Abstract
Disorders of the carnitine cycle and of the beta oxidation spiral impair the ability to obtain energy from fats at time of fasting and stress. This can result in hypoketotic hypoglycemia, cardiomyopathy, cardiac arrhythmia and other chronic medical problems. The in vitro study of fibroblasts from patients with these conditions is impaired by their limited oxidative capacity. Here we evaluate the capacity of valinomycin, a potassium ionophore that increases mitochondrial respiration, to increase the oxidation of fatty acids in cells from patients with inherited fatty acid oxidation defects. The addition of valinomycin to fibroblasts decreased the accumulation of the lipophilic cation tetraphenylphosphonium (TPP+) at low concentrations due to the dissipation of the mitochondrial membrane potential. At higher doses, valinomycin increased TPP+ accumulation due to the increased potassium permeability of the plasma membrane and subsequent cellular hyperpolarization. The incubation of normal fibroblasts with valinomycin increased [14C]-palmitate oxidation (measured as [14C]O2 release) in a dose-dependent manner. By contrast, valinomycin failed to increase palmitate oxidation in fibroblasts from patients with very long chain acyl CoA dehydrogenase (VLCAD) deficiency. This was not observed in fibroblasts from patients heterozygous for this condition. These results indicate that valinomycin can increase fatty acid oxidation in normal fibroblasts and could be useful to differentiate heterozygotes from patients affected with VLCAD deficiency.
Keywords: fatty acid oxidation, valinomycin, carnitine, very long chain acyl CoA dehydrogenase deficiency
INTRODUCTION
Inherited disorders of the carnitine cycle and mitochondrial beta oxidation can impair the oxidation of fatty acids and result in hypoglycemia, liver failure, cardiomyopathy, cardiac arrest and death [1]. They include a number of conditions related either to mitochondrial beta oxidation or the transfer of fatty acids inside mitochondria (disorders of the carnitine cycle) [1,2]. Many fatty acid oxidation defects are clinically silent until an acute illness or prolonged fasting require increased amount of energy from fats and cause acute decompensation. Although many of these conditions can be identified by expanded newborn screening, establishing a definitive diagnosis in affected patients is not always straightforward since biochemical abnormalities might be present only when the patient is acutely stressed [2]. This is the case of very long chain acyl CoA dehydrogenase (VLCAD) deficiency [3] in which biochemical abnormalities may disappear while the patient is well compensated and clinical practice guidelines are based more on expert consensus rather than outcome data. DNA sequencing can identify mutations in affected patients, but some mutations can be missed by the current methodology. In addition, the clinical significance of missense variation not previously identified in other affected patients is sometime difficult to determine. Functional assays, such as enzyme assays, fatty acid fluxes, and acylcarnitine profiling can be performed in fibroblasts derived from affected patients [4,5,6,7]. Some of these methods, however, cannot distinguish among different fatty acid oxidation defects, and some cannot differentiate carriers from affected patients.
Valinomycin is a potassium ionophore that increases respiration in isolated mitochondria and in cultured cells [8]. In human fibroblasts, valinomycin dissipates the potassium electrochemical potential across the inner mitochondrial membrane and, at higher doses, increases potassium permeability of the plasma membrane with a consequent increase in membrane potential [9,10,11]. Here we evaluate the capacity of valinomycin to stress fibroblasts of patients affected by defects of fatty acid oxidation in vitro to mimic the biochemical abnormalities observed in vivo.
MATERIALS AND METHODS
Measurement of the distribution ratio of the lipophilic cation tetraphenylphosphonium (TPP+)
Fibroblasts from patients with fatty acid oxidation defects were obtained by skin biopsy for diagnostic purposes. Patients were referred for diagnostic confirmation either after symptomatic presentation or after identification by newborn screening programs. Their characteristics are presented in Table I. All studies in human cells were approved by the institutional review board (IRB) of the University of Utah. Cells were grown in Dulbecco-modified MEM supplemented with 15% fetal bovine serum. Confluent cells in 24 well plates were washed and incubated for 90 min in Earle’s balanced salt solution supplemented with bovine serum albumin (0.5%) to deplete intracellular amino acids. Cells were then incubated for 60 min in the presence of [3H]-tetraphenylphosphonium (0.2 µM, 0.1 µCi/ml). Cells were then washed three times with ice cold magnesium chloride (0.1 M). Intracellular [3H]-TPP was extracted in 0.5 ml of absolute ethanol and added to 4 ml of scintillation liquid prior to counting in a beta counter [11]. Protein content within each well was determined by a modified Lowry procedure [12]. The accumulation of radioactivity was normalized to proteins and intracellular water content was determined by the equilibrium distribution of 3-O-methyl-D-glucose as previously described [12]. Values are reported as means ± SE of 6 independent determinations obtained in two separate experiments.
