL/min. Optimized source parameters were: gas-1, 55; gas-2, 55; curtain gas, 45; source temperature, 750 °C. Nitrogen collision gas was set at medium for all experiments. Quadrupoles one and three were set to unit resolution. Quantifier and qualifier ion transitions were monitored for THCCOOH and THCCOOH-d9.
Table 1.
Liquid chromatography–tandem mass spectrometry parameters for THCCOOH in oral fluid
| Analyte | Q1 mass (amu) | Q3 mass (amu) | Dwell time (ms) | Declustering potential (V) |
Entrance potential (V) |
Collision energy (V) |
Cell exit potential (V) |
Retention time (min) |
|---|---|---|---|---|---|---|---|---|
| THCCOOH | 342.9 | 245.2 | 20 | −140 | −10 | −38 | −21 | 3.53 |
| 342.9 | 191.0 | 20 | −140 | −10 | −42 | −21 | ||
| THCCOOH-d9 | 351.9 | 254.2 | 20 | −160 | −10 | −38 | −26 | 3.51 |
| 351.9 | 194.0 | 20 | −160 | −10 | −51 | −13 |
Bold masses depict quantification transitions
Q1 quadrupole 1, Q3 quadrupole 3
Data analysis
Peak area ratios of THCCOOH to THCCOOH-d9 were calculated for each concentration to construct daily calibration curves via linear least squares regression with a 1/x2 weighting factor. THCCOOH calibration curves were linear from 12 to 1,020 pg/mL.
Method validation
Specificity, sensitivity, linearity, imprecision, analytical recovery, extraction efficiency, matrix effect, stability, dilution integrity, and carry-over were evaluated during method validation.
Specificity
Analyte peak identification criteria were relative retention time within ±0.1 min of the lowest calibrator and qualifier/quantifier transition peak area ratios ±20 % of mean calibrator transition ratios. Potential endogenous interferences were assessed by analyzing 12 oral fluid specimens from different individuals. In addition, potential interferences from commonly used drugs were evaluated by fortifying drugs into low QC samples prepared along with calibrators in neat solutions. Final interferent concentrations were 1,000 ng/mL cocaine, benzoylecgonine, norcocaine, norbenzoylecgonine, ecgonine ethyl ester, ecgonine methyl ester, ecgonine, anhydroecgonine methyl ester, m-hydroxycocaine, p-hydroxycocaine, m-hydroxybenzoylecgonine, p-hydroxybenzoylecgonine, morphine, normorphine, morphine-3-beta-D-glucuronide, morphine-6-beta-D-glucuronide, codeine, norcodeine, 6-acetylmorphine, 6-acetylcodeine, buprenorphine, norbuprenorphine, methadone, 2-ethyl-5-methyl-3,3-diphenylpyrroline, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, hydrocodone, hydromorphone, oxycodone, diazepam, lorazepam, oxazepam, alprazolam, amphetamine, methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxyamphetamine, clonidine, ibuprofen, pentazocine, caffeine, aspirin, acetaminophen, phencyclidine, nicotine, cotinine, and norcotinine. No interference was noted if all analytes in the low QC sample quantified within ±20 % of target concentrations with acceptable qualifier/quantifier transition ratios.
Sensitivity and linearity
Limit of detection (LOD) was evaluated in triplicate experiments with duplicates from three different oral fluid sources and defined as the lowest concentration producing a peak eluting within ±0.1 min of analyte retention time for the lowest calibrator, Gaussian peak shape, and qualifier/quantifier transition peak area ratios ±20 % of mean calibrator transition ratios for all replicates. Limit of quantification (LOQ) also was evaluated in triplicate experiments with duplicates from three different oral fluid sources and defined as the lowest concentration that met LOD criteria and measured concentration within ±20 % of target. Performance at the LOQ was confirmed in each batch of specimens.
