Abstract
Adults with autism experience significant impairments in social and non-social information processing for which few treatments have been developed. This study conducted an 18-month uncontrolled trial of Cognitive Enhancement Therapy (CET), a comprehensive cognitive rehabilitation intervention, in 14 verbal adults with autism spectrum disorder to investigate its feasibility, acceptability, and initial efficacy in treating these impairments. Results indicated that CET was satisfying to participants, with high treatment attendance and retention. Effects on cognitive deficits and social behavior were also large (d = 1.40 to 2.29) and statistically significant (all p < .001). These findings suggest that CET is a feasible, acceptable, and potentially effective intervention for remediating the social and non-social cognitive impairments in verbal adults with autism.
Keywords: Cognitive Enhancement Therapy, cognitive rehabilitation, cognitive remediation, psychosocial treatment, cognitive therapy, adult treatment, autism, autism spectrum disorder
Autism spectrum disorder (ASD) is characterized by significant impairments in social interaction, verbal and non-verbal communication deficits, and restricted and repetitive interests and behaviors (American Psychiatric Association, 2000). Underlying these broad behavioral impairments are core neurobiologically-based deficits in social and non-social information processing (Minshew, Goldstein, & Siegel, 1997; Minshew & Williams, 2007), which result in significant functional disability throughout the lifespan of individuals with ASD (Gilotty, Kenworthy, Sirian, Black, & Wagner, 2002; Howlin, Goode, Hutton, & Rutter, 2004) and at great cost to society (Ganz, 2007). While advances in early detection and intervention approaches attempting to limit the impact of ASD on individuals and their families have been achieved (e.g., Dawson et al., 2010), surprisingly few efforts have been dedicated to advancing the treatment of adults with ASD (Fitzpatrick, Minshew, & Eack, in press). The majority of intervention efforts have focused on children, yet most individuals encounter significant challenges in adulthood due to ASD, which result in unemployment or underemployment, poor academic performance, limited social functioning, and a poor quality of life (Howlin, Goode, Hutton, & Rutter, 2004).
Growing evidence indicates that the deficits in social and non-social cognition that adults with ASD experience significantly contribute to poor adaptive functioning (Berger, Aerts, van Spaendonck, Cools, & Teunisse, 2003; García-Villamisar, Rojahn, Zaja, & Jodra, 2010). Social-cognitive impairments have been observed in many domains in autism, and include deficits in perspective-taking (Mizuno et al., 2011), theory of mind (Baron-Cohen, 1990), emotion perception (Hobson, Ouston, & Lee, 1988) and emotion management (Samson, Huber, & Gross, 2012), and social context appraisal abilities (Wang, Lee, Sigman, & Dapretto, 2006). While many individuals with autism have intact or elevated intellectual abilities, non-social "neurocognitive" impairments have also been observed in such domains as speed of processing (Mayes & Calhoun, 2007), aspects of working memory (Williams, Goldstein, Carpenter, & Minshew, 2005), planning (Hughes, Russell, & Robbins, 1994), and executive functioning (Ozonoff, 1995). This constellation of social and non-social information processing deficits significantly limits the ability of individuals with ASD to adapt and succeed in adult life, and unfortunately, comprehensive approaches designed to address core neurocognitive and social-cognitive impairments in adults with autism have yet to be developed.
Cognitive rehabilitation represents a potentially effective approach to the remediation of information processing impairments in individuals with ASD without general intellectual disability. Cognitive rehabilitation approaches have demonstrated considerable efficacy in other neurologically impaired populations, including traumatic brain injury and stroke (Cicerone et al., 2005), mild cognitive impairment (Rapp, Brenes, & Marsh, 2002), early stage Alzheimer's disease (Olazaran et al., 2004), dyslexia (Temple et al., 2003), and schizophrenia (Wykes, Huddy, Cellard, McGurk, & Czobor, 2011). Such approaches employ computer-based and/or group-based exercises designed to improve diverse areas of social and non-social cognitive function through repetitive practice and strategic training (Eack, 2012). One particularly promising rehabilitation approach that was originally developed for individuals with schizophrenia with the potential for efficacy in verbal adults with autism is Cognitive Enhancement Therapy (CET; Hogarty & Greenwald, 2006).
Over the course of 18 months, CET integrates computer-based training exercises for pairs of affected individuals in attention, memory, and problem-solving with a small, group-based curriculum designed to facilitate the development of adult social-cognitive milestones. The pair-based neurocognitive training sessions are overseen by therapist-coaches and make use of computer exercises to improve cognition, develop strategic thinking, promote positive peer interaction and socialization, and initially prepare for the social-cognitive group. After several months of neurocognitive training, 3 to 4 participant pairs join together to form a social-cognitive group. Computer training then continues concurrently with these groups throughout the remainder of treatment. The social-cognitive group sessions are structured to provide secondary socialization and experiential learning opportunities to help participants develop perspective-taking, social and emotional wisdom, and gistful thinking and speaking. Each group session contains a psychoeducational talk on a new aspect of social cognition, a cognitive exercise designed to facilitate the development of social-cognitive abilities, and a homework assignment to extend the application of CET to everyday life (see Method and Hogarty & Greenwald, 2006 for more detail).
To date, two NIH-supported randomized-controlled trials of CET encompassing 179 individuals with schizophrenia have been completed. These trials have demonstrated that CET can produce significant differential improvements in neurocognitive (d = .46) and social-cognitive function (range of d = .72 to 1.55) compared to an active supportive therapy control intervention, which ultimately translates into clinically meaningful gains in social adjustment and adaptive function (range of d = .40 to 1.53) (Hogarty et al., 2004; Eack et al., 2009), including improvements in social functioning, instrumental task performance, major role adjustment, work readiness, and competitive employment (Eack, Hogarty, Greenwald, Hogarty, & Keshavan, 2011). Furthermore, many of these functional gains were shown to be maintained for at least 1 year after the completion of treatment (Hogarty, Greenwald, & Eack, 2006; Eack, Greenwald, Hogarty, & Keshavan, 2010), indicating that CET can produce lasting improvements in a neurodevelopmental disorder characterized by broad impairments in social and non-social cognition. It is important to appreciate that CET is not designed to address psychosis in schizophrenia but rather the symptoms that involve impaired social function (behavior and cognition), impaired comprehension and use of language, and impaired planning and problem-solving.
