Abstract
Rationale
Evidence suggests that the progesterone metabolite allopregnanolone (ALLO) decreases cocaine seeking in animal models of relapse.
Objective
The purpose of this study was to examine the effects of ALLO on an animal model of cocaine and sucrose bingeing (escalation). Allopregnanolone's effects on yohimbine-induced sucrose intake were also examined. In a separate group of animals, dose interactions between ALLO and cocaine were examined with an abbreviated procedure, a short access progressive ratio (PR) schedule for cocaine reinforcement.
Methods
Female rats were treated with ALLO (15 mg/kg, s.c.) or vehicle (VEH) and trained to lever press for cocaine infusions (0.4 mg/kg) under an extended-access procedure. In a separate condition, other ALLO- and VEH-treated female rats self-administered orally delivered liquid sucrose. Allopregnanolone and VEH treatment was then discountinued and the sucrose-maintained rats were administered priming injections of saline, yohimbine, or yohimbine+ALLO. For the PR condition, rats were first treated with VEH until reaching stability at four doses of cocaine (0.2, 0.4, 0.8, and 1.6 mg/kg in mixed order). Subsequently, rats re-established their baseline cocaine intake at the four cocaine doses following treatment with each of two counterbalanced doses of ALLO (15 and 30 mg/kg).
Results
ALLO significantly blocked the escalation of cocaine self-administration but did not reliably affect intake of sucrose under a similar condition or affect cocaine intake at several doses under a PR schedule. Yohimbine significantly increased sucrose intake while ALLO failed to attenuate this increase.
Conclusion
These findings indicate that ALLO protects against binge-like patterns of cocaine intake but does not reduce sugar intake that is acutely increased by yohimbine in females.
Keywords: Allopregnanolone, Cocaine, Escalation, Rats, Sucrose, Progressive ratio
Introduction
The transition from steady to dysregulated drug consumption characterizes the escalation phase of the drug abuse process (Ahmed and Koob 1998, 1999, 2005; Kalivas and Volkow 2005). In humans, escalation represents out-of-control drug binging that is linked to overdose and death (Kalivas and Volkow 2005). Escalation also occurs in animals with several drugs (Doherty et al. 2009; Kitamura et al. 2006; Lenoir and Ahmed 2008; Rogers et al. 2008; Specio et al. 2008), and it is typically achieved by increasing the animal's access to the drug (Fitch and Roberts 1993; Lynch et al. 2000; Lynch and Carroll 2001; Morgan and Roberts 2004). Following increased access, drug intake increases over time, and binge and rest patterns replace steady-state patterns of intake.
Growing evidence suggests that gonadal hormones exert a powerful influence on responses to cocaine in females (Anker and Carroll 2010b). Women report increased positive subjective effects following administration of cocaine during the estrogen-dominant follicular phase of the menstrual cycle (Evans and Foltin 2006; Evans et al. 2002; Sofuoglu et al. 1999) while progesterone administration during the follicular phase attenuates this effect (Evans and Foltin 2006; Sofuoglu et al. 2001, 2002). Similar results were demonstrated with the escalation paradigm using rats in a study by Larson et al. (2007). Ovariectomized (OVX) and sham-operated female rats that were treated with vehicle (VEH, peanut oil), estrogen, progesterone, or estrogen+progesterone were compared on the escalation of cocaine self-administration under extended-access conditions (Larson et al. 2007). The results from this study indicated that estrogen treatment in OVX rats increased intake and facilitated escalation while progesterone treatment decreased cocaine intake and attenuated escalation. This study indicated that female gonadal hormones could provide the biological basis for increased vulnerability toward (i.e., estrogen) and protection against (i.e., progesterone) binge-like patterns of cocaine intake in women.
The progesterone metabolite allopregnanolone (ALLO) also exerts an attenuating effect on cocaine-seeking behavior (Anker and Carroll 2010b). For example, in two recent studies ALLO treatment blocked the reinstatement of cocaine-seeking behavior induced by IP injections of cocaine (Anker et al. 2009) and the anxiogenic drug yohimbine, an alpha2-noradrenergic receptor antagonist, (Anker and Carroll 2010a) in female rats. A goal of the present study was to investigate a role for ALLO in suppressing the escalation of cocaine self-administration in female rats. An additional goal was to examine possible dose interactions between ALLO (15 and 30 mg/kg) and cocaine (0.2, 0.4, 0.8, and 1.6 mg/kg) using an abbreviated short-access procedure, a progressive ratio (PR) schedule of reinforcement, as GABA agents such as ALLO have been shown to have dose-dependent effects on several behaviors (Janak et al. 1998; Khisti et al. 2002). In addition, the escalation procedure does not allow an examination of a wide range of doses of cocaine due to escalation the risk of overdose at higher doses. In contrast, the PR schedule has been extensively used to examine the effects of potential treatment medications on the reinforcing effectiveness of drugs of abuse using several dose ranges (Stafford et al. 1998).
Previous studies using animal models have shown that escalation also occurred with behavior reinforced by nondrug rewards such as sucrose (Colantuoni et al. 2001, 2002) and wheel running (Larson and Carroll 2005), and females exhibited higher levels of these behaviors than males (Carroll et al. 2008; Cosgrove et al. 2002). Furthermore, clinical (Edler et al. 2007; Gladis and Walsh 1987; Price et al. 1987) and preclinical (Klump et al. 2008; Leibowitz et al. 2007) research implicates ALLO's precursor progesterone in the modulation of binge-like patterns of food intake in females. Thus, the effects of ALLO on orally delivered sucrose intake under extended-access conditions were examined in a separate group of rats to determine if ALLO would differentially affect bingeing for a nondrug reward.
Stress is a major factor in drug abuse vulnerability in humans. Several clinical reports indicate that stress also contributes to excessive high-caloric food consumption and unhealthy dietary choices that are associated with obesity (Byrne et al. 2003; Grilo et al. 1989; Kayman et al. 1990). Animal models have further confirmed a link between stress and excessive food consumption (Pecoraro et al. 2004). Furthermore, female hormones influence responses to stress. For example, ALLO attenuated biological (Brunton et al. 2009; Guo et al. 1995) and behavioral responses (Frye and Rhodes 2006) to stress and blocked stress-induced reinstatement of cocaine-seeking behavior following the administration of yohimbine in female rats (Anker and Carroll 2010a). Yohimbine elicits a stress-like state in rats (Bremner et al. 1996a, b), and similar to foot shock, administration of yohimbine increases c-fos and corticotropin-releasing factor (CRF) mRNA in areas of the brain associated with the rewarding effects of drugs of abuse and natural rewards (e.g., food) in rats (Angeles-Castellanos et al. 2007; Funk et al. 2006). An additional goal of the present study was to investigate the effects of stress on sucrose self-administration in female rats by administering yohimbine and to determine whether ALLO treatment would attenuate the facilitating effects of yohimbine on sucrose intake.
