Abstract
Vaccines for opioid dependence may provide a treatment that would reduce or slow the distribution of the drug to brain, thus reducing the drug's reinforcing effects. We tested whether a conjugate vaccine against morphine (keyhole limpet hemocyanin-6-succinylmorphine; KLH-6-SM) administered to rats would produce antibodies and show specificity for morphine or other heroin metabolites. The functional effects of the vaccine were tested with antinociceptive and conditioned place preference (CPP) tests. Rats were either vaccinated with KLH-6-SM and received two boosts 3 and 16 weeks later or served as controls and received KLH alone. Anti-morphine antibodies were produced in vaccinated rats; levels increased and were sustained at moderate levels through 24 weeks. Antibody binding was inhibited by free morphine and other heroin metabolites as demonstrated by competitive inhibition ELISA. Vaccinated rats showed reduced morphine CPP, tested during weeks 4 to 6, and decreased antinociceptive responses to morphine, tested at week 7. Brain morphine levels, assessed using gas-chromatography coupled to mass spectrometry (GC–MS) on samples obtained at 26 weeks, were significantly lower in vaccinated rats. This suggests that morphine entry into the brain was reduced or slowed. These results provide support for KLH-6-SM as a candidate vaccine for opioid dependence.
Keywords: Antinociception, Conditioned place preference, Heroin metabolites, Immunotherapy, Opioid dependence
1. Introduction
Opioid dependence is a serious health problem in the United States and world-wide (UNODC, 2010). Relapse rates remain high even though there are approved pharmacological treatments for this disorder (Veilleux et al., 2010). Medications currently approved to treat opioid dependence include methadone, buprenorphine, and naltrexone (Maxwell and Shinderman, 2002; Stotts et al., 2009). These agents act at opioid receptors as an agonist, a partial agonist, or an antagonist, respectively. While each of these treatments shows some therapeutic success, there are numerous limitations to their use, including high cost, limited availability, problems with compliance, and, in the case of the agonists, diversion (Kahan et al., 2001; Kreek et al., 2010; Stotts et al., 2009). An alternative strategy to developing a treatment for opioid dependence is an anti-opioid vaccine (Shen et al., 2012).
The approach for the development of drug addiction vaccines differs from the approach used in the development of the other medications. Whereas the opioidergic treatment agents were designed to target opioidergic effector systems in the brain through pharmacodynamic mechanisms, vaccines act as “pharmocokinetic” antagonists. Upon administration, the vaccine stimulates the production of drug-specific antibodies that can bind to the drug in the circulating blood and extracellular fluid when the drug is ingested. This action should reduce or slow the distribution of the drug to brain and theoretically attenuate the drug's reinforcing or addictive effects. Support for the vaccine approach has been shown by the promising results obtained in clinical trials for vaccines designed for the treatments of cocaine and nicotine addiction (Haney et al., 2010; Hatsukami et al., 2011; Martell et al., 2009).
Heroin, which is arguably the most serious of all opioid addictions, is a pro-drug; that is, its effects occur mainly through its metabolites. Heroin is readily hydrolyzed by serum and liver esterases to the more stable compounds, 6-acetylmorphine (6-AM) and morphine. Morphine itself can then undergo further metabolism into morphine-3-glucuronide (3-GM) and morphine-6 glucuronide (6-GM) via enzymes in liver and kidney (Inturrisi et al., 1983; Selley et al., 2001). In fact, 6-AM is considered to cause the immediate euphoric effects of heroin administration. Thus, a vaccine that can produce antibodies to heroin, morphine, and 6-AM is desirable.
While vaccines for stimulant addictions have been developed fairly recently, vaccines directed at morphine were first tested in animals 40 years ago (Berkowitz and Spector, 1972; Bonese et al., 1974). The vaccine was prepared by conjugating a morphine hapten to bovine serum albumin (BSA) through a 6-succinylmorphine (6-SM) linkage to lysine residues on BSA. These early studies demonstrated that this vaccine could produce antibodies with specificity for heroin and 6-AM,aswell as for morphine. Other studies showed that binding specificity differed depending upon the hapten used (Koida et al., 1974; VanVunakis et al., 1972; Wainer et al., 1973). The study of vaccines for opioid dependence likely fell out of favor after these initial studies due to the introduction of methadone as an effective treatment agent. However, as discussed above, there are limitations and problems with the standard medications for opioid dependence. Thus, the vaccine approach for opioid dependence is being reinvestigated (Anton and Leff, 2006; Anton et al., 2009; Ma et al., 2006; Stowe et al., 2011). For example, a bivalent morphine–heroin vaccine developed using tetanus toxoid as the carrier protein produced antibodies and prevented the acquisition of heroin self-administration in rats (Anton and Leff, 2006; Anton et al., 2009). However, this vaccine required four boosts over 60 days, and biweekly boosts over a year in order to maintain adequate titers. In another study, rats administered a heroin vaccine based on a hapten structure where the linker was attached to the nitrogen of nor-heroin showed reduced heroin-induced antinociception and acquisition of heroin self-administration (Stowe et al., 2011). However, rats administered a vaccine that was also based on nor-morphine did not show changes in heroin self-administration even though both vaccines generated antibodies. This failure to show functional effects of the vaccine may reflect that it had no affinity for 6-AM (Stowe et al., 2011).
The purpose of this study was to evaluate a morphine vaccine consisting of a KLH-6-SM conjugate and aluminum (alum) in rats. We investigated the immunogenicity of KLH-6-SM at its optimal dose for eliciting effective antibodies and tested its ability to attenuate opioid-induced behavioral effects. These behavioral effects included the analgesic and rewarding effects of morphine using hot plate, tail-flick, and place conditioning procedures. In addition, a competitive inhibition ELISA was used to assess antibody binding to free morphine, 6-AM, 3-GM, and 6-GM.
We also hypothesized that the vaccine-induced antibodies would sequester the morphine in the blood, thereby increasing in blood levels and reducing brain levels. This was tested in the present study using gas chromatography combined with mass spectrometry.
