Abstract
The neuroendocrinology of menopause is reviewed from a comparative perspective, with emphasis on laboratory rodent models. These changes are compared by the 2011 STRAW Criteria (Stages of Reproductive Aging Workshop). Ovarian cell loss begins prenatally in all mammals studied, with exponential depletion of primary follicles and oocytes in association with loss of fecundity by midlife. Rodents and humans also share progressively increasing irregularity in ovulatory cycles and increasing fetal aneuploidy as oocyte depletion become imminent. Hypothalamic impairments of the estrogen-induced surge of pituitary gonadotrophins (luteinizing hormone, LH; follicle stimulating hormone, FSH) are prominent in middle-aged rodents, but sporadic in peri-menopausal women. In aging rodents, hypothalamic impairments of the LH surge have been experimentally associated with prolonged phases of sustained estradiol (E2) and very low progesterone (P4) (‘unopposed estradiol’). Although peri-menopausal women also show hyper-estrogenic cycles, there is no indication for irreversible hypothalamic desensitization by E2. Ongoing cognitive assessments in clinical trials of estrogen therapy with and without P4 or other progestins may further inform about possible persisting effects of unopposed estrogens.
Keywords: oocyte loss, hypothalamic impairments, STRAW criteria
1. Introduction
First, ovarian senescence is reviewed from a comparative perspective across vertebrates. Then, the stages of menopause by the STRAW criteria (Stages of Reproductive Aging Workshop) are compared with laboratory rodent models. Past work from my lab in estradiol (E2)-driven rodent neuroendocrine is reviewed, and updated with recent findings that link impaired astrocyte neurotrophic activity to increased expression of estrogen receptor alpha (ERα). Species differences are important in neuroendocrine aging changes which are much less robust in menopausal women than in rodent models.
2. Ovarian senescence
2.1 Oocyte depletion and de novo oogenesis
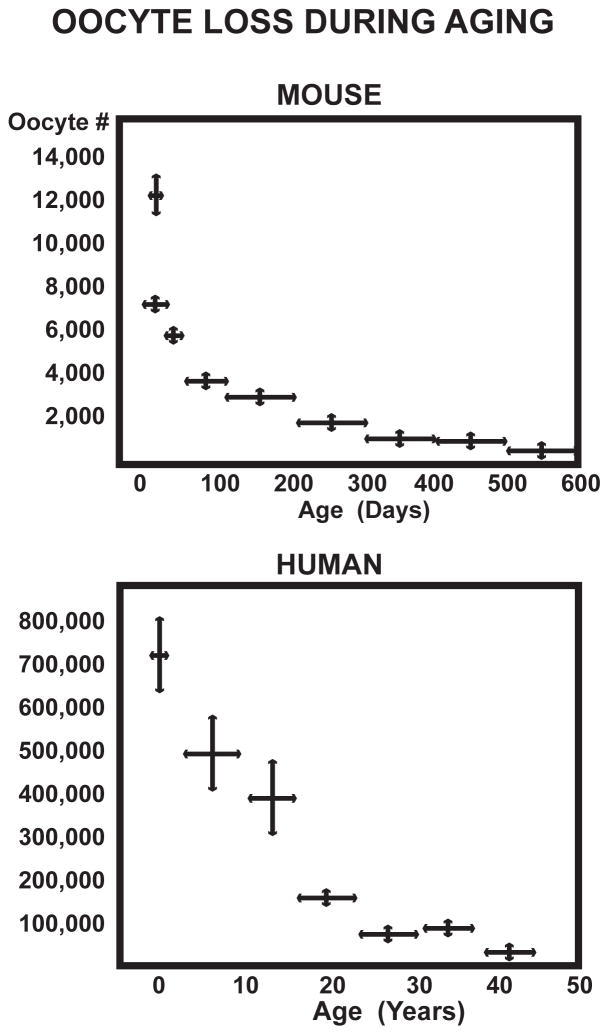
Ovarian primary follicles and oocytes decline exponentially, starting by birth or before in humans, rodents, and all mammals examined (Fig. 1)(Table 1) [1,2,3]. There is some evidence for postnatal de novo oogenesis (neo-folliculogenesis) from Tilly and others [4,5]. However, as the ovarian stock dwindles, there is no evidence for a contribution of de novo oogenesis to active follicular pools. A surgical model of accelerated ovarian aging by hemi-ovariectomy (OVX) advanced the onset of fetal aneuploidy and acyclicity by about one month [6]. Acyclicity is predicted to begin at a threshold of 150 remaining oocytes [3]. As elegantly developed by Nelson and Felicio [7], the onset age of acyclicity can be predicted from the rate of ovarian oocyte loss, which is approximated by a single exponential; like the rate of radioactive decay and ‘zeroeth order’ chemical kinetics, the rate of oocyte loss is independent of the number remaining; also see [8]. The extreme ‘radical ovarian resection’ of removing 90–95% of the follicles in 5 mo old mice accelerated the onset of ayclicity by 5 months, within the limits of the prediction [7]. These findings suggest that de novo oogenesis in mice to compensate for the excised primary follicles is minor after 5 months. Mathematical models of the human oocyte reserve are also consistent with minimal de novo oogenesis [9]. Nonetheless, the potential for recovering oogonial stem cells remains of great interest as women increasingly schedule pregnancies to later ages when natural fecundability is sharply dwindling.
Fig. 1.
Oocyte loss from birth thru midlife in mouse (British strain A) [109] and Caucasian women human [110]. Redrawn from Finch et al. 1984 [49] In both species, the stock of primary follicles and immature oocytes begins to decline exponentially before birth and becomes exhausted during midlife. Mean lifespans in 2010 are much greater than under Darwinian conditions before modern medicine and hygiene.
Table 1.
Stages of Ovarian Aging in Women and Rodent Models
| Ovarian aging, Stages defined by STRAW a | Women | Rodent models b |
|---|---|---|
| −5, early reproductive stages: Variable to regular cycles | 15–20 y | 2–3 mo |
| −3b, late reproduction declining fecundability | 30–50 y irregular cycles, short and long |
7–12 mo, irregular lengthened cycles; rodents are “retired” as breeders |
| −2, early menopausal transition; | 35–50 y elevated FSH; hypo- and hyperestrogenic cycles; increased anovulatory cycles |
9–15 mo, acyclic (constant estrus, with unopposed estrogen; Fig. 3) and hypothalamic desensitization to E2 [49, 55] |
| −1, late menopausal transition | acyclic, anestrous, very low estrogens [57] | |
| 0, final menstrual period no fecundability | amenorrhea, >2 mo; dwindling follicles | no exact equivalent to menopause; dwindling follicles |
| +1a,b early postmenopausal | extinction of follicles; hot flushes, 2 y; increasing FSH and LH | Follicle depletion, 18–24 mo [3]; FSH & LH elevations equivalent to OVX if no pituitary tumors [57] |
| +1c | FSH elevations stabilize, 3–6 y | |
| +2, late postmenopausal | urogenital atrophy | uterine atrophy [57] |
Although Zuckerman’s (1951) [10] concept of finite pools of ovarian oocytes in humans and other mammals is considered the law of the land, the naked-mole rats (rodent family Bathyergidae) may prove to be an exception: these small subterranean species, now laboratory adapted, maintain reproduction to nearly the limit of their prodigious lifespans [11,12]. The current record for Heterocephalus glaber, exceeds 31 years in the lab colony of Rochelle Buffenstein (pers. comm.). Equally remarkable is the increase of litter size in the established breeders older than 15–20 years [11]. We hope for data on ovarian follicle populations by age.
Looking further into long-lived vertebrates, there are a few credible examples of fish and turtles that maintain reproduction into their later years [2, 12]. Two species of rockfish (Sebastes aleutianus, rougheye rockfish and S. alutus, Pacific ocean perch) were examined for follicle counts of individuals of defined ages in a project that I organized [13]. Both fish species showed adult de novo oogenesis with annual crops of mature ova; S. aleutianus maintained follicle and oocyte numbers up to age 80 years. Further studies of these regularly harvested species could evaluate the viability of the oocytes by in vitro fertilization. Other shorter-lived fish show definitive ovarian senescence with definitive postreproductive phases in lab populations [2]. Thus during the last 500 million years of vertebrate evolution, ovarian senescence has arisen independently in different lineages.