Materials and methods
Subjects
A total of 51 sexually mature (90–120 days old) female Wistar rats (Harlan Sprague–Dawley, Madison, WI) were used; 21 rats were used in the extended access procedure for i.v. cocaine (VEH, n=13; ALLO, n=8), 19 rats were used in the extended access condition for sucrose (VEH, n=9; ALLO, n=10), and 11 were used in the PR condition. The data for ten subjects in the vehicle-treated group in the cocaine extended access condition were used in a previous study (Larson et al. 2007), but three additional rats were added to this group for the present study. The experimental procedures in the present study (i.e., duration of experimental conditions, cocaine dose, etc.) were identical to those used previously (Larson et al. 2007). Rats weighed 225–300 g at the beginning of the procedures and were allowed at least 3 days to acclimate upon arrival in the laboratory before experimental testing or i.v. cannulation. During this time they were pair-housed in plastic cages where they had free access to food (Purina Laboratory Chow, Purina Mills, Minneapolis, MN) and water.
Following i.v. cannulation or sham surgery, the rats were placed in operant-conditioning chambers to recover for 3 days, and they remained there for the duration of the study. While in the chambers, they continued to have free access to water, and they were fed 16 g of ground food (Purina Laboratory Chow) at the end of the experimental sessions to maintain consistency in food intake across subjects. These amounts allowed the rats to gain weight slowly during the experiments. All holding rooms were maintained at 24°C and at 40–50% humidity under a light/dark cycle (12/12 h with room lights on at 6:00 am). The experimental protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee (protocol number 0708A15263), and experiments were conducted in accordance with the principles of laboratory animal care (National Research Council 2003). Laboratory facilities used in these studies were accredited by the American Association for the Accreditation of Laboratory Animal Care.
Surgical procedure
Rats were surgically implanted with an indwelling catheter in the right jugular vein following the procedure used by Carroll and Boe (1982). Briefly, the rats were anesthetized with a combination of ketamine (60 mg/kg, IP) and xylazine (10 mg/kg, IP) and administered doxapram (5 mg/kg, IP) and atropine (0.4 mg/ml, 0.15 ml, s.c.) to facilitate respiration. An incision was made lateral to the trachea, the right jugular vein was exposed, and a small incision was made perpendicular to the vein. The beveled end of a polyurethane catheter (MRE-040, Braintree Scientific Inc., Braintree, MA, USA) was inserted and then secured to the vein with silk sutures. The free end of the catheter was guided subcutaneously to the midscapular region of the neck where it exited via a small incision and attached to a metal cannula (C3236, Plastics One, Roanoke, VA, USA) that was embedded in the infusion harness.
Following the surgical procedure, rats were allowed a 3-day recovery period during which antibiotic (gentamicin) and analgesic (buprenorphine) medications were administered. Every 7 days at 3:00 pm, body weights were recorded, and catheter patency was checked by injecting a 0.1-ml solution containing ketamine (60 mg/kg), midazolam (3 mg/kg), and saline (KMS). If a loss of the righting reflex was not manifest upon a KMS catheter patency check, a second catheter was implanted in the left jugular vein following the methods described above and the experiment resumed in 3 days. For the sucrose condition, the rats underwent a sham jugular catheterization surgery to match conditions with the cocaine group, but they were not implanted with a catheter. Following surgery, the rats under this condition were fitted with an infusion harness and tether and allowed to recover for a 3-day period before experimental testing.
Apparatus
Operant chambers were hexagonal in shape with alternating stainless steel and Plexiglas walls. Each operant-conditioning chamber contained slots for insertion of stainless steel wall panels, lights, and operant fixtures. Chambers were also fitted with a food receptacle and a panel that allowed placement of a drinking spout into the operant-conditioning chamber. Each operant-conditioning chamber was enclosed in a sound-attenuating melaminecoated wooden cabinet that had a fan for ventilation. Two operant levers were positioned 2.5 cm above the wire mesh floor on opposite sides of the operant-conditioning chamber, and stimulus lights were situated above each lever. In addition, boxes were illuminated with a house light (4.76 W) located in the upper right corner of each operant-conditioning chamber. An infusion pump (Model PHM-100, Med Associates, St. Albans, VT), used to administer response-contingent cocaine during experimental sessions, was positioned outside of the soundattenuating wooden box. A length of plastic tubing (1.52 mm, outer diameter; 0.51 mm inner diameter, Fisher Scientific, Springfield, NJ) extended from the infusion pump to a small plastic swivel (050-0022, Alice King Chatham, Hawthorne, CA) located at an opening at the top of the operant-conditioning chamber. The swivel attached to a metal spring-covered tether (C313CS, Plastics One, Roanoke, VA) that extended into the operant-conditioning chamber where it was secured to a metal cannula guide (C3236, Plastics One, Roanoke, VA) embedded in the rat's infusion harness. The tether was connected to the rat's infusion harness the day after catheterization, and it remained attached throughout the duration of the study.
Chambers for the sucrose self-administration study were identical to the cocaine self-administration chambers described above with the exception that two lick-operated liquid-delivery devices, activated by a tongue contact, were inserted in place of the levers. Stimulus lights were located above the drinking spouts, and they were illuminated for 3 s following a lick on the spout below. A lick on the active spout delivered a single 0.07 ml delivery of 10% sucrose or water, depending on the experimental condition.
Procedure
Following recovery from surgery, rats were trained to self-administer 0.4 mg/kg/infusion i.v. cocaine during daily sessions under a fixed-ratio 1 (FR 1) schedule of reinforcement. Rats received daily s.c. injections of ALLO or VEH one half hour before the start of each self-administration session (8:30 am) throughout the duration of the study. Self-administration training sessions began daily at 9:00 am with the illumination of the house light and ended at 11:00 am with its termination. During each session, a response on the active lever resulted in a single 0.4 mg/kg cocaine infusion (0.025 ml/100 g body weight) and the simultaneous illumination of three multi-colored stimulus lights located directly above the lever. Responses on the other (inactive) lever produced only the illumination of the stimulus lights for the duration of an infusion, but they did not activate the infusion pump. Responses on the inactive lever were considered a measure of activity, and responses on both levers were recorded using a Med-PC IV software (Med Associates, St. Albans, VT., USA) during each session. During self-administration training, sessions began with three experimenter-administered cocaine infusions each separated by 2 s, and levers were subsequently baited with approximately 0.5 g of food. The criteria for the acquisition of cocaine self-administration consisted of no steadily increasing or decreasing trend in responses, an average of ≥30 infusions over a 3-day period, and a minimum active/inactive lever response ratio of 2:1. Once rats met these criteria, priming injections and placement of food on levers ended, and the subsequent experimental condition commenced.
