1. INTRODUCTION
The outcome of natural infections with pathogenic mycobacteria can range from early asymptomatic clearance through latent infection to clinical disease. Different host and pathogen- specific factors have been implicated in determining the outcome of these infections; however, it is clear that the interaction of mycobacteria with the innate and acquired components of the immune system plays a central role. Specifically, the recognition of mycobacterial components by innate immune cells through different pathogen recognition receptors (PPRs) induces a cytokine response that can promote early control of the infection. In fact, in the majority of individuals that come into contact with mycobacteria, this response is enough to control the infection. Among PRRs, Toll-like receptors (TLRs), Nucleotide Oligomerization Domain (NOD)-like receptors and C-type lectins have all been implicated in recognition of mycobacteria and in the initiation of the cytokine response. Defining the mechanisms by which distinct mycobacterial components and their receptors stimulate the immune response is an area of intense research.
The innate cytokine response is critical to determining the subsequent acquired immune response, which is essential to pathogen control once the infection is established. It is thought that T helper (Th)1 cells, characterized by the secretion of Interferon (IFN)γ are key in the control of mycobacterial infections. However, the magnitude of the Th1 response does not always correlate with bacterial clearance or increased resistance. Th17 cells and regulatory T cells (Tregs) are also induced upon infection; however, their role in the protective immune response is still under investigation. Furthermore, the chronic nature of mycobacterial infection results in constant activation of the immune response which eventually leads to the development of granulomatous structures that allow both containment and transmission of the disease. While we do not fully understand the mechanisms that underlie the generation of the granuloma, cytokines are known to play a central role in the initiation and maintenance of these structures and thus have a critical impact on both the generation of protective immunity and the development of pathological consequences. Therefore, it is important that we define the role and mechanisms of action of different cytokines at different stages of mycobacterial infection. This knowledge will improve our understanding of how host resistance is induced, maintained and regulated, and provide a basis for potential prophylactic and therapeutic interventions.
In this chapter we will provide an overview of the roles of the major cytokine families that are produced during the innate and acquired immune response to mycobacteria, in particular to M. tuberculosis. The modes of action of these cytokines and their impact in the control of infection and development of pathological consequences will also be discussed.
2. THE IL-12 FAMILY: INITIATORS AND REGULATORS OF ACQUIRED IMMUNITY
The IL-12 family of cytokines comprises 4 heterodimeric members: IL-12p70, IL-23, IL-27 and the newest member of the family, IL-35. These cytokines share homology at the subunit, receptor and signaling levels and play distinct roles in the generation and maintenance of acquired immune responses to mycobacteria. Both IL-12p70 and IL-23 share the IL-12p40 subunit that is covalently bound to IL-12p35 to form IL-12p70 or to IL-23p19 to form IL-23. On the other hand, IL-27 is formed by the association of Epstein-Barr Virus-Induced gene 3 (EBI3) and the subunit IL-27p28. While EBI3 resembles IL-12p40 and IL-27p28 is related to IL-12p35, EBI3 and IL-27p28 are not covalently bound and they can be secreted by different cells to heterodimerize in the extracellular compartment. This difference can have important implications in the biological activity of the single subunits as it was recently shown that IL-27p28, when not bound to EBI3, can act as an antagonist for IL-6 signaling (1). Finally, IL-35 is formed by the association of IL-12p35 and EBI3 subunits. This cytokine is the most recent member of the IL-12 family and its function is still under scrutiny. However, recent data point to an immunoregulatory role of IL-35, as regulatory T cells (Tregs) seem to be an important source of this cytokine (2).
2.1. THE IL-12 FAMILY IN THE INITIATION OF AQUIRED IMMUNITY TO MYCOBACTERIA
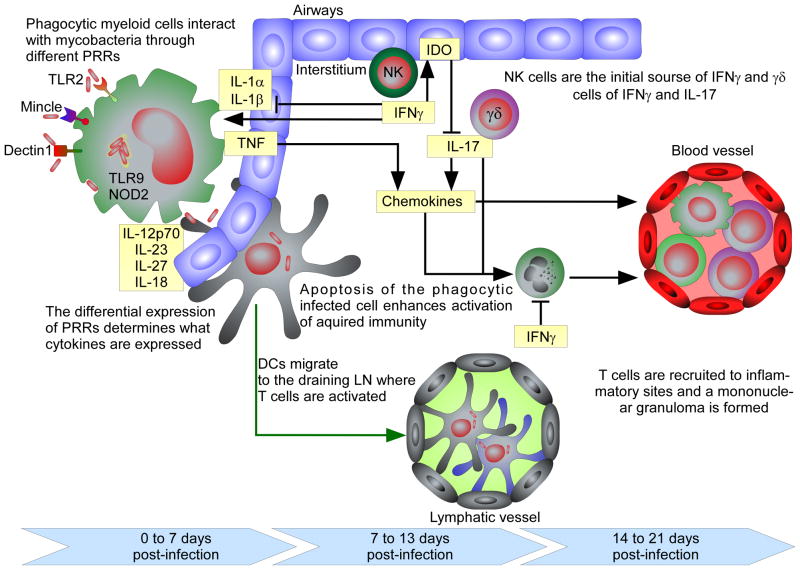
Upon infection with M. tuberculosis, bacilli are deposited in the lower airways where they are thought to be initially phagocytosed by resident myeloid populations, including dendritic cells (DCs) and macrophages. In the mouse aerosol model of infection, M. tuberculosis grows in an unrestricted manner for the first twenty days of infection, followed by growth arrest corresponding to the accumulation of IFNγ-producing T cells in the lung (3–5). Recent data suggest that the T cell response is initiated in the draining lymph node (dLN) of the lung between days 7 and 10 post-infection (6–8). Since the lung is the main site of infection, it is likely that the DCs that pick up bacteria or antigen migrate from the lung to the dLN where they present antigen and initiate the T cell response (figure 1) (9). In the dLN, both IFNγ- and IL-17-producing T cells are induced and these cells then migrate to the lung where they exert effector function.
Figure 1. Cytokines act during the initiation of the immune response and after M. tuberculosis infection has been established.