Humans are unique among primates in a definitive postmenopausal phase of life that may have co-evolved with our exceptional longevity. The chimpanzee age at menopause at about 52 years observed in captivity is close to their maximum lifespan [14, 15]. In natural populations, no postreproductive phase has been observed for chimpanzees, or for shorter lived monkeys, which also have definitive menopause in captivity [16]. The evolution of the extended post-reproductive phase of the human life history is a definitive human innovation, and may be understood in terms of ‘inclusive fitness’ for the role of grandmothers and other sources of social capital [15,17]. Nonetheless, ovarian senescence has also evolved in species across the vertebrate phyletic radiations that entirely lack multi-generational social systems [2].
Loss of oocytes and primary follicles begins before birth at an astonishing rate [8, 18]: Starting at 7 million oocytes at 20 weeks of gestation, one million remain at birth, and the loss continues postnatally. At most, about 350 oocytes could be ovulated during the human reproductive lifespan, which is 1/20,000th of the initial 7 million potential eggs. The mechanisms of oocyte attrition involve apoptotic processes intrinsic to the ovary. Because aneuploidy increases as the pool is exhausted (see above), some have argued that oocyte attrition is evolutionarily adaptive to eliminate defective oocytes with chromosomal abnormalities or damaged mitochondria [19].
Human oocyte loss curves show more complexity than the rodent, with some acceleration of loss in the last decade [20, 21, 22, 23]. Nonetheless, the age at menopause is still determined by the initial oocyte and follicular pool, with a threshold number of primary follicles before the last cycle estimated at 1000 for women [22] and 100 for mice [3]. In postmenopausal ovaries, “follicles were virtually absent” [20].
In women, the rates of follicle recruitment fall sharply as menopause approaches [23], but ovulation is still possible, even after menopause [24, 25]. In mice the progressive deficits in ova were clearly shown by superovulation with injected gonadotrophins [26]. Postnatal oocyte loss is under systemic physiological regulation, and is slowed by hypophysectomy [27, 28] and by caloric restriction [29, 30]. After restoration of ad lib feeding, mice regained regular cycling [29] and retained fecundity up to 6 months beyond their usual ages [31]. Conversely in women, tobacco smokers tend to have early menopause by about 1 year [32], in association with dose-response for pack-years and second hand (passive) inhalation of smoke [33]. No oocyte or follicle counts are yet available for smokers.
2.2. Defective oocytes and birth defects
Birth defects from chromosomal meiotic abnormalities (aneuploidy) increase sharply as the follicular pool diminishes. The risk of Down syndrome (trisomy 21) increases exponentially with maternal age 100-fold between age 25 and 50 [35, 36]. In mice, oocyte aneuploidy increased with age >5-fold (Table 2) [26]. The increase of abnormal fetal karyotypes is accelerated by premature follicular depletion, in mouse models with partial OVX and in Turner’s syndrome with ovarian dysgenesis, a subset of which retain sufficient follicles for adult fertility [6, 37]. Remarkably, in oocytes from aging mice, aneuploidy and abnormal mitochondrial aggregates were attenuated by caloric restriction [30], which as mentioned above, also slowed the rate of oocyte loss. While caloric restriction in mice and other animal models does not readily translate into human benefits, we must remain open to the possibility that human ovarian aging is modulated by systemic factors, such as tobacco smoke toxins.
Table 2.
Ovarian ova production and oocyte aneuploidy in aging mice
| Age, month | # Ova | Aneuploidy |
|---|---|---|
| 2 | 40 ± 7 | 4.2% |
| 6 | 31 ± 4 | |
| 12 | 17 ± 6 | |
| 16 | 4.4 ± 3.6 | 25% |
B6D2F1 mice (C57BL/6J x DBA2/J mice) oocytes arrested at metaphase II [26].
The basis for increasing aneuploidy in mice includes abnormalities in meiotic spindle formation and chromosome segregation at anaphase I [26, 38, 39]. In women, deacetylation defects increase with maternal age and correlate with chromosome misalignment [40]. Gene expression profiling of oocytes in aging mice [26] and humans [41] showed selective changes in mRNA for functions (GO and KEGG categories) related to chromosome alignment and spindle formation. A bioinformatics could identify shared RNA changes across rodents and humans.
2.3. Perimenopausal cycles and sex steroid transitions
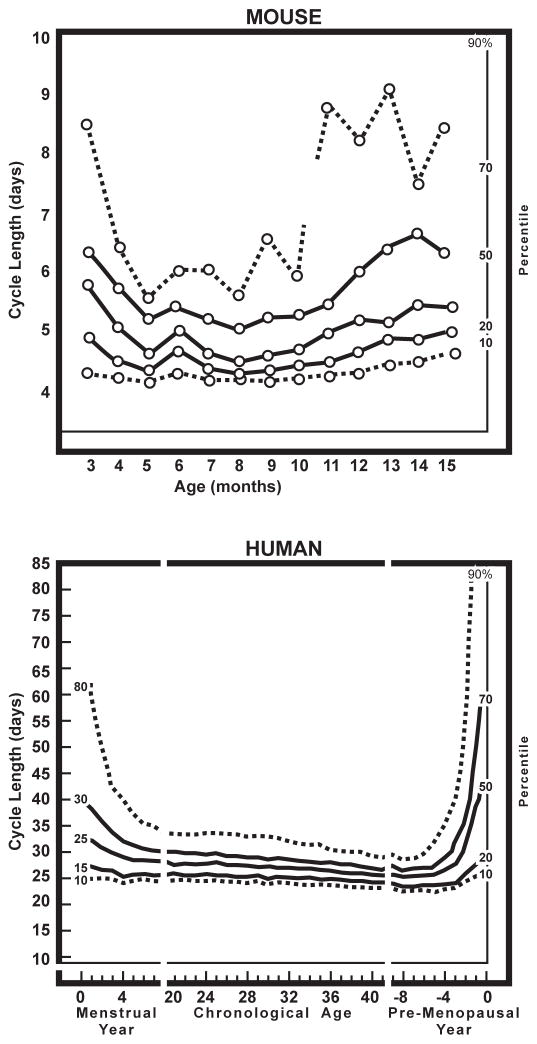
As the follicular pool approaches exhaustion, ovarian cycle irregularity and average cycle length increases in humans and rodent models (Fig. 2). Table 1 compares rodent changes with the stages of menopause according to 2012 STRAW Criteria (Stages of Reproductive Aging Workshop) [42]. Table 1 notes species differences as well as similarities.
Fig. 2.
Ovarian cycle regularity, defined by the frequency distribution of cycle lengths in mice and women. Redrawn from Nelson et al., 1982 [51], from longitudinal data of cycle lengths of C57BL/6J mice [51] and Treloar’s landmark study of self-reported menstrual cycles (alumna, University of Minnesota) [43]. Both show U-shaped curves of the variance in cycle length. During early adulthood, variance decreases, followed by maximum regularity during the most fertile phase of life, and then increased irregularity during follicular depletion. Perimenopausal stages are compared in Table 1.
Longitudinal studies of women show the increased prevalence of longer, as well as shorter than normal cycles, both interspersed with normative ca. 30 d cycles [23, 43, 44, 45]. Anovulatory cycles increase. There are also hyperestrogenic cycles, discussed below. Total estrogen production eventually diminishes towards OVX levels after menopause (STRAW Stage 1, Table 1) [25, 45]. However, there are wide individual variations during the peri-menopause (Stages −2 and −1; Table 1) such that “Unpredictability is the norm” [47]. Even identical twins vary in age at menopause: mean MZ co-twin difference at menopause is 2 y, with a range 1–12 y [48]. Heterogeneity in age at menopause may arise in part from individual variations in the numbers of oocytes formed during development, as also observed in inbred mice [3, 8].
