Abstract
Objectives
To assess sex-differences in ventricular-arterial interactions.
Background
Heart failure with preserved ejection fraction (HFpEF) is more prevalent in women than men, but the basis for this difference remains unclear.
Methods
Echocardiography and arterial tonometry were performed to quantify arterial and ventricular stiffening and interaction in 461 participants without heart failure (189 men, age 67±9 years; 272 women, age 65±10 years). Aortic characteristic impedance (Zc), total arterial compliance (TAC, pulsatile load) and systemic vascular resistance index (SVRI, steady load) were compared between men and women, and sex-specific multivariable regression analyses were performed to assess associations of these arterial parameters with diastolic dysfunction and ventricular-arterial coupling (effective arterial elastance/left ventricular end-systolic elastance, Ea/Ees) after adjustment for potential confounders.
Results
Zc was higher and TAC was lower in women, whereas SVRI was similar between sexes. In women but not men, higher Zc was associated with E/A ratio (β±SE: −0.17±0.07), diastolic dysfunction (OR 7.8; 95% CI: 2.0, 30.2) and Ea/Ees (β±SE: 0.13±0.0) (P≤0.01 for all). Similarly, TAC was associated with E/A ratio (β± SE: 0.12±0.04), diastolic dysfunction (OR 0.33; 95% CI: 0.12, 0.89) and Ea/Ees (β± SE: −0.09±0.03) in women only (P≤0.03 for all). SVRI was not associated with diastolic dysfunction or Ea/Ees.
Conclusions
Proximal aortic stiffness (Zc) is greater in women than men, and women may be more vulnerable to the deleterious effects of greater pulsatile and early arterial load on diastolic function and ventricular-arterial interaction. This may contribute to the greater risk of HFpEF in women.
Keywords: aortic stiffness, diastolic dysfunction, echocardiography, sex-specific, ventricular-arterial interaction
Introduction
Heart failure with preserved ejection fraction (HFpEF) is associated with high morbidity and mortality, and its prevalence is increasing (1). Women outnumber men with HFpEF by a 2:1 ratio (1–4). One hypothesis proposed for this discrepancy is based on sex differences in ventricular-arterial mechanics, since women display increased arterial and ventricular stiffening and deranged ventricular-arterial coupling compared to men, particularly with aging (5). This may impair cardiac performance in the presence of normal ejection fraction by increasing blood pressure lability, reducing cardiac efficiency, prolonging diastolic relaxation (6) and increasing diastolic chamber stiffness (7). In addition, the association of increased arterial stiffness with mortality is almost twofold higher in women than men (8). Thus, investigation of sex differences in arterial stiffness and its association with cardiac function is needed to better understand the pathophysiology of HFpEF and the health burden associated with arterial aging.
The hemodynamic (arterial) load on the left ventricle can be divided into steady (systemic vascular resistance) and pulsatile components (total arterial compliance [TAC], aortic characteristic impedance [Zc]). Given the increase in aortic stiffening with aging and the potential impact of proximal aortic properties on left ventricular loading and performance, we hypothesized that increased aortic characteristic impedance (Zc) (and therefore greater pulsatile hemodynamic load on the left ventricle) would be more strongly associated with diastolic dysfunction and with altered ventricular-arterial coupling in women than men.
To this end, in a large, well-characterized cohort of community-dwelling subjects without heart failure, we evaluated sex differences in Zc and investigated whether the associations of Zc with diastolic dysfunction and systolic ventricular-arterial coupling were different in men and women. Our secondary objectives were to determine whether: (a) the pulsatile (Zc, TAC) vs. the steady (systemic vascular resistance index [SVRI]) components of hemodynamic load were more strongly associated with diastolic dysfunction and systolic ventricular-arterial coupling in men and women, and (b) assess whether the associations of central pulse pressure (PP), PP amplification, carotid-femoral pulse wave velocity (cfPWV) and augmentation index (AIx) with diastolic dysfunction and systolic ventricular-arterial coupling differed by sex.
Methods
Study participants and assessment of baseline characteristics
The study participants consisted of community-based non-Hispanic whites from the Genetic Epidemiology Network of Arteriopathy (GENOA) study (9) and belonged to sibships with at least 2 family members diagnosed with hypertension before the age of 60 years. Hypertension was defined based on a prior diagnosis of hypertension and/or current treatment with medications for hypertension. The study was approved by the Mayo Clinic’s Institutional Review Board and participants gave informed consent. Between October 2009 and December 2010, 493 participants completed the study protocol. We excluded 16 participants with inadequate tonometry or echocardiographic data, 3 with a history of heart failure, 2 with low ejection fraction, 8 with history of valve surgery or more than mild aortic stenosis, and 3 with atrial fibrillation, leaving 461 participants for the final analyses. The methods for assessing baseline characteristics of the participants are outlined in the Supplemental File.