At very early stages of infection (0 – 7 days in the mouse model) the interaction of mycobacteria or mycobacterial products with myeloid phagocytic cells through distinct PRRs induces the expression of innate cytokines. TNF and other inflammatory cytokines promote the expression of chemokines that recruit inflammatory cells to the infection foci. Between days 7 and 13 of infection, after DCs migrate to the draining lymph node and T cells are activated. At the infection site, innate source of IL-17 and IFNγ counter-regulate each other. While IL-17 recruits neutrophils, IFNγ regulates the response of the interstitium to promote a regulatory environment, possibly dependent upon induction of IDO. From day 14 onwards the inflammation generated at the site of initial infection attracts newly activated T cells that, upon accumulation restrain bacterial growth in IFNγ- and TNF-dependent and independent ways.
IL-12p70 is the critical factor that drives the generation of IFNγ-producing T cells that are thought to be essential for bacterial control (10). Indeed, deficiencies in the IL-12 or IFNγ signaling pathways have been associated with human susceptibility to tuberculosis (11). Similarly, in the mouse model, the absence of IL-12p35 (and therefore IL-12p70) is associated with increased susceptibility to infection, corresponding with a significant reduction in the number of antigen-specific, IFNγ-producing T cells in the lung (12). However, these mice still have an adaptive IFNγ response, which is virtually nonexistent in mice that lack both IL-12p35 and IL-23p19 (IL-12p70 and IL-23 double deficient mice) or IL-12p40 (12, 13) suggesting that, IL-23 can compensate for the absence of IL-12p70 and induce IFNγ-producing T cells. This response is not potent enough to maintain long term control of the infection, supporting an important role of IL-12p70 in maintenance of long-term Th1 responses. The main role for IL-23 during mycobacterial infection is the maintenance of the IL-17 response (14). Indeed, mice deficient in IL-23p19 are unable to maintain IL-17-producing cells or IL-17 mRNA expression in the lung throughout infection (13). However, there is still an IL-17 response in the absence of IL-23p19 in the dLN suggesting that, as in other models of disease, IL-23 is not required to initiate the Th17 response but it is critical to sustain it (15, 16).
Unlike IL-12p70 and IL-23, IL-27 appears to play mostly a regulatory role during mycobacterial infections. IL-27 was originally described as a Th1 differentiating factor in vitro (17); however, in vivo, in distinct infection models, including the M. tuberculosis aerosol infection model, the absence of IL-27 activity does not significantly compromise the generation of protective IFNγ-producing cells (18–21). In contrast, during M. tuberculosis infections in the absence of IL-27 signaling, T cells express less IFNγ on a per cell basis (18), suggesting that, IL-27 may be critical to maximize IFNγ production by these cells. Interestingly, mice deficient in IL-27 signaling are more resistant to M. tuberculosis infection (18, 19). Since IL-27 has been shown to inhibit Th17 differentiation (22) and to induce IL-10 production from activated T cells (23, 24), it is likely that, during M. tuberculosis infections, IL-27 function is to suppress excessive T cell activation. Indeed, although bacterial burdens are reduced in mice that lack IL-27 signaling, these mice also display greater inflammatory responses and reduced survival when compared to wild-type mice (19). It will be important to further dissect the mechanisms whereby IL-27 activity reduces protection against M. tuberculosis as this may be a pathway induced to tolerate the pathogen in order to protect the infected organ from immunopathological consequences.
2.2. IMPACT OF IL-12-RELATED CYTOKINES IN THE CHRONIC T CELL RESPONSE
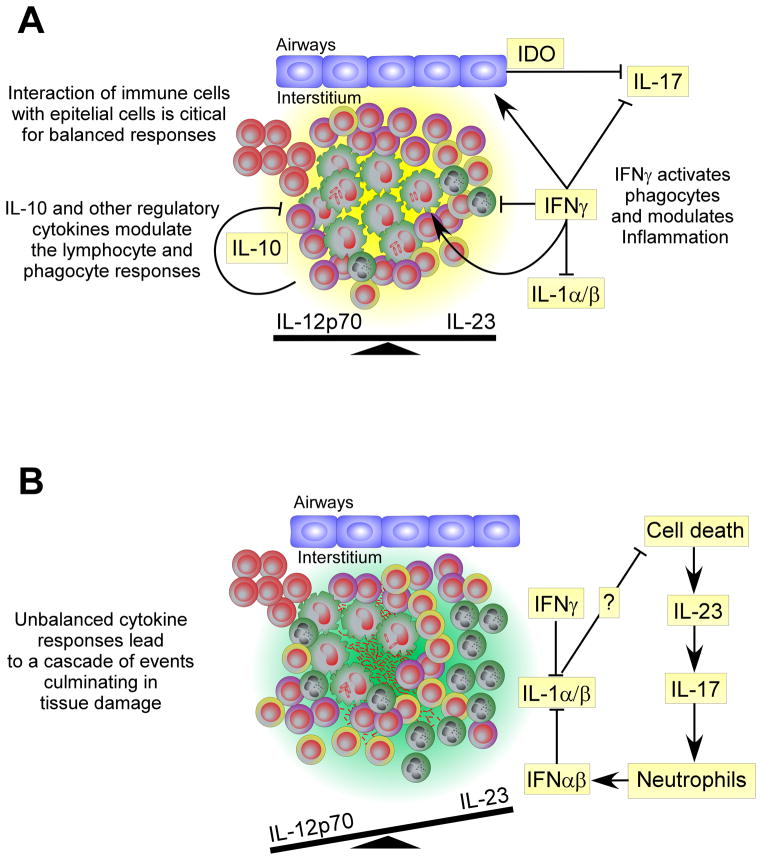
Chronic exposure to IL-12 and IL-23 in the site of infection can have an important impact in the T cell response, specifically in circumstances where these cytokines are over expressed. Indeed, it has long been known that repeated exposure to high level of antigens in M. tuberculosis-infected hosts can lead to exacerbated inflammatory responses, known as the Koch Phenomenon. Recently, it was shown that the local expression of IL-23 is important for this response, as this cytokine promotes further expansion of the ongoing IL-17 response and shifts the chemokine profile resulting in enhanced granulocytic inflammation without impacting the IFNγ protective response (25). In line with these data, it was also recently shown that IFNγ signaling by lung stromal cells is critical to regulate IL-17-dependent immunopathology during tuberculosis in mice (26). Indeed, stromal cells that lack the ability to signal IFNγ, have impaired expression of the enzyme indoleamine 2,3-dioxygenase (IDO) and a reduced accumulation of byproducts of the tryptophan catabolism (26). These products were shown to reduce the immunopathological consequences caused by the M. tuberculosis infection, by restraining the IL-23-dependent IL-17 response (26). These data show that IL-23 plays an important role in the maintenance of the IL-17 response at the site of infection and that elevated expression of IL-23 further expands the ongoing IL-17 response, causing extensive recruitment of neutrophils with important pathological consequences. IFNγ appears to play a central role in the regulation of these responses, both directly in the differentiation of IL-17-producing cells (27) and indirectly by inducing IDO activation by non-hematopoietic cells (26) (figure 2).