Rodents also have extensive individual heterogeneity of ovarian cycle aging, e.g. in our studies of the C57BL/6J mouse [49, 50, 51, 52, 53, 54]. Young lab mice and rats typically have 4–5-d cycles, which become increasingly irregular after 7 mo, with long cycles (6–10 d) interspersed with normal cycles of 4- to 5-d length (Fig. 2 and Fig. 3). However, unlike humans, aging rodents do not show cycle shortening, with no cycles <4 d. Nonetheless, aging rodents have been widely used as convenient models for the perimenopause because of ovarian cycle irregularity during depletion of the ovarian follicle pool, increased risk of fetal aneuploidy, and eventual reduction of plasma estrogen and progesterone to OVX levels [1, 49, 52, 55, 56]. While rodents show definitive ovarian senescence, by convention the endpoint is not termed menopause because rodents lack vaginal discharges equivalent to menstrual flow (the menses).
Fig. 3.
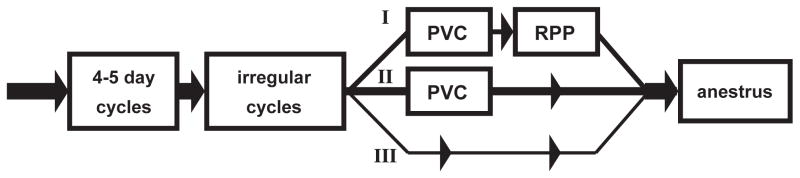
Rodent reproductive senescence proceeds on three alternate pathways to anestrus (terminal acyclicity), based on vaginal smear cytology; redrawn from Finch et al. 1984 [49]. Regular 4–5 day cycles during peak fecundability across 3–7 months are followed by pathway I: irregular cycles (prolonged cycles >5 day interspersed with 4–5 day cycles) and then PVC (persistent vaginal cornification; alternate name, constant estrus, CE) and lastly to anestrus (the ovarian equivalent of the human post menopause with extremely low plasma E2 and P4, and elevated gonadotropins, ovariectomy equivalent) [57] (Table 1 above). PVC cytology is due to persistently elevated blood E2 and negligible P4 from polyfollicular anovulatory ovaries lacking corpora lutea [50]. The duration of PVC varies inversely with age of onset [53]. Pathway II: PVC may be followed by 10–14 day cycles resembling repetitive pseudo-pregnancy (RPP), with growing follicles and corpora lutea (prolonged luteal phases) and intermittent ovulation [60]; the PVC-RPP pathway may be more common aging rats than mice, (Table 7 in [1]). The minor pathway III is a direct transition from irregular cycles to anestrus.
The onset of rodent perimenopause is marked by a phase of interspersed longer cycles (irregular cycles), identified by vaginal smear cytology followed by one of three alternate pathways of ovarian activity (rodent pathways I, II, III, Fig. 3) [49]. In pathway I, rodents transition from irregular cycles to ‘constant estrus’ or persistent vaginal cornification, an anovulatory state with polyfollicular; eventually, constant estrus fades to anestrus with thin smears (few cells) indicative of low estrogens and progesterone from the imminent or complete depletion of growing follicles [57]. Aging rodent anestrus is a model for the STRAW postmenopausal Stages +1 and +2, with OVX levels of plasma estradiol (E2) and progesterone (P4) and uterine atrophy [57]. In pathway II, constant estrus is followed or interrupted by prolonged diestrus-like vaginal cytology with intermittent ovulation, also known as repetitive pseudo-pregnancy (RPP) [58, 59, 60], again ultimately followed by anestrus. In the minor pathway III, irregular cycles are directly followed by anestrus. In practice, rodent studies of the peri-menopause require laborious daily tracking of vaginal cytology in large groups for at least 10 consecutive days to define the cycling status.
The major changes in menstrual flow in women, and vaginal cytology in women and rodents, result from lower estrogen and progesterone production as the follicular pool diminishes. All mammals characterized show the end-point of ovarian senescence from the depletion of growing follicles with major decreases in blood estrogen and progesterone that approach the equivalent of OVX in young adults. However, in the approach to follicular exhaustion, women show hyperestrogenic cycles with elevated plasma E2:P4 (see below), which may be modeled in the constant estrus stage of rodents. However, rodents differ in their regular development of hypothalamic desensitization with impaired pre-ovulatory E2-induced LH surges (Fig. 4), for which there is no clear equivalent in women. Rodents also differ from humans and monkeys in their very low levels of the adrenal androgens (Section 3.4).
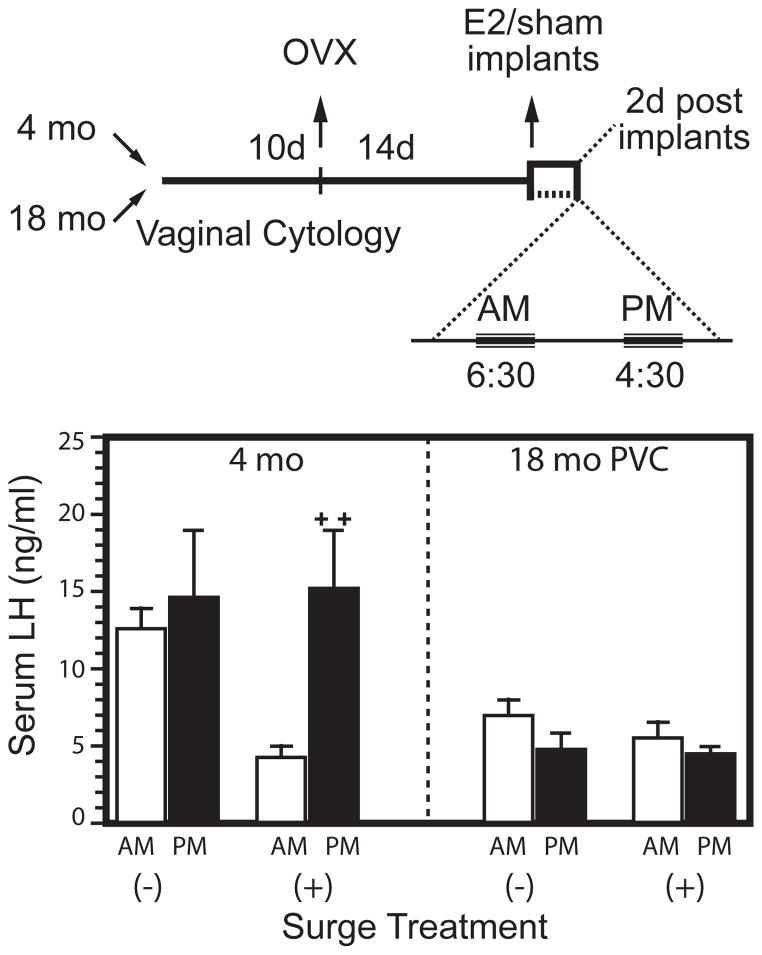
Fig. 4.
Rodent age impairments in the E2-induced gonadotrophin surge. Redrawn from Anderson et al. 2002 [78]. Hypothalamic-pituitary impairments in the induced LH surge in acyclic rats (18 month, PVC). Top panel: experimental design, with E2 implants to ovariectomized rats (OVX). Bottom panel: Young cycling rats OVX and given E2 implants had a nocturnal LH surge which models the pre-ovulatory blood LH surge at proestrus, whereas middle-aged acyclic rats did not respond. Notably, LH was elevated in the young OVX rats without E2 (−), but not in the old, showing impaired negative feedback of low E2 on pituitary secretion of LH.