2. Methods
2.1. Animals and housing
Male, Sprague Dawley rats were purchased from Charles River Laboratories (Wilmington, MA, USA) at about 8 weeks of age and 250 g body weight. They were group-housed (3 per cage) under temperature- and humidity-controlled conditions with a 12:12 h light/dark cycle (lights on from 0600). Food and water were available ad libitum. Procedures were approved by the Institutional Animal Care and Use Committee in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (NIH, 1996).
2.2. Groups
A total of 54 rats were employed in the studies. Half of the rats were vaccinated with KLH-6-SM as described below and the other half served as naïve, non-vaccinated controls. The 27 vaccinated rats consisted of three groups (n = 9 ea) that were administered one of three amounts of vaccine conjugate at the second boost session (see below). One group (n = 9) of vaccinated rats was tested for antinociception and compared to a sub-group (n = 9) of naïve rats and both of these groups were used at the termination of the protocol to provide blood and tissue samples. All of the vaccinated and naïve rats were used for the conditioned place preference (CPP) study.
2.3. Drug
Morphine sulfate was purchased from Noramco Inc. (Wilmington, DE). 6-AM, 3-GM, and 6-GM were obtained from the National Institute on Drug Abuse (Chemistry and Physiological Systems Research Branch, Bethesda, MD). All drug doses and concentrations were expressed as salt free base. Morphine was administered at a dose of 2 mg/kg (S.C.) in a volume of 5 ml/kg for the hot plate and tail flick tests. A dose of 1 and 2 mg/kg (S.C.) in a volume of 4 ml/kg was used for the CPP study.
2.4. Vaccine preparation and administration
2.4.1. Preparation of protein conjugates
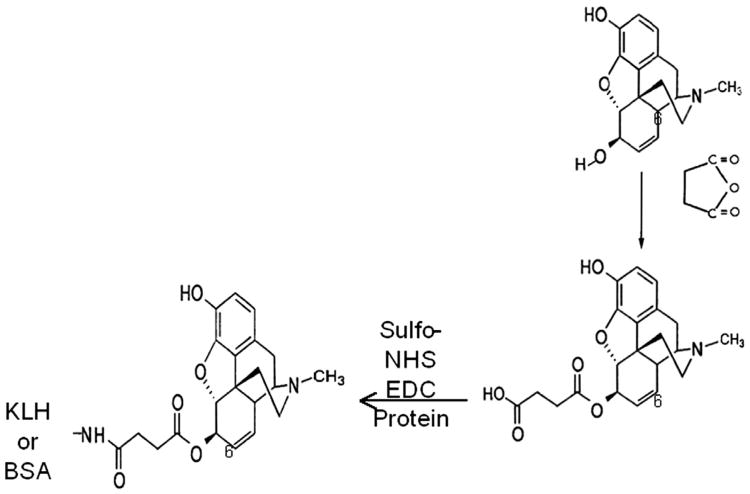
6-succinyl morphine (6-SM) was prepared according to a previously published method (Simon et al., 1972). To prepare the KLH conjugate, 14 mg (0.07 mmol, 1.2 M equivalents over 6-SM) of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC, Sigma-Aldrich, Milwaukee WI) and 20 mg (0.09 mmol, 1.5 M equivalents over 6-SM) of N-hydroxysulfosuccinimide (sulfoNHS Sigma-Aldrich, Milwaukee WI) were added to 0.5 mL phosphate-buffered saline (PBS) and the solution stirred for 20 min at room temperature. 6-SM (23 mg, 0.06 mmol) was added and stirring continued for 1 h. KLH (2 mL,10 mg/mL) was prepared by adding 2 mL H2O and 89 mg NaCl to a vial containing freeze-dried Imject KLH/PBS (Thermo-Fisher Scientific, Rockford, Ill). The solution of activated 6-SM was added to the KLH solution and the pH adjusted to 7.4 with 2 M NaOH. The resulting clear solution was stirred overnight and purified over a NAP-25 column (GE Healthcare, Piscataway, NJ) equilibrated with PBS, following the manufacturer's instructions. The final KLH-6-SM conjugate (3.5 mL, 5.7 mg/mL) was filtered through a 0.45 μm filter (Millipore, Cork, Ireland) and stored at 4 °C until use. The BSA-6-SM conjugate was synthesized using the same method as for KLH, except that NaCl was not added to the 2 mL of 10 mg/mL Imject KLH/PBS BSA. The morphine conjugate structure is depicted in Fig. 1.
Fig. 1.
The structure shows the synthesis of keyhole limpet hemocyanin-6-succinyl morphine (KLH-6-SM). The KLH-6-SM protein conjugate is produced by the reaction between 6-SM and keyhole limpet hemocyanin or bovine serum albumin (BSA) using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (sulfoNHS).
2.4.2. Vaccination schedule
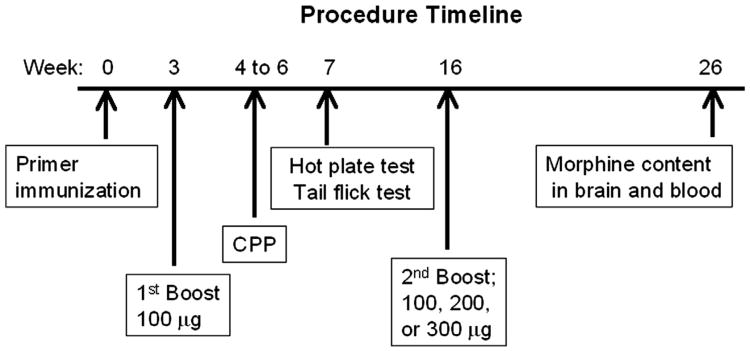
The experimental procedure schedule is shown in Fig. 2. Rats were vaccinated via intramuscular (IM) injection with 100 μg of KLH-6-SM mixed with 1500 μg alum (Sigma-Aldrich) at week 0. All rats were boosted with 100 μg of the KLH-6-SM conjugate at 3 weeks. At 16 weeks, rats received another boost injection using 100 μg, 200 μg, and 300 μg of KLH-6-SM morphine conjugate with 1500 μg, 3000 μg or 4500 μg alum, respectively (n = 9 ea). The control rats received an injection of KLH alone at the same time as the vaccinated rats. The CPP study was conducted between weeks 4 to 6 and the hot plate and tail flick tests were performed at week 7 as shown in Fig. 2. Finally, blood and brain tissue samples were obtained at week 26.