Preliminary experiments with five sets of calibrators determined the most appropriate calibration model comparing goodness of fit for unweighted linear least squares, linear least squares employing 1/x and 1/x2 weighting. Calibration curves were fit by linear least squares regression with six concentrations across the linear dynamic range for THCCOOH. Calibrators were required to quantify within ±15 %, except ±20 % at LOQ, and correlation coefficients (R2) were required to exceed 0.995.
Analytical recovery and imprecision
Intra-day and inter-day analytical recovery (bias) and imprecision were determined from four replicates at three different QC concentrations across the linear dynamic range of the assay. Analytical recovery was determined by comparing the mean result for all analyses to the nominal concentration value (i.e., mean percent expected concentration). Inter-day imprecision and analytical recovery were evaluated on five different runs with four replicates in each run, analyzed on five separate days (n=20). Imprecision was expressed as percent coefficient of variation (% CV) of the calculated concentrations. The guidelines detailed by Krouwer and Rabinowitz [24] were employed to calculate pooled intra-day, inter-day, and total imprecision.
Extraction efficiency and matrix effect
Extraction efficiency and matrix effect were evaluated via three sets of samples as described by Matuszewski et al. (n=10 for each set) [25]. In the first set, oral fluid samples from ten individuals were fortified with analytes and internal standards prior to SPE. In set 2, oral fluid samples from ten individuals were fortified with analytes and internal standards after SPE, and the third set contained analytes and internal standards in mobile phase. Extraction efficiency, expressed as a percentage, was calculated by dividing analyte mean peak areas of set 1 by set 2. Absolute matrix effect was calculated by dividing the mean peak area of the analyte in set 2 by the mean analyte area in set 3. The value was converted to a percentage and subtracted from 100 to represent the amount of signal suppressed by the presence of matrix. As an additional evaluation of matrix effect, ten blank oral fluid lots were fortified with low QC solution and internal standard and were processed along with calibrators prepared using a separate lot of blank oral fluid to verify accurate quantification.
Stability
Stability was evaluated with blank oral fluid fortified with analytes of interest at low and high QC concentrations (n=3). Short-term temperature stability was evaluated for fortified oral fluid stored in the dark in polypropylene cryovials for 16 h at room temperature, 96 h at 4 °C, and after three freeze–thaw cycles at −20 °C. On the day of analysis, internal standard was added to each specimen and analyzed as described. Autosampler stability was assessed by re-injecting QC specimens after 72 h on the autosampler (4 °C) and comparing calculated concentrations to values obtained against the original calibration curve.
Dilution integrity
Dilution integrity was evaluated by diluting a fortified oral fluid sample (n = 3) containing 900 pg/mL THCCOOH in blank oral fluid/Quantisal buffer to achieve 1:5 (v/v) dilution. Internal standards were added and samples extracted as described. Dilution integrity was maintained if specimens quantified within ±20 % of expected diluted concentration.
Carry-over
Carry-over was investigated in triplicate by injecting extracted blank oral fluid samples containing internal standards immediately after samples containing target analytes at twice the ULOQ. Blank oral fluid specimen injections could not meet LOD criteria to document absence of carry-over.
Clinical study
Oral fluid was collected with Quantisal devices from a single healthy cannabis smoker that provided written informed consent to participate in a National Institute on Drug Abuse Institutional Review Board-approved protocol comparing Sativex oromucosal spray versus oral Marinol pharmacokinetics and pharmacodynamics following controlled administration. The participant resided on a secure research unit during the study. Oral fluid was collected with Quantisal devices from −0.5 to 10.5 h after 5 and 15 mg Marinol oral administration. The device collects 1.0±0.1 mL oral fluid with an absorptive cellulose pad. Pads were placed into a plastic tube containing 3 mL elution/stabilization buffer for at least 24 h to elute drugs. The oral fluid/buffer mixture was decanted into Nunc® cryotubes and was stored at −20 °C until analysis. Participants were required to rinse their mouth with water after eating during the study, and collection devices were visually inspected after collection for presence of blood or other material prior to placement in storage buffer.