TABLE I.
Mutations in patients with fatty acid oxidation defects. Phase of mutations when more than one mutation was present was confirmed by parental analysis. The genes affected and the reference sequence are indicated. Nucleotide sequencing start from the A (+1) of the ATG start codon. “C” denoted carrier status.
| Patient | Mutation 1 | Mutation 2 | Gene | NCBI reference sequence |
|---|---|---|---|---|
| CACT | c. 713A>G, p.Q238R |
c. 713A>G, p.Q238R |
SLC25A20 | NM_000387.5 |
| VLCAD-01 | c.343delG, p.E114Kfs*1 |
c.1146G>C, p.K382N |
ACADVL | NM_000018.2 |
| VLCAD-02 | c.1066A>G, p.I356V |
c.1504C>G, p.L502V |
ACADVL | NM_000018.2 |
| VLCAD-C-03 | c.1322G>A, p.G441D |
ACADVL | NM_000018.2 | |
| VLCAD-C-04 | c.552delC, p.I184Mfs*32 |
ACADVL | NM_000018.2 | |
| VLCAD-C-05 | c.1127C>T, p.F376S |
ACADVL | NM_000018.2 |
Fatty acid oxidation
For fatty acid oxidation studies, confluent fibroblasts in 24 well plates were washed twice with phosphate buffered saline solution and incubated in 0.3 ml of Earle’s balanced salt Solution (EBSS) buffered by Hepes (30 mM) at pH 7.4 containing 0.5% bovine serum albumin and [1–14C]-Palmitic acid (55 mCi/mmol, 0.5 mCi/ml, Moravek MC121) for a final concentration of 4 μM (0.22 µCi/ml). Glucose and amino acids were omitted from the incubation medium. Each well was capped by 2 caps, of which the bottom one was perforated to allow CO2 released by the cells to be captured (Fig. 2A). The perforated cap contained a 13 mm fiber filter (Pall Corporation 600 South Wagner Rd, Ann Arbor MI 48103) previously soaked in 2 N NaOH to capture CO2. At the end of the incubation, the cap was removed and the filter paper was added to the scintillation fluid for counting. Cells were then washed with magnesium chloride (0.1M) and extracted with ethanol to determine intracellular palmitic acid concentration. Protein content was determined within each well by a modified Lowry procedure [12]. The release of CO2 and the accumulation of palmitic acid were normalized to proteins and intracellular water, respectively. Values are reported as means ± SE of 6 independent determinations obtained in two separate experiments.
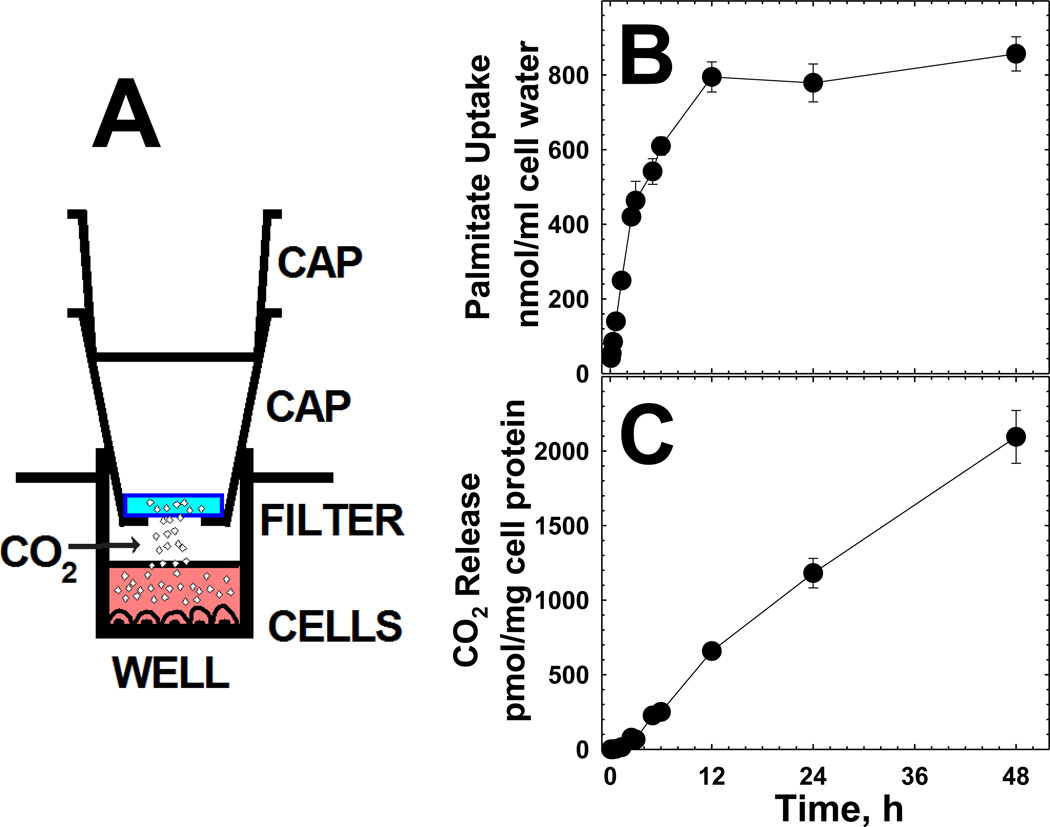
Fig. 2. Schematic of the system for measuring CO2 release by cultured cells (A) and time course of palmitate uptake (B) and CO2 release (C) by normal human fibroblasts.