Fig. 2.
The timeline of procedures is shown by week beginning with the primer immunization (week 0) when rats were administered either the morphine vaccine (100 μg KLH-6-SM; n = 27) or the carrier protein (KLH) in order to serve as non-vaccinated controls (n = 27). The first vaccine booster of the same amount was given at week 3. The CPP study was conducted between weeks 4 to 6 and the hot plate and tail flick tests were conducted at week 7. At week 16, three vaccine amounts (100, 200, or 300 μg) were administered (n = 9/amount) as the second boost. Finally, morphine content was determined using GC–MS from blood and brain samples obtained at week 26.
2.5. Anti-morphine antibody assessments
2.5.1. Determination of serum antibody levels
Blood samples were collected at weeks 2, 4, 6, 8, 12, 18, 20 and, 24 weeks after the initial vaccination and allowed to clot at room temperature for 2-h. Samples were centrifuged (4000 rpm for 15 min) and sera collected and stored at −80 °C until ELISAs were performed. To measure specific anti-morphine antibody, ELISA plates (Immulon 2HB, Daigger, Vernon Hills, IL) were coated overnight in 100 μl carbonate buffer (0.05 M, pH 9.6, Sigma-Aldrich, St. Louis, MO) containing 0.02 μg/ml of BSA-6-SM, a conjugate using heterologous carrier protein from KLH-6-SM. The serum samples (100 μl per well) were added to the plates in threefold serial dilutions starting at 1:1000 in PBS-0.1% Tween and incubated for 2 h. After washing with PBS-Tween, goat anti-rat IgG conjugated to horseradish peroxidase (1:10,000, Southern Biotech, Birmingham, AL) was then incubated in the plates for 35 min. The plates were again washed and then 100 μl substrate (Tetramethylbenzidine, Sigma-Aldrich, Milwaukee WI) was incubated in the plates for 35 min. Reactions were stopped with 1 M HCl and the optical density was measured on a microplate reader (LabX, Midland, ON, Canada). Background antibody binding to BSA alone was subtracted from every sample to ensure that the results reflect antibodies specific for morphine. Optical density readings in the linear range of the standard curve obtained from rat IgG (Sigma-Aldrich, Milwaukee WI) were used to calculate the concentration of antibody from appropriate dilutions.
2.5.2. Antibody specificity test
A competitive inhibition ELISA was conducted using pooled sera obtained from rats 22 weeks after initial vaccination. ELISA plates were pre-incubated with morphine or with other heroin metabolites including 6-AM, 3-GM, or 6-GM, prior to conducting ELISA as described above. The concentrations used in pre-incubation ranged from 1 nM to 10 mM for all chemicals used. Serum samples were also tested against xylocaine as a negative control. The drug concentrations that produced 50% inhibition of maximum binding of drug to anti-morphine antibodies, IC50, were calculated using non-linear fit analysis.
2.6. Conditioned place preference test
In this study, 27 vaccinated rats and 27 naïve rats were non-systematically assigned to one of three morphine doses (0, 1 or 2 mg/kg) training groups (n = 9 ea). Morphine doses were chosen based on our previous work and on published reports (Carr et al., 1989; Cicero et al., 2000; Reid et al., 1985). Rats were administered the same dose of morphine on all drug training days. This study was conducted during weeks 4 to 6 of the vaccination protocol.
2.6.1. Place conditioning apparatus
Four custom-made place preference apparatuses (25″W × 7″ D × 15″H) constructed of acrylic, were used. One of the two compartments of each apparatus had black walls and coarse, wire mesh floors. The other conditioning compartment had black and white horizontal striped walls and fine mesh floors. A small middle section had wide aluminum bars on the floor. Lighting (no-heat LED units) in each apparatus had been adjusted during pilot work to optimize an unbiased situation (e.g., rats spent similar amounts of time in each compartment). Pixxo digital video cameras (Pixxo, City of Industry, CA) connected to standard Windows laptops using AMCap video software were placed above each apparatus. Baseline and test sessions were video-recorded and analyzed later using ANY-maze software (Stoelting Co., Wood Dale, IL).
2.6.2. Place conditioning procedure
We utilized an unbiased procedure. That is, the compartment assigned to be the drug-paired side was non-systematic across rats and was independent of the times spent on the side prior to conditioning trials. On conditioning days, an acrylic barrier was inserted to separate the compartments and confine the animal to one side. On baseline and test days, the barrier was not in place to allow the animal access to both compartments. The animal was placed in the small middle section facing a wall at the start of these 30-min test sessions. All conditioning sessions were 60 min in duration and all test and conditioning sessions were conducted between 1000 and 1700 h. The room lights were off during all sessions. Each compartment was cleaned thoroughly after each session with Clidox (Pharmacal Research Laboratories Inc., Naugatuck CT, USA).
The first session, the Baseline session, provided data on times spent in each compartment. If any rat showed a bias towards one compartment (i.e., spent >80% time on one side), it was excluded from the study. Drug conditioning trials started the day after the Baseline session. There was one conditioning trial per day alternating between vehicle and drug trials beginning with a drug training trial (e.g., D, V, D, V) for eight consecutive days, excluding weekends. During drug conditioning trials, the rat was immediately confined to one compartment of the apparatus after either morphine or vehicle injection. Rats assigned to the 0 mg/kg morphine group received saline on all training trials although their placement in the two compartments alternated each conditioning session in the same way as for the rats in the two active morphine dose training groups. Following the last conditioning trial, a test session was conducted in the same manner as the Baseline test (i.e., it was conducted with no drug injections).