Results
Evaluation of potential SPE elution solvents
Initial experiments using the validated SPE columns and procedure but 2 mL 5 % ammonium hydroxide in methanol instead of hexane/ethyl acetate/acetic acid (78:20:2, v/v) elution solvent yielded better THCCOOH recoveries (77.6–83.1 %, n=6) but more matrix suppression (−99.4 to −99.5 %, n=6) than we found with the validated approach (see Table 2).
Table 2.
Mean THCCOOH extraction efficiency and matrix effect for Quantisal oral fluid devices, with authentic oral fluid fortified at 36 (low) and 900 (high) pg/mL concentrations
| Analyte | Extraction
efficiency (%, N=10) |
Matrix effect (% of
signal suppressed, N=10) |
||
|---|---|---|---|---|
| Low | High | Low | High | |
| THCCOOH | 44.6 | 64.5 | −2.4 | 10.8 |
| THCCOOH-d9 | 40.8 | 65.1 | −1.2 | 11.5 |
Specificity
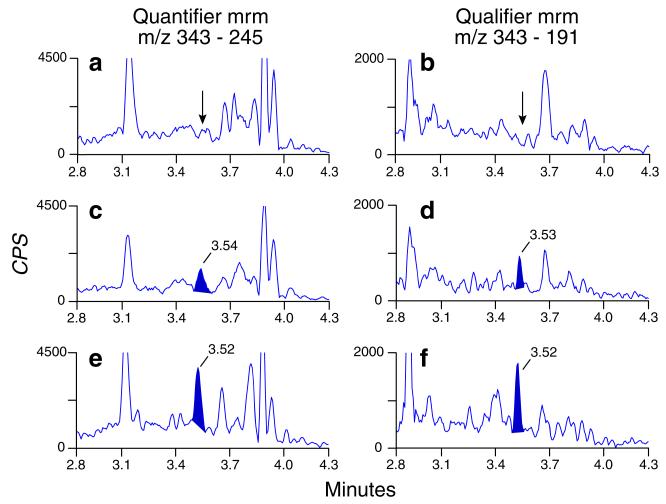
There were no interfering peaks in oral fluid from 12 cannabis-abstinent individuals when mixed with device buffer. None of the 50 potential exogenous interferences fortified at 1,000 ng/mL into neat low QC samples produced transition ratio or quantification criteria failure except for 11-OH-THC and THCCOOH-glucuronide which produced higher than expected QC concentrations. 11-OH-THC and THCCOOH-glucuronide did not significantly alter low QC concentrations (within 80–120 % of expected concentration) when fortified at 10 and 1 ng/mL, respectively. Multiple reaction monitoring ion chromatograms from Quantisal-collected blank oral fluid, Quantisal-collected blank oral fluid fortified at the LOQ, and an authentic specimen collected after 5 mg Marinol oral administration are shown in Fig. 1.
Fig. 1.
Multiple reaction monitoring ion chromatograms for THCCOOH quantifier and qualifier transitions: from a blank oral fluid sample (A and B), blank oral fluid fortified at the 12 pg/mL limit of quantification (C and D), and an authentic specimen containing 26 pg/mL THCCOOH collected 0.25 h after 5 mg oral Marinol (E and F)
Sensitivity and linearity
Initial experiments were conducted with five sets of calibration curves fit via unweighted linear least squares and linear least squares with 1/x and 1/x2 weighting factor to identify the most appropriate calibration model. Inspection of residuals indicated linear least squares with 1/x2 weighting factor produced the best fit for the calibration data. All correlation coefficients exceeded 0.995.
LOD and LOQ were 9 and 12 pg/mL, respectively; THCCOOH linear range was 12 to 1,020 pg/mL. Mean calibration curve slopes were 1.24 (SD=0.08), y intercepts were 0.02 (SD=0.02), and all correlation coefficients (R2) exceeded 0.996.