Figure 2. Balanced cytokine responses during chronic mycobacterial infection limits tissue damage.
(A) During chronic infection, IFNγ activates phagocytic cells and modulates the inflammatory environment by regulating IL-17 and IL-1 from inflammatory macrophages while IFNγ regulates IL-1 in a more general manner. IL-10 produced by highly activated T cells and Tregs limits lymphocyte and phagocyte responses. (B) When the cytokine balance is shifted or excessive cell death occurs (absence of IL-1 or high antigen availability), elevated IL-23 expression can enhance ongoing IL-17 responses, culminating in excessive the neutrophil recruitment and tissue damage.
Overall, these data show that the relative balance of each IL-12 family member during T cell priming and at the site of infection can have important implications for disease control and pathological consequences (figure 2). Understanding how these cytokines are regulated during infection will have important implications in our ability to modulate the immune response to promote bacterial control with minimal pathological consequences.
3. THE IL-1 CYTOKINE FAMILY: MEDIATORS AND REGULATORS OF INFLAMMATION
The IL-1 family of cytokines comprises 11 members of which IL-1α, IL-1β, IL-18 and IL-33 have been studied during mycobacterial infections. Recent data support an important role for both IL-1α and IL-1β during M. tuberculosis infections, whereas the role of IL-18 is still under investigation. On the other hand, IL-33 is a Th2-related cytokine with little impact in the immune response to mycobacteria as demonstrated by the similar inflammatory response and bacterial burdens of mice deficient in the IL-33 receptor chain st2 and wild-type mice (28).
3.1. REGULATION OF IL-1β PRODUCTION DURING MYCOBACTERIAL INFECTION
IL-1β signaling is mediated through the adaptor molecule MyD88, shared by most TLRs. Interestingly, the first observations regarding the high susceptibility of MyD88 deficient mice to mycobacteria were interpreted as a requirement for TLR signaling (29, 30). However, mice deficient in different TLRs were not as susceptible to mycobacterial infection as MyD88 deficient mice. Recently, the susceptibility of IL-1β and IL-1R deficient mice was shown to be indistinguishable from that of MyD88 deficient mice, suggesting that, the signals conveyed by MyD88 that required for host survival during tuberculosis are from the IL-1R (31, 32).
M. tuberculosis is a strong inducer of both IL-1α and IL-1β at the site of infection (31, 32). Unlike IL-1α, IL-1β is produced in the form of a pro-cytokine, i.e., in a non-active form. In order to become active, a multi-protein complex known as the inflammasome is required to trigger the activation of caspase-1, the enzyme that converts pro-IL-1β into mature IL-1β (33). In vitro, macrophages produce mature IL-1β through the NOD-like receptor family, pyrin domain containing 3 (NLRP3)-inflammasome mediated caspase-1 activation (34–37). Interestingly, the ESAT-6 secretion system 1 (ESX-1), a system that mediates the secretion of virulence factors encoded by the region of difference 1 (RD1), is required for mature IL-1β secretion (34, 37). Accordingly, macrophages and DCs that are deficient in the apoptosis-associated spek-like protein containing a caspase recruitment domain (ASC), a critical component of the NLRP3 inflammasome, are unable to secrete IL1β upon stimulation with M. tuberculosis in vitro (31, 34, 35, 37). However, mice deficient in caspase 1, ASC or NLRP3 do not show the same susceptibility to M. tuberculosis as IL-1β or IL-1R deficient mice (31, 35) suggesting that, there are alternative pathways for IL-1β cleavage in vivo.
Recent data shows that the recognition of some species of mycobacteria though Dectin 1 triggers the activation of a noncanonical caspase-8-dependent inflammasome resulting in the processing of pro-IL-1β (38). Indeed, the release of IL-β by in vitro cultured DCs in response to M. leprae was completely dependent on this pathway whereas M. tuberculosis stimulated the processing of pro-IL-1β via the canonical caspase-1 pathway (38). It is likely that, in vivo, M. tuberculosis induces the cleavage of pro-IL-1β through an inflammasome-independent, or caspase-1- and caspase-8-independent mechanism. Candidate cleavage enzymes include other caspases, chymases, cathepsins and elastases (39). Recent data suggest that inflammatory monocytes/macrophages and DCs are the major sources of both IL-1α and IL-1β in the lungs of M. tuberculosis infected mice (32). It will be important to determine the mechanisms that lead to the cleavage of pro-IL-1β by these populations in vivo and the effector function of these cytokines in the immune response to mycobacteria.
3.2. THE ROLE OF IL-1α AND IL-1β IN MYCOBACTERIAL CONTROL
Studies conducted in gene-deficient mice clearly support a critical role for IL-1α and IL-1β in the immunity to mycobacteria. However, the mechanism by which these cytokines impact bacterial control and ensure host survival is still not completely understood. It is clear that the high susceptibility of mice deficient in IL-1α, IL-1β or IL-1R to M. tuberculosis is not associated with impaired Th1 responses (31). IL-1β was previously implicated as a cofactor for the generation of Th17 cells, and it is likely that this is also the case during mycobacterial infections. Indeed, it was shown that M. tuberculosis-induced IL-17 response by human cells was strongly dependent on IL-1β signaling (40). In this system, TLR4 and dectin 1 were the main receptors responsible for mediating IL-17 production (40). Whether or not IL-1β is critical for Th17 generation in vivo, these data does not explain the high susceptibility of IL-1β deficient mice to M. tuberculosis, as the absence of IL-17 does not significantly impact control of infection (41). On the other hand, extensive granuloma necrosis is a hallmark of M. tuberculosis infection in IL-1β and IL-1R deficient mice (31), and it is possible that in vivo, IL-1 activity regulates host resistance by modulating cell death (figure 2B).