The time (seconds) spent on the drug-paired side on the Test day was subtracted from the time spent on this side at Baseline for each rat. This number represents their conditioned place preference (CPP) value in which greater, positive numbers reflect an increase in appetitive behavior. These data were analyzed using a 2 × 3 ANOVA for the between group factors of Vaccine (naïve, vaccinated) and Morphine dose (0, 1, and 2 mg/kg) with the p value set at 0.05. Significant main effects or interaction effect were followed by post-hoc, Fisher LSD tests.
2.7. Antinociceptive tests
Nine vaccinated rats and nine, naïve controls were employed in this study. Morphine-induced antinociception was assessed using both tail-flick and hot-plate assays. These tests were conducted during week 7 of the protocol on the same day in succession, 30-min after the morphine administration (2 mg/kg), with the tail-flick test performed first.
2.7.1. Antinociception apparatuses
The antinociceptive effects of morphine were tested with the tail flick and hot plate apparatuses in this order. For the tail flick test, radiant heat was applied to the tail 3 cm from the tip using a tail flick apparatus (Ugo Basile, Collegeville, PA). The intensity of the radiant heat had been adjusted previously so that baseline latencies would fall between 2 and 4 s. The time from the onset of the heat to the withdrawal of the tail (latency) was measured. To avoid tissue damage, the heat stimulus was discontinued after 14 s (cut-off latency). For the hot plate test, rats were placed on a 50 °C hot plate (Columbus Instruments, Columbus, OH) and the latency to paw lick was recorded. The cut-off time for this assay was 20 s.
2.7.2. Antinociception procedure
Baseline latencies for both assays were obtained three times for each animal prior to the morphine administration. Then, the response latencies of each animal were determined three times at the time of peak drug effect (30-min post-morphine administration) with the tail flick test performed first. The mean of each set of three measurements (baseline and post-morphine administration) were determined for each animal. These latency data from both assays were converted to percent of Maximal Possible Effect (%MPE = [cut-off time – baseline time] * 100%). Percent MPEs for both assays were compared between naïve and vaccinated groups using two separate t-tests with the p value set at 0.05.
2.8. Morphine brain and blood levels
Control (n = 7) and vaccinated (n = 6) rats were injected with morphine (4 mg/kg, SC) at 26 weeks. Sixty minutes later, the rats were euthanized deeply and decapitated. Trunk blood was collected into a K2 EDTA blood collection tube (BD, Franklin Lakes, NJ, USA). The brain was removed and rinsed three times in PBS. All samples were placed immediately on dry ice and then stored at −80° until assays were performed.
Carbonate buffer (pH 9.5) was added to both blood and pre-weighted brain samples. The samples were homogenized for 30–40 s using a disperser (IKA® Works, Inc., NC, USA), and subjected to liquid/liquid extraction using a standard solvent mixture (Toluene 50, Ethyl Acetate 30, Butyl Chloride 15, and Isopropanol 5). The extracted morphine was converted to a trimethylsilyl derivative with the addition of N,O-bis-trimethylsilyl-trifluoroacetamide/Trimethylchlorosilane (BSTFA/TMCS), and quantified by gas-chromatography coupled to mass spectrometry (GC–MS, Agilent Technologies, Santa Clara CA, USA). Standard curves were prepared using bovine serum (Equitech-Bio, Kerrville, TX) and were linear in the range of 5–500 ng/ml for morphine. The internal standards were the deuterated analogs of morphine (Cerilliant Analytical Reference Standards, Round Rock, TX). Samples were analyzed on a Agilent Technologies 7890 GC coupled to 5975 MS. Total run time was 10 min. Validation was based on a six point standard curve run in duplicate.
Brain and serum levels of morphine were compared between control and vaccinated groups in two, separate t-tests with the p value set at 0.05.
3. Results
3.1. Antibody assessments
3.1.1. Serum anti-morphine antibody levels
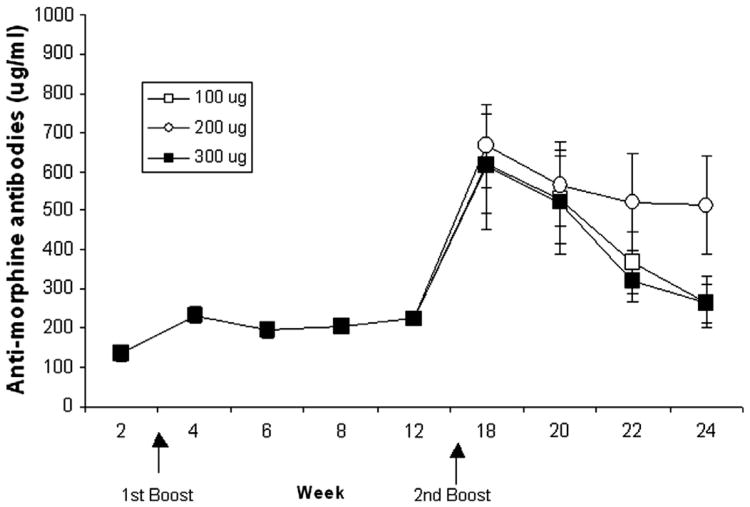
Anti-morphine antibody levels were determined by ELISA. As shown in Fig. 3, anti-morphine antibody levels were low initially but increased (∼200 μg/ml) after the first boost at week 3 and were maintained in that range until week 12 of the protocol. The second boost, performed at week 16, tested three vaccine amounts (100, 200, and 300 μg of vaccine) in separate groups of rats. Antibody levels increased 3-fold after this boost, peaked at week 18 and then the levels of antibodies declined over the following weeks. The decline in antibody levels over these weeks was significant, F(3, 63) = 15.85; P < 0.0001. As showed in Fig. 3, there was no significant difference in antibody levels across vaccine amount groups, P > 0.10.