While important differences exist between autism and schizophrenia (e.g., age of onset, psychosis, restricted repetitive behavior), convergence in the cognitive manifestations of these conditions is becoming increasingly recognized as the number of individuals with ASD without intellectual disability has increased and aged into adulthood. Both autism and schizophrenia are well-known to be characterized by significant impairments in neurocognitive and social-cognitive functioning (Penn, Corrigan, Bentall, Racenstein, & Newman, 1997; Volkmar, Lord, Bailey, Schultz, & Klin, 2004). Indeed, numerous direct comparisons of the two conditions have found similar degrees of impairment in social and non-social cognitive domains, including theory of mind (Pilowsky, Yirmiya, Arbelle, & Mozes, 2000), gaze orientation (Sasson et al., 2007), emotion perception (Couture et al., 2010), speed of processing (Goldstein, Minshew, Allen, & Seaton, 2002; Schneider & Asarnow, 1987), and executive functioning (Schneider & Asarnow, 1987). CET is one of the only cognitive rehabilitation interventions that systematically targets both social and non-social cognitive impairments. These deficits in social and non-social cognition are known to be related (and perhaps dependent), such that challenges in a non-social domain (e.g., slow speed of processing) can negatively affect performance in a social domain (e.g., identifying social cues) (e.g., Sergi, Rassovsky, Nuechterlein, & Green, 2006). Given the interrelationships between these areas and challenges that verbal adults with ASD have in both of these domains, the comprehensive nature of CET may afford the greatest opportunity to these individuals for cognitive improvement that results in meaningful gains in functional outcome. Further, many of the specific targets of CET (processing speed, perspective-taking, social context appraisal, emotion perception, emotion management) are among the most common and challenging areas for adults with autism, suggesting a congruence between the targets of the approach and the areas of greatest need for treatment in the ASD population.
The pathophysiology of autism and schizophrenia has also begun to show considerable overlap, as studies have reported similar neurobiologic and genetic pathways affected in both conditions. Shared genetic abnormalities in regions of the genome coding for synaptic formation and neurotransmission have been found in both disorders (e.g., Guilmatre et al., 2009), and studies have noted similar functional abnormalities in affected brain regions (Pinkham, Hopfinger, Pelphrey, Piven, & Penn, 2007; Sugranyes, Kyriakopoulos, Corrigall, Taylor, & Frangou, 2011), particularly in those areas associated with social cognition. Finally, recent neuroimaging findings from a CET trial in early course schizophrenia have shown that at least some of the beneficial effects of the treatment are due to a neuroprotective effect of CET on brain structures (e.g., amygdala, fusiform gyrus) commonly implicated in social-cognitive impairment in both schizophrenia and autism (Eack et al., 2010b), thus providing initial evidence that the approach may target underlying neural pathways that are shared between these disorders. Taken together, these observations suggest that a treatment that effectively addresses the neural basis of information processing deficits in schizophrenia is likely to have promise for treating similar impairments in autism.
To examine the feasibility, acceptability, and potential efficacy of CET in adults with ASD, two initial cohorts of verbal adults with these conditions were recruited to participate in an uncontrolled, 18-month trial of CET adapted for ASD. Primary outcomes included treatment adherence and satisfaction, and secondary outcomes included impact on cognition and social adjustment. We hypothesized that CET could be feasibly applied to verbal adults with ASD once appropriate adaptations were made, and that the intervention would be well-tolerated and acceptable to these individuals as evidenced by high treatment attendance (≥ 70% of sessions), high retention (≥ 70% of participants would complete the entire 18 months of treatment), and high satisfaction (average satisfaction scores of "mostly satisfied" or greater). In addition, we hypothesized that the application of CET to adults with ASD in this feasibility study would provide preliminary evidence of benefits to cognition and adaptive behavior in this population, as evidenced by at least medium-sized (d = .50) or greater improvements on measures of neurocognition, social cognition, and social adjustment.
Method
Participants
Participants included 14 verbal adults enrolled in a feasibility study of CET for ASD. Individuals were included if they met expert clinical opinion and research criteria for autistic disorder or autism spectrum disorder using the Autism Diagnostic Observation Schedule (Lord et al., 2000), met autism cutoffs on the Autism Diagnostic Interview-R (Lord, Rutter, & Couteur, 1994), were age 18–45 years, had an IQ ≥ 80 as assessed by the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), had not abused substances in the 3 months prior to enrollment, did not exhibit behavioral problems that would negatively impact other participants in the program, and demonstrated cognitive and social disability on the Cognitive Style and Social Cognition Eligibility Interview (Hogarty et al., 2004). This semi-structured interview has been validated in previous studies of CET for patients with schizophrenia, and is used to provide a clinical assessment of cognitive dysfunction and social impairment indicative of the need for treatment.
Enrolled participants were mostly young adults, with an average age of 25.29 (SD = 5.72) years, predominantly male (n = 12), and all Caucasian. Over half (n = 8) of the participants met criteria for autism, with the remaining meeting criteria for ASD. Psychiatric, learning, and other developmental comorbidities were common (n = 7) and included anxiety disorders (n = 4), depressive disorders (n = 4), personality disorders (n = 1), developmental dysgraphia (n = 1), learning disorder NOS (n = 1), mathematics disorder (n = 1), and motor coordination disorder (n = 1); four participants had more than one significant comorbidity. Although the majority (n = 12) of individuals had attended some college and the average full scale IQ for the sample was above average (M = 117.70, SD = 16.77, range = 92 to 157), only half (n = 7) of the participants were employed, and all participants, except for one, were living with their family. Of those individuals employed, all were in jobs below levels commensurate with their education and intellectual level; every employed participant had received at least some college education and had higher levels of intellectual functioning (range of IQ = 107 to 157), yet none were employed in positions greater than clerical work. These findings are commensurate with Leo Kanner’s early report about adults with autism who had the best outcomes (Kanner, Rodriguez, & Ashenden, 1972). All participants provided written, informed consent prior to participation and the study had the approval of the institutional review board for human subject research protection.
Measures
Treatment acceptability and adherence
Measures of treatment acceptability and adherence represented the primary outcome measures for this initial feasibility study of CET in verbal adults with ASD. Treatment acceptability and satisfaction was measured using the Client Satisfaction Questionnaire-8 (CSQ-8; Larsen, Attkisson, Hargreaves, & Nguyen, 1979) with wording adapted for CET, which is a field standard measure of treatment satisfaction that has been widely employed to assess the acceptability of psychotherapy programs. This measure consists of 8 items rated between 1 ("quite dissatisfied") and 4 ("very satisfied") to assess self-reported satisfaction with treatment programs. The CSQ-8 has been shown to be a reliable and valid measure of treatment acceptability (Larsen, Attkisson, Hargreaves, & Nguyen, 1979), and was completed during the first quarter of treatment and at the end of treatment by participants. Research staff independent of the treating clinicians providing CET were available to participants during the completion of this questionnaire to help record responses and to answer any questions regarding the instrument. Treatment adherence was assessed throughout the course of the study by the treating clinician using attendance logs for neurocognitive training and social-cognitive group session appointments. These logs were kept and recorded in real-time, and their accuracy was checked when necessary by reviewing neurocognitive session scoring sheets and social-cognitive group session videotapes.