Analytical recovery and imprecision
Analytical recovery and imprecision were evaluated at three concentrations across the linear dynamic range. Analytical recovery ranged from 85.0 to 113.3 % of expected concentrations for intra-day and inter-day analytical recoveries (Table 3). Pooled intra-day, inter-day, and total imprecision were 4.1–6.6, 0–7.3, and 6.6–8.4 % CV, respectively (Table 3).
Table 3.
Analytical recovery and imprecision data for THCCOOH in oral fluid by liquid chromatography-tandem mass spectrometry
| Concentration (pg/mL) | Analytical recovery (% of
expected concentration) |
Imprecision (% coefficient of
variation, N=20) |
||||||
|---|---|---|---|---|---|---|---|---|
| Intra-day, N
=4 |
Inter-day, N
=20 |
Pooled Intra-day | Inter-day | Total | ||||
| Mean | Range | Mean | Range | |||||
| THCCOOH | 36 | 96.3 | 87.8–110.8 | 97.1 | 85.0–110.8 | 6.6 | 0 | 6.6 |
| 225 | 97.2 | 91.1–104.9 | 100.4 | 89.3–112.4 | 4.1 | 5.7 | 7.0 | |
| 900 | 90.9 | 88.6–96.9 | 98.2 | 87.2–113.3 | 4.2 | 7.3 | 8.4 | |
Extraction efficiency and matrix effect
Extraction efficiencies and matrix effects for THCCOOH in oral fluid are presented in Table 2. Mean extraction efficiencies were 44.6–64.5 %, and mean matrix effects (percent suppressed signal) were −2.4 to 10.8 % (n=10, Table 2).
Stability, dilution integrity, and carry-over
Analytes at low and high QC concentrations were stable for 72 h at 4 °C in the autosampler (Table 4). THCCOOH was stable for 16 h at room temperature, 96 h at 4 °C, and after three freeze/thaw cycles (Table 4).
Table 4.
THCCOOH stability in Quantisal-collected oral fluid, fortified at 36 (low) and 900 (high)pg/mL concentrations
| Analyte | 72 h autosampler (% difference, n=4) |
16 h room temperature (% difference, n=4) |
96 h, 4 °C (% difference, n=4) |
3 Freeze/thaw cycles (% difference, n=4) |
||||
|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | Low | High | |
| THCCOOH | 6.1 | −8.7 | 4.7 | −0.5 | −18.9 | −14.5 | −16.0 | −12.3 |
Dilution integrity was acceptable (within ±20 % of expected diluted concentration) after diluting 1:5 with blank oral fluid/device buffer. There was no evidence of carry-over; negative specimens injected after samples containing twice the ULOQ did not contain analyte peaks satisfying assay LOD criteria (n=3).
Proof of method
The method was applied to measurement of THCCOOH specimens collected with Quantisal devices after administering 5 (Fig. 1) and 15 mg (Table 5) oral synthetic THC (Marinol) to a single participant.
Table 5.