(A) Two caps were used for each well in which the bottom one was hollow to sustain a filter soaked in 2 N NaOH (to absorb CO2). At the end of incubation, the caps were removed and the radioactivity collected in the filter was quantified by scintillation counting. (B) Confluent fibroblasts were incubated in Earle balanced salt solution in the presence of [1–14C]-palmitic acid (4 µM). At the indicated time, the CO2 captured in filter paper soaked in sodium hydroxide was measured by scintillation counting (C). The radioactivity accumulated inside the cells was also measured to determine the accumulated palmitic acid (B). Values are reported as means ± SE of 6 independent determinations obtained in two separate experiments.
RESULTS
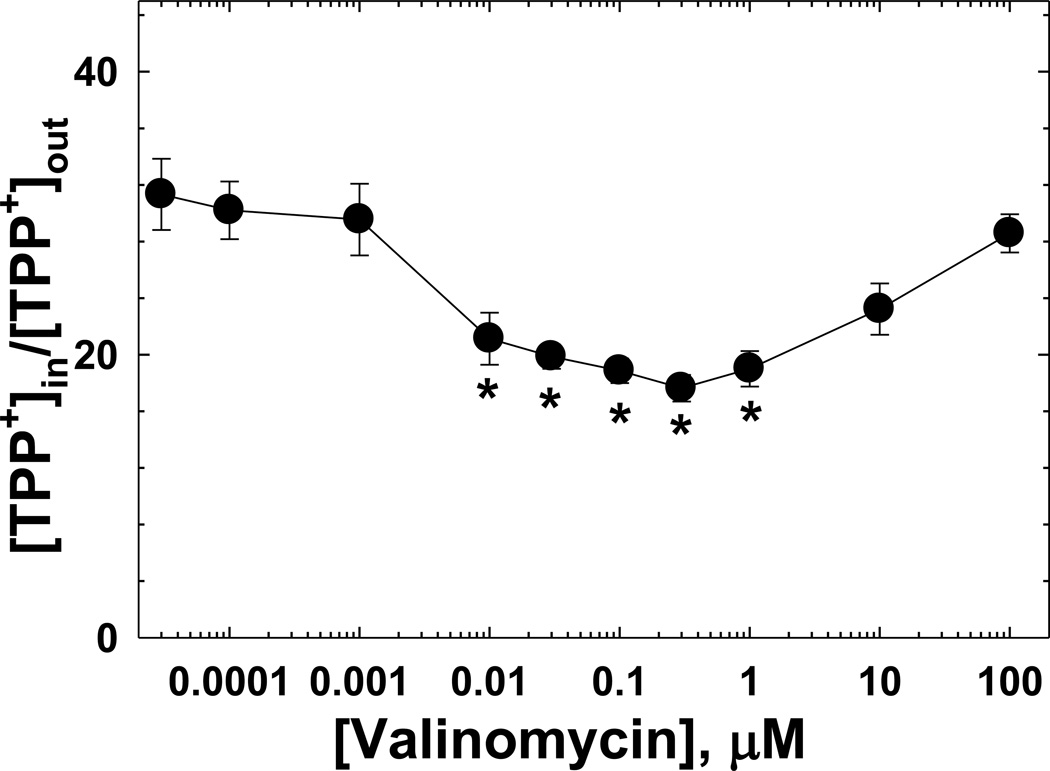
In order to determine the concentration of valinomycin necessary to cause cellular stress and increase mitochondrial respiration, we first evaluated its effects on the equilibrium distribution of the lipophilic cation tetraphenylphosphonium (TPP+). This cation crosses biological membranes and its equilibrium distribution depends on the membrane potential [11]. Fig. 1 shows the equilibrium distribution of TPP+ in normal human fibroblasts incubated for 60 min in the presence of increasing concentrations of valinomycin. A significant reduction in the distribution ratio of TPP+ was observed with 10 nM valinomycin, with half maximal effect obtained at 4.2±1 nM of valinomycin. The distribution ratio of TPP+ remained relatively constant until a concentration of 1 µM after which it increased significantly. Based on the estimation of membrane potential by the distribution ratio and influx of arginine [9,10], the first decline in TPP distribution ratio was due to the effect of valinomycin on the mitochondrial potential [11]. The subsequent increase in the TPP distribution ratio with valinomycin concentrations >1 µM was due to the increased permeability of the plasma membrane to potassium that increased membrane potential [9,10,11]. A dose of valinomycin between 0.1 and 1 µM caused a stable depression in TPP+ accumulation and was likely to cause cellular stress, a result in line with a recent report of a human fibroblasts study [13].
Fig. 1. Distribution ratio of tetraphenylphosphonium (TPP+) in the presence of increasing concentrations of valinomycin (0–100 µM).
Confluent cultures of normal human fibroblasts were washed and incubated for 60 min in Earle balanced salt solution in the presence of the indicated concentrations of valinomycin and [3H] TPP+ (0.2 µM). Cells were then washed three times with ice cold magnesium chloride (0.1 M) and intracellular content extracted in 0.5 ml of absolute ethanol. Intracellular radioactivity as determined by scintillation counting and values were normalized to cell proteins. Values are reported as means ± SE of 6 independent determinations obtained in two separate experiments.