Cognitive and behavioral outcomes
An abbreviated battery of measures of cognition and behavior were included in this research to provide an initial assessment of the efficacy of CET adapted for adults with ASD. Neurocognition was assessed using the NIMH MATRICS Consensus Cognitive Battery (Green et al., 2004), which is a battery of standardized neuropsychological tests originally compiled to assess the efficacy of cognitive enhancing medication in patients with schizophrenia. This battery assesses neurocognitive dysfunction in a variety of domains relevant to the treatment of ASD, including processing speed, attention/vigilance, verbal and non-verbal working memory, verbal learning, visual learning, reasoning and problem-solving, and social cognition. Since the MATRICS battery does not include an assessment of cognitive flexibility, which is a critical domain of impairment in ASD, the battery was expanded to include the Wisconsin Card Sorting Test (Heaton, Chelune, Talley, Kay, & Curtiss, 1993). To avoid the effects of repeated testing on neurocognitive measures, testing intervals were long (9 months) and alternate versions of cognitive tests were used when available, particularly for those tests likely to have high re-test effects (i.e., verbal learning and problem-solving assessments).
Multiple additional measures of social cognition, which is minimally assessed in the MATRICS battery, included the full Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT; Mayer, Salovey, Caruso, & Sitarenios, 2003), the Penn Emotion Recognition Test-40 (Kohler et al., 2003), and the Social Cognition Profile (Hogarty et al., 2004). The MSCEIT is a 141-item performance-based measure of emotional intelligence that has been validated for assessing the domains of emotion perception, facilitation, understanding, and management (Mayer, Salovey, Caruso, & Sitarenios, 2003). The Penn Emotion Recognition Test is a 40-item test of facial emotion recognition, which has been shown to assess brain functions supporting emotion perception (Gur et al., 2002a). The Social Cognition Profile is a 50-item clinician-rated measure of social-cognitive behaviors used in previous studies of CET (Hogarty et al., 2004; Eack et al., 2009), which assesses the domains of tolerant (e.g., accepting, cooperative, flexible), perceptive (e.g., foresightful, gistful, sensitive to others' feelings), supportive (e.g., empathic, reciprocal, friendly), and self-confident (e.g., comfortable, assertive, involved) behaviors indicative of adequate social cognition. To ensure these measures provided an assessment of generalizable cognitive improvement, all cognitive measures used to assess treatment outcomes in this research were different from the neurocognitive exercises upon which participants were trained during the course of CET.
Finally, dysfunctional cognitive style and social adjustment were repeatedly assessed using the Cognitive Style and Social Cognition Eligibility Interview (Hogarty et al., 2004), which is a semi-structured interview designed, in part, to elicit responses and behaviors from participants about cognitive and functional challenges in their lives that reflect the core cognitive profiles or "styles" that become key treatment targets in CET. Items are rated based on behavioral adjectives from the interview on a 1 ("rare") to 5 ("very severe") scale and provide a dimensional assessment of impoverished (e.g., reduced affect, lack of motivation, difficulty planning), disorganized (e.g., difficulty maintaining attention/staying on task, ineffective inhibition, chaotic/imprecise planning), and inflexible (e.g., obsessive/repetitive thinking, fixed cognitive schema, preoccupation with details) cognitive functioning. Measurement of functional outcome was purposely limited in this pilot study, and was assessed by separate interview areas on the Cognitive Style and Social Cognition Eligibility Interview, which included assessments of vocational ineffectiveness, interpersonal ineffectiveness, and adjustment to disability. Interview questions for these domains covered current employment, school, and household activities (vocational ineffectiveness); the quality and quantity of interactions with friends and family members (interpersonal ineffectiveness); and knowledge of autism and the ability to adapt to its challenges (adjustment to disability). After the interview, items covering these domains were rated from 1 ("rare") to 5 ("very severe"), and together they provided a basic assessment of social adjustment and adaptive function.
Cognitive Enhancement Therapy
Cognitive Enhancement Therapy (CET) is a comprehensive, developmental approach to the treatment of social and non-social cognitive impairments that was originally developed for patients with schizophrenia (Hogarty et al., 2004). Over the course of 18 months, CET integrates 60 hours of computer-based neurocognitive training in attention, memory, and problem-solving with a structured 45-session social-cognitive group curriculum designed to facilitate the achievement of adult social-cognitive milestones, particularly perspective-taking and social context appraisal. Neurocognitive training is strategic in nature, and is designed to help individuals improve core deficits in basic information processing that contribute to poor social cognition and social adjustment. A CET coach pairs and guides two individuals to participate in computer-based cognitive exercises for 1 hour each week to develop and practice strategies for improving cognition, including increasing processing speed, sustaining attention, developing a schematization or categorizing capacity, increasing cognitive flexibility, managing frustration, becoming more strategic and foresightful in planning, and increasing their ability to engage in conversations and give support to each other.
Neurocognitive training exercises are all computer-based and are divided into three modules: attention, memory, and problem-solving. A hierarchical approach to training is taken such that lower-order cognitive abilities are trained first (e.g., processing speed, sustained attention) followed by more complex, higher-order cognitive abilities (e.g., working memory, planning, and executive function). This staging of training is based on models of information processing that indicate that basic, fundamental aspects of cognition support higher-order cognitive processes (Simon, 1979). Attention training makes use of the Orientation Remediation Module developed by Ben-Yishay, Piasetsky, & Rattok (1985), and memory and problem-solving training uses the PSSCogRehab software developed by Bracy (1994). An example of an early neurocognitive training exercise is the Attention Reaction Conditioner, where participants must respond to a critical stimulus (center target light) on the computer screen by pressing the space bar as quickly as possible. If participants respond within the critical stimulus window (170, 300, or 450 ms), they will illuminate all 9 feedback lights on the screen indicating they were successful for that trial; fewer feedback lights will illuminate the farther outside the critical stimulus window participants respond. A constant 5 second delay is always present between the time when the computer prompts participants that the exercise is beginning and when the critical stimulus illuminates. Initially, auditory cues (beeps) are presented 1 per second for each of these five seconds until the critical stimulus is presented. Gradually these cues are faded such that participants must sustain their attention and keep track of the timing of the critical stimulus presentation on their own. Additionally, as participants master the exercise, the window for responding to the critical stimulus is reduced (e.g., from 300 to 170 ms), requiring a very rapid speed of processing to succeed. The use of cueing and fading, and the adaptive nature of this exercise are illustrative of the neurocognitive training processes used in CET. In total, there are 3 attention, 7 memory, and 6 problem-solving neurocognitive computer exercises (see Hogarty & Greenwald, 2006 for more detail).