Non-invasive assessment of aortic characteristic impedance and other hemodynamic parameters
A comprehensive non-invasive hemodynamic evaluation including arterial tonometry and transthoracic echocardiography, with simultaneous ECG recording, was performed during a single visit to the Echocardiography Laboratory at the Mayo Clinic. Characteristic impedance (Zc) is a major property of the aorta, representing aortic opposition to pulsatile inflow from the contracting left ventricle, and is calculated as the ratio between aortic pulsatile pressure and flow. To estimate Zc, arterial tonometry (NIHem, Cardiovascular Engineering Inc, Norwood, MA) of the right carotid artery was performed to obtain a surrogate of central aortic pressure, followed immediately by 2-dimensional Doppler echocardiography to measure the left ventricular outflow tract (LVOT) diameter (parasternal long axis view) and time velocity integral (apical long axis view). LVOT area was multiplied by LVOT velocity time integral to calculate aortic flow. Zc was then calculated in the time domain as the ratio of increase in central pressure by the corresponding increase in aortic flow in early systole, using a software capable of Fourier analysis of the pressure and flow data obtained (NIHem, Cardiovascular Engineering Inc., Norwood, MA) (10). This has been shown correlate well with invasively-obtained aortic impedance (r=0.92) (11).
Arterial load can be divided into steady (systemic vascular resistance) and a pulsatile (TAC) components. Systemic vascular resistance is the resistance to blood flow offered by all of the systemic vasculature, excluding the pulmonary vasculature, and is mainly determined by the resistance of the small peripheral arteries, arterioles and capillaries. TAC is the change in arterial blood volume due to a given change in pulsatile arterial blood pressure. Since most of the compliance of the arterial tree resides in the aorta, TAC mostly represents aortic compliance, although smaller arteries also contribute. The techniques utilized to obtain TAC, SVRI, cfPWV and AIx are described in the Supplemental File.
Assessment of diastolic function
Transthoracic 2-dimensional and Doppler echocardiography (ACUSON Sequoia c512, Siemens Medical Solutions USA Inc., Malvern, PA) was performed during the same visit to assess diastolic function according to American Society of Echocardiography recommendations (12). Methods for assessing diastolic function and cardiac structure are detailed in the Supplemental File. Diastolic function was categorized based on the algorithm proposed by Kane et al (13), except that left atrial volume index (LAVI) ≥ 32 cc/m2 was used as the second measure of increased filling pressures as it has been shown to be a marker of diastolic dysfunction (14) (Supplemental Table S1). We then grouped the patients with grades 1–4 diastolic dysfunction into one unifying variable called “diastolic dysfunction”.
Systolic ventricular-arterial coupling assessment
Left ventricular end systolic pressure was calculated as 0.9*SBP (15). Effective arterial elastance (Ea), a global marker of arterial stiffness that encompasses both steady and pulsatile arterial load, was calculated as end systolic pressure divided by stroke volume (15). The left ventricular end-systolic elastance (Ees) describes the slope and volume intercept of the left ventricular end-systolic pressure volume relationship. Ees is sensitive to contractility, chamber geometry and passive ventricular stiffening, and was determined using the single-beat technique (16) based upon measured arterial pressure, left ventricular stroke volume, ejection fraction and systolic time intervals.
As arterial stiffening increases and Ea rises, the elastance of the left ventricle (Ees) rises to match it, in order to ensure adequate systolic ventricular-arterial coupling and optimize the transfer of blood from heart to arteries. When ventricular-arterial coupling is altered, heart failure symptoms may ensue. As such, we quantified systolic ventricular-arterial coupling by calculating the coupling ratio Ea/Ees (6).
Statistical analyses
Continuous variables are reported as mean ± standard deviation. Differences across groups were compared by t-test (normally distributed variables) or by Wilcoxon rank-sum test (skewed variables). Categorical variables were reported as n and %, and differences across groups were assessed with the Chi-square test. Sex differences in Zc were further assessed by linear regression with hierarchical adjustment for potential confounders. All regression analyses were performed using generalized estimating equations to account for the presence of sibships in the cohort. First, we adjusted for age (Model 1). Next, we adjusted for potential confounders that have been shown to influence arterial stiffness and/or diastolic dysfunction - body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), history of hypertension, diabetes and smoking, and estimated glomerular filtration rate (eGFR) (Model 2). In addition, since aortic size influences Zc, models were further adjusted for ascending aortic diameter.
To reduce skewness, Zc, LAVI and mitral inflow E/A ratio were log-transformed. Sex-specific linear and logistic regression analyses were then performed to assess the associations of Zc with diastolic function parameters (log LAVI, log E/A ratio, medial and lateral e’ velocities), ventricular-arterial coupling parameters (Ea, Ees, Ea/Ees), and with the presence of diastolic dysfunction, respectively, after adjustment for the variables in Models 1 and 2 above. In addition, to assess whether the associations of Zc with diastolic dysfunction and ventricular-arterial coupling were independent of left ventricular remodeling, we repeated the multivariable regression analyses to include relative wall thickness as an independent variable. We also constructed sex-specific receiver-operating characteristic curves and calculated the c-statistics for the models described above. To assess whether sex modified the associations of Zc with diastolic dysfunction and with ventricular-arterial coupling, we included the interaction term sex * Zc in the models.