Estrogens gradually decline very late in the human perimenopause as the follicular pool becomes exhausted (STRAW Stage minus 1, Table 1) [24, 42, 44, 61]. However, there is a trend for increased follicular phase plasma E2 and higher E2:P4 ratio during the perimenopause relative to young cycling women [25, 45, 62, 63, 64]. From Prior’s 1998 meta-analysis of 12 studies [61], I calculate that average follicular phase blood E2 was 30% higher in perimenopausal vs the premenopausal women1. Subsequent studies confirm these trends [25, 63, 65]. Prior [61] describes ‘the paradox of endogenous ovarian hyperstimulation”, which is paradoxic because declines, not increases, are the larger trend in estrogen production as menopause approaches. Hyper-estrogenic cycles may be interspersed (flanked by) with cycles with lower estrogens, suggested by the 7-fold higher frequency of episodes of high unopposed estrogens in perimenopausal than regularly cycling younger women [66]. There are also anomalous cycles with secondary peaks of estrogen during the luteal phase (‘LOOP cycles’, luteal out-of- phase follicular event) [67]. Sometimes a dominant follicle may also emerge from the cohorts (‘waves’) of anovulatory follicles that precede the ovulatory wave [68]. I note the important lack of hormone data for successive cycles in sequences (strings) of cycles of varying length, as discussed below.
Unopposed estrogens are known risk factors in cancers of the uterus [64, Clear definition of the ‘window of risk’ is frustrated by uncertainties about the total duration of unopposed estrogens because cycle length transitions and hormone levels vary widely, both within and between individuals. A model for phases of ‘unopposed estrogen’ in perimenopausal women may be found in constant estrus aging rodents, with sustained modest E2 and low P4 from polyfollicular ovaries without corpora lutea.
Plasma FSH and LH remain elevated at later STRAW Stages 0 to+2, after eventual depletion of the follicular reserve. Similarly, aging mice may show elevated FSH and LH [57, 70], particularly those with uterine atrophy indicative of low estrogens [57]. However, there is an important interaction with age-related pathology, because plasma LH elevations are not seen in aging mice with pituitary tumors or some other gross organ lesions [57]. Female rodents older than 18 mo have a high incidence (ca. 50%) of pituitary tumors that hypersecrete prolactin [58, 71, 72, 73]. The lower plasma LH in tumor-bearing mice is attributable to their prolactinemia, which can directly suppress gonadotrophin release [74]. In contrast, pituitary tumors are relatively uncommon in postmenopausal women, and as well as in aging male mice.
3. Neuroendocrine changes
3.1. Rodent models
Impairment of the E2-induced pre-ovulatory gonadotrophin surge in aging constant estrus mice and rats is a major landmark of reproductive senescence [49, 55, 75, 76]. Hypothalamic-pituitary desensitization was demonstrated by my lab and others by short-term OVX followed by acute E2 replacements that induce vigorous, circadian timed LH surges in young adults, whereas constant estrus rodents were unresponsive [60,75,77,78] (Fig. 4B).
Hypothalamic-pituitary impairments were further shown by ovarian transplantation to beneath the kidney capsule (heterotopic transplantation). The control group of young ovaries transplanted to young hosts (Y to Y) had about 20 cycles, close to the number in intact hosts (Fig. 5 & Fig. 6). In contrast, ovaries from middle-aged donors (MA to Y) supported <10 cycles (Fig. 6, MA-INT to Y), consistent with their smaller pool of remaining follicles [3]. Paralleling the impaired E2-induced LH surge (Fig. 4B), ovaries from young cycling (Yc) donors transplanted to middle-aged acyclic hosts incompletely restored cyclicity for a limited duration (Fig. 5, Yc to MAc; Fig. 6, Y to MA-INT) [49, 54].
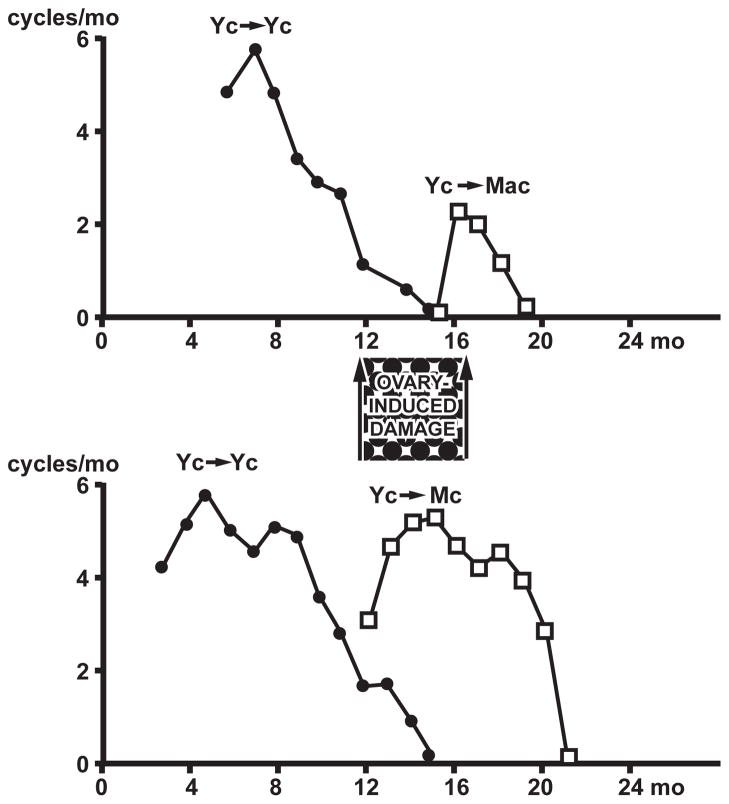
Fig. 5.
Ovarian transplantation. C57BL/6J mice were given young (3–4 mo) ovarian transplants (heterotopic kidney capsule); ovarian cycles were followed by vaginal cytology. Redrawn from Finch et al. 1984 [49]. Top: Young to young cycling mice (Yc to Yc) controls cycled nearly as long as intact controls, with acyclicity complete by 16 mo. However, middle-aged acyclic mice if given young ovaries (Yc to Mac) had limited ability to cycle and became acyclic within 3 mo. Bottom: Middle-aged hosts that were still cycling at grafting of young ovaries (Yc-Mc) maintained regular cycles for the next 6 months. This suggests that the onset of acyclicity (PVC, Fig. 3 and Table 1) with prolonged exposure to E2 with low progesterone (‘unopposed estrogen’) can cause ‘ovary-induced damage’ (hypothalamic desensitization).
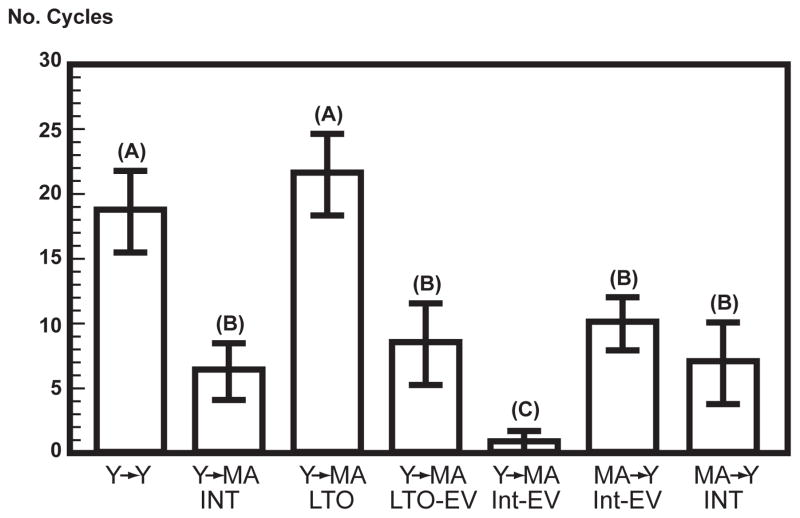
Fig. 6.
Test of the hypothesis that prolonged exposure to unopposed estrogens induces hypothalamic desensitization using ovarian transplantation, redrawn from Mobbs et al. 1985 [84]. Letters above bars (A,B,C) identify groups that differ statistically. The two groups from the left show that middle-aged hosts given young ovaries (Y-MA, INT, intact) supported fewer cycles than young (Y-Y) (replication of findings in Fig. 5). The third group from the left was OVX when young and after 6 months (long-term OVX, LTO) received young ovaries at middle-age (Y to MA-LTO) and had as many cycles as Y-Y controls. This finding suggests that aging in the absence of endogenous ovarian steroids protects against hypothalamic desensitization from prolonged cycles and PVC [49]. The fourth and fifth groups were given E2 valerate (EV) that caused sustained elevations of E2; when middle-aged, their ovaries were replaced by young ovarian grafts; EV treatment blocked the ability to support cycles in the intact group, but supported slightly more cycles in the LTO, which is consistent with shorter duration exposure to unopposed estrogens. In the two right-most groups, MA ovaries were transplanted to Y hosts, and supported about 60% fewer cycles than the Y-Y controls, consistent with imminent depletion of ovarian follicles at middle-age.