Following the acquisition of cocaine self-administration in the extended access to cocaine condition, a separate group of rats was allowed to continue self-administering i.v. cocaine (0.4 mg/kg/infusion) during three short access (ShA) sessions (2 h/day, 9:00–11:00 am) under a FR 1, 20-sec timeout schedule of reinforcement. Rats were then given long access (LgA) (6 h/day, 9:00 am–3:00 pm) to cocaine self-administration (0.4 mg/kg/infusion, i.v.) for 21 days under a FR 1, 20-sec timeout schedule of reinforcement. The 0.4 mg/kg dose of cocaine was selected as it produces robust escalation of cocaine self-administration under similar extended-access conditions (Larson et al. 2007). Self-administration under the 3-day ShA condition was retested after the LgA phase in order to determine if escalation led to changes in ShA cocaine intake.
Escalation of cocaine self-administration occurs under a narrow range of doses, and it precludes an analysis of large doses of cocaine due to the risk of overdose. In order to examine dose interactions between ALLO and cocaine, a separate group of rats was allowed to self-administer four doses of cocaine under a ShA PR schedule following treatment with two doses of ALLO. Initially, the rats were trained to lever press following the same acquisition procedure detailed above (i.e., 0.4 mg/kg i.v. cocaine, FR 1, 2 h sessions) with the exception that injections of VEH and ALLO were not given. Following acquisition under the FR 1 schedule, the rats were allowed to self-administer four doses of cocaine (0.2, 0.4, 0.8, and 1.6 mg/kg in mixed order) during the 2-h sessions (9:00 am–11:00 am) under a PR schedule. During this time, VEH was administered 30 min prior to each session (8:30 am). Following stable intake at each dose (3 days with no increase or decrease in cocaine infusions) the rats were treated with either 15 or 30 mg/kg ALLO (8:30 am), and dose-response curves were re-established. Dose-response curves were then established following administration of an ALLO dose alternated in counterbalanced order.
For the sucrose self-administration procedure, following the stabilization of water intake after surgery (≥20 ml water consumed for three consecutive days), the rats were trained to drink water through the lick-operated spouts. The next morning, the rats achieved stable water intake from the water bottle; water bottles were removed from the operant chambers, the 500-ml reservoir was filled with tap water, and the rats subsequently had 24-h access (8:00 am to 8:00 am the next day) to water contingent on contact with the active spout. During this time, a contact on the inactive spout was recorded, and it produced the same stimulus conditions as a contact on the active spout, with the exception that it did not deliver water. The next day the water bottle was returned to the operant-conditioning chamber. If a rat obtained 100 or more deliveries during the 24-h training session, the water in the 500-ml reservoir was replaced with a 10% (w/v) sucrose solution, the session length was changed to 2 h (9:00 am to 11:00 am), and the rats began treatment with VEH or ALLO (s.c. at 8:30 am). After meeting this criterion, the water bottle was returned to the operant-conditioning chamber, and the rats were once again allowed 24-h access to water throughout the remainder of the study. If a rat did not meet the acquisition criteria during the 24-h training session, the water bottle was returned for at least 3 days until water intake again stabilized, and the 24-h training session resumed the following day. If a rat did not achieve acquisition after three training sessions, it was excluded from the study. Following acquisition, the rats self-administered sucrose during three 2-h ShA sessions (9:00 am to 11:00 am), and session length was extended to 6 h (LgA) (9:00 am to 3:00 pm) for 21 days. Sucrose intake under the ShA condition (3 days) was then reassessed following LgA. A 10% sucrose solution was used in this experiment as it has previously been shown to reliably produce escalation of sucrose intake under extended-access conditions (Rada et al. 2005).
Following the last post-LgA ShA session, ALLO and VEH treatments were discontinued, and the rats were allowed to continue self-administering 10% sucrose during ShA sessions for at least seven additional days to allow sucrose intake to stabilize and the ALLO levels to dissipate. Subsequently, the rats were administered saline, VEH+ yohimbine (Y, 2.5 mg/kg), or ALLO (A, 15 mg/kg)+Y (2.5 mg/kg) priming injections on separate days using a within-subjects procedure described previously (Anker and Carroll 2010a). Injections occurred in the following sequence: S, V+Y, S, A+Y, S, V+Y. Yohimbine or S was injected immediately before the beginning of the session, and on the A+Y day ALLO was administered 30 min before the session (8:30 am).
Drugs
Cocaine HCl was provided by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC), dissolved in a sterile 0.9% saline solution at concentrations of 0.8, 1.6, 3.2, 6.4 mg cocaine HCl/ml saline and refrigerated. To extend catheter patency, the anticoagulant heparin (1 ml heparin/200 ml of saline; 190 USP units of heparin per kilogram) was added to the cocaine solution. The flow rate of each infusion was 0.025 ml/s, and the duration of pump activation (1 s/100 g body weight) was adjusted weekly following catheter patency checks to reflect changes in weight and maintain a constant cocaine dose.
ALLO (20 mg/ml) was dissolved in peanut oil. ALLO and peanut oil were purchased from Sigma-Aldrich (St. Louis, MO). A 15 mg/kg dose of ALLO was used as it had been shown to produce relatively stable brain concentrations of ALLO 2 h following an s.c. injection that are within the physiological range during pregnancy (Lancel et al. 1997), and it attenuated cocaine-and stress-induced reinstatement of cocaine seeking in female rats (Anker and Carroll 2010a; Anker et al. 2009). In addition, in a previous study we demonstrated that an ALLO dose two-fold higher had no effect on baseline measures of locomotor activity or food maintained responding in female rats (Anker et al. 2009). Injections of yohimbine (2 mg/ml; Yohbine Injection®, Lloyd Laboratories, Shenandoah, IA) were administered IP at a dose (2.5 mg/kg) that has been shown to reliably produce reinstatement of cocaine-seeking behavior (Anker and Carroll 2010a; Feltenstein and See 2006).
Estrous cycle phase determination
Samples of the vaginal mucosa were taken at 8:00 am before each self-administration session for the rats in the sucrose condition to determine if sucrose intake changed across different phases of the estrous cycle. The estrous cycle was not monitored in rats self-administering cocaine, as cycle progression is severely disrupted following extended access to cocaine self-administration precluding a direct comparison of drug intake across estrous phase (Larson et al. 2007). Cells were collected from the rats via a saline-dampened cotton applicator, placed on microscope slides stained with methylene blue, and promptly cover-slipped. The diestrus, proestrus, estrus, and metestrus phases were identified from the prototypic cytologic morphology described by Montes and Luque (1988).