To determine whether the pulsatile (Zc and TAC) vs. steady (SVRI) components of hemodynamic load were associated with diastolic dysfunction and ventricular-arterial coupling in men and women, we performed additional sex-specific multivariable linear and logistic regression analyses to predict diastolic function parameters, presence of diastolic dysfunction, and Ea/Ees, respectively, using TAC and SVRI as independent variables. Models were adjusted as for the Zc models above, except that blood pressure was not included in SVRI models due to collinearity.
Lastly, cfPWV was log-transformed to reduce skewness, and multivariate linear and logistic regression analyses were used to assess the associations of additional arterial stiffness parameters (central PP, PP amplification, log cfPWV, AIx) with diastolic and ventricular-arterial coupling variables as described in the models above. Since heart rate can influence PP and AIx, central PP, PP amplification and AIx models were additionally adjusted for heart rate.
Statistical analyses were performed with SPSS® version 20.0 (IBM Corporation, Armonk, NY, USA). A P value ≤0.05 was considered to be statistically significant.
Results
Background characteristics
Mean age was 65 years in women and 67 years in men (Table 1). 76% of women and 83% of men were hypertensive; means for SBP and DBP did not differ between the two sexes. The prevalences of diabetes and smoking were higher in men. Although mean arterial pressure did not differ between sexes, brachial and central PP were significantly higher in women. Pulse pressure amplification was attenuated in women, with greater central PP, amplitude of forward and reflected pressure waves, higher AIx, and lower TAC, all consistent with greater vascular stiffness in women than in men. Both arterial (Ea) and left ventricular end-systolic elastances (Ees) were significantly higher in women, but the Ea/Ees ratio was similar between sexes (Table 1).
Table 1.
Baseline characteristics of the participants
| Variable (mean±SD), or n (%) | Men (n= 189) | Women (n=272) | P-value |
|---|---|---|---|
| Age, years | 67.2±9.3 | 65.0±9.5 | 0.99 |
| Hypertension, n (%) | 154 (82%) | 197 (72%) | 0.07 |
| Diabetes, n (%) | 46 (24%) | 35 (13%) | 0.02 |
| Smoking, n (%) | 108 (57%) | 95 (35%) | <0.0001 |
| SBP, mmHg | 136±17 | 138±19 | 0.10 |
| DBP, mmHg | 71±9 | 69±8 | 0.97 |
| Heart rate, bpm | 60±9 | 63±10 | 0.003 |
| Fasting glucose, mmol/L | 5.9±1.5 | 5.3±1.1 | <0.0001 |
| Total cholesterol, mmol/L | 4.3±0.9 | 4.8±1.0 | <0.0001 |
| HDL cholesterol, mmol/L | 1.1±0.3 | 1.5±0.5 | <0.0001 |
| Triglycerides, mmol/L | 1.5±0.7 | 1.5±0.8 | 0.63 |
| Serum creatinine, µmol/L | 92.0±20.0 | 73.0±18.0 | <0.0001 |
| eGFR, mL/min/1.73m2 | 80.3±18.3 | 78.0±17.5 | 0.90 |
| Body mass index, kg/m2 | 31.0±4.9 | 30.4±9.6 | 0.81 |
| Arterial stiffness variables | |||
| Mean arterial pressure, mmHg | 97±11 | 98±12 | 0.10 |
| Brachial PP, mmHg | 66±16 | 70±18 | 0.007 |
| Central SBP, mmHg | 134±21 | 140±23 | 0.01 |
| Central DBP, mmHg | 71±9 | 69±8 | 0.97 |
| Central PP, mmHg | 64±20 | 71±21 | 0.0006 |
| PP amplification | 1.05±0.13 | 1.00±0.14 | 0.99 |
| cfPWV, m/s | 11.9±3.8 | 10.5±3.4 | 0.0001 |
| Zc, dyne × sec/cm5 | 172±64 | 211±75 | <0.0001 |
| Total arterial compliance, mL/mmHg | 1.9±0.7 | 1.4±0.5 | <0.0001 |