After several months of neurocognitive training in attention, 6 to 8 participants (3 to 4 pairs) come together to form a social-cognitive group. Through the use of in vivo cognitive exercises and psychoeducation, the weekly 1.5 hour social-cognitive group sessions provide rich secondary socialization experiences that target a broad, theoretically-driven array of social-cognitive abilities ranging from abstracting the "gist" from spontaneous, unrehearsed social interactions to understanding the perspectives of others, accurately appraising novel social contexts, and managing emotions. Generalization of these abilities to everyday life is a key emphasis of the CET group and is supported through homework assignments, individually-tailored recovery/treatment plans, and generalization exercises designed to consolidate learning. Social-cognitive group sessions are designed to make effective use of the group context to provide secondary socialization opportunities to participants (e.g., learning from observing peers and coaches), which is a fundamental avenue for higher-order social-cognitive development (Selman & Schultz, 1990). Each CET group session is highly structured and generally includes a Welcome Back introduction to the session; a Homework Presentation that is chaired by one of the group members; a Cognitive Exercise designed to facilitate the development of social-cognitive abilities, usually involving two group members; Feedback from group members and therapists/coaches on the performance of individuals participating in the exercise; a brief Psychoeducational Lecture on a new social-cognitive topic; and a Homework Assignment based on the lecture.
The CET group cognitive exercises are not computer-based, but performed in vivo center stage in the group, and as in everyday life, purposely integrate multiple aspects of social cognition. Condensed Message is an example of a CET group cognitive exercise where participants are presented with a social problem (e.g., a son learns that his father has left his wallet at an airport restaurant), and must send a brief message (e.g., a 10-word page over the airport public address system) from one person in the scenario (e.g., the son) to the other (e.g., the father) to get the recipient of the message to act a certain way (e.g., retrieve the wallet before boarding the plane). This requires participants to identify the perspectives of both the sender and receiver of the message, including their intentions and emotions; to construct a gistful, but meaningful message that will urge the recipient to act; and to be sensitive to the social context when constructing the message (e.g., it may not be advisable to announce to the entire airport that a wallet is available). As with most CET group exercises, Condensed Message is performed in pairs, and thus participants must also work collaboratively to resolve discrepancies and arrive at a mutually agreed upon solution. Neurocognitive training proceeds concurrently with the social-cognitive groups throughout the remaining course of treatment, and content from the two modalities are continuously integrated. The practice principles and methods of the treatment originally developed for patients with schizophrenia are described in detail elsewhere (Hogarty & Greenwald, 2006).
The targets of CET are the cognitive abilities that underlie successful interpersonal interactions and problem-solving in daily life that can be applied to novel, unrehearsed social exchanges. This is important to distinguish the methods of CET from those of social skills training, which uses behavioral rehearsal to target specific behaviors (e.g., how to greet a family member, how to behave appropriately at the dinner table) in specific, rehearsed social situations. In CET, the training of cognitive abilities central to all interpersonal encounters is expected to lead to greater generalization and thus, improved adaptive function. The range of abilities addressed is purposively broad, and includes both social (e.g., perspective-taking, social context appraisal, reciprocity) and non-social (e.g., processing speed, planning, strategic thinking) aspects of cognition. The ability to take the perspective of others and identify their thoughts, feelings, and intentions is hypothesized to be the central unifying focus of CET, and training in other aspects of cognition (e.g., improving speed of processing to assess the perspective of others quickly, learning to identify emotional and other non-verbal cues in others to assess a person's emotional state) support the development of these abilities.
Several adaptations to CET were made to ensure the applicability of the treatment to the unique needs of adults with ASD. The largest adaptations occurred with regard to the early components of the social-cognitive group curriculum, which originally focused on psychoeducation about schizophrenia. Such content was removed and replaced with the latest knowledge and understanding of ASD and its impact upon cognition, information processing, social cognition, sensory perception, and emotion management. In addition, some of the computer exercises in the neurocognitive training produced sounds that were uncomfortable to some participants, and these exercises were altered to mute such sounds. Coaches also had to alter their approach in working with participants with ASD, who unlike individuals with schizophrenia, often do not ask for help and commonly needed greater clinical outreach and engagement. A more guided, repetitive, and elaborated approach was also employed in the training of some advanced abilities (e.g., providing support, perspective-taking) in the social-cognitive groups, as the impairments in social cognition experienced by individuals with ASD who have not had normal early periods of social development were at times considerably greater than those observed in schizophrenia. Overall, however, we found the need for adaptations to be surprisingly minimal compared to initial expectations, as the majority of the content in CET was perceived as highly applicable by both ASD participants and clinicians. These adaptations are being collated in a supplement to the existing CET treatment manual.
Procedures
Participants were recruited from support groups, community colleges and universities, previous research studies, specialty clinics, and local advocacy groups for an 18-month study of CET for verbal adults with ASD. Upon recruitment, participants were assessed for diagnostic and IQ eligibility by trained research staff from the University of Pittsburgh Autism Center of Excellence who have extensive experience with adults with ASD and disorders with which it can be confused. Staff were supervised by a study psychologist. A member of the clinical team then conducted a videotaped interview of the participant using the Cognitive Style and Social Cognition Eligibility Interview (Hogarty et al., 2004). Final eligibility determinations were made in consensus meetings based on review of all available diagnostic, testing, and interview data. Eligible participants were then assigned to 18 months of active treatment with CET, and administered cognitive and behavioral outcome measures prior to initiating treatment and every 9 months thereafter. Cognitive assessments were administered by master's-level neuropsychological testers supervised by a study psychologist, and clinical and behavioral assessments were completed by the treating CET clinician. CET was provided by master's and doctoral-level clinicians who were experts in its use in schizophrenia and had been trained in the treatment of ASD. This research was conducted between August, 2009 and December, 2011.
Results
Treatment Acceptability and Adherence
The primary goal of this study was to assess the feasibility of recruiting an initial sample of verbal adults with ASD and treating them with CET. The community response to recruitment and intake was largely positive. Within a 6-month period, 25 individuals were referred for potential participation in the study, 14 of whom met full study inclusion criteria. Among those who were not enrolled, the majority failed to meet inclusion criteria with 2 individuals not meeting research diagnostic criteria for ASD, 2 demonstrating an IQ < 80, 1 experiencing active substance use problems, and 1 demonstrating behavioral problems that were contraindicated to group participation. In addition, 4 individuals were not interested in participating in an experimental treatment study despite their parents contacting the study to express interest, and 1 individual was interested but could not feasibly participate due to distance from the program.
Of the 14 individuals who enrolled in the study, 11 (79%) completed the entire 18 months of treatment. One participant withdrew at 9 months due to increased hours of employment; 1 was administratively terminated at 9 months due to personality disorder instability; and 1 completed the entire 18 months of the study, but could not attend the social-cognitive groups due to persistent family and transportation problems, and thus was not considered to have completed treatment. Treatment adherence was high across both neurocognitive training (89%) and social-cognitive group (85%) sessions, with an 87% average overall attendance rate at treatment sessions. In addition, treatment satisfaction among all participants (treatment completion ratings for completing participants and interim ratings for partial completers) was also high with average CSQ-8 total and overall satisfaction scores for the program of 3.27 (SD = .46) and 3.57 (SD = .51) out of 4.00, respectively. These ratings indicate that individuals were "mostly satisfied" to "very satisfied" with CET (see Table 1).