As mentioned above, IL-1α does not need proteolytic cleavage and can function as a nuclear transcription factor. In viral models, it has been suggested that the constitutive expression of IL-1α is critical for the antiviral effects of IFNγ (42). During tuberculosis, IFNγ is critical to induce the expression of nos2 to produce nitric oxide and restrain bacterial growth (discussed below). As mice deficient in IL-1α, IL-1β or IL-1R have equivalent expression of effector molecules important to control M. tuberculosis as wild-type mice (31, 32), it is likely that the IL-1 cytokines act in concert with other inflammatory mediators to regulate inflammation and induce bacterial growth arrest by an, as yet, undefined mechanism.
Finally, the role of IL-1 signaling in human disease is supported by different studies suggesting associations of polymorphisms in the Il1 or Il1r1 genes with susceptibility to tuberculosis (43, 44). Therefore, it is important that we continue investigating the role of IL-1 during mycobacterial infections. Specifically, if IL-1 is as central in controlling M. tuberculosis in humans as it is in mice, blockade of IL-1 may have important implications in tuberculosis progression or reactivation.
3.3. THE ROLE OF IL-18
IL-18 like IL-1β is produced as a pro-cytokine that was originally identified as a Th1 differentiating factor (45). Early reports in the mouse model of tuberculosis confirmed the role for IL-18 in the IFNγ response, as mice deficient in IL-18 had lower IFNγ responses when compared to wild-type mice (46, 47). Despite this, the control of M. tuberculosis bacterial burdens was only modestly impaired in the absence of this cytokine (46, 47).
On the other hand, in a more recent study it was shown that mice deficient in IL-18 were extremely and acutely susceptible to aerosol infection with M. tuberculosis, to a similar extent as MyD88 and IL-1β deficient mice (48). As shown in the earlier studies, there was a reduced IFNγ response in the absence of IL-18, corresponding to a reduced expression of effector genes downstream IFNγ, and an elevated accumulation of granulocytes (48). It is intriguing however that, mice deficient in the IL-18 receptor were not more susceptible to M. tuberculosis (48). The discrepancy in terms of susceptibility in the absence of IL-18 versus its receptor suggests redundancy in receptor usage. However, this hypothesis requires experimental demonstration.
While these data suggest a potentially important role for IL-18 in the control of M. tuberculosis, it is possible that the different susceptibility observed in different studies is related with the dose of infection and/or virulence of the M. tuberculosis strain. It will be important to determine the factors behind these differences, as IL-18 may have a central, not yet identified role in the protective immune response to M. tuberculosis.
4. TUMOR NECROSIS FACTOR: FROM PHAGOCYTE ACTIVATION TO GRANULOMA FORMATION
The potential for using TNF blocking agents to treat inflammatory diseases, such as rheumatoid arthritis, has reinvigorated the interest in the role of TNF during intracellular infections, as in some cases TNF blockade caused reactivation of tuberculosis (49). Indeed, mice deficient in this cytokine, together with mice deficient in IFNγ or IL-12p40 are the most susceptible to M. tuberculosis infection. The most striking characteristic of TNF deficiency is poor phagocyte activation; however, the mechanisms underlying the activity of TNF during mycobacterial infections are not restricted to phagocyte activation, but also to a deficiency in chemokine expression with important implications in granuloma organization (50).
The complex role of TNF during mycobacterial infections may, in part, be associated with the different forms of the cytokine and the receptors it engages. Indeed, TNF is produced primarily as a type II transmembrane protein that can be cleaved by the metalloprotease TNF alpha converting enzyme to become soluble TNF. Both soluble and transmembrane TNF bind to the TNFRI whereas the TNFRII can only be fully activated by transmembrane TNF. Both receptors can transduce pro-inflammatory and anti-apoptotic signals by activating the NF-κB pathway and the mitogen-activated protein kinase. However, TNFRI can also transduce apoptotic and anti-inflammatory signals by recruiting the Fas-associated death domain and caspase 8.
It has been shown that mice that are deficient in the soluble form of TNF can control acute infections by M. tuberculosis; however, long-term control requires the full complement of TNF activity (51). TNF deficient mice are also more susceptible and succumb earlier to M. avium infections however the reduced survival of these mice is associated with an exacerbated immune response that is characterized by an elevated accumulation of IFNγ-producing T cells and disintegration of the granuloma (52). It will be important to determine whether this is caused by absence of TNF signaling in the myeloid population, or directly on the T cells, as it is likely that TNF also acts on the T cells in order to regulate their function and immunopathological potential.
Eicosanoids have been shown to have an impact in the TNF pathway, and therefore in the control of infection. A recent study shows that mutations in the lta4h locus encoding leukotriene A4 hydrolase, which catalyzes the final step in the synthesis of leukotriene B4, were extremely susceptible to mycobacterial infection, caused by a redirection of eicosanoid substrates to anti-inflammatory lipoxins (53). The resultant anti-inflammatory state permits increased mycobacterial proliferation by limiting the production of TNF (53). These data highlight the importance of innate-derived TNF in the control of mycobacterial infections in the absence of acquired immunity.
5. THE IFN CYTOKINE FAMILY: EFFECTORS AND REGULATORS OF AQUIRED IMMUNITY
IFNs have long been recognized as important mediators of immunity to mycobacteria. Of extreme importance is the type II IFN, IFNγ, which is essential to activation of phagocytic cells to kill mycobacteria. However, the role of IFNγ during mycobacterial infections is not as straightforward as it appears to be.