| SVRI, dyne·m2/s·cm−5 | 2941±598 | 2921±561 | 0.62 |
| Forward pressure wave, mmHg | 52±15 | 56±16 | 0.02 |
| Reflected pressure wave, mmHg | 19±6 | 21±7 | 0.005 |
| Augmentation index, % | 11.7±10.8 | 18.2±11.0 | <0.0001 |
| Reflected wave arrival time, ms | 147.5±22.4 | 128.6±24.7 | <0.0001 |
| Echocardiographic variables | |||
| LV septal thickness, mm | 12±2 | 10±1 | <0.0001 |
| LV posterior wall thickness, mm | 11±2 | 10±1 | <0.0001 |
| LV end-diastolic diameter, mm | 49±5 | 45±4 | <0.0001 |
| LV end-systolic diameter, mm | 31±5 | 27±4 | <0.0001 |
| LV mass index, g/m2 | 99.9±24.4 | 86.7±19.0 | <0.0001 |
| LV relative wall thickness | 0.48±0.07 | 0.46±0.07 | 0.99 |
| LV ejection fraction, % | 61±7 | 65±5 | <0.0001 |
| Left atrial volume index (cc/m2) | 30±9 | 29±8 | 0.51 |
| Mitral inflow E/A ratio | 0.91±0.27 | 0.96±0.29 | 0.07 |
| Deceleration time (ms) | 227±48 | 216±42 | 0.99 |
| Tissue Doppler medial E’ velocity (m/s) | 0.08±0.02 | 0.09±0.03 | 0.01 |
| Tissue Doppler lateral E’ velocity (m/s) | 0.10±0.03 | 0.10±0.03 | 0.11 |
| Medial E/e’ ratio | 8.0±2.3 | 8.8±3.4 | 0.05 |
| Lateral E/e’ ratio | 6.7±2.3 | 7.4±2.7 | 0.01 |
| Normal diastolic function, n (%) | 59 (31%) | 95 (35 %) | 0.43 |
| Grade 1 diastolic dysfunction, n (%) | 61 (33%) | 77 (29%) | 0.37 |
| Grade 2 diastolic dysfunction, n (%) | 13 (7%) | 28 (10%) | 0.23 |
| Grades 3–4 diastolic dysfunction, n (%) | 0 | 0 | N/A |
| Any diastolic dysfunction, n (%) | 74 (39%) | 105 (39 %) | 0.86 |
| Indeterminate diastolic function, | 49 (26 %) | 67 (25 %) | 0.66 |
| n (%)* | |||
| RVSP, mmHg | 29.7±6.8 | 29.7±5.9 | 0.66 |
| Ascending aortic diameter, mm | 35.6±3.7 | 32.6±3.7 | <0.0001 |
| LV outflow tract diameter, mm | 2.3±0.2 | 2.0±0.2 | <0.0001 |
| Ventricular- arterial coupling variables † (n=380) | |||
| Ea (mmHg/mL) | 1.30±0.28 | 1.57±0.36 | <0.0001 |
| Ees (mmHg/mL) | 1.42±0.38 | 1.73±0.47 | <0.0001 |
| Ea/Ees | 0.94±0.17 | 0.93±0.19 | 0.71 |
Includes participants with missing diastolic function variables or those whose diastolic function did not meet criteria for normal diastolic function or for diastolic dysfunction.
Available in 380 participants.
AIx : augmentation index. cfPWV : carotid-femoral pulse wave velocity. DBP: diastolic blood pressure. Ea: effective arterial elastance. Ees: end-systolic elastance. Ea/Ees: ventricular-arterial coupling ratio. eGFR: estimated glomerular filtration rate. LAVI : left atrial volume index. LV: left ventricle. PP: pulse pressure. RVSP: right ventricular systolic pressure. SBP: systolic blood pressure. SVRI: systemic vascular resistance index. Zc: aortic characteristic impedance
The higher Ea in women was due to the increased pulsatile components of arterial load (higher Zc, lower TAC) and slightly higher heart rate (63 vs. 60 bpm), and not due to the steady component of arterial load, since SVRI was similar between sexes (Table 1).
Sex differences in Zc and its association with diastolic dysfunction and ventricular-arterial coupling
Zc was significantly higher in women than men (Table 1). In a multivariable linear regression model adjusted for age, BMI, SBP, DBP, eGFR, ascending aortic diameter, and history of hypertension, diabetes and smoking, female sex remained independently associated with higher Zc, (P≤0.0001).