THCCOOH concentrations in oral fluid collected from a single participant with Quantisal device after 15 mg oral synthetic THC (Marinol) administration. The LOQ was 12 pg/mL
| Time since administration (h) | THCCOOH (pg/mL) |
|---|---|
| −0.5 | <LOQ |
| 0.3 | 29.1 |
| 4.5 | <LOQ |
| 7.5 | 55.5 |
| 10.5 | 27.2 |
Discussion
A validated and sensitive LCMSMS method for quantifying THCCOOH in oral fluid is necessary for workplace, pain management, drug treatment, and forensic drug testing programs. Monitoring THCCOOH is critical for distinguishing between passive environmental exposure and active cannabis smoking. THC was identified in oral fluid after passive cannabis exposure with concentrations from 0.3 to 1.2 [13] and 0.7 to 17 ng/mL [14]. THCCOOH was not present following passive cannabis exposure at an LOQ of 2 pg/mL [14]. Another advantage of THCCOOH monitoring is that this analyte exceeded 7.5 pg/mL in oral fluid from abstinent chronic cannabis smokers for up to 29 days of sustained monitored abstinence [15]. Therefore, THCCOOH provides adequate windows of detection for workplace, pain management, drug treatment, and forensic applications, while distinguishing passive exposure from active cannabis intake. THCCOOH also provides more effective monitoring of oral THC administration than THC since THCCOOH was detected in oral fluid for at least 10.5 h after 15 mg oral THC, while THC was undetectable in most participants [16]. THCCOOH was present in 98.2 % of oral fluid samples collected during and after 37 oral 20 mg THC doses, and profiles were similar to THCCOOH plasma profiles; THC was only present in 20.7 % of oral fluid samples, and concentrations actually decreased during oral THC dosing [17, 26]. THCCOOH analytical sensitivity is a significant challenge for oral fluid testing since median oral fluid concentrations were less than 100 pg/mL 1 h after smoking a single 6.8 % THC cigarette [10, 11]. We present a validated method measuring THCCOOH in oral fluid collected with Quantisal device achieving a linear range of 12–1,020 pg/mL of neat oral fluid after solid phase extraction and direct injection onto an LCMSMS triple quadrupole instrument that will be useful for high-throughput drug treatment, pain management, and forensic and workplace testing laboratories.
We demonstrated the utility of the method’s sensitivity and linearity by analyzing oral fluid specimens collected via Quantisal after administration of 15 mg Marinol to a single participant (Table 5). It is interesting that THCCOOH was less than the method LOQ at 4.5 h but was 29.1 and 55.1 pg/mL at 0.3 and 7.5 h, respectively, after Marinol administration. It is important to note that the data are from a single session for a single participant. These specimens from a single participant illustrate the usefulness of our method. Analysis of additional specimens from more participants should reveal whether a decreasing THCCOOH trend at 4.5 h after Marinol is significant. THCCOOH-d9 peak areas were consistent for all specimens, indicating variable SPE recovery is not confounding the participant’s THCCOOH concentration profile. Variable analyte recovery from collection devices may also explain variable THCCOOH concentrations. However, Moore et al. previously reported consistent 80 % THCCOOH recovery from Quantisal devices with an 8.2 % coefficient of variation (n=6) [18].
Most oral fluid collection devices employ elution/stabilization buffers containing detergents that can cause problematic matrix effects during LCMSMS analysis [2, 5]. During method development, we achieved 77.6–83.1 % THCCOOH recoveries but observed greater than 95 % matrix suppression, unacceptable accuracy, and imprecision with a 5 % ammonium hydroxide in methanol elution solvent. Selecting a hexane/ethyl acetate/acetic acid (78:20:2; v/v/v) elution solvent produced lower THCCOOH recoveries but yielded less matrix effect (−2.4 to 10.8 %), accuracies within ±15 % of target concentration, and <10 % imprecision. We achieved our desired assay sensitivity despite lower recovery, and the reduced matrix effect enabled acceptable THCCOOH accuracy and imprecision.
Two reports describe 2D-GCMS methods for THCCOOH in Quantisal-collected oral fluid with a specimen volume equivalent to 250 μL oral fluid, similar to our method, achieving LOQs of 2 and 7.5 pg/mL [18, 19]. Day et al. presented a THCCOOH GCMSMS method for 100 μL oral fluid collected via Intercept device achieving an LOQ of 10 pg/mL [20]. All three GCMS methods employed solid phase extraction and derivatization with trifluoracetic acid [18, 19] or pentafluoroacetic acid anhydride [20] and hexafluoropropanol. Our THCCOOH LCMSMS assay LOQ is similar or slightly less sensitive than these GCMS methods using similar oral fluid specimen volumes while affording time and cost savings by avoiding derivatization.