5.1. CD4 T CELLS AS THE MAIN CELLULAR SOURCE OF IFNγ
Antigen-specific CD4 T cells are thought to be the most important source of IFNγ in vivo. Indeed, upon aerosol infection with M. tuberculosis bacterial control correlates with the accumulation of IFNγ-producing CD4 T cells into the lung (5). However, there is still no experimental proof that IFNγ production by CD4 T cells, is required to control bacterial proliferation (54). Recent data suggest that, CD4 T cells unable to secrete IFNγ are equally capable of inducing bacterial control as wild-type CD4 T cells (55). In fact, antigen-specific CD4 T cells within the lungs of M. tuberculosis infected mice produce very low amounts of IFNγ, even at the peak of the response (56, 57); yet, bacterial control is maintained for long periods of time. It is likely that the cause for this apparent inability of the antigen-specific cells to secrete IFNγ is a very low level of cognate antigen. Indeed, it has been shown that the frequency of IFNγ-producing CD4 T cells correlates with the expression of the cognate antigen by M. tuberculosis, and that delivery of the cognate peptide results in greatly increased frequency of IFNγ production (56). The location of the T cells in the infection site is also important to induce bacterial control. During mycobacterial infection, it was recently shown that both antigen specific and non specific cells migrate very rapidly through the granuloma, with very few cells showing migration arrest, a hallmark of antigen recognition and presentation (57). In combination with the observation that antigen-specific cells express low levels of cytokine in real time, these data suggest that antigen availability at the infection site is limited and this limits migration arrest and cytokine production (57).
These data suggest that the classical mechanism in which T cell-derived IFNγ activates the macrophage to restrain M. tuberculosis growth may not provide the full mechanism of control. There is also the potential for T cells to mediate their effector function independently of cytokine-secretion. As our ability to measure effector function is limited by measuring bacterial arrest, we cannot exclude the possibility that the appropriate effector functions are being expressed, and this does not require migration arrest or cytokine production.
5.2. THE IMPACT OF IFNγ IN CELL SURVIVAL IN THE INFLAMMATORY ENVIRONMENT
If T cells are capable of limiting bacterial growth, even when they are unable to secrete IFNγ, why is that both mice and humans with deficiencies in the IFNγ signaling pathway are so susceptible to tuberculosis? The hallmark of M. tuberculosis infection in IFNγ deficient mice is the accumulation of polymorphonuclear granulocytes in the infection site (58). IFNγ is known to limit the IL-17 response during mycobacterial infections (26, 27) and thus IFNγ also inhibits the inflammatory programs initiated by IL-17 that culminate in neutrophil influx to the infected lungs (59). In a recent study, IFNγ was also shown to have a direct and negative impact in the survival of neutrophils in the infected lung (59). Also in M. avium and M. leprae infected IFNγ-deficient mice show mild increases in bacterial burdens, but have robust granulocyte recruitment to the infection site (60). As neutrophil accumulations are associated with a poor disease outcome, these data suggest that neutrophilic lesions during tuberculosis are probably caused by impaired IFNγ responses or IFNγ signaling and predispose to poor bacterial control.
The impact of IFNγ in cell survival is not restricted to neutrophils. Indeed, IFNγ has also an important impact in the survival of CD4 T cells in the inflammatory environment. This is demonstrated in the mouse model of M. avium infections, known to induce a strong lymphocyte depletion during the chronic stages of infection which is mediated by IFNγ (61). However, it is still not clear whether these effects occur directly in the T cell or indirectly, via the induction of inflammatory mediators that are detrimental for the survival of T cells in the inflammatory environment.
While there has long been appreciation for IFNγ as a regulator of the inflammatory response during mycobacterial infection (60) this appreciation has increased dramatically with the recent papers demonstrating the mechanisms whereby IFN mediates these anti-inflammatory effects (figure 2). Keeping in mind both the anti-bacterial and the anti-inflammatory roles of IFNγ when considering interventions with this chronic inflammatory disease will be critical to successful outcomes.
5.3. THE IMMUNOREGULATORY PROPERTIES OF TYPE I IFNs
Contrary to IFNγ, type I IFNs appear to have a largely detrimental role during mycobacterial infections. Indeed, type I IFN receptor-deficient mice are more resistant to M. tuberculosis infection and display significantly reduced bacterial burdens during the chronic stage of infection when compared to wild-type animals (62). Interestingly, the virulence of M. tuberculosis clinical isolates has been correlated with the induction of type I IFN, which was associated with impaired Th1 responses (63). Furthermore, macrophages express type I IFN-associated genes and IFNγ in response to virulent M. tuberculosis but not to a less virulent strain with an inactive ESX-1 secretion system (64). This response was found to be independent of the TLR adaptor TRIF and the adaptors for NOD1 and NOD2, but dependent on the activity of the TANK-binding kinase 1 (64), which is also necessary for type I IFN induction by Listeria monocytogenes (65).
In line with the detrimental role of type I IFN, it was recently shown that M. tuberculosis-infected mice treated with the type I IFN-inducer poly-nosinic-polycytidylic acid have exacerbated lung pathology and bacterial burdens (62). The elevated susceptibility was not associated with impaired T cell responses, but with the accumulation of a myeloid population that was permissive to mycobacterial growth when compared to the same population isolated from non-treated mice (62). Also recently, type I IFNs were shown to be strong inhibitors of IL-1α and IL-1γ production by macrophages and DCs in the lungs of M. tuberculosis infected mice (figure 2) (32). This inhibition was shown to occur directly in the IL-1α/β producing cell as, in the same environment, IL-1 α/ β expression by Ifnar1 deficient cells was not affected (32). As discussed above, IL-1 α and IL-1β cytokines are strong inflammatory mediators and thus it is likely that this is a negative feedback mechanism that prevents extensive generation of pathological consequences during infection.
Type I IFNs have also been suggested as important factors in determining the outcome of human tuberculosis. Indeed, a recent study shows that most patients with active tuberculosis display an expression signature associated with type I IFN genes in neutrophils (66). This same profile is also present in some of the asymptomatic patients, suggesting that these are at higher risk to develop active tuberculosis (66).
Overall the IFN family of cytokines appears to be critical to the outcome of mycobacterial infection with roles in containment of bacterial growth as well as regulation of immunopathological consequences (figure 2).