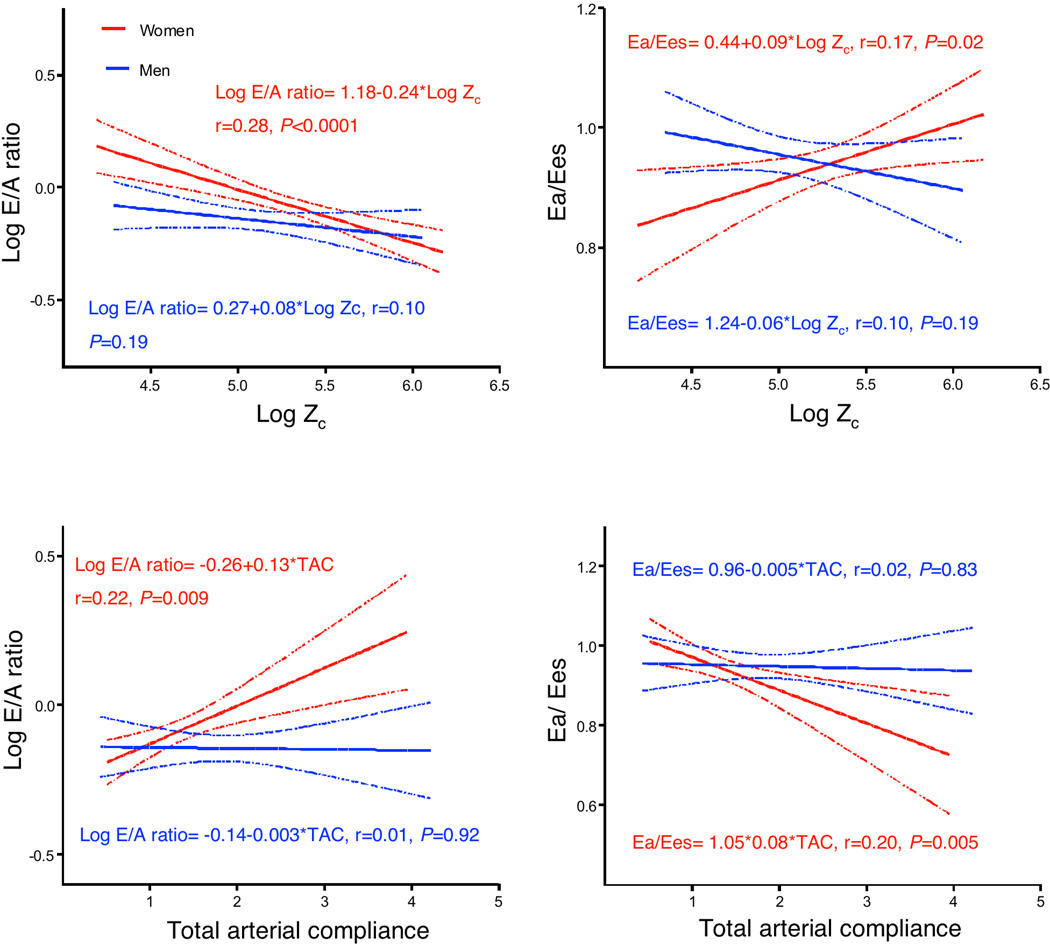
Zc was higher in women with diastolic dysfunction than men with diastolic dysfunction (237.4±80.5 vs. 181.2±60.3 dyne × sec / cm5, P<0.0001). The sex-specific associations of Zc with LAVI, E/A ratio, medial and lateral e’ velocities, presence of diastolic dysfunction, Ea, Ees, and Ea/Ees are summarized in Table 2. The unadjusted associations of Zc with E/A ratio and Ea/Ees in men and women are represented graphically in Figure 1. After adjustment for age, higher Zc was associated with lower mitral inflow E/A ratio, higher medial and lateral e’ velocities, higher Ea/Ees and greater odds of diastolic dysfunction in women but not in men. After further adjustment for confounders, greater Zc remained significantly associated with lower mitral inflow E/A ratio, higher Ea/Ees and greater odds of diastolic dysfunction in women only (Table 2). Inferences remained unchanged when models were adjusted for mean arterial pressure rather than SBP and DBP (analyses not shown). The associations of Zc with E/A ratio (β±SE:−0.23±0.07, P=0.002), diastolic dysfunction (OR: 11.43, 95% CI: 2.23, 58.46, P=0.003), and Ea/Ees (β±SE: 0.14±0.04, P=0.001) in women remained significant after adjustment for relative wall thickness, indicating that LV remodeling alone was not responsible for the sex differences. In the pooled sample, interaction term analyses confirmed that sex was a significant effect modifier of the associations of Zc with E/A ratio, diastolic dysfunction, and Ea/Ees (P≤0.01 for all interactions).
Table 2.
Sex-specific associations of aortic characteristic impedance (Zc) with measures of diastolic function and ventricular-arterial coupling
| Age-adjusted model | ||
|---|---|---|
| Men | Women | |
| log LAVI (cc/m2) | 0.09±0.08 | 0.06±0.06 |
| log E/A ratio | 0.05±0.05 | −0.14±0.06 * |
| Medial e’ (m/s) | 0.002±0.004 | −0.009±0.005 * |
| Lateral e’ (m/s) | −0.008±0.006 | −0.010±0.005 * |
| Diastolic dysfunction (OR [95% CI]) | 0.92 (0.34, 2.48) | 2.94 (1.21, 7.14) * |
| Ea (mmHg/mL) | 0.34±0.07 ‡ | 0.42±0.07 ‡ |
| Ees (mmHg/mL) | 0.46±0.11 ‡ | 0.26±0.09 † |
| Ea/Ees | −0.05±0.04 | 0.13±0.04 ‡ |
| Multivariable model § | ||
| log LAVI (cc/m2) | −0.02±0.12 | −0.09±0.07 |
| log E/A ratio | 0.04±0.07 | −0.17±0.07 † |
| Medial e’ (m/s) | 0.009±0.007 | −0.008±0.006 |
| Lateral e’ (m/s) | −0.001±0.008 | −0.009±0.006 |
| Diastolic dysfunction (OR [95% CI]) | 0.55 (0.10, 3.11) | 7.76 (1.99, 30.24) † |
| Ea | 0.30±0.08 ‡ | 0.38±0.07 ‡ |
| Ees | 0.39±0.11 ‡ | 0.23±0.10 * |
| Ea/Ees | −0.04±0.04 | 0.13±0.04 † |
Results are reported as β± standard error unless otherwise specified.