Fig. 3.
Mean (±S.E.M.) anti-morphine antibody concentrations (μg/ml) in sera obtained from vaccinated rats over weeks are shown. All rats were immunized and boosted at week 3 with 100 μg of KLH-6-SM. The second boost was given at week 16 using 100 μg (open circle, n = 9), 200 μg (open square, n = 9), or 300 μg (solid square, n = 9) of vaccine (arrows indicate weeks of boosts). There was no group difference in antibody levels, P > 0.10, although concentrations decreased significantly from weeks 18 to 24, P < 0.0001.
3.1.2. Specificity of antibodies
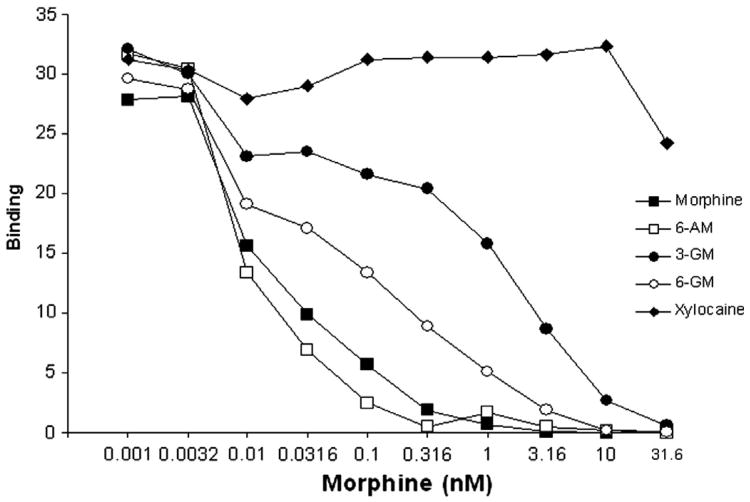
Competitive inhibition ELISA was used to determine the specificity of the antibodies produced. As seen in Fig. 4, antibody binding to morphine was competitively inhibited by morphine, 6-AM, 3-GM, and 6-GM across increasing concentrations of the drugs. As expected, the unrelated drug, xylocaine, did not inhibit morphine antibody binding. The IC50 values are presented in Table 1. These results showed that the anti-morphine antibodies displayed high binding affinity for morphine 6-AM and had moderate specificity for 6-GM. 3-GM showed 10-fold lower affinity binding than 6-GM although it was recognized more specifically than the control drug, xylocaine.
Fig. 4.
Results from the competitive inhibition ELISA using sera samples obtained from 22-week vaccinated rats show that the anti-morphine antibodies displaced binding to morphine and other heroin metabolites. Antibody binding to the ELISA plate coated with bovine serum albumin-6-succinylmorphine (BSA-6-SM) was inhibited by pre-incubation of increasing doses of morphine, 6-acetyl morphine (6-AM), morphine-3-glucuronide (3-GM), and morphine-6-glucuronide (6-GM). In contrast, the anti-morphine antibodies did not recognize the dissimilar compound, xylocaine.
Table 1.
The IC50 (concentration that produced 50% inhibition of maximum binding) values of antibody responses to morphine and morphine-related compounds are shown. Values were derived by non-linear fit analysis.
| Compound | IC50 (nM) |
|---|---|
| Morphine | 19.3 |
| 6-acetyl morphine (6-AM) | 11.2 |
| Morphine-6-glucuronide (6-GM) | 60.3 |
| Morphine-3-glucuronide (3-GM) | 614.7 |
| Xylocaine | ND1 |
IC50 value could not be determined by non-linear fit analysis.
3.2. Conditioned place preference
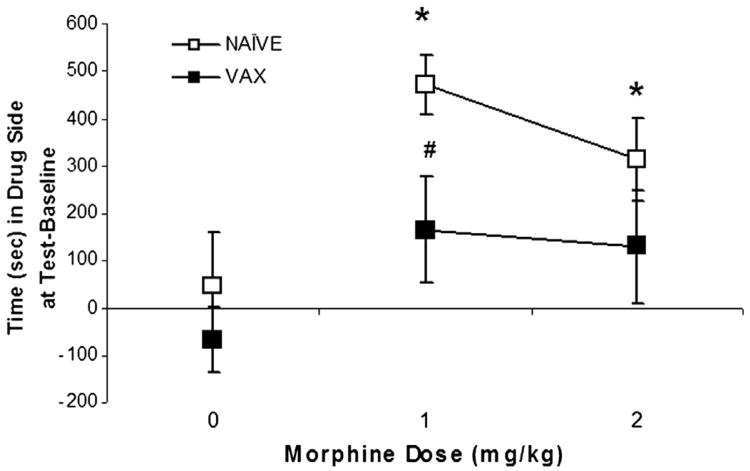
One rat (from the vaccinated group trained with 0 mg/kg morphine) was removed from the study due to side bias exhibited on the baseline test. There was a significant main effect of Vaccine, F(1, 47) = 6.14; P < 0.02, and a significant Vaccine × Morphine interaction, F(2, 47) = 5.72; P < 0.01. That is, morphine was able to support conditioned place preference (CPP). The naive rats conditioned with either 1 or 2 mg/kg of morphine showed increased times spent on the drug-paired side at test relative to baseline compared to naïve rats conditioned with vehicle (0), P′s < 0.05. Morphine CPP was significantly attenuated in the vaccinated groups as seen in Fig. 5. Post-hoc tests revealed that the vaccinated group conditioned with 1 mg/kg morphine differed significantly from the naïve group conditioned with this dose, P < 0.05.
Fig. 5.