Palmitic acid (16 carbon atoms) is one of the most abundant fats in human serum and is the typical substrate used in fatty acid oxidation studies in fibroblasts [6]. Little is known, however, about the amount that it is actually oxidized by these cells. Fig. 2 shows the accumulation of palmitic acid (panel B) and the release of CO2 by normal fibroblasts (panel C). Palmitic acid accumulation was detected at the shortest time point (5 min) after the beginning of the incubation and reached an apparent plateau after 12 hours (panel B). Prolonging the incubation beyond 24 hours decreased cell survival as indicated by a significant decline in the amount of protein remaining inside the well at the end of the incubation (data not shown). CO2 release was negligible up to 40 min of incubation after which it increased linearly up to about 12 hours (Panel C). After this time, there was a lower rate of CO2 release that continued until the longest incubation point (48 h). To further compare cells of normal controls to those of patients with fatty acid oxidation defects, an incubation time between 6 and 12 h was selected to remain within the linear increase of CO2 release.
Fig. 3 shows a dose-response curve of the effect of valinomycin on CO2 release from palmitic acid in cells from two controls and patient VLCAD-01 (see Table 1 for specific mutations) who had a childhood phenotype presenting with cardiomyopathy and hypoglycemia at 7 years of age. The accumulation of palmitic acid was slightly higher in cells from the patient with VLCAD deficiency, but did not change in a consistent manner with the addition of valinomycin (panel A). By contrast, valinomycin stimulated CO2 release from control cells with a consistent effect observed between 10 and 1000 nM valinomycin (panel B) and half-maximal effect observed at 3 nM valinomycin. In different experiments, CO2 release increased between 1.5 and 4 fold in normal fibroblasts at the highest valinomycin concentration (data not shown). Baseline CO2 release was similar in control cells and cells from the patient with VLCAD deficiency, although there was variability in the baseline among different experiments. Valinomycin failed to stimulate CO2 release in cells from the patient with VLCAD deficiency and a significant decrease in CO2 release was seen at higher valinomycin concentrations (Fig. 3).