P≤0.05
P≤0.01
P≤0.001
Multivariable models were adjusted for: age, body mass index, estimated glomerular filtration rate, ascending aorta diameter, systolic and diastolic blood pressure, and history of hypertension, diabetes and smoking.
CI: confidence interval. OR: odds ratio. Other abbreviations as in Table 1.
Figure 1. Unadjusted associations of aortic characteristic impedance with mitral inflow E/A ratio and ventricular-arterial coupling.
Aortic characteristic impedance and total arterial compliance are associated with E/A ratio and with ventricular-arterial coupling in women, but not in men.
Ea/Ees: ventricular-arterial coupling ratio. TAC: total arterial compliance. Zc: aortic characteristic impedance.
Results of the receiver-operating curves to predict presence of diastolic dysfunction are summarized in Supplemental Table S2. Age was the main predictor of diastolic dysfunction in both sexes. Addition of Zc to a model that included relevant clinical variables increased the c-statistic from 0.81 to 0.84 in women (P=0.01), whereas no significant increase in the c-statistic was noted in men (P=0.35) once Zc was added to the model.
Associations of different components of hemodynamic load with diastolic dysfunction and ventricular-arterial coupling
Although SVRI was similar between the sexes, TAC was lower in women than men (Table 1). In multivariable analyses, SVRI was not associated with any of the diastolic parameters in men or women (P >0.05 for all). However, TAC was independently associated with E/A ratio (β±SE: 0.12±0.04, P=0.004) and Ea/Ees (β±SE: −0.09±0.03, P=0.002) in women. The unadjusted associations of TAC with E/A ratio and Ea/Ees in men and women are represented graphically in Figure 1. Interestingly, the association of higher TAC with the presence of diastolic dysfunction differed based on sex, being inverse in women (OR: 0.33, 95% CI: 0.12, 0.89, P=0.03 and direct in men (OR: 2.79, 95% CI: 1.09, 7.13, P=0.03). Inferences remained unchanged when models were adjusted for mean arterial pressure rather than SBP and DBP (analyses not shown). The association of higher TAC with E/A ratio (β±SE: 0.17±0.04, P≤0.001), diastolic dysfunction (OR: 0.33, 95% CI: 0.12, 0.90, P=0.03) and Ea/Ees (β±SE: −0.09±0.03, P=0.002) in women remained significant despite further adjustment for relative wall thickness. There was a significant interaction between sex and TAC in the prediction of E/A ratio, diastolic dysfunction and Ea/Ees (P≤0.05 for all).
Sex differences in the associations of additional arterial stiffness parameters with diastolic dysfunction and ventricular-arterial coupling
Central PP was associated with higher log LAVI in men (β±SE: 0.002±0.001. P=0.04) and women (β±SE: 0.004±0.001, P=0.01), and with lower Ea/Ees in women (β±SE: −0.001 ±0.0007. P=0.04), independent of age and other potential confounders. However, there was no interaction between central PP and sex in the prediction of LAVI or Ea/Ees (P>0.05 for both). Higher PP amplification was associated with lower Ea/Ees in women (β±SE: −0.24 ±0.12, P=0.05), and there was a trend towards an interaction between sex and PP amplification in the prediction of Ea/Ees (P=0.08). Log cfPWV was associated with higher medial e’ velocity in men but not in women (β±SE: 0.02±0.009, P=0.02), but the interaction term cfPWV*sex was not significant (P=0.37). The remainder of the associations between central PP, PP amplification and cfPWV with diastolic function and ventricular-arterial coupling were not statistically significant (analyses not shown). AIx was not associated with any of the diastolic function or ventricular-arterial coupling variables in men or women (P >0.05 for all).
Discussion
To better understand how sex differences in ventricular and arterial stiffness and ventricular-arterial coupling might contribute to the greater risk of HFpEF in women, we performed a comprehensive non-invasive hemodynamic evaluation of arterial stiffness and cardiac function in a large, well-characterized sample of community-dwelling subjects without heart failure but with multiple risk factors for HFpEF. To our knowledge, this is the first study dedicated to the assessment of the sex-specific associations of Zc with diastolic dysfunction and altered ventricular-arterial coupling. In addition, our findings confirm the hypothesis that pulsatile hemodynamic load on the left ventricle is significantly associated with diastolic dysfunction and altered systolic ventricular-arterial coupling in women, but not men. These findings provide novel insights into the relationship between arterial and ventricular function in women and men without heart failure, while also highlighting differences in aortic impedance to flow and arterial compliance as potential explanations for the higher prevalence of HFpEF in women.