Data analysis
Active and inactive lever responses and cocaine infusions during LgA were averaged into seven blocks of 3 days each and analyzed using a two-factor repeated-measures ANOVA (group × block). Responses and infusions during the first through sixth hour of LgA were also analyzed with two-factor repeated-measures ANOVA for days 1, 7, 14, and 21 of LgA (group × hour). Seven-day increments were selected as escalation of intake was steady and we wanted to determine hourly intake at several time points during the escalation procedure. The number of cocaine infusions during ShA sessions before and after LgA were analyzed using a two-factor repeated-measures ANOVA (group × phase). The same statistical procedures to analyze cocaine deliveries during the ShA and LgA conditions were used to analyze sucrose deliveries in the sucrose self-administration condition. A two-factor ANOVA (group × estrous cycle phase) was used to compare sucrose deliveries during the estrus, proestrus, and metestrus/diestrus phases of the estrous cycle. A two-factor repeated-measures ANOVA (ALLO dose × cocaine dose) was used to analyze active and inactive responses, breakpoints, and cocaine infusions earned under the PR schedule.
Responses following S, Y, or A+Y injections in the sucrose condition were compared using a single-factor repeated-measures ANOVA. After a significant main effect, post-hoc tests were conducted using Fisher's LSD protected t tests. All statistical analyses were conducted using GB Stat (Dynamic Microsystems, Inc., Silver Spring, MD).
Results
Cocaine self-administration
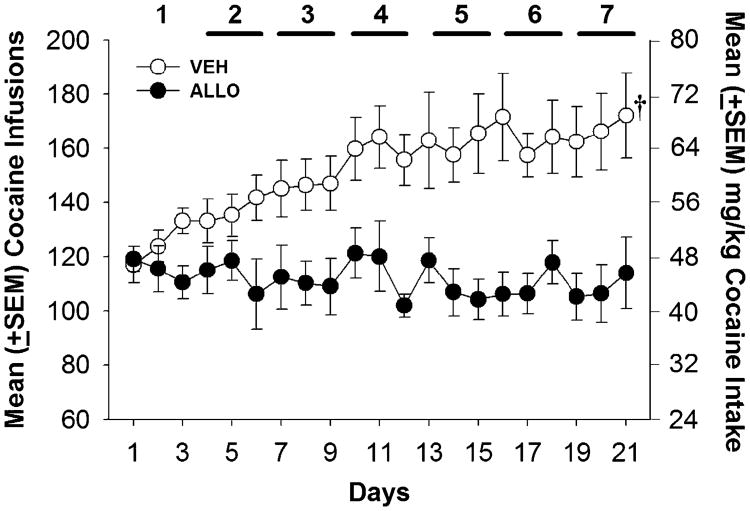
Mean body weights for VEH- and ALLO-treated rats self-administering cocaine did not differ across the LgA condition (VEH: day 1=283±10.17, day 21=280±6.98; ALLO: day 1=264±6.89, day 21=264±8.23). Figure 1 illustrates the mean (± SEM) number of cocaine infusions (0.4 mg/kg) self-administered under the FR 1 schedule during each day of LgA (6 h/day) to cocaine self-administration. There was a significant main effect of group (F1,146=12.313, p<0.01), and a significant group × block interaction (F6,146=5.126, p<0.01), but no main effect of block (F6,146=2.046) on the number of cocaine infusions self-administered during LgA. Post-hoc comparisons indicated that compared to the first 3-day block, VEH-treated rats increased (escalated) cocaine self-administration compared to blocks 3–7 (ps<0.05), while ALLO-treated rats maintained stable cocaine intake throughout the escalation procedure. Group comparisons revealed that the VEH-treated group self-administered significantly more cocaine than the ALLO group in blocks 2–7 but not block 1. Thus, ALLO blocked the escalation of cocaine self-administration; however, these differences were not apparent until the fourth day (second block) of extended access. ALLO-treated rats earned significantly fewer infusions than VEH-treated rats across all 6 h of the session on days 14 and 21 (ps<0.05) (Fig. 2). There were no within-group differences between hours 1–6 during the first, seventh, 14th, and 21st LgA sessions in either group.
Fig. 1.
Data represent the mean (±SEM) cocaine infusions self-administered by female rats each day of the LgA phase. Horizontal lines indicate 3-day intervals during which VEH-treated rats earned more infusions than ALLO-treated rats (p<0.05). (†) p<0.05 block 1 <blocks 3–7 in the VEH group
Fig. 2. Mean (+SEM) hourly cocaine infusions (per 6-h session) self-administered by VEH- and ALLO-treated rats during the 1st, 7th, 14th, and 21st days of LgA. Horizontal lines indicate significant group differences across all of the hours during the 14th and 21st days of LgA.
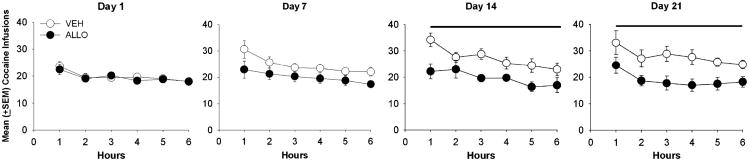
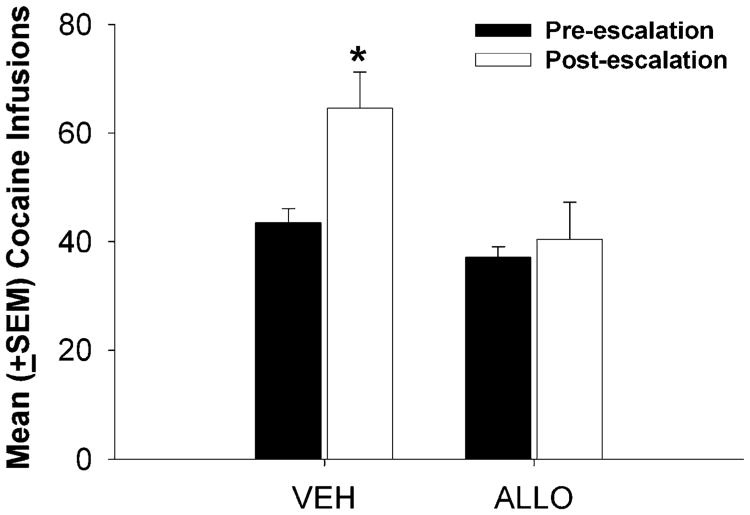
Figure 3 illustrates the mean (±SEM) number of cocaine infusions self-administered under ShA (2 h/day) conditions, assessed either before (black bars) or after (white bars) exposure to LgA conditions. There was a significant effect of group (F1,41=7.113, p<0.05) and condition (before or after LgA) (F1,41=9.050, p<0.01) on cocaine infusions, but there was not a significant group × phase interaction. There were no significant main effects or interactions for active and inactive lever presses during the LgA and ShA conditions.
Fig. 3.