Lee et al. reported a LCMSMS method for THCCOOH in 250 μL expectorated oral fluid that employed acetonitrile precipitation, derivatization with dansyl chloride, and liquid-liquid extraction prior to LC triple quadrupole MS achieving an LOQ of 5 pg/mL [22]. Coulter et al. recently reported a LCMSMS method for THCCOOH in a Quantisal-collected oral fluid with a specimen volume equivalent to 250 μL oral fluid, similar to our current method, employing solid phase extraction prior to derivatization with triphenylphosphine and 2-picolylamine prior to LC triple quadrupole MS achieving a 10 pg/mL LOQ [21]. Our current LCMSMS method achieves similar LOQs with identical oral fluid specimen volumes while avoiding costly derivatization required for these other two LCMSMS methods [21, 22].
He et al. describe a microflow LC high-resolution MS method for THCCOOH in OralEze-collected samples with a specimen volume including 133 μL oral fluid achieving a 7.5 pg/mL LOQ [23]. This method employed an elaborate DRUF sample pretreatment and online trapping prior to LCMSMS analysis. Samples were dried down and reconstituted in 30 % methanol, centrifuged at 17,000×g for 5 min, and filtered through hydrophilic PTFE filters before injection onto an aQ trapping column and ultimately chromatographed on an aQ LC column with a total runtime of 12.5 min with data acquired at 40,000 resolution during MSMS analysis [23]. Although this method achieves sensitivity appropriate for monitoring THCCOOH in oral fluid, it requires extensive sample pretreatment steps that are not amenable to automation and requires high-resolution instrumentation that is cost prohibitive in many drug testing laboratories. Our current method employs solid phase extraction that could be automated and employs standard triple quadrupole MS instrumentation that is available in most oral fluid testing laboratories.
Quintela et al. reported a THCCOOH high-resolution LC quadrupole-time-of-flight MS method using 167 μL Intercept device-collected oral fluid [27]. This method achieved a 500 pg/mL THCCOOH LOQ that is inadequate for cannabis oral fluid testing.
This is the first validated oral fluid THCCOOH LCMSMS method employing LCMSMS triple quadrupole instrumentation, not requiring derivatization to achieve an LOQ below 15 pg/mL. This method provides an approach appropriate for high-throughput oral fluid drug testing in routine workplace, pain management, drug treatment, and forensic testing laboratories. THCCOOH recoveries were greater than 41 % and matrix effect less than 12 %. Intra- and inter-day accuracy were within ±15 %, with pooled intra-day, inter-day, and total imprecision better than 9.4 % CV. THCCOOH linear range was 12-1,020 pg/mL, which is appropriate for monitoring oral fluid THCCOOH since reports indicate THCCOOH oral fluid concentrations below 763 pg/mL [10, 11, 15, 18, 20, 22, 23, 28]. This LCMSMS method provides a rapid and reliable means of differentiating passive environmental cannabis exposure from active cannabis intake.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Contributor Information
Karl B. Scheidweiler, Chemistry and Drug Metabolism, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Biomedical Research Center, 251 Bayview Boulevard Suite 200 Room 05A-721, Baltimore, MD 21224, USA
Sarah K. Himes, Chemistry and Drug Metabolism, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Biomedical Research Center, 251 Bayview Boulevard Suite 200 Room 05A-721, Baltimore, MD 21224, USA
Xiaohong Chen, ABSciex, 353 Hatch Drive, Foster City, CA 94404, USA.
Hua-Fen Liu, ABSciex, 353 Hatch Drive, Foster City, CA 94404, USA.