Sex differences in arterial stiffness are also relevant for prognosis, as greater central arterial stiffness (defined as an attenuation of the natural carotid-brachial pressure augmentation) has been shown to be associated with mortality, and this association was almost two-fold higher in women than men. (8) In our study, women had greater proximal aortic stiffness (Zc) than men, consistent with three previous studies in the general population (17–19). Aortic characteristic impedance varies inversely with aortic size, but female sex remained associated with higher Zc after adjusting for ascending aorta diameter; suggesting that greater aortic stiffening in women may be due to differences in the material properties of the aorta. Waddell et al (20) demonstrated that age-related increases in aortic impedance are more pronounced in women than men, and that circulating levels of estradiol were inversely correlated with aortic impedance, suggesting a hormonal basis for the sex differences in proximal aortic stiffness.
Our results indicate that Zc is associated with diastolic dysfunction and deranged ventricular-arterial coupling (relative afterload mismatch) in women but not men. Increased concentric remodeling in women is thought to contribute to sex differences in HFpEF.(4) However, the associations of Zc with diastolic dysfunction and ventricular-arterial coupling in women were independent of left ventricular remodeling, suggesting an additional contribution of aortic impedance to flow in the pathophysiology of diastolic dysfunction in women. Further, addition of Zc to age, conventional risk factors and relative wall thickness lead to a mild improvement in the prediction of diastolic dysfunction in women but not in men. The current data confirm and extend observations from prior studies (21) showing that pulsatile arterial load (Zc, TAC) rather than the non-oscillatory load has the greatest impact on diastolic dysfunction and deranged ventricular-arterial coupling. Zc is also considered to be the major determinant of early systolic load, highlighting a possible contribution of early hemodynamic load on the left ventricle to diastolic dysfunction and altered ventricular-arterial coupling in women. In contrast to earlier studies (21,22), we did not observe a relationship between diastolic dysfunction and measures of late systolic loading (AIx), likely due to the older age of our participants. In the Framingham Heart Study, AIx was shown to plateau and subsequently fall starting at age 50 (23). This pattern was also observed in our cohort (analyses not shown). Thus, the fall in AIx with age in older adults may explain the differences between previous studies and ours. In addition, although both cfPWV and Zc are measures of arterial stiffness, we found them to be only modestly correlated (r=0.36). Given the parallel transmission of flow to the carotids and to the aortic arch, cfPWV does not fully represent stiffness of the proximal ascending aorta, which is the site of determination of Zc, and where the ventricular-arterial interaction initially occurs. This may explain why Zc was associated with diastolic dysfunction and altered ventricular-arterial coupling in women, while cfPWV was not.
In the normal state, the elastance (stiffness) achieved by the left ventricle during systole is closely coupled to the elastance of the arterial system (i.e.: normal coupling ratio, Ea/Ees), and in the setting of a compliant aorta and left ventricle, there is enhanced forward transmission of flow during ejection, with only minimal increases in blood pressure. The majority of the compliance of the arterial system resides in the proximal thoracic aorta, which serves as an elastic reservoir that not only to conducts blood to the periphery but also buffers the ample pulsatile energy generated by the heart with each beat. As such, ascending aortic characteristic impedance represents the pressure/ flow relationship at the level of the proximal aorta, precisely at the site of its interaction with the heart. Increased aortic characteristic impedance translates into a greater rise in pressure due to increase in flow during left ventricular ejection. The rate of left ventricular diastolic pressure decay during diastole is directly related to the peak aortic pressure generated by the preceding systole (24). Thus, it is possible that lower aortic compliance in women leads to greater impedance to flow during early ejection, greater pulsatile hemodynamic load on the left ventricle, relative afterload mismatch, and impaired left ventricular diastolic relaxation, which may promote progression from asymptomatic hypertensive heart disease (ACC/AHA stage A/B) to symptomatic HFpEF (ACC/AHA Stage C). Drug trials in stage C HFpEF to date have been uniformly disappointing (25) and intervention at an earlier stage may be needed to prevent disease progression. Identification of women at risk for HFpEF using novel risk markers such as Zc might be useful to test preventative strategies moving forward.
Shim and colleagues assessed sex-specific associations of central hemodynamics with diastolic function (26), and found that lower PP amplification was associated with lower tissue Doppler e’ velocity in women only. In contrast, we did not find PP amplification to be associated with diastolic dysfunction in either sex. There are several differences between the present study and that of Shim et al; the latter study (26) did not measure Zc, did not assess the impact of different components of load on cardiac function, included a referral population (referred for echocardiography due to dyspnea), had a relatively small sample size (n=158), and subjects were younger (mean age 58 years, compared to 66 years in our study), less obese (mean BMI 25 kg/m2, compared to 31 kg/m2 in our study), less often hypertensive (71% vs. 78%), and, importantly, displayed less aortic stiffening (mean cfPWV was 8 m/s, normal for the age enrolled (27), as compared to greater burden of aortic stiffening in the current study).