Mean (±SEM) cocaine infusions self-administered by female rats during ShA sessions before (black bars) and after (white bars) the escalation phase. (Aterisk) p<0.05 post LgA vs. pre LgA within group
Table 1 shows the mean (±SEM) number of 0.2, 0.4, 0.8, and 1.6 mg/kg cocaine infusions earned under the PR condition following VEH, 15 mg/kg ALLO, and 30 mg/kg ALLO. ALLO at the 15 and 30 mg/kg doses did not significantly disrupt cocaine intake across all doses of cocaine. However, at the higher dose of ALLO and lowest dose of cocaine there was a trend toward fewer cocaine infusions earned (Fe).
Table 1. Mean (±SEM) number of cocaine infusions earned under a PR schedule following administration with VEH, 15 mg/kg ALLO or 30 mg/kg ALLO.
| Cocaine dose (mg/kg) | ||||
|---|---|---|---|---|
|
| ||||
| 0.2 | 0.4 | 0.8 | 1.6 | |
| VEH | 13.1 (0.75) | 13.7 (1.00) | 13.3 (0.96) | 12.0 (0.71) |
| 15 mg/kg ALLO | 11.3 (1.22) | 14.0 (1.24) | 13.0 (1.59) | 11.75 (1.02) |
| 30 mg/kg ALLO | 9.53 (1.25) | 13.92 (1.71) | 12.92 (1.58) | 11.96 (0.69) |
Sucrose self-administration
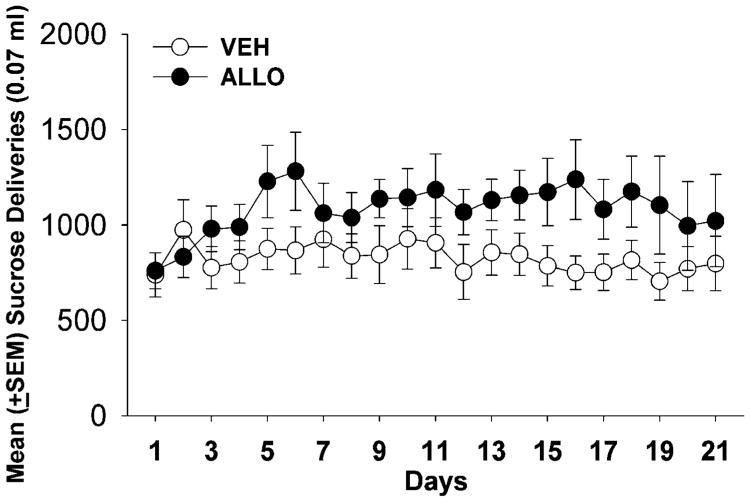
Mean body weights for VEH- and ALLO-treated rats self-administering sucrose were higher than those for the cocaine condition, but they did not differ across the LgA condition (VEH: day 1=306 g±6.9 g, day 21=329 g± 8.01 g; ALLO: day 1=305±7.74, day 21=331±9.75). There were no significant main effects or interactions for sucrose deliveries, active spout licks, and inactive spout licks during LgA and ShA to sucrose self-administration in ALLO- and VEH-treated rats (data not shown). However, there was a trend toward increased sucrose intake in the ALLO-treated group, suggesting that ALLO facilitated sucrose intake (F1,132=2.500) (Fig. 4).
Fig. 4.
Data represent the mean (±SEM) sucrose deliveries self-administered by VEH- and ALLO-treated female rats each day of the LgA phase
Sucrose intake was also elevated in both groups during the first hour of the session compared to the subsequent hours across all days (Fig. 5). There were no differences in sucrose intake across different phases of the estrous cycle in either group (data not shown).
Fig. 5. Mean (+SEM) hourly sucrose deliveries (per 6 h session) self-administered by VEH- and ALLO-treated rats during the 1st, 7th, 14th, and 21st days of LgA.
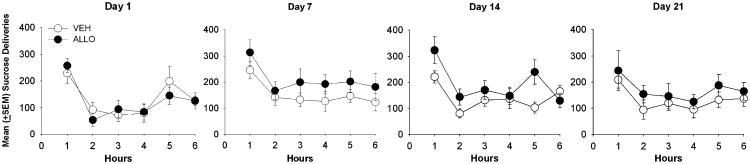
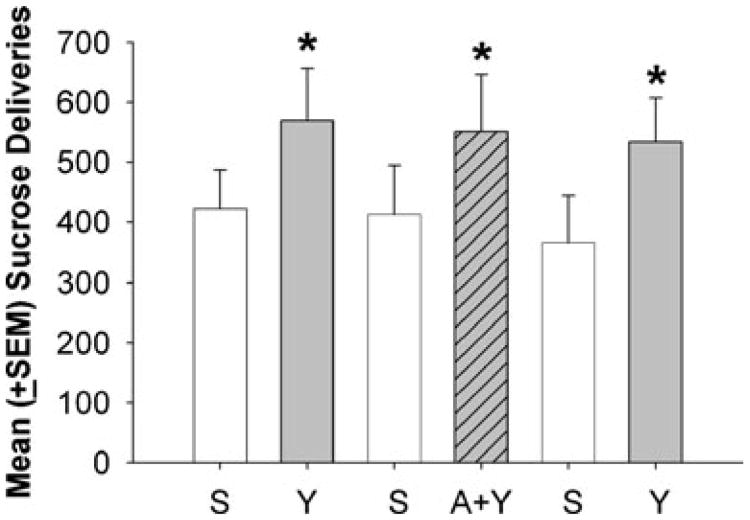
Figure 6 illustrates the mean sucrose deliveries following saline, yohimbine+VEH, or yohimbine+ALLO injections. There were no significant differences in sucrose intake between rats that were previously treated with ALLO or VEH under this condition; therefore, these data were combined. On the first, second, and third day of yohimbine administration, the rats earned significantly more sucrose deliveries than on the preceding days when saline was administered (ps<0.01). However, there were no differences in sucrose intake when ALLO was administered on the second day of yohimbine administration (A+Y) compared to when yohimbine was administered with VEH. These results indicated that ALLO did not reduce yohimbine-induced sucrose intake.
Fig. 6.
Mean (± SEM) sucrose deliveries following saline (S) or yohimbine (Y) priming injections during 2-h sessions. Asterisks indicate significantly greater responding following Y compared to S priming injections (p<0.05)
Discussion
The results of the present experiment showed that ALLO attenuated the escalation of cocaine self-administration in female rats. These results are similar to those reported for other phases of the drug abuse process. For example, ALLO treatment decreased cocaine- (Anker et al. 2009) and yohimbine- (Anker and Carroll 2010a) primed reinstatement of cocaine seeking in female rats and blocked acute convulsions (Gasior et al. 1999) and kindling (Kaminski et al. 2003) following the administration of a large dose of cocaine in male mice. Together these results indicate that ALLO attenuated the effects of cocaine across different phases of the drug abuse process.