Marilyn A. Huestis, Chemistry and Drug Metabolism, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Biomedical Research Center, 251 Bayview Boulevard Suite 200 Room 05A-721, Baltimore, MD 21224, USA
References
- 1.Drummer OH. Drug testing in oral fluid. Clin Biochem Rev. 2006;27(3):159. [PMC free article] [PubMed] [Google Scholar]
- 2.Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. Clin Chem. 2009;55(11):1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langel K, Engblom C, Pehrsson A, Gunnar T, Ariniemi K, Lillsunde P. Drug testing in oral fluid-evaluation of sample collection devices. J Anal Toxicol. 2008;32(6):393–401. doi: 10.1093/jat/32.6.393. [DOI] [PubMed] [Google Scholar]
- 4.Huestis MA, Verstraete A, Kwong TC, Morland J, Vincent MJ, De La Torre R. Oral fluid testing: promises and pitfalls. Clin Chem. 2011;57(6):805–810. doi: 10.1373/clinchem.2010.152124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood M, Laloup M, Ramirez Fernandez Mdel M, Jenkins KM, Young MS, Ramaekers JG, De Boeck G, Samyn N. Quantitative analysis of multiple illicit drugs in preserved oral fluid by solid-phase extraction and liquid chromatography-tandem mass spectrometry. Forensic Sci Int. 2005;150(2-3):227–238. doi: 10.1016/j.forsciint.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration . Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. U.S. Department of Health and Human Services; Rockville: 2012. NSDUH Series H-44. [Google Scholar]
- 7.Matsunaga T, Iwawaki Y, Watanabe K, Yamamoto I, Kageyama T, Yoshimura H. Metabolism of delta-9-tetrahydrocannabinol by cytochrome P450 isozymes purified from hepatic microsomes of monkeys. Life Sci. 1995;56:2089–2095. doi: 10.1016/0024-3205(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007;80(15):1415–1419. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Huestis MA, Cone EJ. Relationship of delta 9-tetrahydrocannabinol concentrations in oral fluid and plasma after controlled administration of smoked cannabis. J Anal Toxicol. 2004;28(6):394–399. doi: 10.1093/jat/28.6.394. [DOI] [PubMed] [Google Scholar]
- 10.Lee D, Schwope DM, Milman G, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid disposition in oral fluid after controlled smoked cannabis. Clin Chem. 2012;58(4):748–756. doi: 10.1373/clinchem.2011.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milman G, Schwope DM, Gorelick DA, Huestis MA. Cannabinoids and metabolites in expectorated oral fluid following controlled smoked cannabis. Clin Chim Acta. 2012;413(7-8):765–770. doi: 10.1016/j.cca.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niedbala S, Kardos K, Salamone S, Fritch D, Bronsgeest M, Cone EJ. Passive cannabis smoke exposure and oral fluid testing. J Anal Toxicol. 2004;28(7):546–552. doi: 10.1093/jat/28.7.546. [DOI] [PubMed] [Google Scholar]
- 13.Niedbala RS, Kardos KW, Fritch DF, Kunsman KP, Blum KA, Newland GA, Waga J, Kurtz L, Bronsgeest M, Cone EJ. Passive cannabis smoke exposure and oral fluid testing. II. Two studies of extreme cannabis smoke exposure in a motor vehicle. J Anal Toxicol. 2005;29(7):607–615. doi: 10.1093/jat/29.7.607. [DOI] [PubMed] [Google Scholar]
- 14.Moore C, Coulter C, Uges D, Tuyay J, van der Linde S, van Leeuwen A, Garnier M, Orbita J., Jr Cannabinoids in oral fluid following passive exposure to marijuana smoke. Forensic Sci Int. 2011;212(1-3):227–230. doi: 10.1016/j.forsciint.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Lee D, Milman G, Barnes AJ, Goodwin RS, Hirvonen J, Huestis MA. Oral fluid cannabinoids in chronic, daily cannabis smokers during sustained, monitored abstinence. Clin Chem. 2011;57(8):1127–1136. doi: 10.1373/clinchem.2011.164822. [DOI] [PubMed] [Google Scholar]