Further transplantation studies evaluated cycle length [54]. In MA hosts with young ovary transplants, we observed longer cycles, but not 4 d cycles. In contrast, the Y to Y transplants had predominantly 4 d cycles. Thus, there may be a neuroendocrine component in the transition to longer cycles during aging, as well as in the ultimate failure of cyclicity.
Evidence that these mechanisms involve the hypothalamic-pituitary exposure to ovarian steroids came from attenuation of neuroendocrine impairments by long-term ovariectomy (LTO) when young, followed by young ovarian grafts. Middle-aged LTO mice (MA-LTO) had predominantly 4 d cycles if OVX when young and allowed to reach middle-age before receiving young ovaries. The Y ovary to MA-LTO hosts had the same number of cycles as Y to Y controls (Fig. 6). Moreover, LTO mice did not show the usual age decline in negative feedback sensitivity of E2 on LH [79].
We hypothesized [49] that neuroendocrine impairments with E2-desensitization arise from cumulative exposure to estrogen from elevated blood E2:P4 ratios during the initial lengthening of cycles and acyclicity. This hypothesis was tested in two ways. First, we transplanted young ovaries into middle-aged hosts that were still cycling and observed more cycles than in slightly older mice that were acyclic (Fig. 5). A second approach exposed young OVX mice to sustained lowl levels of E2, administered in the drinking fluid, which were sufficient to suppress the post-OVX elevations of LH [80,81]: as few as 6 weeks of unopposed low E2 caused premature loss of cyclicity, as assessed with young ovary transplants [80, 81]. We also compared the effects of depot injections of E2 valerate (EV) that maintain supraphysiological high levels of exogenous plasma E2 for 4–5 weeks in young LTO and intact mice: the EV had larger effects on cycling duration in the LTO than intact mice (Fig. 6) [82]. The ovarian capacity for cycling was not altered by the effects of EV (Fig. 6, MA to Y, INT-EV vs INT). The importance of unopposed E2 was shown by the ability of P4 implants to block the effects of E2 on hypothalamic desensitization [81].
Further aspects of postmaturational E2 exposure are shown in the delayed anovulatory syndrome (DAS) of rodents, which indicates interactions of pre- and postpubertal steroid exposure. If neonatal females are given a large single dose of testosterone (T), the adults lack ovarian cycles in association with impaired hypothalamic responses in the lack of E2-induced surges of LH and FSH. Because male rodents also do not respond to E2 with a gonadotrophin surge, this is known as hypothalamic masculinization, and represents an ‘organizing effect’ of neonatal steroids on the developing brain [83]. However, with a smaller ‘submasculinizing’ dose of T given neonatally, young adult females initially have ovulatory cycles, but become prematurely anovulatory (DAS). Further we showed that DAS effects can be caused by small neonatal doses of E2 and that the loss of cyclicity is due to hypothalamic desensitization to E2 [84]. These and other findings suggest a continuum model for irreversible effects of E2 (and other sex steroids) in which postpubertal exposure was additive with earlier exposure [49].
Lastly, the number of hypothalamic GnRH neurons was not altered during normal aging up through late middle-age [85] or in the DAS [86]. This was important in early discussions of neuron loss during non-pathological brain aging as an example of a major function change without neuronal loss.
3.2. Peri-menopausal women
Peri-menopausal women also show sporadic impaired hypothalamic functions [24, 44, 76], but to a much milder degree than in aging rodents. On one hand, the negative feedback of E2 on LH and FSH has not shown impairment in postmenopausal women [87, 88]. Moreover, the pulsatile LH patterns of postmenopausal women show no change in frequency and even less variability than in younger women [89]. On the other hand, peri-menopause includes sporadic, transient impairments of the LH surge. In the SWAN Study, Weiss et al. [90] followed 159 women of average age 49 daily for ovarian and pituitary hormones, with hormones assayed in urine (estrone conjugates, E1c) and LH (Fig. 7). Group I had normal ovulatory cycles with LH surges at the peak of estrogen production. Group II showed hypothalamic-pituitary insensitivity with the absence of LH-FSH surges, despite equivalent estrogen elevations, while Group III lacked surges of estrogens and LH. Given the evidence from rodents that unopposed estrogens impair the pre-ovulatory LH surge, I suggest that the impaired peri-menopausal LH-FSH surges in Group II resulted from transient E2-desensitization by a prior sustained hyperestrogenic follicular phase within that cycle. Current data on individual cycles does not address this possibility.
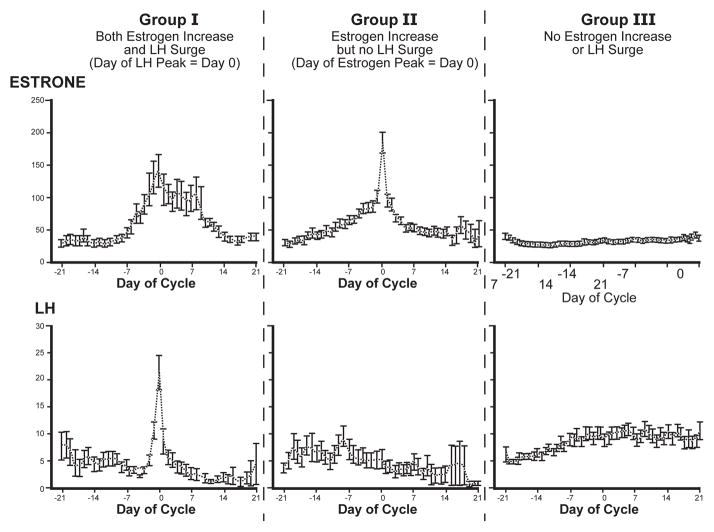
Fig. 7.
Perimenopausal cycles from women aged 48–49 years show divergent hypothalamic-pituitary functions; redrawn from Weiss et al. 2004 [90]. Three hormonal subgroups were defined from daily urinary levels of estrone conjugates (E1c/mg creatinine) and luteinizing hormone (LH/mg creatinine): Group I (18%) had mid-cycle elevations of E1c and normative LH surges at Day 0; Group II (20%) had E1c elevations at all days, but no LH surge. The authors concluded that thisshowed “unresponsiveness of the hypothalamic pituitary axis to an estrogen peak…. supporting the hypothesis that there is a relative insensitivity to estrogen… manifested by both positive and negative feedback”. The majority Group III (62%) lacked elevations of E1c or LH surges, but had higher FSH levels than the other groups (not shown).
3.3. Open questions about persisting estrogen effects
There is no evidence for enduring hypothalamic impairments in humans or primates from adult exposure to unopposed estrogens. In a unique study, young OVX monkeys given 30 month of equine estrogens (Premarin) had no hypothalamic microglial activation or evident neurotoxicity [91]. However, it is unclear if this exposure was sufficiently long, because 30 months represent about 12% of the 30 year monkey lifespan, and is less in proportion than the 8 mo of exposure that activated hypothalamic glia in rats that live 30 months. Thus, the question of persisting sex steroid effects on primate and human brain cells should remain open.
Recent MRI studies of the pubertal human brain suggest that the cerebral cortex, amygdala, and other regions are subject to sex-specific influences during adolescence. Sex differences in white and grey matter regional volumes emerge after puberty in correlation with individual blood levels of sex steroids [92, 93]. Girls showed 1–2 year earlier onset of brain morphological changes, corresponding to their earlier puberty. These emerging findings suggest that a need to revisit the classic 1959 concept of Phoenix et al. [94] of a ‘critical window’ of limited duration in early postnatal life when sex steroids ‘organize’ brain circuits [95, 96, 97].