Escalated cocaine intake or bingeing is hypothesized to be regulated by an increasing negative emotional or withdrawal state. The negative emotional state involves recruitment of the CRF system following chronic and sustained cocaine use (Koob 1999; Mantsch et al. 2007, 2008; Mantsch and Katz 2007). Agents that normalize CRF activation and alleviate this negative emotional state may be useful in treating episodes of cocaine bingeing. For example, in a recent study by Specio et al. (2008), administration of CRF antagonists decreased cocaine intake under extended-access conditions but were less effective in attenuating intake under short-access conditions. Interestingly, previous work indicates that ALLO attenuates the release of CRF following exposure to stress (Drugan et al. 1993; Frye et al. 2006; Owens et al. 1992; Patchev et al. 1994; Purdy et al. 1991); and in the present study, ALLO administration blocked the development of escalation in rats self-administering cocaine under LgA. Similar to the results with CRF antagonists (Specio et al. 2008), ALLO had no effect on cocaine self-administration during 2-h sessions under an FR 1 (pre-escalation) and PR schedule of reinforcement in the present study. Thus, ALLO may attenuate cocaine bingeing by normalizing CRF levels induced by chronic cocaine use.
ALLO is a potent positive allosteric modulator of the GABAA receptor, and this may also account for its attenuation of cocaine self-administration. Drugs that facilitate GABAA neurotransmission antagonize the effects of CRF (Heberlein et al. 2008) and inhibit cocaine-induced extracellular dopamine in the nucleus accumbens and striatum in rodents (Dewey et al. 1997; Finlay et al. 1992; Giorgetti et al. 1998; Morgan and Dewey 1998). Furthermore, administration of GABAergic drugs (e.g., topiramate and baclofen) attenuated cocaine self-administration in rats (Campbell et al. 1999, 2002) and have shown some similar results in treating drug dependence in humans (Kalivas 2007; Karila et al. 2008; Ling et al. 1998; Reis et al. 2008; Shoptaw et al. 2003).
There are several alternative explanations that may account for attenuating effects of ALLO on the escalation of cocaine self-administration. It is possible that ALLO blocked tolerance to cocaine thereby attenuating escalation. The putative mechanisms by which drug intake escalates (tolerance, sensitization, reward allostasis, etc.) have been reviewed elsewhere (Zernig et al. 2007). Another possible explanation of ALLO's mechanism may involve interaction with cocaine pharmacokinetics. However, previous findings indicate little to no effect of the menstrual cycle on plasma levels of cocaine or its metabolites following smoked and i.v. cocaine in women (Evans and Foltin 2004; Mendelson et al. 1999; Sofuoglu et al. 1999) and nonhuman primates (Evans and Foltin 2004). In addition, several studies with female rats indicate that plasma levels of cocaine's metabolite benzoylecgonine are not altered in progesterone-treated vs. VEH-treated OVX rats (Niyomchai et al. 2006; Perrotti et al. 2001; Quinones-Jenab et al. 2000). These results suggest that the mechanism by which progesterone and/or ALLO attenuates cocaine-seeking behavior does not include alteration of cocaine's pharmacokinetic profile.
Currently there are a few studies that have examined the role of ALLO in drug abusing human populations. Alcohol, a substance that is consumed in a binge-like pattern, has been the most extensively studied, and findings suggest a role for ALLO in the suppression of withdrawal symptoms from alcohol. In two studies, decreased biosynthesis of ALLO increased the severity of withdrawal symptoms in alcoholics (Hill et al. 2005; Romeo et al. 1996), while the reinstatement of baseline levels of ALLO alleviated alcohol withdrawal symptoms (Hill et al. 2005). In another study, baseline plasma levels of ALLO were far lower in smokers compared to nonsmoking controls (Childs and de Wit 2009). More work is needed to examine the influence of ALLO on withdrawal symptomatology that may drive continued abuse of drugs.
Palatable substances and drugs of abuse share several common features (Avena et al. 2008; Carroll et al. 2008). In one study, rats given 12-h access to a 10% sucrose solution exhibited binge-like patterns of sucrose intake (Rada et al. 2005) that resembled the escalated patterns typically found in rats with extended (6 h) access to i.v. cocaine. Despite a noticeable increase in sucrose intake during the first compared to subsequent days under extended access, this result was not significant in the present study. One explanation for the discrepancy between these studies may be that the 6-h session was not an adequate amount of time for rats to escalate their 10% sucrose intake. For example, in other studies examining sucrose escalation a 12- or 24-h extended-access procedure was used. An additional discrepancy that may account for the differences in results is that an automatic drinking device was used to deliver sucrose solution in the present study, while in previous studies that have demonstrated escalation of sucrose intake, a standard drinking spout was used (Rada et al. 2005). This is especially pertinent given that the form of operant response modulates the development of escalation during extended-access sessions for food reinforcement (Goeders et al. 2009).
In the present study, ALLO did not have an effect on sucrose deliveries. This lack of a significant effect of ALLO on liquid sucrose intake was also reported under ShA (1 h) conditions in a previous study (Janak and Michael Gill 2003). These results suggest that ALLO selectively reduces drug intake. The trend toward increased sucrose intake in the present study was consistent with previous work demonstrating that ALLO increased food consumption in male and female rats (Chen et al. 1996; Higgs and Cooper 1998; Reddy and Kulkarni 1999). These findings suggest that ALLO has opposite effects on drug- vs. food-maintained behavior.
An additional feature that is shared between palatable substances and drugs of abuse is that both are consumed at a higher rate under stressful conditions. In the present study, rats that were administered the anxiogenic drug yohimbine significantly increased sucrose intake under a 2-h access condition. This result was similar to previously reported findings indicating that yohimbine (Ghitza et al. 2006; Richards et al. 2008) or other stressful stimuli (Silveira et al. 2000) dramatically increased palatable food intake and food-seeking behavior in rats. Similar patterns were also found in cocaine seeking under similar stressful conditions (Anker and Carroll 2010a).
In a previous study we demonstrated that ALLO blocked yohimbine-induced reinstatement of cocaine seeking (Anker and Carroll 2010a). This result did not extend to sucrose self-administration in the present study, as there were no differences between responding for sucrose following the administration of ALLO+yohimbine compared to when yohimbine was administered with VEH. These results suggest that the suppressant effects of ALLO are specific to yohimbine-induced cocaine seeking and do not affect yohimbine-induced responding for a nondrug reward. It is possible that different results would have occurred had the ALLO+Y day preceded VEH+Y. However, the purpose of this component was to examine the effect of ALLO on yohimbine-induced sucrose self-administration in a within-subjects procedure. Therefore, the ABA design (VEH+Y, ALLO+Y, VEH+Y), also used in Anker and Carroll (2010a), was used to investigate the interaction of ALLO and yohimbine while ruling out a time effect. Since responding on the first VEH+Y day, the Y+ALLO day, and the second VEH+Y day did not differ, it is unlikely that the order of the injections or their position in sequence over time contributed to the results seen.