Morphine conditioned place preference (CPP) by morphine training dose (0, 1, and 2 mg/kg) is shown for the non-vaccinated (open squares) and vaccinated (closed squares) groups. CPP is defined as the difference in time (seconds) spent on the morphine-paired side on the test day relative to the baseline session. Mean (±S.E.M.) CPP was minimal in groups trained with 0 mg/kg morphine but increased significantly in the naïve, non-vaccinated groups trained with 1 or 2 mg/kg morphine, P < 0.05 (*). Morphine CPP conditioned with 1 mg/kg was attenuated in vaccinated rats compared to non-vaccinated control rats, P < 0.05 (#).
3.3. Antinociceptive tests
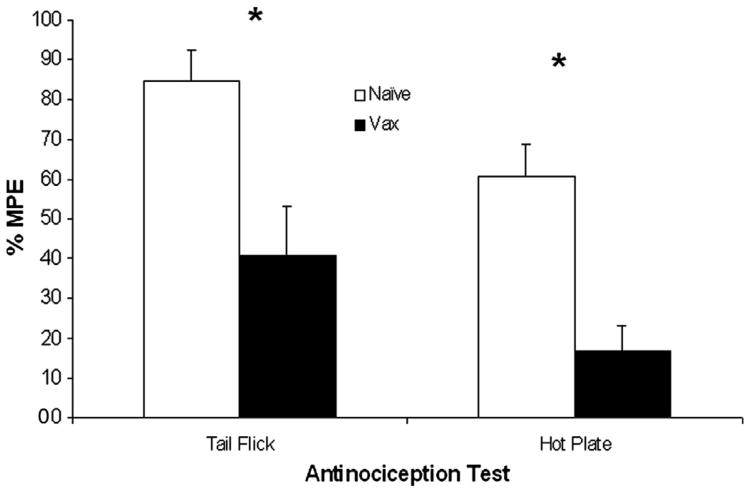
Morphine-induced antinociception effects in control and vaccinated rats are shown in Fig. 6. The antinociceptive effects of morphine were significantly reduced in vaccinated rats in both the tail flick t(16) = 4.04; P < 0.001; and hotplate, t(16) = 5.34; P < 0.0005, tests.
Fig. 6.
The morphine-induced antinociception was measured by tail flick and hot plate tests at week 7, with the tail-flick test performed first. Morphine (2 mg/kg) was administered 30 min prior to the tests. Data shown are the mean (±S.E.M.) Maximal Possible Effect (MPE; %). The morphine-induced antinociception was attenuated in vaccinated rats in both tests compared to non-vaccinated control rats, P′s < 0.05.
3.4. Morphine levels in brain and blood
Morphine levels in brain and blood obtained 60 min after an injection of morphine (4 mg/kg) are shown in Table 2. Vaccinated rats tended to have higher blood morphine levels compared to control rats, t(11) = 1.88; P < 0.08, and had significantly lower morphine brain levels, t(11) = 2.44; P < 0.05.
Table 2.
Mean (±S.E.M.) morphine content determined using GC–MS in brain and blood of control, non-vaccinated rats (n = 9) and vaccinated rats (n = 7) obtained 60 min after morphine (4 mg/kg; SC) administration.
P < 0.05.
P < 0.08.
4. Discussion
The results of the present study demonstrate that the KLH-6-SM vaccine elicited antibodies that bound to morphine, 6-AM and 6-GM. A vaccination schedule that included two boosts was capable of producing high and sustained anti-morphine antibody levels in the rats. This vaccine was also associated with attenuating the functional effects of morphine as assessed by two antinociceptive tests and by conditioned place preference (CPP). Moreover, vaccinated rats exhibited 25% reduced brain levels of morphine and tended to show enhanced blood levels of morphine compared to control rats. This suggests that the anti-morphine antibodies sequestered morphine in the blood, thereby reducing the level in the brain. Overall, these data show strong support for the KLH-6-SM morphine vaccine as a candidate vaccine for opioid dependence.
Three vaccine administrations of KLH-6-SM were capable of producing and sustaining anti-morphine antibodies in the rats for 24 weeks. The second boost greatly enhanced antibody levels to about a 3-fold increase over the levels measured after the first boost. The second boost was given in three different amounts but our results suggest that the lowest amount (100 μg) was as effective in boosting antibody levels as the highest amount (300 μg, see Fig. 3). This suggests that as long as a minimum dose of vaccine is administered, an adequate immunologic response will be reached. Further, antibody levels did not correlate with analgesic responses (data not shown). Although the antinociceptive tests were conducted at week 7, prior to the second boost and when antibody levels were more modest, it is likely that once a critical threshold of antibody production is reached, it will have similar functional consequences. Indeed, we retested analgesic responses at week 23 after the second boost, and the antinociceptive effect was not enhanced (data not shown).
We tested the functional effects of the KLH-6-SM vaccine by assessing its ability to reduce morphine antinociception and morphine reward. Antinociception was tested with two methods, tail flick and hot plate, and both responses were highly and significantly attenuated in the vaccinated rats (see Fig. 6). The percent maximal percent effect (%MPE) of morphine was decreased by two- to three-fold in both assays. These findings are in line with those reported upon in a recent study that tested heroin and morphine vaccines in rats (Stowe et al., 2011). In that study, a vaccine constructed against heroin fully blocked the effects of heroin (1 mg/kg) in the hot plate test and a vaccine constructed against morphine resulted in a partial blockade of this effect. Neither vaccine altered the antinociceptive effects of oxycodone at a dose of 2.5 mg/kg. The antinociceptive tests conducted in the present study utilized a modest dose of morphine (2 mg/kg).
Whether the KLH-6-SM vaccine would be effective with lower or higher doses of morphine is not known. However, the KLH-6-SM vaccine is effective in reducing the place conditioning effects of morphine, particularly at the 1 mg/kg dose. These results suggest that the vaccine attenuates the rewarding effects of morphine. This finding is consistent with prior studies showing that opioid vaccines reduce opioid self-administration in rats and primates (Anton and Leff, 2006; Bonese et al., 1974; Stowe et al., 2011).