Several important questions may be considered. Do sex differences in brain regional volume require continued exposure to plasma sex steroids during adulthood? How does the human brain respond to changing sex steroid levels during middle-age in both genders, and hormone therapy. Ongoing large trials of hormone therapy may inform about hazards and possible benefits of unopposed estrogens to normal cognitive aging, as well as to Alzheimer disease and cerebrovascular pathology.
The subcellular mechanisms of persistent estrogen perturbations (Fig. 5 and Fig. 6) could involve high affinity estrogen receptors (ERs). We recently reported that primary astrocyte cultures from the cerebral cortex have impaired neurotrophic activity if obtained from middle-aged acyclic (constant estrus) rats [99]; in contrast, cultures from regularly cycling rats of the same age (10 month) had normal neurotrophic activity. We hypothesize that astrocyte neurotrophic activity assayed in culture carried a persistent effect from the in vivo steroidal milieu of irregular cycles and constant estrus. There is a precedent for persisting effects of E2 in human hepatocarcinoma HepG2 cells which was described as “estrogen memory” [98]. Exposure of HepG2 cells to 20 nM E2 for 48 hours induced a “moderate affinity” E2-binding site that persisted for 10 cell generations, whereas 6 hours of E2 exposure had no lingering effect. This lower affinity E2 binding site is unlikely to include the high affinity estrogen receptors, ERα or ERβ. In possible parallel to HepG2 cells, brain astrocytes had elevated ERα bit not ERβ in astrocytes cultured from acyclic but not regularly cycling rats, both in primary culture and in vivo in the cerebral cortex [99]. Manipulations of ERα levels in astrocytes from different age groups showed reciprocal relationships between elevated ERα and neurotrophic activity via the upstream promoter of GFAP. We do not consider the term estrogen memory from the HepG2 study is appropriate for brain astrocytes which have specific roles in synaptic functions of learning and memory. We are studying the possibility that the unopposed estrogen milieu of constant estrus (see above) induces excess ERα expression as an outcome of inflammatory cascades that diminish neurotrophic activity during the loss of regular cycling. Both ERα or ERβ are implicated in maintaining synaptic functions during aging and in regulating synaptic plasticity. The selective increases of astrocyte ERα during aging, in contrast to decreased ERα in rat hippocampal neurons [100] give a basis for considering brain cell-type specificities of estrogenic drugs.
3.4 Adrenal androgens
Adrenal androgens transiently increase in blood during the perimenopause, among which dehydroepiandrosterone-sulfate (DHEA-S) and delta-5 androstenediol are receiving particular attention [101,102]. There are wide variations of basal plasma DHEA-S levels between and within and ethnic groups, which are considered greater than the perimenopausal variations in plasma E2. While average DHEA-S increases are about 15%, androstenediol may be elevated 5-fold in some women, yet others comprising “possibly 15%” do not incur any elevations of adrenal androgens [103]. The extent of concurrence of adrenal androgens with hyperestrogenic cycles has not been defined, and longitudinal studies are needed. The levels of estrogens and androgens may vary independently because of the distinct steroidogenic pathways of adrenal and ovary and their different hormonal inputs. Plasma levels of sex hormone binding globulin (SHBG) a hepatically secreted glycoprotein, may also be important in the menopausal transitions. The level of free androgens in plasma varies with SHBG levels, which binds both androgens and estrogens with high affinity [101,104]. Depending on the degree of SHBG binding saturation, which is generally lower in women than men, elevations of androgens or estrogens can displace the other steroid class. SHBG levels are also influenced by individual variables (e.g. obesity lowers, whereas oral contraceptives increase) [104]. The variations of adrenal androgens are being discussed in relation to the individual postmenopausal health outcomes.
Lab rodents are not good models for the manifestly complex role of adrenal androgens during aging, because of their very low plasma DHEA [105]. However, macaque monkeys which have a well-defined menopause, also show increased DHEA-S [106]. Nonetheless, rodents may still be useful experimental models for gene expression responses to adrenal androgens, which show stereoselective ligand binding to ERα or ERβ and transcriptional modulation of ER-targeted genes [107,108]. Moreover, Baker notes importantly that blood 27-hydroxycholesterol can interact with steroid binding by SHBG, despite its 1000-fold smaller Kd, because of its 1000-fold higher concentration [107].
4. Conclusions
This comparative perspective on menopause is ovarian centered. Humans and laboratory mouse and rat models undergo exponential decay in the numbers of oocytes and follicles, with total loss of fecundity during midlife. As the follicular pool wanes, cycles become longer and birth defects increase. Human and rodent stages of reproductive senescence are compared in Table 1, based on the 2012 STRAW Criteria. The peri-menopausal hyperestrogenic cycles may be modeled in constant estrus rodent. However, there is no rodent equivalent of the peri-menopausal increase of adrenal androgens. The hypothalamic mechanisms that support ovarian cycles show modest sporadic impairments in peri-menopausal women. In apparent contrast, female rodents develop major hypothalamic impairments during the peri-menopause that are linked to elevated blood levels of E2 with low P4 (“unopposed estrogens”). The impact on brain cells of unopposed estrogens is well documented in adult rodents for which there is no clear human clinical condition. Nonetheless, the human brain has evidenced persisting effects of sex steroids during puberty with gender-specificity. Ongoing clinical trials of hormone therapy may further inform about this question. We face many challenges in the expanding physiological and pharmacological complexity of menopause to optimize HT for brain, metabolic, skeletal, and vascular health.
Highlights.
The STRAW Criteria for the menopause correspond to some rodent aging changes
Ovarian cell loss begins prenatally in all mammals
Hypothalamic impairments in aging rodentsare robust, but sporadic in perimenopausal women
In aging rodents, hypothalamic impairments are associated with chronic unopposed estrogens
Acknowledgments
These studies are supported by the NIH (1-P01 AG-026572, R.D. Brinton PI; Project 2, C.E. Finch). Valuable critical comments were given by Todd Morgan (U.S.C. Davis School of Gerontology), Kathleen O’Connor (U. of Washington, Dept. Anthropology) and Frank Stanczyk (U.S.C. Keck School of Medicine). Figures were expertly redrawn by Troy Palmer-Hughes from the cited primary sources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.vom Saal FS, Finch CE, Nelson JF. The natural history of reproductive aging in humans, laboratory rodents, and selected other vertebrates. In: Knobil E, Neill JD, editors. Physiology of Reproduction. 2. Vol. 2. New York: 1994. pp. 1213–1314. [Google Scholar]
- 2.Finch CE, Holmes DJ. Ovarian aging in developmental and evolutionary contexts. Ann N Y Acad Sci. 2010;1204:82–94. doi: 10.1111/j.1749-6632.2010.05610.x. [DOI] [PubMed] [Google Scholar]
- 3.Gosden RG, Laing SC, Felicio LS, Nelson JF, Finch CE. Imminent oocyte exhaustion and reduced follicular recruitment mark the transition to acyclicity in aging C57BL/6J mice. Biol Reprod. 1983;28:255–260. doi: 10.1095/biolreprod28.2.255. [DOI] [PubMed] [Google Scholar]