Fig. 3. Dose-response curve of the effect of valinomycin on palmitate uptake (A) and CO2 release (B) from palmitic acid in cells from two controls and patient VLCAD-01.
Confluent cultures of normal human fibroblasts were washed and incubated for 12 hours in Earle balanced salt solution in the presence [1–14C]-palmitic acid (4 µM) and of the indicated concentrations of valinomycin. After 12 h, the CO2 captured in filter paper soaked in sodium hydroxide was measured by scintillation counting. The radioactivity accumulated inside the cells was also measured to determine the accumulated palmitic acid. Values are reported as means ± SE of 6 independent determinations obtained in two separate experiments.
Fig. 4 shows the effect of valinomycin on palmitate oxidation in fibroblasts from patients with VLCAD deficiency (VLCAD) and heterozygous carriers of VLCAD deficiency (VLCAD-C) (see Table 1 for specific mutations). Valinomycin stimulated CO2 release from palmitate in the 4 control cells, but failed to do so in cells from two patients with VLCAD deficiency. A significant stimulation of CO2 release was observed in cells from all 3 patients who were carriers of VLCAD deficiency (heterozygotes). CO2 release was consistently low in cells from one patient with a severe form of carnitine acylcarnitine translocator (CACT) deficiency and the increased release with valinomycin in this case was not statistically significant.
Fig. 4. Effect of valinomycin on palmitate oxidation by cultured fibroblasts.
Confluent cultures of normal human fibroblasts were washed and incubated for 12 hours in Earle balanced salt solution in the presence [1–14C]-palmitic acid (4 µM) with and without valinomycin (0.1 µM). After 12 h, the CO2 captured in filter paper soaked in sodium hydroxide was measured by scintillation counting. Values are reported as means ± SE of 6 independent determinations obtained in two separate experiments.
DISCUSSION
VLCAD deficiency is a defect of fatty acid oxidation that can result in cardiomyopathy, arrhythmias, hypoglycemia, sudden death or late-onset intermittent rhabdomyolysis [3]. Affected patients can be detected by newborn screening using tandem mass spectrometry with elevated C14:1 carnitine, but biochemical testing alone cannot distinguish carriers from affected patients [5]. For these reasons, molecular and functional testing is necessary to confirm or exclude the diagnosis in patients with abnormal newborn screening [5,14,15]. Most disorders of fatty acid oxidation cause symptoms and more evident biochemical abnormalities when the organism is stressed by fasting and there is increased need for mitochondrial beta oxidation [1,2]. Here we use valinomycin, a potassium ionophore, to stimulate mitochondrial respiration in cultured cells and to reproduce the stress that triggers symptoms in patients with fatty acid oxidation defects [8].
Valinomycin had a dual effect in human fibroblasts [9,10,11]: at relatively low concentrations (10 nM-1μM) it dissipated the membrane potential across the inner mitochondrial membrane reducing the TPP+ distribution ratio (Fig. 1). At higher concentrations (above 1 µM), it increased the TPP+ distribution ratio by increasing potassium permeability at the cell membrane level. In this study, valinomycin at relatively low concentrations (0.1–1 µM) was therefore used to stress mitochondria of fibroblasts from normal controls and patients with fatty acid oxidation defects (Table I).