Table 1.
Acceptability and Adherence of Cognitive Enhancement Therapy in Adults with Autism Spectrum Disorder (N = 14).
| Measure | N | % |
|---|---|---|
| Adherence | ||
| Number completing first 9 months of CET | 14 | 100 |
| Number completing entire 18 months of CET | 11 | 79 |
| Average percent of neurocognitive training sessions attended (M / SD) | 89 | 15 |
| Average percent of social-cognitive group sessions attended (M / SD) | 85 | 14 |
| Acceptability | ||
| Average overall satisfaction with CET (M / SD)a | 3.57 | .51 |
| Average CSQ-8 total satisfaction score (M / SD)a | 3.27 | .46 |
| Number "mostly satisfied" with CET | 14 | 100 |
Note. CET = Cognitive Enhancement Therapy; CSQ-8 = Client Satisfaction Questionnaire 8
Rated on a 1 to 4 scale, with higher scores indicating greater satisfaction
Effects on Cognition and Behavior
Although the emphasis of this study was to assess the feasibility of adapting and applying CET to verbal adults with ASD, preliminary cognitive and behavioral outcome data were examined to provide an initial assessment of treatment efficacy. Efficacy analyses made use of intent-to-treat linear mixed-effects models predicting outcome from study timepoint that included all 14 individuals who received any exposure to CET, and allowed unequal variances across study timepoints to account for heteroscedasticity (Raudenbush & Bryk, 2002). The statistical significance of change in outcome measures was evaluated using t-tests, two-tailed, of the linearly-coded fixed-effect time regression coefficient from these models. Unsuccessful attempts were made to collect reliable 18-month data on the two individuals who either withdrew early or were administratively terminated at 9 months. Missing data were therefore handled using the expectation-maximization approach to facilitate intent-to-treat analyses (Dempster, Laird, & Rubin, 1977).
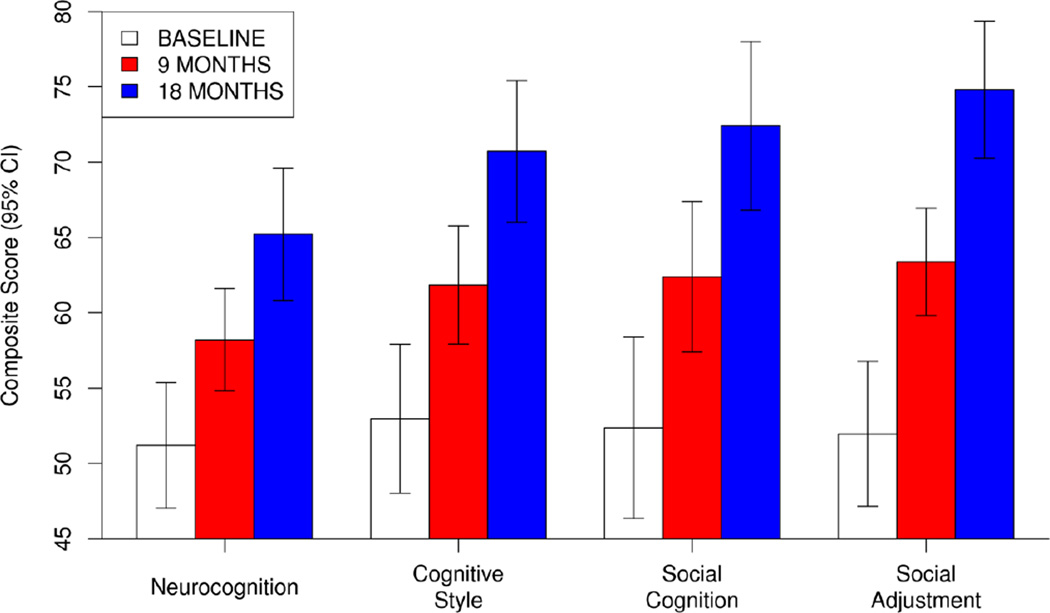
As can be seen in Figure 1, highly significant (all p < .001) and large (d = 1.40 to 2.29) levels of improvement were observed across composite domains of neurocognition, cognitive style, social cognition, and social adjustment. Neurocognitive improvement was particularly large in the domain of processing speed, which was also the greatest area of non-social cognitive impairment in the sample prior to treatment, and significant levels of improvement were observed in all neurocognitive domains, with the exception of attention/vigilance (see Table 2). In addition, all clinician-rated aspects of dysfunctional cognitive style showed significant levels of improvement.
Figure 1.
Effects of Cognitive Enhancement Therapy on Composite Indexes of Cognition and Behavior in Adults with Autism Spectrum Disorder (N = 14).
Table 2.
Univariate Effects of Cognitive Enhancement Therapy on Cognition and Behavior in Adults with Autism Spectrum Disorder (N = 14).