- 4.Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol Reprod. 2009;80:2–12. doi: 10.1095/biolreprod.108.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virant-Klun I, Stimpfel M, Skutella T. Stem cells in adult human ovaries: from female fertility to ovarian cancer. Curr Pharm Des. 2012;18:283–92. doi: 10.2174/138161212799040394. [DOI] [PubMed] [Google Scholar]
- 6.Brook JD, Gosden RG, Chandley AC. Maternal ageing and aneuploidy embryos--evidence from the mouse that biological and not chronological age is the important influence. Hum Genet. 1984;66:41–5. doi: 10.1007/BF00275184. [DOI] [PubMed] [Google Scholar]
- 7.Nelson JF, Felicio LS. Radical ovarian resection advances the onset of persistent vaginal cornification, but only transiently disrupts hypothalamic-pituitary regulation of cyclicity in C57BL/6J mice. Biol Reprod. 1986;35:957–964. doi: 10.1095/biolreprod35.4.957. [DOI] [PubMed] [Google Scholar]
- 8.Finch CE, Kirkwood TLB. Chance, Development, and Aging. Oxford; New York: 2000. [Google Scholar]
- 9.Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5:e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res. 1951;6:63–108. [Google Scholar]
- 11.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol B. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 12.Finch CE. Update on slow aging and negligible senescence—a mini-review. Gerontology. 2009;55:307–313. doi: 10.1159/000215589. [DOI] [PubMed] [Google Scholar]
- 13.de Bruin JP, Gosden RG, Finch CE, Leaman BM. Ovarian aging in two species of long-lived rockfish, Sebastes aleutianus and S. alutus. Biol Reprod. 2004;71:1036–1042. doi: 10.1095/biolreprod.103.019919. [DOI] [PubMed] [Google Scholar]
- 14.Videan EN, Fritz J, Heward CB, Murphy J. The effects of aging on hormone and reproductive cycles in female chimpanzees (Pan troglodytes) Comp Med. 2006;56:291–299. [PubMed] [Google Scholar]
- 15.Hawkes K, Kim PS, Coxworth JE, Hawkes K. Increased longevity evolves from grandmothering. Proc Biol Sci. 2012;279:4880–4884. doi: 10.1098/rspb.2012.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarkson TB, Meléndez GC, App SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause. 2013;20:342–353. doi: 10.1097/GME.0b013e3182843aad. [DOI] [PubMed] [Google Scholar]
- 17.Kachel AF, Premo LS, Hublin JJ. Grandmothering and natural selection. Proc Biol Sci. 2011;278:384–391. doi: 10.1098/rspb.2010.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krysko DV, Diez-Fraile A, Criel G, Svistunov AA, Vandenabeele P, D’Herde K. Life and death of female gametes during oogenesis and folliculogenesis. Apoptosis. 2008;13:1065–1087. doi: 10.1007/s10495-008-0238-1. [DOI] [PubMed] [Google Scholar]
- 19.Krakauer DC, Mira A. Mitochondria and germ-cell death. Nature. 1999;400:125–126. doi: 10.1038/22026. [DOI] [PubMed] [Google Scholar]
- 20.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 21.Faddy MJ, Gosden RG. A model conforming the decline in follicle numbers to the age of menopause in women. Hum Reprod. 1996;11:1484–1486. doi: 10.1093/oxfordjournals.humrep.a019422. [DOI] [PubMed] [Google Scholar]
- 22.Faddy MJ. Follicle dynamics during ovarian ageing. Mol Cell Endocrinol. 2000;163:43–48. doi: 10.1016/s0303-7207(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 23.Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5:e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause. 2008;15:603–612. doi: 10.1097/gme.0b013e318174ea4d. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor KA, Ferrell R, Brindle E, Trumble B, Shofer J, Holman DJ, Weinstein M. Progesterone and ovulation across stages of the transition to menopause. Menopause. 2009;16:1178–1187. doi: 10.1097/gme.0b013e3181aa192d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol. 2008;316:397–407. doi: 10.1016/j.ydbio.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingram DL. The effect of hypophysectomy on the number of oocytes in the adult albino rat. J Endocrinol. 1953;9:307–311. doi: 10.1677/joe.0.0090307. [DOI] [PubMed] [Google Scholar]
- 28.Jones EC, Krohn PL. The effect of hypophysectomy on age changes in the ovaries of mice. J Endocrinol. 1961;21:497–509. doi: 10.1677/joe.0.0210497. [DOI] [PubMed] [Google Scholar]
- 29.Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging. 1995;16:837–843. doi: 10.1016/0197-4580(95)00072-m. [DOI] [PubMed] [Google Scholar]
- 30.Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci U S A. 2011;108:12319–12324. doi: 10.1073/pnas.1018793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7:622–629. doi: 10.1111/j.1474-9726.2008.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun L, Tan L, Yang F, Luo Y, Li X, Deng HW, Dvornyk V. Meta-analysis suggests that smoking is associated with an increased risk of early natural menopause. Menopause. 2012;19:126–132. doi: 10.1097/gme.0b013e318224f9ac. [DOI] [PubMed] [Google Scholar]
- 33.Mikkelsen TF, Graff-Iversen S, Sundby J, Bjertness E. Early menopause, association with tobacco smoking, coffee consumption and other lifestyle factors: a cross-sectional study. BMC Public Health. 2007;7:149. doi: 10.1186/1471-2458-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hornak M, Jeseta M, Musilova P, Pavlok A, Kubelka M, Motlik J, Rubes J, Anger M. Frequency of aneuploidy related to age in porcine oocytes. PLoS One. 2011;6:e18892. doi: 10.1371/journal.pone.0018892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hook EB. Rates of chromosome abnormalities at different maternal ages. Obstet Gynecol. 1981;58:282–285. [PubMed] [Google Scholar]
- 36.Munné S, Held KR, Magli CM, Ata B, Wells D, Fragouli E, Baukloh V, Fischer R, Gianaroli L. Intra-age, intercenter, and intercycle differences in chromosome abnormalities in oocytes. Fertil Steril. 2012;97:935–942. doi: 10.1016/j.fertnstert.2012.01.106. [DOI] [PubMed] [Google Scholar]
- 37.Abir R, Fisch B, Nahum R, Orvieto R, Nitke S, Ben Rafael Z. Turner’s syndrome and fertility: current status and possible putative prospects. Hum Reprod Update. 2001;7:603–610. doi: 10.1093/humupd/7.6.603. [DOI] [PubMed] [Google Scholar]
- 38.Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online. 2004;8:45–58. doi: 10.1016/s1472-6483(10)60497-x. [DOI] [PubMed] [Google Scholar]
- 39.Eichenlaub-Ritter U, Staubach N, Trapphoff T. Chromosomal and cytoplasmic context determines predisposition to maternal age-related aneuploidy: brief overview and update on MCAK in mammalian oocytes. Biochem Soc Trans. 2010;38:1681–1686. doi: 10.1042/BST0381681. [DOI] [PubMed] [Google Scholar]
- 40.van den Berg IM, Eleveld C, van der Hoeven M, Birnie E, Steegers EA, Galjaard RJ, Laven JS, van Doorninck JH. Defective deacetylation of histone 4 K12 in human oocytes is associated with advanced maternal age and chromosome misalignment. Hum Reprod. 2011;26:1181–1190. doi: 10.1093/humrep/der030. [DOI] [PubMed] [Google Scholar]
- 41.Andersen CG, Bogstad J, Nielsen FC, Meinertz H, Borup R. Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod. 2010;25:957–968. doi: 10.1093/humrep/deq014. [DOI] [PubMed] [Google Scholar]
- 42.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ. STRAW + 10 Collaborative Group, Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 12(1967):77–126. [PubMed] [Google Scholar]; Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause. 2008;15:603–612. doi: 10.1097/gme.0b013e318174ea4d. [DOI] [PubMed] [Google Scholar]
- 44.Butler L, Santoro N. The reproductive endocrinology of the menopausal transition. Steroids. 2011;76:627–635. doi: 10.1016/j.steroids.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prior JC. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine. 2005;26:297–300. doi: 10.1385/ENDO:26:3:297. [DOI] [PubMed] [Google Scholar]