- 16.Lee D, Karschner EL, Milman G, Barnes AJ, Goodwin RS, Huestis MA. Can oral fluid cannabinoid testing monitor medication compliance and/or cannabis smoking during oral THC and oromucosal Sativex administration? Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.10.011. doi:10.1016/j.drugalcdep.2012.10.011, epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milman G, Barnes AJ, Schwope DM, Schwilke EW, Darwin WD, Goodwin RS, Kelly DL, Gorelick DA, Huestis MA. Disposition of cannabinoids in oral fluid after controlled around-the-clock oral THC administration. Clin Chem. 2010;56(8):1261–1269. doi: 10.1373/clinchem.2009.141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore C, Coulter C, Rana S, Vincent M, Soares J. Analytical procedure for the determination of the marijuana metabolite 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid specimens. J Anal Toxicol. 2006;30(7):409–412. doi: 10.1093/jat/30.7.409. [DOI] [PubMed] [Google Scholar]
- 19.Milman G, Barnes AJ, Lowe RH, Huestis MA. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J Chromatogr A. 2010;1217(9):1513–1521. doi: 10.1016/j.chroma.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day D, Kuntz DJ, Feldman M, Presley L. Detection of THCA in oral fluid by GC-MS-MS. J Anal Toxicol. 2006;30(9):645–650. doi: 10.1093/jat/30.9.645. [DOI] [PubMed] [Google Scholar]
- 21.Coulter C, Garnier M, Moore C. Analysis of tetrahydrocannabinol and its metabolite, 11-nor-delta(9)-tetrahydrocannabinol-9-carboxylic acid, in oral fluid using liquid chromatography with tandem mass spectrometry. J Anal Toxicol. 2012;36(6):413–417. doi: 10.1093/jat/bks039. [DOI] [PubMed] [Google Scholar]
- 22.Lee PD, Chang YJ, Lin KL, Chang YZ. Simultaneous determination of delta9-tetrahydrocannabinol and 11-nor-9-carboxy-delta9-tetrahydrocannabinol in oral fluid using isotope dilution liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2012;402(2) doi: 10.1007/s00216-011-5439-8. [DOI] [PubMed] [Google Scholar]
- 23.He X, Kozak M, Nimkar S. Ultra-sensitive measurements of 11-nor-delta(9)-tetrahydrocannabinol-9-carboxylic acid in oral fluid by microflow liquid chromatography-tandem mass spectrometry using a benchtop quadrupole/orbitrap mass spectrometer. Anal Chem. 2012;84(18):7643–7647. doi: 10.1021/ac3019476. [DOI] [PubMed] [Google Scholar]
- 24.Krouwer JS, Rabinowitz R. How to improve estimates of imprecision. Clin Chem. 1984;30(2):290–292. [PubMed] [Google Scholar]
- 25.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75(13):3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 26.Milman G, Schwope DM, Schwilke EW, Darwin WD, Kelly DL, Goodwin RS, Gorelick DA, Huestis MA. Oral fluid and plasma cannabinoid ratios after around-the-clock controlled oral delta9-tetrahydrocannabinol administration. Clin Chem. 2011;57(11):1597–1606. doi: 10.1373/clinchem.2011.169490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quintela O, Andrenyak DM, Hoggan AM, Crouch DJ. A validated method for the detection of delta(9)-tetrahydrocannabinol and 11-nor-9-carboxy-delta(9)-tetrahydrocannabinol in oral fluid samples by liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. J Anal Toxicol. 2007;31(3):157–164. doi: 10.1093/jat/31.3.157. [DOI] [PubMed] [Google Scholar]
- 28.Moore C, Rana S, Coulter C, Day D, Vincent M, Soares J. Detection of conjugated 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid. J Anal Toxicol. 2007;31(4):187–194. doi: 10.1093/jat/31.4.187. [DOI] [PubMed] [Google Scholar]