| Baseline |
9 Months |
18 Months |
Analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | M | SE | M | SE | M | SE | t | p | d |
| Neurocognitiona | 51.20 | 2.13 | 58.21 | 1.73 | 65.22 | 2.24 | 5.23 | .000 | 1.40 |
| Processing speedb | 38.69 | 8.26 | 57.33 | 6.96 | 75.98 | 8.30 | 4.16 | .000 | 1.22 |
| Attention/vigilanceb | 49.28 | 8.90 | 50.97 | 8.08 | 52.66 | 8.93 | .45 | .657 | .12 |
| Working memoryb | 55.59 | 8.32 | 71.66 | 6.84 | 87.72 | 7.57 | 3.96 | .001 | .81 |
| Verbal learningb | 53.20 | 8.10 | 60.12 | 7.55 | 67.03 | 8.23 | 2.24 | .034 | .43 |
| Visual learningb | 41.03 | 6.28 | 55.47 | 4.26 | 69.92 | 6.15 | 3.19 | .004 | 1.13 |
| Problem-solvingb | 45.82 | 8.93 | 56.85 | 8.18 | 67.88 | 7.99 | 5.00 | .000 | .63 |
| Cognitive Flexibility | |||||||||
| WCST: Perseverative errors (log) | 2.23 | .16 | 1.78 | .08 | 1.32 | .19 | −2.80 | .010 | −1.33 |
| WCST: Non-perseverative errors (log) | 2.10 | .19 | 1.69 | .10 | 1.27 | .19 | −2.54 | .018 | −.93 |
| Cognitive Stylea | 52.98 | 2.52 | 61.85 | 2.00 | 70.72 | 2.40 | 6.15 | .000 | 1.77 |
| Impoverished stylec | 9.54 | .44 | 8.34 | .37 | 7.14 | .42 | −5.27 | .000 | −1.01 |
| Disorganized stylec | 8.71 | .57 | 7.68 | .50 | 6.65 | .55 | −4.22 | .000 | −.95 |
| Rigid stylec | 10.71 | .52 | 9.49 | .44 | 8.26 | .46 | −5.81 | .000 | −1.42 |
| Total impairment, disability, and social handicapd |
28.77 | .99 | 25.32 | .76 | 21.87 | .94 | −5.79 | .000 | −1.69 |
| Highest cognitive style scorec | 11.59 | .46 | 10.13 | .40 | 8.67 | .45 | −6.28 | .000 | −1.70 |
| Social Cognitiona | 52.37 | 3.07 | 62.39 | 2.55 | 72.41 | 2.86 | 6.60 | .000 | 2.00 |
| Social Cognition Profilee | |||||||||
| Tolerant factor | 3.35 | .11 | 3.73 | .09 | 4.11 | .10 | 8.02 | .000 | 1.75 |
| Supportive factor | 2.45 | .14 | 3.05 | .13 | 3.65 | .14 | 9.79 | .000 | 2.39 |
| Perceptive factor | 2.58 | .13 | 3.15 | .08 | 3.72 | .11 | 7.09 | .000 | 2.04 |
| Confident factor | 2.62 | .13 | 3.10 | .09 | 3.58 | .10 | 6.38 | .000 | 1.36 |
| MSCEIT | |||||||||
| Emotion facilitation (z) | .21 | .28 | .16 | .23 | .11 | .23 | −.44 | .661 | −.10 |
| Emotion understanding (z) | −.11 | .31 | .26 | .25 | .63 | .28 | 2.35 | .027 | .73 |
| Emotion management (z) | −.02 | .27 | .30 | .20 | .61 | .21 | 2.19 | .038 | .62 |
| Penn Emotion Recognition Test-40f | 30.80 | 1.09 | 31.32 | 1.08 | 31.85 | 1.12 | 2.01 | .055 | .24 |
| Social Adjustmenta | 51.97 | 2.45 | 63.39 | 1.82 | 74.82 | 2.32 | 7.43 | .000 | 2.29 |
| Cognitive Style and Social Cognition Eligibility Interview |
|||||||||
| Vocational ineffectivenessg | 3.77 | .16 | 3.24 | .13 | 2.71 | .17 | −5.53 | .000 | −1.52 |
| Interpersonal ineffectivenessg | 4.03 | .13 | 3.43 | .12 | 2.82 | .16 | −7.48 | .000 | −2.54 |
| Adjustment to disabilityg | 3.12 | .15 | 2.52 | .09 | 1.92 | .08 | −7.22 | .000 | −1.82 |
Note. Means and standard errors are adjusted from linear mixed-effects intent-to-treat models. Effect sizes, t-tests, and p-values reflect the results of these mixed-effects models evaluating the size and significance of change over the entire 18-months of treatment in reference to the null hypothesis of no change during this time period.
Composite score scaled with a mean (SD) of 50 (10), with higher scores indicating better cognitive and behavioral functioning
Percentile score
Scores range from 3 to 15, with higher scores indicating greater cognitive dysfunction
Scores range from 9 to 45, with higher scores indicating greater impairment from cognitive dysfunction
Scores range from 1 to 5, with higher scores indicating better social-cognitive functioning
Scores range from 0 to 40, with higher scores indicating better social-cognitive functioning
Scores range from 1 to 5, with higher scores indicating worse social adjustment
MSCEIT = Mayer-Salovey-Caruso Emotional Intelligence Test, WCST = Wisconsin Card Sorting Test
Social cognition and social functioning proved to be the largest domains of improvement in this study (see Figure 1). Social cognition was significantly improved across both clinician-rated and performance-based measures, particularly with regard to emotion understanding and management. A trend-level (p = .055) effect was observed for improvements in emotion perception, which was primarily due to an improvement in accuracy in the perception of sad faces, t(25) = 2.43, p = .023, d = .61. Importantly, these social-cognitive gains generalized to broader improvements in adaptive function and social adjustment, as large and highly significant levels of improvement were observed in vocational effectiveness, interpersonal effectiveness, and participants' ability to adjust to their condition, as measured by the Cognitive Style and Social Cognition Eligibility Interview (see Table 2). Taken together, such findings suggest that CET is a feasible, acceptable, and potentially effective approach to the treatment of cognitive impairments in adults with ASD that can confer substantial benefits to social and adaptive function in these individuals.
Discussion
Adults with ASD experience significant impairments in social and non-social cognition that place profound limitations on their ability to function adaptively. Treatment development efforts for autism have focused primarily on childhood (Kasari & Lawton, 2010), and interventions designed to address the vast array of core neurocognitive and social-cognitive deficits that limit functional outcome in adults with these conditions have yet to be developed. This is the first study to examine the feasibility and applicability of CET, a comprehensive cognitive rehabilitation intervention, in adults with ASD. Results revealed that CET was well tolerated by participants, who were not compensated for attending treatment. Rates of neurocognitive and social-cognitive training session attendance were consistently high, and 79% of the sample was retained for the entire 18-month course of treatment. In addition, when asked about their experience in the program by an independent rater, participants reported high degrees of satisfaction with CET. The results of efficacy analyses were also positive, with large and highly significant levels of improvement observed across all cognitive and behavioral domains assessed. These findings provide the first evidence of the feasibility, acceptability, and initial efficacy of long-term cognitive rehabilitation with CET for verbal adults with ASD.
The results of this feasibility study have several important potential implications for the treatment of verbal adults with autism. Despite having above-average intelligence scores and being labeled as "high-functioning," it was clear that this sample experienced substantial disability that would warrant the need for cognitive rehabilitation; all participants met study criteria for significant social and cognitive disability. Furthermore, half of this working-age sample of participants were not employed, those who were employed held jobs well below their academic qualifications, and the majority of the sample was dependent upon their families. The high levels of satisfaction and treatment attendance observed in this study are indicative not only of the feasibility of CET for this population, but also confirm that verbal adults with ASD are interested in continuing to receive treatment in adulthood and are willing to devote a substantial amount of time and effort to participating in interventions that they find beneficial.
Findings regarding treatment efficacy have implications for the plasticity of the adult autism brain. Given that many of these cognitive impairments have been present since early childhood, the large levels of improvement in cognition observed in this preliminary study suggest that there remains a window of opportunity to capitalize on neuroplasticity and positively affect cognition in these conditions well into adulthood. Neuroimaging studies are currently in progress to characterize the neuroplastic effects of CET on the brain in autism, and to define the neural mechanisms underlying these improvements. The efficacy analyses also support the need for long-term treatment in this population, as the effects of CET at 18 months of treatment were considerably larger than those observed at 9 months. The need for long-term treatment is consistent with the conceptualization of autism as a long-term condition that begins very early in life, and evidence that many of the adults with this condition receive little autism-specific treatment (Shattuck, Wagner, Narendorf, Sterzing, & Hensley, 2011).