In summary, the results of this experiment demonstrated that ALLO blocked the escalation of cocaine self-administration but did not affect cocaine intake under a PR schedule or sucrose intake over a 21-day extended-access condition. In addition, injection of the anxiogenic drug yohimbine increased sucrose intake under a subsequent ShA condition and similar to sucrose intake under extended access, ALLO failed to disrupt yohimbine-induced sucrose intake. Taken together, these results indicate that fluctuations of endogenous levels of ALLO may correspond with increased or decreased vulnerability to engage in binge cocaine use in women.
Acknowledgments
This research was supported by the National Institute on Drug Abuse grants, R01 DA 003240-26, R01 DA019942-3, K05 015267-08 (MEC), and F31 DA 023301-02 (JJA). The authors would like to thank Luke Gliddon, Nathan Holtz, Emily Kidd, Brandon Knight, Kinner Patel, Paul Regier, Amy Saykao, Matthew Starr, Rachael Turner, Troy Velie, and Jeremy Williams for their technical assistance.
Contributor Information
Justin J. Anker, Email: anke0022@umn.edu, Department of Psychiatry, University of Minnesota, MMC 392, Minneapolis, MN 55455, USA.
Natalie E. Zlebnik, Department of Psychiatry, University of Minnesota, MMC 392, Minneapolis, MN 55455, USA
Marilyn E. Carroll, Department of Psychiatry, University of Minnesota, MMC 392, Minneapolis, MN 55455, USA
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Mendoza J, Escobar C. Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience. 2007;144:344–355. doi: 10.1016/j.neuroscience.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010a;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci Biobehav Rev. 2010b doi: 10.1016/j.neubiorev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology (Berl) 2009;203:63–72. doi: 10.1007/s00213-008-1371-9. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, McKay AJ, Ochedalski T, Piastowska A, Rebas E, Lachowicz A, Russell JA. Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J Neurosci. 2009;29:6449–6460. doi: 10.1523/JNEUROSCI.0708-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne S, Cooper Z, Fairburn C. Weight maintenance and relapse in obesity: a qualitative study. Int J Obes Relat Metab Disord. 2003;27:955–962. doi: 10.1038/sj.ijo.0802305. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Lac ST, Carroll ME. Effects of baclofen on maintenance and reinstatement of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1999;143:209–214. doi: 10.1007/s002130050937. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Morgan AD, Carroll ME. Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug Alcohol Depend. 2002;66:61–69. doi: 10.1016/s0376-8716(01)00185-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Boe IN. Increased intravenous drug self-administration during deprivation of other reinforcers. Pharmacol Biochem Behav. 1982;17:563–567. doi: 10.1016/0091-3057(82)90319-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–460. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Chen SW, Rodriguez L, Davies MF, Loew GH. The hyperphagic effect of 3 alpha-hydroxylated pregnane steroids in male rats. Pharmacol Biochem Behav. 1996;53:777–782. doi: 10.1016/0091-3057(95)02142-6. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology (Berl) 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and muopioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Chaurasia CS, Chen CE, Volkow ND, Clarkson FA, Porter SP, Straughter-Moore RM, Alexoff DL, Tedeschi D, Russo NB, Fowler JS, Brodie JD. GABAergic attenuation of cocaine-induced dopamine release and locomotor activity. Synapse. 1997;25:393–398. doi: 10.1002/(SICI)1098-2396(199704)25:4<393::AID-SYN11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Doherty J, Ogbomnwan Y, Williams B, Frantz K. Age-dependent morphine intake and cue-induced reinstatement, but not escalation in intake, by adolescent and adult male rats. Pharmacol Biochem Behav. 2009;92:164–172. doi: 10.1016/j.pbb.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugan RC, Park R, Kaufman L, Holmes PV. Etiology of the sexual dimorphism in renal peripheral benzodiazepine receptor response to stress in rats. Horm Behav. 1993;27:348–365. doi: 10.1006/hbeh.1993.1026. [DOI] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med. 2007;37:131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Pharmacokinetics of intravenous cocaine across the menstrual cycle in rhesus monkeys. Neuropsychopharmacology. 2004;29:1889–1900. doi: 10.1038/sj.npp.1300486. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Damsma G, Fibiger HC. Benzodiazepine-induced decreases in extracellular concentrations of dopamine in the nucleus accumbens after acute and repeated administration. Psychopharmacology (Berl) 1992;106:202–208. doi: 10.1007/BF02801973. [DOI] [PubMed] [Google Scholar]
- Fitch TE, Roberts DC. The effects of dose and access restrictions on the periodicity of cocaine self-administration in the rat. Drug Alcohol Depend. 1993;33:119–128. doi: 10.1016/0376-8716(93)90053-s. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5alpha-pregnan-3alpha-ol-20-one (3alpha, 5alpha-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3alpha, 5alpha-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006;18:960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O'Malley BW, Pfaff DW, Rhodes ME. Progesterone's effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology (Berl) 2006;186:312–322. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Gasior M, Carter RB, Witkin JM. Neuroactive steroids: potential therapeutic use in neurological and psychiatric disorders. Trends Pharmacol Sci. 1999;20:107–112. doi: 10.1016/s0165-6147(99)01318-8. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychophar-macology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti M, Javaid JI, Davis JM, Costa E, Guidotti A, Appel SB, Brodie MS. Imidazenil, a positive allosteric GABAA receptor modulator, inhibits the effects of cocaine on locomotor activity and extracellular dopamine in the nucleus accumbens shell without tolerance liability. J Pharmacol Exp Ther. 1998;287:58–66. [PubMed] [Google Scholar]
- Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. Am J Psychiatry. 1987;144:1592–1595. doi: 10.1176/ajp.144.12.1592. [DOI] [PubMed] [Google Scholar]
- Goeders JE, Murnane KS, Banks ML, Fantegrossi WE. Escalation of food-maintained responding and sensitivity to the locomotor stimulant effects of cocaine in mice. Pharmacol Biochem Behav. 2009;93:67–74. doi: 10.1016/j.pbb.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Shiffman S, Wing RR. Relapse crises and coping among dieters. J Consult Clin Psychol. 1989;57:488–495. doi: 10.1037//0022-006x.57.4.488. [DOI] [PubMed] [Google Scholar]
- Guo AL, Petraglia F, Criscuolo M, Ficarra G, Nappi RE, Palumbo MA, Trentini GP, Purdy RH, Genazzani AR. Evidence for a role of neurosteroids in modulation of diurnal changes and acute stress-induced corticosterone secretion in rats. Gynecol Endocrinol. 1995;9:1–7. doi: 10.3109/09513599509160184. [DOI] [PubMed] [Google Scholar]