6. IL-17 AND TH17 RELATED CYTOKINES
Early studies clearly established IL-17 as a critical cytokine in the protective immune response to rapidly growing extracellular pathogens with the protective response mediated by rapid neutrophil recruitment and tissue repair (67–69). On the other hand, in infections caused by intracellular pathogens the role of IL-17 is not as clear. In some infection models, IL-17 appears to play a mostly protective role, however not as dramatically as observed for extracellular pathogens. For instance, during L. monocytogenes infections, mice have increased bacterial burdens and defective granuloma generation in the absence of IL-17 (70). As for mycobacteria, the data is equivocal as to whether IL-17 is required to control infection. In the low dose aerosol infection model, IL-17 is not required (41) although following slightly higher intratracheal infection with M. tuberculosis or BCG (71), IL-17 does have a protective role. This apparent discrepancy suggests that the circumstances of infection are critical for defining the role of IL-17. A possible mechanism for this impact may be that neutrophils have recently been implicated in limiting the early activation of acquired immunity to M. tuberculosis (72) suggesting that the level of IL-17, and potentially neutrophil recruitment, very early after infection may impact the ability of the bacteria to limit the initiation of immunity (figure 1).
As IL-17 acts mostly by inducing inflammatory programs associated with neutrophil recruitment, it is possible that in the low dose aerosol infection model IL-17 acts to maintain granuloma integrity independently of the protective immune response. Indeed, depletion of neutrophils later in M. tuberculosis infection has been shown to delay granuloma formation with little impact on bacterial burden (73). Neutrophil-mediated regulation of granuloma formation has been shown to be mediated by CXCR3-ligating chemokines, specifically CXCL9 (73). Indeed, neutrophils are an important source of this chemokine early after infection, and antibody blockade of CXCL9 results in defective granuloma formation (73). It is thought that neutrophils and macrophages can co-operate to limit mycobacterial survival (74) and this may be via macrophage phagocytosis of apoptotic neutrophils (72); although it appears that virulent mycobacteria may limit this process (72).
Further complexity in the role of IL-17 during mycobacterial infections may be associated with the Th17 related cytokine IL-22. In recent studies, it was shown that IL-22 deficient mice or IL-22 neutralization did not have a significant impact in the ability of mice to control infection (75, 76). However, IL-22 can activate inflammatory programs similar to those activated by IL-17. Furthermore, in vitro and in vivo generation of Th17 cells can lead to the development of cell expressing only IL-17, IL-22 and cell expressing both cytokines (77). This is important because, in the infected tissue, IL-17 and IL-22 may be secreted and act independently of each other and have redundant roles. Indeed, in healthy humans exposed to M. tuberculosis, IL-22-expressing CD4 T cells were reported to be distinct form Th17 and Th1 cells (78). It will be important to address the redundancy of IL-17 and IL-22 during infection as they can be produced and act independently of each other.
7. IL-10 AND OTHER IMMUNOSUPPRESSIVE CYTOKINES
As discussed above, the chronic nature of mycobacterial infections requires constant activation of the immune system in order to maintain control over bacterial proliferation. At the same time, it is important to control the inflammatory response to ensure survival of the host. In this respect, IL-10 may be important in the protective immune response to mycobacteria on two fronts (figure 2). On one hand, IL-10 has been shown to modulate the activity of phagocytes in the lung by negatively impacting their ability to secrete TNF and IL-12p40 (79, 80) and by blocking the maturation of the phagosome (81). Indeed, mice strains that express high levels of IL-10 upon infection, such as the CBA, are naturally more susceptible to tuberculosis and when IL-10 activity is neutralized, these mice are better able to control M. tuberculosis (80, 82). Recent data also suggest that IL-10 can have a negative impact in the recruitment of T cells into the lung by inhibiting the expression of T cell recruiting chemokines (82). IL-10 may also act to prevent strong activation of T cells thereby ensuring their survival and possibly limiting immunopathological consequences. Interestingly, IL-27 has been shown to be a critical factor in induction of IL-10 by activated T cells (23, 24). The role of IL-27 in inducing IL-10 is yet to be demonstrated during tuberculosis but as discussed before, mice deficient in IL-27 signaling are more resistant to infection, at the cost of greater inflammatory responses (18, 19).
Mycobacteria are strong inducers of Th1 immunity, but it is still not clear whether Th2 responses or Th2 derived cytokines can have a negative impact in the control of infection. In the mouse model of tuberculosis, IL-4 is very low or undetectable in the site of infection. In humans however, IL-4 can be detected in some lesions (83). It is also relevant to understand the impact of Th2 responses in the context of mycobacterial infections, even when these responses are not directed to mycobacterial antigens. In this respect, it was recently shown that pre-exposure of mice to the intestinal helminth Nippostrongylus brasiliensis is detrimental for the control of a subsequent aerosol infection with M. tuberculosis (84). Indeed, the Th2 response induced by N. brasiliensis skewed macrophage activation to the alternative state, without affecting the protective T cell response (84). Alternatively activated macrophages express high levels of the arginine hydrolytic enzyme arginase 1, which competes with iNOS for the same substrate, arginine, and impairs the production of nitric oxide (85). In the above discussed co-infection model, arginase I was induced by IL-4 (84); however, it has been shown that the expression of this enzyme can be induced in mycobacterial infections in a TLR-dependent way (85) or in settings where IL-10 expression is high (86).
While we generally associate immunopathology during tuberculosis to Th1 and Th17 responses, Th2 responses and Th2-derived cytokines are in fact the major cause of pulmonary fibrosis in different diseases, such as systemic sclerosis, idiopathic pulmonary fibrosis, and radiation induced pulmonary fibrosis and chronic lung allograft rejection (83). We cannot discard the hypothesis that fibrosis during tuberculosis may be, at least in part, dependent on Th2 cytokines.
Overall, immunosuppressive cytokines such as IL-10 seems to be mostly detrimental at early stages of infection, but may be required to control long term inflammatory responses. In addition, Th2 derived cytokines may have a negative impact in high incidence areas where infections by helminths can skew macrophage activation to the alternative state.