Despite the implications of this research for understanding and advancing the treatment of adults with ASD, these findings need to be interpreted in the context of a number of limitations. This study was characterized by a small sample size, which was appropriate for a first feasibility study, but also limits inferences regarding the generalizability of these results. The repeated use of cognitive tests could have also introduced testing effects that resulted in some gains in cognition, although the magnitude of cognitive improvements observed in this study are unlikely to be fully accounted for by assessments repeated on a 9-month basis. The presence of statistical regression toward the mean could have also accounted for some improvements in outcome. In addition, some behavioral assessments were completed by study clinicians involved in the treatment of participants, although large and significant levels of improvement were also observed on more objective performance-based measures of social and non-social cognition. Further, assessment of changes in adaptive function was limited in this feasibility study, and it will be important to evaluate the effects of CET on functional outcome more comprehensively. CET is also only appropriate for individuals with ASD who are verbal and do not experience a comorbid intellectual disability, and it will be important for researchers to develop alternative approaches for those who have not developed speech and experience intellectual disability. Finally, the absence of a treatment control condition limits inferences regarding the specificity of the effects of CET compared to usual care or other active treatment approaches. A treatment control condition was not included because this initial study focused first on establishing the feasibility of applying CET to verbal adults with ASD, before proceeding with a costly clinical trial. Future studies should incorporate active control interventions that account for the potential non-specific effects of CET (e.g., provision of support, attention by a skilled therapist, development of a good therapeutic relationship). A randomized-controlled trial of CET compared to an appropriately-matched active treatment control is in progress, which will help address the limitations of this pilot study. Further conclusions regarding the efficacy of CET in verbal adults with ASD will therefore be reserved until the completion of this new controlled trial.
In summary, this research provides the first evidence of the feasibility of CET, a comprehensive neurocognitive and social-cognitive remediation approach, in verbal adults with ASD. Such cognitive rehabilitation interventions have been available and highly successful with individuals with other neurological disorders, and although these results are limited by a modest sample size and the absence of a treatment control condition, findings suggest that CET is an acceptable and satisfying treatment for verbal individuals with ASD that may have substantial benefits for cognitive and functional outcomes in this population.
Acknowledgments
This work was supported by NIH grants MH-85851 (NJM and SME), RR-24154 (SME), and HD-55748 (NJM), as well as grants from Autism Speaks (NJM and SME), the Department of Defense (NJM and SME), and the Pennsylvania Department of Health (NJM).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Baron-Cohen S. Autism: A Specific Cognitive Disorder of 'Mind-Blindness’. International Review of Psychiatry. 1990;2(1):81–90. [Google Scholar]
- Ben-Yishay Y, Piasetsky EB, Rattok J. A systematic method for ameliorating disorders in basic attention. In: Meir MJ, Benton AL, Diller L, editors. Neuropsychological rehabilitation. New York: Guilford Press; 1985. pp. 165–181. [Google Scholar]
- Berger HJC, Aerts FHTM, van Spaendonck KPM, Cools AR, Teunisse JP. Central Coherence and Cognitive Shifting in Relation to Social Improvement in High-Functioning Young Adults with Autism. Journal of Clinical and Experimental Neuropsychology. 2003;25(4):502–511. doi: 10.1076/jcen.25.4.502.13870. [DOI] [PubMed] [Google Scholar]
- Bracy OL. PSSCogRehab [computer software] Indianapolis, IN: Psychological Software Services Inc; 1994. [Google Scholar]
- Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, Ellmo W, Kalmar K, Giacino JT, Harley JP, et al. Evidence-Based Cognitive Rehabilitation: Updated Review of the Literature From 1998 Through 2002. Archives of Physical Medicine and Rehabilitation. 2005;86(8):1681–1692. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Losh M, Adolphs R, Hurley R, Piven J. Comparison of social cognitive functioning in schizophrenia and high functioning autism: more convergence than divergence. Psychological Medicine. 2010;40(4):569–579. doi: 10.1017/S003329170999078X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125(1):e17. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data using the EM algorithm. Journal of the Royal Statistical Society Series B (Methodological) 1977;39(1):1–38. [Google Scholar]
- Eack SM. Cognitive remediation: A new generation of psychosocial interventions for people with schizophrenia. Social Work. 2012;57(3):235–246. doi: 10.1093/sw/sws008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Greenwald DP, Hogarty SS, Keshavan MS. One-year durability of the effects of Cognitive Enhancement Therapy on functional outcome in early schizophrenia. Schizophrenia Research. 2010;120(1):210–216. doi: 10.1016/j.schres.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Greenwald DP, Hogarty SS, Cooley SJ, DiBarry AL, Montrose DM, Keshavan MS. Cognitive Enhancement Therapy for early-course schizophrenia: Effects of a two-year randomized controlled trial. Psychiatric Services. 2009;60(11):1468–1476. doi: 10.1176/appi.ps.60.11.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Cho RY, Prasad KMR, Greenwald DP, Hogarty SS, Keshavan MS. Neuroprotective effects of Cognitive Enhancement Therapy against gray matter loss in early schizophrenia: Results from a two-year randomized controlled trial. Archives of General Psychiatry. 2010;67(7):674–682. doi: 10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Greenwald DP, Hogarty SS, Keshavan MS. Effects of Cognitive Enhancement Therapy on employment outcomes in early schizophrenia: Results from a two-year randomized trial. Research on Social Work Practice. 2011;21(3):32–42. doi: 10.1177/1049731509355812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick LB, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-012-1615-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz ML. The Lifetime Distribution of the Incremental Societal Costs of Autism. Archives of Pediatrics and Adolescent Medicine. 2007;161(4):343–349. doi: 10.1001/archpedi.161.4.343. [DOI] [PubMed] [Google Scholar]
- García-Villamisar D, Rojahn J, Zaja RH, Jodra M. Facial emotion processing and social adaptation in adults with and without autism spectrum disorder. Research in Autism Spectrum Disorders. 2010;4(4):755–762. [Google Scholar]