Limitations
We did not have invasive hemodynamic data to corroborate the diagnosis of diastolic dysfunction, or to invasively estimate arterial parameters. However, the non-invasive arterial measures we used have been validated and are well accepted by the scientific community. Since our study was restricted to hypertensive sibships of non-Hispanic whites, further studies will be necessary to determine whether the associations found are also present in other ethnic groups and in normotensive individuals. Lastly, the cross-sectional nature of our study does not allow us to make inferences about the causality or temporality of the associations found.
Conclusions
In a cohort of subjects from the community with multiple risk factors for HFpEF, women had greater aortic stiffening evidenced by increased aortic characteristic impedance (Zc) and lower total arterial compliance than men. Furthermore, these measures were independently associated with diastolic dysfunction and relative afterload mismatch in women but not men, suggesting that increased aortic stiffness and pulsatile load during early systole may decrease the efficiency of the cardiovascular system in women and therefore predispose to HFpEF. These results support further investigation of the impact of proximal aortic stiffening as a potential link to the greater risk of HFpEF in women. Longitudinal studies will help clarify whether increased Zc at baseline is associated with future development of HFpEF, and whether Zc is a suitable therapeutic target for preventing onset of HFpEF in women.
Supplementary Material
Acknowledgments
None.
Funding Sources: This work was supported by Grants HL89354 and M01 RR00585 from the National Institutes of Health.
Abbreviations
- AIx
Augmentation index
- cfPWV
arotid-femoral pulse wave velocity
- DBP
Diastolic blood pressure
- Ea
Effective arterial elastance
- Ees
Left ventricular end-systolic elastance
- Ea/Ees
Ventricular-arterial coupling ratio
- HFpEF
Heart failure with preserved ejection fraction
- PP
Pulse pressure
- SBP
Systolic blood pressure
- SVRI
Systemic vascular resistance index
- TAC
Total arterial compliance
- Zc
Aortic characteristic impedance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Masoudi FA, Havranek EP, Smith G, et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–2013. doi: 10.1161/CIRCULATIONAHA.110.954388. discussion 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Current opinion in cardiology. 2011;26:562–568. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]
- 5.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Cardiology clinics. 2011;29:447–459. doi: 10.1016/j.ccl.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–969. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regnault V, Thomas F, Safar ME, Pellegrin MO, Khalil RA, Pannier B, Lacolley P. Sex Differences in Cardiovascular Risk: Role of Pulse Pressure Amplification. J Am Coll Cardiol. 2012;59:1771–1777. doi: 10.1016/j.jacc.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kardia SL, Rozek LS, Krushkal J, et al. Genome-wide linkage analyses for hypertension genes in two ethnically and geographically diverse populations. Am J Hypertens. 2003;16:154–157. doi: 10.1016/s0895-7061(02)03249-1. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell GF, Izzo JL, Jr, Lacourciere Y, et al. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation. 2002;105:2955–2961. doi: 10.1161/01.cir.0000020500.77568.3c. [DOI] [PubMed] [Google Scholar]
- 11.Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- 12.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Kane GC, Karon BL, Mahoney DW, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. Jama. 306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–1289. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 15.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell GF, Lacourciere Y, Ouellet JP, et al. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation. 2003;108:1592–1598. doi: 10.1161/01.CIR.0000093435.04334.1F. [DOI] [PubMed] [Google Scholar]
- 18.Segers P, Rietzschel ER, De Buyzere ML, et al. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. 2007;49:1248–1255. doi: 10.1161/HYPERTENSIONAHA.106.085480. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility-Reykjavik Study. Hypertension. 2008;51:1123–1128. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens. 2001;19:2205–2212. doi: 10.1097/00004872-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Borlaug BA, Melenovsky V, Redfield MM, et al. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. doi: 10.1016/j.jacc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 22.Weber T, O'Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008;21:1194–1202. doi: 10.1038/ajh.2008.277. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell GF, Wang N, Palmisano JN, et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. The Journal of clinical investigation. 1976;58:751–760. doi: 10.1172/JCI108522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shim CY, Park S, Choi D, et al. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. J Am Coll Cardiol. 57:1226–1233. doi: 10.1016/j.jacc.2010.09.067. [DOI] [PubMed] [Google Scholar]
- 27.Khoshdel AR, Thakkinstian A, Carney SL, Attia J. Estimation of an age-specific reference interval for pulse wave velocity: a meta-analysis. J Hypertens. 2006;24:1231–1237. doi: 10.1097/01.hjh.0000234098.85497.31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.