CONCLUSIONS
Mycobacterial infections are of enormous clinical importance. It is clear that the cytokine response induced by mycobacteria have a critical impact in the development of disease both by limiting bacterial growth and by regulating inflammation. Only by determining the pathways by which specific cytokines modulate the immune response to infection and by defining the specific cell types that produce each cytokine will we be able to effectively intervene. It is also important that we consider the interaction between different cell populations and how these interactions impact the cytokine balance and the inflammatory environment. These interactions are not restricted to immune cells, as recent data shows an important role of non-hematopoietic cells in modulating the inflammatory environment. It is therefore important that we understand how each cytokine acts during the initiation of the immune response and after infection has been established and disease is ongoing. This will allow us to generate better preventive and prophylactic strategies that enforce balanced immune responses and allow containment of infection with minimal pathological consequences.
References
- 1.Stumhofer J, Tait E, Quinn Wr, Hosken N, Spudy B, Goenka R, et al. A role for IL- 27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. 2010;11:1119–26. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collison L, Workman C, Kuo T, Boyd K, Wang Y, Vignali K, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 3.Cooper AM. Cell mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper A. T cells in mycobacterial infection and disease. Curr Opin Immunol. 2009;21:378–84. doi: 10.1016/j.coi.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008 Dec;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf A, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–15. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiley W, Calayag M, Wittmer S, Huntington J, Pearl J, Fountain J, et al. ESAT-6- specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in mediastinal lymph nodes. Proc Natl Acad Sci USA. 2008;105:10961–6. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallegos A, Pamer E, Glickman M. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J Exp Med. 2008;205:2359–68. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–19. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 10.Cooper A, Solache A, Khader S. Interleukin-12 and tuberculosis: An old story revisited. Curr Opin Immunol. 2007;19:441–7. doi: 10.1016/j.coi.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–61. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–7. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 13.Khader S, Pearl J, Sakamoto K, Gilmartin L, Bell G, Jelley-Gibbs D, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-g responses if IL-12p70 is available. J Immunol. 2005;175:788–95. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 14.Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21:455–62. doi: 10.1016/j.cytogfr.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wozniak T, Ryan A, Britton W. Interleukin-23 restores immunity to Mycobacterium tuberculosis infection in IL-12p40-deficient mice and is not required for the development of IL-17-secreting T cell responses. J Immunol. 2006;177:8684–92. doi: 10.4049/jimmunol.177.12.8684. [DOI] [PubMed] [Google Scholar]
- 16.Khader S, Bell G, Pearl J, Fountain J, Rangel-Moreno J, Cilley G, et al. IL-23 and IL-17 in establishment of protective pulmonary CD4+ T cell responses upon vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 17.Pflanz S, Timans J, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 18.Pearl JE, Shabaana AK, Solache A, Gilmartin L, Ghilardi N, deSauvage F, et al. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173:7490–6. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 19.Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T, et al. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3534–44. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 20.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–55. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 21.Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, et al. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:641–4. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 22.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J Mol Med. 2007;85:661–77. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–9. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 24.Stumhofer J, Silver J, Laurence A, Porrett P, Harris T, Turka L, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–71. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 25.Cruz A, Fraga A, Fountain J, Rangel-Moreno J, Torrado E, Saraiva M, et al. Pathological role of Interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med. 2010;207:1609–16. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desvignes L, Ernst JD. Interferon-g-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31:974–85. doi: 10.1016/j.immuni.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz A, Khader S, Torrado E, Fraga A, Pearl J, Pedrosa J, et al. Cutting edge: IFN-g regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J Immunol. 2006;177:1416–20. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 28.Wieland CW, van der Windt GJ, Florquin S, McKenzie AN, van der Poll T. ST2 deficient mice display a normal host defense against pulmonary infection with Mycobacterium tuberculosis. Microbes Infect. 2009;11:524–30. doi: 10.1016/j.micinf.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Scanga C, Bafica A, Feng C, Cheever A, Hieny S, Sher A. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect Immun. 2004;72:2400–4. doi: 10.1128/IAI.72.4.2400-2404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fremond C, Yeremeev V, Nicolle D, Jacobs M, Quesniaux V, Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. Journal of Clinical Investigations. 2004;114:1790–9. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer-Barber K, Barber D, Shenderov K, White S, Wilson MS, Cheever A, et al. Cutting Edge: Caspase-1 independent IL-1b production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–30. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, et al. Innate and adaptive interferons suppress IL-1a and IL-1b production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–34. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 34.Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, et al. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol. 2010;12:1046–63. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 35.McElvania Tekippe E, Allen IC, Hulseberg PD, Sullivan JT, McCann JR, Sandor M, et al. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS One. 2010;5:e12320. doi: 10.1371/journal.pone.0012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter K, Holscher C, Tschopp J, Ehlers S. NALP3 is not necessary for early protection against experimental tuberculosis. Immunobiol. 2010;215:804–11. doi: 10.1016/j.imbio.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS, Brown EJ. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol. 2008;10:1866–78. doi: 10.1111/j.1462-5822.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1b via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–54. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 39.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 40.van de Veerdonk F, Teirlinck A, Kleinnijenhui J, Jan Kullberg B, van Creval R, van der Meer J, et al. Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J Leukocyte Biol. 2010;88:227–32. doi: 10.1189/jlb.0809550. [DOI] [PubMed] [Google Scholar]