The reinforcing strengths of drugs are greater with shorter injection or infusion times (deWit et al., 1992; Marsch et al., 2001; Woolverton and Wang, 2004). For example, rhesus monkeys self-administer more cocaine intravenously if the infusion rate is shortened even though the dose is held constant (Woolverton and Wang, 2004). Human drug users report greater effects of morphine (e.g., “High”; “Drug effect”) with faster IV infusion rates (Marsch et al., 2001). The reduction in morphine reward seen in the present study and the attenuation of heroin self-administration reported in the prior studies are consistent with the notion that an anti-opioid vaccine is effective because it slows the entry of the drug into the brain. Indeed, we showed that morphine levels were significantly decreased in brains of the KLH-6-SM vaccinated rats and there was a trend towards higher blood serum levels.
Current concepts that attempt to explain rewarding effects of drug actions of addictive substances combine aspects of “rate” and equilibrium binding. The pharmacokinetics of antibody-bound morphine and other heroin metabolites relate to metabolism, tissue distribution, and elimination pathways of the drug, as well as to the intrinsic half-life of the antibodies. The antibodies elicited by KLH-6-SM had specific binding to morphine, 6-AM, 3-GM, and 6-MG with nanomolar affinity (Table 1). This cross-reactivity is critically important because heroin is rapidly converted to the pharmacologically active opiates, 6-acetyl morphine (6-AM) and morphine (Inturrisi et al., 1983). Indeed, antibody binding prolongs the terminal half-life of morphine in animals by 2–3-fold, but has little effect on its metabolism (Hill et al., 1975).
Construction of an effective anti-morphine vaccine involves several choices in the design of the hapten and carrier protein. Studies of different morphine hapten linker sites to a carrier protein have been conducted. For example, the morphine derivative 3-O-carboxymethyl-morphine (Gross et al., 1974; VanVunakis et al., 1972) and the morphine-6-hemisuccinate (Akbarzadeh et al., 1999; Anton and Leff, 2006; Bonese et al., 1974; Ma et al., 2006; Wainer et al., 1972, 1973) used shorter spacer linker-arms of either 8 or 12 Å in length, respectively, that linked morphine derivatives to BSA or KLH-protein carriers. Notably, the 3 hydroxyl construct did not bind well to the highly active opioid 6-GM. In contrast, antibodies produced by the 6-SM hapten bind well to 6-GM and show a 10-fold lower affinity to 3-GM, an inactive metabolite.
The morphine vaccine we evaluated, KLH-6-SM, has the hapten, 6-succinylmorphine, linked to lysine groups on the protein. This hapten structure is similar to ones used in earlier studies (Bonese et al., 1974; Wainer et al., 1973). In the current study, the hapten was conjugated to KLH instead of BSA, as was used in earlier studies, because KLH has high immunogenicity and has the potential to be used in humans. A previous report showed that a vaccine constructed by linking 6-SM to a derivative of tetanus toxoid produced antibodies and prevented the acquisition of heroin self-administration in rats. However, this vaccine required four boosts over 60 days, and biweekly boosts over the period of a year in order to keep adequate titers (Anton and Leff, 2006). Recently, Janda et al. found that the polyclonal antibodies produced by a vaccine with a heroin-like hapten linked to KLH had micromolar affinities to 6-acetyl morphine (6-AM), heroin and morphine, but were nonetheless able to prevent the acquisition of heroin self-administration and the antinociceptive effects of heroin in rodents. Conversely, antibodies generated by a morphine-like KLH-vaccine only had adequate affinity for morphine and reduced binding for heroin, but no affinity for 6-acetyl morphine; in addition, the morphine-like the vaccine was not effective for prevention of heroin administration acquisition (Stowe et al., 2011). In comparison, our study has shown a vaccination schedule that included three administrations was capable of producing high and sustained anti-morphine antibody levels for 6 months in the rats. The vaccine construct we employed is similar although not identical to those used in previous studies. However, we believe this is the first report that examined the functional effects of a morphine vaccine using conditioned place preference. This expands upon our recent study that tested an anti-methamphetamine vaccine (Shen et al., 2013).
5. Conclusion
Anti-addiction vaccines have distinctly different mechanisms from current medications for heroin and morphine abuse. They do not rely on inhibiting drug binding at specific receptors within the brain; rather, the antibodies serve as pharmacokinetic antagonists, altering the concentration–time course of drug in multiple organ systems, especially reducing drug concentrations in the brain. We have shown that a morphine vaccine, KLH-6-SM is capable of producing sustained anti-morphine antibody levels. Further, the functional consequences of the vaccine are demonstrated by the reduction in the ability of morphine to support conditioned place preference, have analgesic effects, and lower morphine brain levels. These characteristics support its potential as a treatment agent for opioid dependence. A key test regarding the potential therapeutic effects of this vaccine or any other construct for opioid dependence will be investigating its ability to alter morphine or heroin self-administration behavior.
Acknowledgments
Financial support was provided by a grant from the National Institute on Drug Abuse, DA026859, and by a grant from the VA Merit Review Program. The authors gratefully acknowledge the excellent technical assistance provided by A. Lopez and Y. Wu and thank Dr. Reetakshi Arora for her valuable input.