The oxidation of palmitate was measured with a simple system to collect CO2 released from the oxidation of [14C]-palmitate (Fig. 2A). Palmitate entered human fibroblasts very rapidly, reaching an apparent plateau after 12 h of incubation (Fig. 2B). The release of [14C]O2 became measurable after 40 min and continued linearly until 12 h and beyond (Fig. 2C). Valinomycin stimulation of palmitate oxidation by normal human fibroblasts reached maximal effect at doses higher than 10 nM (Fig. 3). The dose of valinomycin required to half-maximally stimulate palmitate oxidation (Fig. 3) was similar to the one required to half-maximally inhibit TPP+ accumulation (Fig. 1), suggesting that this effect was related to dissipation of the mitochondrial membrane potential and induction of mitochondrial stress. Cells from a patient with VLCAD deficiency identified because of symptomatic presentation at 7 years of age maintained residual capacity for palmitate oxidation (Fig. 3). Complete impairment of VLCAD activity results in a more severe phenotype, usually associated with infantile death [3]. Due to the presence of residual enzyme activity, it is not always simple to differentiate patients with mild forms of VLCAD deficiency from carriers of these conditions (with 50% residual enzyme activity). Valinomycin at any dose failed to stimulate palmitate oxidation in cells from a patient with VLCAD deficiency (Fig. 3). By contrast, valinomycin retained the capacity to stimulate palmitate oxidation in cells from heterozygotes (carriers) for VLCAD deficiency (Fig. 4), effectively discriminating between affected and unaffected patients. Cells from a patient with a severe form of CACT deficiency [16] had a marked decrease in the baseline rate of palmitate oxidation (Fig. 4). This is likely a result of the defective transfer of acylcarnitines inside mitochondria caused by the mutations in this patient (Table I).
Several methods based on acylcarnitine profiling or fatty acid fluxes are available to confirm or exclude the diagnosis in patients with fatty acid oxidation defects (reviewed in [17]). However, most cannot differentiate between heterozygotes (who do not need therapy) from patients with VLCAD deficiency (who require treatment). The results reported here show that valinomycin can cause mitochondrial stress, mimicking the stress that triggers metabolic decompensation in affected patients. The use of valinomycin (or possibly other stressors) can effectively differentiate between affected and unaffected VLCAD patients. These results suggest that the lack of metabolic adaptation and failed ability to increase fatty acid oxidation upon stress, rather than a decreased rate in stable conditions, is the basis of metabolic decompensation in patients with fatty acid oxidation defects with residual enzyme activity.
Highlights.
Valinomycin can cause mitochondrial stress and stimulate fatty acid oxidation.
Cells with VLCAD deficiency fail to increase fatty acid oxidation in response to valinomycin.
Response to valinomycin can help in the diagnosis of VLCAD deficiency.
Acknowledgements
Funding: Supported in part by grant R01 DK 53824 from the National Institutes of Health. UCNE is supported by NIH supplement 3R01DK053824-08S1. FI is supported by a fellowship from the “Dottorato in Morfobiologia Applicata e Citometabolismo dei Farmaci”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Longo N, Amat di San Filippo C, Pasquali M. Disorders of carnitine transport and the carnitine cycle, American journal of medical genetics. Part C. Seminars in medical genetics. 2006;142C:77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiekerkoetter U. Mitochondrial fatty acid oxidation disorders: clinical presentation of long-chain fatty acid oxidation defects before and after newborn screening. J Inherit Metab Dis. 2010;33:527–532. doi: 10.1007/s10545-010-9090-x. [DOI] [PubMed] [Google Scholar]
- 3.Leslie ND, Tinkle BT, Strauss AW, Shooner K, Zhang K. Very Long-Chain Acyl-Coenzyme A Dehydrogenase Deficiency. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, editors. GeneReviews. Seattle (WA): 1993. [Google Scholar]