- 41.Aujla S, Dubin P, Kolls J. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–82. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hurgin V, Novick D, Werman A, Dinarello CA, Rubinstein M. Antiviral and immunoregulatory activities of IFN-g depend on constitutively expressed IL-1a. Proc Natl Acad Sci USA. 2007;104:5044–9. doi: 10.1073/pnas.0611608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellamy R, Ruwende C, Corrah T, McAdam KPWJ, Whittle HCA. V.S. H. Assessment of the Interleukin-1 gene cluster and other candidate gene polymorphisms in host susceptibility to tuberculosis. Tuberc Lung Dis. 1998;79:83–9. doi: 10.1054/tuld.1998.0009. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson RJ, Patel P, Llewelyn M, Hirsch CS, Pasvol G, Snounou G, et al. Influence of polymorphism in the genes for the interleukin (IL)-1 receptor antagonist and IL-1b on tuberculosis. J Exp Med. 1999;189:1863–74. doi: 10.1084/jem.189.12.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamura H, Tsutsui H, Komatsu T, Yatsudo M, Hakura A, Tanimoto T, et al. Cloning of a new cytokine that induces IFN-g production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 46.Sugawara I, Yamada H, Kaneko H, Mizuno S, Takeda K, Akira S. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect Immun. 1999 May;67:2585–9. doi: 10.1128/iai.67.5.2585-2589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinjo Y, Kawakami K, Uezu K, Yara S, Miyagi K, Koguchi Y, et al. Contribution of IL-18 to Th1 response and host defense against infection by Mycobacterium tuberculosis. J Immunol. 2002;169:323–9. doi: 10.4049/jimmunol.169.1.323. [DOI] [PubMed] [Google Scholar]
- 48.Schneider BE, Korbel D, Hagens K, Koch M, Raupach B, Enders J, et al. A role for IL-18 in protective immunity against Mycobacterium tuberculosis. Eur J Immunol. 2010;40:396–405. doi: 10.1002/eji.200939583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Eng J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 50.Roach D, Bean A, Demangel C, France M, Briscoe H, Britton W. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–7. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 51.Saunders B, Tran S, Ruuls S, Sedgwick J, Briscoe H, Britton W. Transmembrane TNF is sufficient to initiate cell migration and granuloma formation and provide acute, but not long- term, control of Mycobacterium tuberculosis infection. J Immunol. 2005;174:4852–9. doi: 10.4049/jimmunol.174.8.4852. [DOI] [PubMed] [Google Scholar]
- 52.Flórido M, Appelberg R. Characterization of the deregulated immune activation occurring at late stages of mycobacterial infection in TNF-deficient mice. J Immunol. 2007;179:7702–8. doi: 10.4049/jimmunol.179.11.7702. [DOI] [PubMed] [Google Scholar]
- 53.Tobin DM, Vary JC, Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–30. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torrado E, Cooper AM. What do we really know about how CD4 T cells control Mycobacterium tuberculosis? PLoS Pathog. 2011;7:e1002196. doi: 10.1371/journal.ppat.1002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallegos AM, van Heijst JWJ, Samstein M, Su X, Pamer EG, Glickman MS. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 2011;7:e1002052. doi: 10.1371/journal.ppat.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bold TD, Banaei N, Wolf AJ, Ernst JD. Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog. 2011;7:e1002063. doi: 10.1371/journal.ppat.1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egen JG, Rothfuchs AG, Feng CG, Horwitz MA, Sher A, Germain RN. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity. 2011;34:807–19. doi: 10.1016/j.immuni.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nandi B, Behar SM. Regulation of neutrophils by interferon-g limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251–62. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cooper AM, Adams LB, Dalton DK, Appelberg R, Ehlers S. IFN-g and NO in mycobacterial disease: new jobs for old hands. Trends Microbiol. 2002;10:221–6. doi: 10.1016/s0966-842x(02)02344-2. [DOI] [PubMed] [Google Scholar]
- 61.Florido M, Pearl J, Solache A, Borges M, Haynes L, Cooper A, et al. Gamma interferon-induced T-cell loss in virulent Mycobacterium avium infection. Infect Immun. 2005;73:3577–86. doi: 10.1128/IAI.73.6.3577-3586.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antonelli LR, Gigliotti Rothfuchs A, Goncalves R, Roffe E, Cheever AW, Bafica A, et al. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest. 2010;120:1674–82. doi: 10.1172/JCI40817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc Natl Acad Sci USA. 2001;98:5752–7. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178:3143–52. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- 65.O’Connell RM, Vaidya SA, Perry AK, Saha SK, Dempsey PW, Cheng G. Immune activation of type I IFNs by Listeria monocytogenes occurs independently of TLR4, TLR2, and receptor interacting protein 2 but involves TNFR-associated NF kappa B kinase-binding kinase 1. J Immunol. 2005;174:1602–7. doi: 10.4049/jimmunol.174.3.1602. [DOI] [PubMed] [Google Scholar]
- 66.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–7. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of Interleukin-17 receptor signalling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recriutment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Crit Care Med. 2001;25:335–40. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 69.Happel K, Dubin P, Zheng M, Ghilardi N, Lockhart C, Quinton L, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–9. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–63. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okamoto Yoshida Y, Umemura M, Yahagi A, O’Brien R, Ikuta K, Kishihara K, et al. Essential role of IL-17A in the formation of a mycobacterial Infection-induced granuloma in the lung. J Immunol. 2010;184(8):4414–22. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 72.Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seiler P, Aichele P, Bandermann S, Hauser A, Lu B, Gerard N, et al. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur J Immunol. 2003;33:2676–86. doi: 10.1002/eji.200323956. [DOI] [PubMed] [Google Scholar]
- 74.Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukocyte Biol. 2010;87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- 75.Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Fallert Junecko BA, Fountain JJ, et al. IL-23 Is Required for Long-Term Control of Mycobacterium tuberculosis and B Cell Follicle Formation in the Infected Lung. J Immunol. 2011;187:5402–7. doi: 10.4049/jimmunol.1101377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, et al. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol. 2010;184:4378–90. doi: 10.4049/jimmunol.0903416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang S, Tan X, Luxenberg D, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–70. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turner J, Gonzalez-Juarrero M, Ellis D, Basaraba R, Kipnis A, Orme I, et al. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169:6343–51. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- 80.Beamer G, Flaherty D, Assogba B, Stromberg P, Gonzalez-Juarrero M, de Waal Malefyt R, et al. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J mice. J Immunol. 2008;181:5545–50. doi: 10.4049/jimmunol.181.8.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Leary S, O’Sullivan M, Keane J. IL-10 Blocks Phagosome Maturation in Mycobacterium tuberculosis-infected Human Macrophages. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2010-0319OC. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 82.Redford P, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, et al. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur J Immunol. 2010;40(8):2200–10. doi: 10.1002/eji.201040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rook GA, Hernandez-Pando R, Dheda K, Teng Seah G. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 2004;25:483–8. doi: 10.1016/j.it.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 84.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med. 2011;208:1863–74. doi: 10.1084/jem.20091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9:1399–406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schreiber T, Ehlers S, Heitmann L, Rausch A, Mages J, Murray PJ, et al. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J Immunol. 2009;183:1301–12. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]