Abbreviations
- CPP
conditioned place preference
- KLH
keyhole limpet hemocyanin
- BSA
bovine serum albumin
- 6-SM
6-succinylmorphine
- KLH-6-SM
keyhole limpet hemocyanin-6-succinylmorphine
- alum
aluminum hydroxide gel
- 6-AM
6-acetyl morphine
- 3-GM
morphine-3-glucuronide
- 6-GM
morphine-6-glucuronide
- EDC
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
- sulfo-NHS
N-hydroxysulfosuccinimide
- ELISA
enzyme-linked immunosorbent assay
- GC-MS
gas-chromatography coupled to mass spectrometry
- IC50
50% inhibition of maximum binding
- MPE
maximal possible effect
References
- Akbarzadeh A, Mehraby M, Zarbakhash M, Farzaneh H. Design and synthesis of morphine-6-succinyl-bovine serum albumin hapten for vaccine development. Biotechnol Appl Biochem. 1999;30:139–46. [PubMed] [Google Scholar]
- Anton B, Leff P. A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine. 2006;24:3232–40. doi: 10.1016/j.vaccine.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Anton B, Salazar A, Flores A, Matus M, Marin R, Hernandezet JA. Vaccines against morphine/heroin and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccin. 2009;5:214–29. doi: 10.4161/hv.5.4.7556. [DOI] [PubMed] [Google Scholar]
- Berkowitz B, Spector S. Evidence for active immunity to morphine in mice. Science. 1972;22:1290–2. doi: 10.1126/science.178.4067.1290. [DOI] [PubMed] [Google Scholar]
- Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature. 1974;252:708–10. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. In: Liebman J, Cooper S, editors. The neuropharmacological basis of reward. New York: Oxford University Press; 1989. pp. 264–319. [Google Scholar]
- Cicero TJ, Ennis T, Ogden J, Meyer ER. Gender differences in the reinforcing properties of morphine. Pharmacol Biochem Behav. 2000;65:91–6. doi: 10.1016/s0091-3057(99)00174-4. [DOI] [PubMed] [Google Scholar]
- deWit H, Bodker B, Amber J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology. 1992;107:352–8. doi: 10.1007/BF02245161. [DOI] [PubMed] [Google Scholar]
- Gross SJ, Grant JD, Wong SR, Schuster CR. Critical antigenic determinants for production of antibody to distinguish morphine from heroin, codeine, and dextromethorphan. Immunochemistry. 1974;11:453–6. doi: 10.1016/0019-2791(74)90079-2. [DOI] [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67:59–65. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Jorenby DE, Donzales G, Aigotti RN, Glover ED, Oncken CA, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011;89:392–9. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JH, Wainer BH, Fitch FW, Rothberg RM. Delayed clearance of morphine from the circulation of rabbits immunized with morphine-6-hemisuccinate bovine serum albumin. J Immunol. 1975;114:1363–8. [PubMed] [Google Scholar]
- Inturrisi CE, Schultz M, Shin S, Umans JG, Simons EJ. Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci. 1983;33:773–6. doi: 10.1016/0024-3205(83)90616-1. [DOI] [PubMed] [Google Scholar]
- Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2001;57:281–9. [PMC free article] [PubMed] [Google Scholar]
- Koida M, takahashi M, Muraoka S, Kaneto H. Antibodies to BSA conjugates of morphine derivatives: strict dependency of the immunological specificity on the hapten structure. Jpn J Pharmacol. 1974;24:165–7. doi: 10.1254/jjp.24.165. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Borg L, Ducat E, Ray B. Pharmacotherapy in the treatment of addiction: methadone. J Addict Dis. 2010;29:200–16. doi: 10.1080/10550881003684798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LX, Zhou Q, Zheng HB, Li SB. Preparation and characterization of anti-morphine vaccine antibody. Chinese Cell Mol Immunol. 2006;22:368–70. [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Rathmell JP, Swedberg MDB, Jonzon B, et al. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther. 2001;299:1056–65. [PubMed] [Google Scholar]
- Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66:1116–23. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S, Shinderman MS. Optimizing long-term response to methadone maintenance treatment: a 152-week follow-up using higher-dose methadone. J Addict Dis. 2002;21:1–12. doi: 10.1300/J069v21n03_01. [DOI] [PubMed] [Google Scholar]
- NIH. Guide for the care and use of laboratory animals. Washington D.C.: National Academy Press; 1996. [Google Scholar]
- Reid LD, Hunter GA, Beaman CM, Hubbell CL. Toward understanding ethanol's capacity to be reinforcing: a conditioned place preference following injections of ethanol. Pharmacol Biochem Behav. 1985;22:483–7. doi: 10.1016/0091-3057(85)90051-6. [DOI] [PubMed] [Google Scholar]
- Selley DE, Cao CC, Sexton T, Schwegel JA, Martin TJ, Childers SR. m-Opioid receptor-mediated G-protein activation by heroin metabolites: evidence for greater efficacy of 6-monacetylmorphine compared to morphine. Biochem Pharmacol. 2001;62:447–55. doi: 10.1016/s0006-2952(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Shen XY, Orson FM, Kosten TR. Vaccines against drug abuse. Clin Pharmacol Ther. 2012;91:60–70. doi: 10.1038/clpt.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XY, Kosten TA, Lopez AY, Kinsey BM, Kosten TR, Orson FM. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129:41–8. doi: 10.1016/j.drugalcdep.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon EJ, Dole WP, Hiller JM. Coupling of a new, active morphine derivative to sepharose for affinity chromatography. Proc Natl Acad Sci. 1972;69:1835–7. doi: 10.1073/pnas.69.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10:1727–40. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, et al. A vaccine strategy that induces protective immunity against heroin. J Med Chem. 2011;54:5195–204. doi: 10.1021/jm200461m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC. United Nations Office on Drugs and Crime: World Drug Report. United Nations Publication; 2010. Vol. Sales No. E.10.XI.13. [Google Scholar]
- VanVunakis H, Wasserman E, Levine L. Specificities of antibodies to morphine. J Pharmacol Exp Ther. 1972;180:514–21. [PubMed] [Google Scholar]
- Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2010;30:155–66. doi: 10.1016/j.cpr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Wainer BH, Fitch FW, Rothberg RM, Fried J. Morphine-3-succinyl-bovine serum albumin: an immunogenic hapten–protein conjugate. Science. 1972;176:1143–5. doi: 10.1126/science.176.4039.1143. [DOI] [PubMed] [Google Scholar]
- Wainer BH, Fitch FW, Fried J, Rothberg RM. A measurement of the specificities of antibodies to morphine-6-succinyl-BSA by competitive inhibition of 14 C–morphine binding. Clin Psychol Rev. 1973;30:155–66. [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486:251–7. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]