- 4.Houten SM, Wanders RJ. A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J Inherit Metab Dis. 2010;33:469–477. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liebig M, Schymik I, Mueller M, Wendel U, Mayatepek E, Ruiter J, Strauss AW, Wanders RJ, Spiekerkoetter U. Neonatal screening for very long-chain acyl-coA dehydrogenase deficiency: enzymatic and molecular evaluation of neonates with elevated C14: 1-carnitine levels. Pediatrics. 2006;118:1065–1069. doi: 10.1542/peds.2006-0666. [DOI] [PubMed] [Google Scholar]
- 6.Roe DS, Yang BZ, Vianey-Saban C, Struys E, Sweetman L, Roe CR. Differentiation of long-chain fatty acid oxidation disorders using alternative precursors and acylcarnitine profiling in fibroblasts. Mol Genet Metab. 2006;87:40–47. doi: 10.1016/j.ymgme.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Okun JG, Kolker S, Schulze A, Kohlmuller D, Olgemoller K, Lindner M, Hoffmann GF, Wanders RJ, Mayatepek E. A method for quantitative acylcarnitine profiling in human skin fibroblasts using unlabelled palmitic acid: diagnosis of fatty acid oxidation disorders and differentiation between biochemical phenotypes of MCAD deficiency. Biochimica et biophysica acta. 2002;1584:91–98. doi: 10.1016/s1388-1981(02)00296-2. [DOI] [PubMed] [Google Scholar]
- 8.Heinen A, Camara AK, Aldakkak M, Rhodes SS, Riess ML, Stowe DF. Mitochondrial Ca2+-induced K+ influx increases respiration and enhances ROS production while maintaining membrane potential, American journal of physiology. Cell physiology. 2007;292:C148–C156. doi: 10.1152/ajpcell.00215.2006. [DOI] [PubMed] [Google Scholar]
- 9.Bussolati O, Laris PC, Nucci FA, Dall'Asta V, Franchi-Gazzola R, Guidotti GG, Gazzola GC. Influx of L-arginine is an indicator of membrane potential in human fibroblasts. The American journal of physiology. 1989;256:C930–C935. doi: 10.1152/ajpcell.1989.256.4.C930. [DOI] [PubMed] [Google Scholar]
- 10.Bussolati O, Laris PC, Nucci FA, Dall'Asta V, Longo N, Guidotti GG, Gazzola GC. Dependence of L-arginine accumulation on membrane potential in cultured human fibroblasts. The American journal of physiology. 1987;253:C391–C397. doi: 10.1152/ajpcell.1987.253.3.C391. [DOI] [PubMed] [Google Scholar]
- 11.Bussolati O, Laris PC, Longo N, Dall'Asta V, Franchi-Gazzola R, Guidotti GG, Gazzola GC. Effect of extracellular potassium on amino acid transport and membrane potential in fetal human fibroblasts. Biochimica et biophysica acta. 1986;854:240–250. doi: 10.1016/0005-2736(86)90116-1. [DOI] [PubMed] [Google Scholar]
- 12.Longo N, Griffin LD, Elsas LJ. Influx and efflux of 3-O-methyl-D-glucose by cultured human fibroblasts. The American journal of physiology. 1988;254:C628–C633. doi: 10.1152/ajpcell.1988.254.5.C628. [DOI] [PubMed] [Google Scholar]
- 13.Rakovic A, Grunewald A, Seibler P, Ramirez A, Kock N, Orolicki S, Lohmann K, Klein C. Effect of endogenous mutant and wild-type PINK1 on Parkin in fibroblasts from Parkinson disease patients. Human molecular genetics. 2010;19:3124–3137. doi: 10.1093/hmg/ddq215. [DOI] [PubMed] [Google Scholar]
- 14.Lindner M, Hoffmann GF, Matern D. Newborn screening for disorders of fatty-acid oxidation: experience and recommendations from an expert meeting. J Inherit Metab Dis. 2010;33:521–526. doi: 10.1007/s10545-010-9076-8. [DOI] [PubMed] [Google Scholar]
- 15.Arnold GL, Van Hove J, Freedenberg D, Strauss A, Longo N, Burton B, Garganta C, Ficicioglu C, Cederbaum S, Harding C, Boles RG, Matern D, Chakraborty P, Feigenbaum A. A Delphi clinical practice protocol for the management of very long chain acyl-CoA dehydrogenase deficiency. Mol Genet Metab. 2009;96:85–90. doi: 10.1016/j.ymgme.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iacobazzi V, Pasquali M, Singh R, Matern D, Rinaldo P, Amat di San Filippo C, Palmieri F, Longo N. Response to therapy in carnitine/acylcarnitine translocase (CACT) deficiency due to a novel missense mutation. American journal of medical genetics. Part A. 2004;126A:150–155. doi: 10.1002/ajmg.a.20573. [DOI] [PubMed] [Google Scholar]
- 17.Law LK, Tang NL, Hui J, Ho CS, Ruiter J, Fok TF, Wanders RJ, Lam CW. A novel functional assay for simultaneous determination of total fatty acid beta-oxidation flux and acylcarnitine profiling in human skin fibroblasts using (2)H(31)-palmitate by isotope ratio mass spectrometry and electrospray tandem mass spectrometry. Clinica chimica acta; international journal of clinical chemistry. 2007;382:25–30. doi: 10.1016/j.cca.2007.03.011. [DOI] [PubMed] [Google Scholar]