- Gilotty L, Kenworthy L, Sirian L, Black DO, Wagner AE. Adaptive skills and executive function in autism spectrum disorders. Child Neuropsychology. 2002;8(4):241–248. doi: 10.1076/chin.8.4.241.13504. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Minshew NJ, Allen DN, Seaton BE. High-functioning autism and schizophrenia A comparison of an early and late onset neurodevelopmental disorder. Archives of Clinical Neuropsychology. 2002;17(5):461–475. [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biological Psychiatry. 2004;56(5):301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Guilmatre A, Dubourg C, Mosca AL, Legallic S, Goldenberg A, Drouin-Garraud V, Layet V, Rosier A, Briault S, Bonnet-Brilhault F, Laumonnier F, Odent S, Le Vacon G, Joly-Helas G, David V, Bendavid C, Pinoit J, Henry C, Impallomeni C, Germano E, Tortorella G, Di Rosa G, Barthelemy C, Andres C, Faivre L, Frébourg T, Veber PS, Campion D. Recurrent rearrangements in synaptic and neurodevelopmental genes and shared biologic pathways in schizophrenia, autism, and mental retardation. Archives of general psychiatry. 2009;66(9):947–956. doi: 10.1001/archgenpsychiatry.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, Alsop D, Maldjian J, Gur RE. Brain Activation during Facial Emotion Processing. Neuroimage. 2002;16(3PA):651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources Inc; 1993. [Google Scholar]
- Hobson RP, Ouston J, Lee A. Emotion recognition in autism: coordinating faces and voices. Psychological Medicine. 1988;18(4):911–923. doi: 10.1017/s0033291700009843. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Greenwald DP. Cognitive Enhancement Therapy: The Training Manual. Authors: University of Pittsburgh Medical Center; 2006. Available through www.CognitiveEnhancementTherapy.com. [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, Keshavan M, Cooley S, DiBarry AL, Garrett A, Parepally H, Zoretich R. Cognitive enhancement therapy for schizophrenia. Effects of a 2-year randomized trial on cognition and behavior. Archives of General Psychiatry. 2004;61(9):866–876. doi: 10.1001/archpsyc.61.9.866. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Greenwald DP, Eack SM. Durability and mechanism of effects of Cognitive Enhancement Therapy. Psychiatric Services. 2006;57(12):1751–1757. doi: 10.1176/ps.2006.57.12.1751. [DOI] [PubMed] [Google Scholar]
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. Journal of Child Psychology and Psychiatry. 2004;45(2):212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32(4):477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Kanner L, Rodriguez A, Ashenden B. How far can autistic children go in matters of social adaptation? Journal of Autism and Developmental Disorders. 1972;2(1):9–33. doi: 10.1007/BF01537624. [DOI] [PubMed] [Google Scholar]
- Kasari C, Lawton K. New directions in behavioral treatment of autism spectrum disorders. Current Opinion in Neurology. 2010;23(2):137–143. doi: 10.1097/WCO.0b013e32833775cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, Gur RE, Gur RC. Facial Emotion Recognition in Schizophrenia: Intensity Effects and Error Pattern. American Journal of Psychiatry. 2003;160:1768–1774. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Evaluation and Program Planning. 1979;2(3):197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule—Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G. Measuring emotional intelligence with the MSCEIT V2.0. Emotion. 2003;3(1):97–105. doi: 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL. Learning, attention, writing, and processing speed in typical children and children with ADHD, autism, anxiety, depression, and oppositional-defiant disorder. Child Neuropsychology. 2007;13(6):469–493. doi: 10.1080/09297040601112773. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism. Archives of Neurology. 2007;64(7):945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: profile of a complex information processing disorder. Journal of the International Neuropsychological Society. 1997;3(4):303–316. [PubMed] [Google Scholar]
- Mizuno A, Liu Y, Williams DL, Keller TA, Minshew NJ, Just MA. The neural basis of deictic shifting in linguistic perspective-taking in high-functioning autism. Brain. 2011;134(8):2422–2435. doi: 10.1093/brain/awr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazaran J, Muniz R, Reisberg B, Pena-Casanova J, del Ser T, Cruz-Jentoft AJ, Serrano P, Navarro E, Garcia de la Rocha ML, Frank A, et al. Benefits of cognitive-motor intervention in MCI and mild to moderate Alzheimer disease. Neurology. 2004;63(12):2348–2353. doi: 10.1212/01.wnl.0000147478.03911.28. [DOI] [PubMed] [Google Scholar]
- Ozonoff S. Executive functions in autism. In: Schopler E, Mesibov GB, editors. Learning and cognition in autism. New York: Plenum Press; 1995. pp. 3–27. [Google Scholar]
- Penn DL, Corrigan PW, Bentall RP, Racenstein J, Newman L. Social cognition in schizophrenia. Psychological Bulletin. 1997;121(1):114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- Pilowsky T, Yirmiya N, Arbelle S, Mozes T. Theory of mind abilities of children with schizophrenia, children with autism, and normally developing children. Schizophrenia Research. 2000;42(2):145–155. doi: 10.1016/s0920-9964(99)00101-2. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophrenia Research. 2007;99(1–3):164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp S, Brenes G, Marsh AP. Memory enhancement training for older adults with mild cognitive impairment: a preliminary study. Aging and Mental Health. 2002;6(1):5–11. doi: 10.1080/13607860120101077. [DOI] [PubMed] [Google Scholar]
- Raudenbush DSW, Bryk DAS. Hierarchical Linear Models: Applications and data analysis methods. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Samson AC, Huber O, Gross JJ. Emotion regulation in Asperger's syndrome and high-functioning autism. Emotion. 2012;12(4):659–665. doi: 10.1037/a0027975. [DOI] [PubMed] [Google Scholar]
- Sasson N, Tsuchiya N, Hurley R, Couture SM, Penn DL, Adolphs R, Piven J. Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia. 2007;45(11):2580–2588. doi: 10.1016/j.neuropsychologia.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SG, Asarnow RF. A comparison of cognitive/neuropsychological impairments of nonretarded autistic and schizophrenic children. Journal of Abnormal Child Psychology. 1987;15(1):29–45. doi: 10.1007/BF00916464. [DOI] [PubMed] [Google Scholar]
- Selman RL, Schultz LH. Making a Friend in Youth. Chicago, IL: University of Chicago Press; 1990. [Google Scholar]
- Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social Perception as a Mediator of the Influence of Early Visual Processing on Functional Status in Schizophrenia. American Journal of Psychiatry. 2006;163(3):448–454. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- Shattuck PT, Wagner M, Narendorf S, Sterzing P, Hensley M. Post-high school service use among young adults with an autism spectrum disorder. Archives of Pediatrics and Adolescent Medicine. 2011;165(2):141–146. doi: 10.1001/archpediatrics.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon HA. Information processing models of cognition. Annual review of psychology. 1979;30(1):363–396. doi: 10.1146/annurev.ps.30.020179.002051. [DOI] [PubMed] [Google Scholar]
- Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: Meta-analysis of the neural correlates of social cognition. PloS One. 2011;6(10) doi: 10.1371/journal.pone.0025322. e25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JDE. Neural deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. Proceedings of the National Academy of Sciences. 2003;100(5):2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. Journal of Child Psychology and Psychiatry. 2004;45(1):135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006;129(4):932–943. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Intelligence Scale. San Antonio, T: The Psychological Corporation; 1999. [Google Scholar]
- Williams DL, Goldstein G, Carpenter PA, Minshew NJ. Verbal and spatial working memory in autism. Journal of Autism and Developmental Disorders. 2005;35(6):747–756. doi: 10.1007/s10803-005-0021-x. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. American Journal of Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]