- Heberlein A, Bleich S, Kornhuber J, Hillemacher T. Neuroendocrine pathways in benzodiazepine dependence: new targets for research and therapy. Hum Psychopharmacol. 2008;23:171–181. doi: 10.1002/hup.911. [DOI] [PubMed] [Google Scholar]
- Higgs S, Cooper SJ. Antineophobic effect of the neuroactive steroid 3alpha-hydroxy-5beta-pregnan-20-one in male rats. Pharmacol Biochem Behav. 1998;60:125–131. doi: 10.1016/s0091-3057(97)00562-5. [DOI] [PubMed] [Google Scholar]
- Hill M, Popov P, Havlikova H, Kancheva L, Vrbikova J, Kancheva R, Pouzar V, Cerny I, Starka L. Altered profiles of serum neuroactive steroids in premenopausal women treated for alcohol addiction. Steroids. 2005;70:515–524. doi: 10.1016/j.steroids.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Janak PH, Michael Gill T. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Janak PH, Redfern JE, Samson HH. The reinforcing effects of ethanol are altered by endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–1112. [PubMed] [Google Scholar]
- Kalivas PW. Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am J Addict. 2007;16:71–78. doi: 10.1080/10550490601184142. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Gasior M, Carter RB, Witkin JM. Protective efficacy of neuroactive steroids against cocaine kindled-seizures in mice. Eur J Pharmacol. 2003;474:217–222. doi: 10.1016/s0014-2999(03)02086-7. [DOI] [PubMed] [Google Scholar]
- Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lepine JP. New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol. 2008;11:425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- Kayman S, Bruvold W, Stern JS. Maintenance and relapse after weight loss in women: behavioral aspects. Am J Clin Nutr. 1990;52:800–807. doi: 10.1093/ajcn/52.5.800. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Deshpande LS, Chopde CT. The neurosteroid 3agr-hydroxy-5agr-pregn-20-one affects dopamine-mediated behavior in rodents. Psychopharmacology. 2002;161:120–128. doi: 10.1007/s00213-002-1006-5. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: exploring associations in community samples. Psychol Med. 2008;38:1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Ann NY Acad Sci. 1999;897:27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Schiffelholz T, Romeo E, Di Michele F, Holsboer F, Rupprecht R. Allopregnanolone affects sleep in a benzodiazepine-like fashion. J Pharmacol Exp Ther. 1997;282:1213–1218. [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Wheel running as a predictor of cocaine self-administration and reinstatement in female rats. Pharmacol Biochem Behav. 2005;82:590–600. doi: 10.1016/j.pbb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Akabayashi A, Wang J, Alexander JT, Dourmashkin JT, Chang GQ. Increased caloric intake on a fat-rich diet: role of ovarian steroids and galanin in the medial preoptic and paraventricular nuclei and anterior pituitary of female rats. J Neuroendocrinol. 2007;19:753–766. doi: 10.1111/j.1365-2826.2007.01584.x. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Supply of a nondrug substitute reduces escalated heroin consumption. Neuropsychopharmacology. 2008;33:2272–2282. doi: 10.1038/sj.npp.1301602. [DOI] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Majewska D. Baclofen as a cocaine anticraving medication: a preliminary clinical study. Neuropsychopharmacology. 1998;18:403–404. doi: 10.1016/S0893-133X(97)00128-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Katz ES. Elevation of glucocorticoids is necessary but not sufficient for the escalation of cocaine self-administration by chronic electric footshock stress in rats. Neuropsychopharmacology. 2007;32:367–376. doi: 10.1038/sj.npp.1301077. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2007;1167:101–111. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Montes GS, Luque EH. Effects of ovarian steroids on vaginal smears in the rat. Acta Anat. 1988;133:192–199. doi: 10.1159/000146639. [DOI] [PubMed] [Google Scholar]
- Morgan AE, Dewey SL. Effects of pharmacologic increases in brain GABA levels on cocaine-induced changes in extracellular dopamine. Synapse. 1998;28:60–65. doi: 10.1002/(SICI)1098-2396(199801)28:1<60::AID-SYN7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Morgan D, Roberts DC. Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev. 2004;27:803–812. doi: 10.1016/j.neubiorev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. The National Academies Press; Washington DC: 2003. p. 209. [PubMed] [Google Scholar]
- Niyomchai T, Akhavan A, Festa ED, Lin SN, Lamm L, Foltz R, Quinones-Jenab V. Estrogen and progesterone affect cocaine pharmacokinetics in female rats. Brain Res Bull. 2006;68:310–314. doi: 10.1016/j.brainresbull.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Ritchie JC, Nemeroff CB. 5 alpha-pregnane-3 alpha, 21-diol-20-one (THDOC) attenuates mild stress-induced increases in plasma corticosterone via a non-glucocorticoid mechanism: comparison with alprazolam. Brain Res. 1992;573:353–355. doi: 10.1016/0006-8993(92)90788-b. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–271. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Russo SJ, Fletcher H, Chin J, Webb T, Jenab S, Quinones-Jenab V. Ovarian hormones modulate cocaine-induced locomotor and stereotypic activity. Ann NY Acad Sci. 2001;937:202–216. doi: 10.1111/j.1749-6632.2001.tb03566.x. [DOI] [PubMed] [Google Scholar]
- Price WA, Torem MS, DiMarzio LR. Premenstrual exacerbation of bulimia. Psychosomatics. 1987;28:378–379. doi: 10.1016/s0033-3182(87)72511-0. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Jenab V, Perrotti LI, Ho A, Jenab S, Schlussman SD, Franck J, Kreek MJ. Cocaine affects progesterone plasma levels in female rats. Pharmacol Biochem Behav. 2000;66:449–453. doi: 10.1016/s0091-3057(00)00213-6. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Sex and estrous cycle-dependent changes in neurosteroid and benzodiazepine effects on food consumption and plus-maze learning behaviors in rats. Pharmacol Biochem Behav. 1999;62:53–60. doi: 10.1016/s0091-3057(98)00126-9. [DOI] [PubMed] [Google Scholar]
- Reis AD, Castro LA, Faria R, Laranjeira R. Craving decrease with topiramate in outpatient treatment for cocaine dependence: an open label trial. Rev Bras Psiquiatr. 2008;30:132–135. doi: 10.1590/s1516-44462008005000012. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, Sasso GF, Spalletta G, Troisi A, Pasini A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clin Neuropharmacol. 1996;19:366–369. doi: 10.1097/00002826-199619040-00011. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, Ling W. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64:1440–1448. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- Silveira PP, Xavier MH, Souza FH, Manoli LP, Rosat RM, Ferreira MB, Dalmaz C. Interaction between repeated restraint stress and concomitant midazolam administration on sweet food ingestion in rats. Braz J Med Biol Res. 2000;33:1343–1350. doi: 10.1590/s0100-879x2000001100013. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav. 2001;69:299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Specio SE, Wee S, O'Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin E, Vezina P, Negus SS, Crespo JA, Stockl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Psychopharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]