- 46.Skurnick JH, Weiss G, Goldsmith LT, Santoro N, Crawford S. Longitudinal changes in hypothalamic and ovarian function in perimenopausal women with anovulatory cycles: relationship with vasomotor symptoms. Fertil Steril. 2009;91:1127–1134. doi: 10.1016/j.fertnstert.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 47.Metcalf MG, Donald RA, Livesey JH. Classification of menstrual cycles in pre-and perimenopausal women. J Endocrinol. 1981;91:1–10. doi: 10.1677/joe.0.0910001. [DOI] [PubMed] [Google Scholar]
- 48.Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman’s reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab. 1998;83:1875–1880. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- 49.Finch CE, Felicio LS, Mobbs CV, Nelson JF. Ovarian and steroidal influences on neuroendocrine aging processes in female rodents. Endocr Rev. 1984;5:467–497. doi: 10.1210/edrv-5-4-467. [DOI] [PubMed] [Google Scholar]
- 50.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod. 1981;24:784–794. doi: 10.1095/biolreprod24.4.784. [DOI] [PubMed] [Google Scholar]
- 51.Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- 52.Nelson JF, Felicio LS. Reproductive aging in the female: an etiological perspective. Rev Biol Res Aging. 1985;2:251–314. [Google Scholar]
- 53.Felicio LS, Nelson JF, Finch CE. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod. 1984;31:446–453. doi: 10.1095/biolreprod31.3.446. [DOI] [PubMed] [Google Scholar]
- 54.Felicio LS, Nelson JF, Finch CE. Prolongation and cessation of estrous cycles in aging C57BL/6J mice are differentially regulated events. Biol Reprod. 1986;34:849–858. doi: 10.1095/biolreprod34.5.849. [DOI] [PubMed] [Google Scholar]
- 55.Wise PM, Smith MJ, Dubal DB, Wilson ME, Krajnak KM, Rosewell KL. Neuroendocrine influences and repercussions of the menopause. Endocr Rev. 1999;20:243–248. doi: 10.1210/edrv.20.3.0364. [DOI] [PubMed] [Google Scholar]
- 56.Brinton RD. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology. 2012;153:3571–3578. doi: 10.1210/en.2012-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gee DM, Flurkey K, Finch CE. Aging and the regulation of luteinizing hormone in C57BL/6J mice: impaired elevations after ovariectomy and spontaneous elevations at advanced ages. Biol Reprod. 1983;28:598–607. doi: 10.1095/biolreprod28.3.598. [DOI] [PubMed] [Google Scholar]
- 58.Huang HH, Marshall S, Meites J. Capacity of old versus young female rats to secrete LH, FSH and prolactin. Biol Reprod. 1976;14:538–543. doi: 10.1095/biolreprod14.5.538. [DOI] [PubMed] [Google Scholar]
- 59.Fayein NA, Aschheim P. Age-related temporal changes of levels of circulating progesterone in repeatedly pseudopregnant rats. Biol Reprod. 1980;23:616–620. doi: 10.1095/biolreprod23.3.616. [DOI] [PubMed] [Google Scholar]
- 60.Lu JK, Damassa DA, Gilman DP, Judd HL, Sawyer CH. Differential patterns of gonadotropin responses to ovarian steroids and to LH-releasing hormone between constant-estrous and pseudopregnant states in aging rats. Biol Reprod. 1980;23:345–351. doi: 10.1095/biolreprod23.2.345. [DOI] [PubMed] [Google Scholar]
- 61.Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev. 1998;19:397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- 62.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 63.Miro F, Parker SW, Aspinall LJ, Coley J, Perry PW, Ellis JE. FREEDOM study, Origins and consequences of the elongation of the human menstrual cycle during the menopausal transition: the FREEDOM Study. J Clin Endocrinol Metab. 2004;89:4910–4915. doi: 10.1210/jc.2003-031731. [DOI] [PubMed] [Google Scholar]
- 64.Hale GE, Hughes CL, Cline JM. Endometrial cancer: hormonal factors, the perimenopausal “window of risk,” and isoflavones. J Clin Endocrinol Metab. 2002;87:3–15. doi: 10.1210/jcem.87.1.8132. [DOI] [PubMed] [Google Scholar]
- 65.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92:3060–3067. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 66.Metcalf MG, Mackenzie JA. Menstrual cycle and exposure to oestrogens unopposed by progesterone: relevance to studies on breast cancer incidence. J Endocrinol. 1985;104:137–141. doi: 10.1677/joe.0.1040137. [DOI] [PubMed] [Google Scholar]
- 67.Hale GE, Hughes CL, Burger HG, Robertson DM, Fraser IS. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause. 2009;16:50–59. doi: 10.1097/GME.0b013e31817ee0c2. [DOI] [PubMed] [Google Scholar]
- 68.Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update. 2012;18:73–91. doi: 10.1093/humupd/dmr039. [DOI] [PubMed] [Google Scholar]
- 69.Pike MC. Age-related factors in cancers of the breast, ovary, and endometrium. J Chronic Dis. 1987;40(Suppl):59S–69S. doi: 10.1016/s0021-9681(87)80009-7. [DOI] [PubMed] [Google Scholar]
- 70.Parkening TA, Collins TJ, Smith ER. Plasma and pituitary concentrations of LH,FSH and prolactin in aged female C57BL/6 mice. J Reprod Fertil. 1980;58:377–386. doi: 10.1530/jrf.0.0580377. [DOI] [PubMed] [Google Scholar]
- 71.Schechter JE, Felicio LS, Nelson JF, Finch CE. Pituitary tumorigenesis in aging female C57BL/6J mice: a light and electron microscopic study. Anat Rec. 1981;199:423–432. doi: 10.1002/ar.1091990310. [DOI] [PubMed] [Google Scholar]
- 72.Clayton CJ, Schechter J, Finch CE. The development of mammotroph adenomas in pituitaries of aging female C57BL/6J mice. Exp Gerontol. 1984;19:313–320. doi: 10.1016/0531-5565(84)90004-4. [DOI] [PubMed] [Google Scholar]
- 73.Gordon MN, Schechter JE, Felicio LS, Finch CE. Spontaneous tumors in aging female mice are more prevalent in the lateral pituitary zones. Neurobiol Aging. 1987;8:67–70. doi: 10.1016/0197-4580(87)90060-1. [DOI] [PubMed] [Google Scholar]
- 74.Bartke A, Morgan RW, Clayton RN, Banerji TK, Brodie AM, Parkening TA, Collins TJ. Neuroendocrine studies in hyperprolactinaemic male mice. J Endocrinol. 1987;112:215–220. doi: 10.1677/joe.0.1120215. [DOI] [PubMed] [Google Scholar]
- 75.Lu JK, Anzalone CR, LaPolt PS. Relation of neuroendocrine function to reproductive decline during aging in the female rat. Neurobiol Aging. 1994;15:541–544. doi: 10.1016/0197-4580(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 76.Kermath BA, Gore AC. Neuroendocrine control of the transition to reproductive senescence: lessons learned from the female rodent model. Neuroendocrinology. 2012;96:1–12. doi: 10.1159/000335994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gee DM, Flurkey K, Mobbs CV, Sinha YN, Finch CE. The regulation of luteinizing hormone and prolactin in C57BL/6J mice: effects of estradiol implant size, duration of ovariectomy, and aging. Endocrinology. 1984;114:685–693. doi: 10.1210/endo-114-3-685. [DOI] [PubMed] [Google Scholar]
- 78.Anderson CP, Rozovsky I, Stone DJ, Song Y, Lopez LM, Finch CE. Aging and increased hypothalamic glial fibrillary acid protein (GFAP) mRNA in F344 female rats. Dissociation of GFAP inducibility from the luteinizing hormone surge. Neuroendocrinology. 2002;76:121–130. doi: 10.1159/000064429. [DOI] [PubMed] [Google Scholar]
- 79.Mobbs CV, Cheyney D, Sinha YN, Finch CE. Age-correlated and ovary-dependent changes in relationships between plasma estradiol and luteinizing hormone, prolactin, and growth hormone in female C57BL/6J mice. Endocrinology. 1985;116:813–820. doi: 10.1210/endo-116-2-813. [DOI] [PubMed] [Google Scholar]
- 80.Kohama SG, Anderson CP, Osterburg HH, May PC, Finch CE. Oral administration of estradiol to young C57BL/6J mice induces age-like neuroendocrine dysfunctions in the regulation of estrous cycles. Biol Reprod. 1989;41:227–232. doi: 10.1095/biolreprod41.2.227. [DOI] [PubMed] [Google Scholar]
- 81.Kohama SG, Anderson CP, Finch CE. Progesterone implants extend the capacity for 4-day estrous cycles in aging C57BL/6J mice and protect against acyclicity induced by estradiol. Biol Reprod. 1989;41:233–244. doi: 10.1095/biolreprod41.2.233. [DOI] [PubMed] [Google Scholar]
- 82.Mobbs CV, Finch CE. Estrogen-induced impairments as a mechanism in reproductive senescence of female C57BL/6J mice. J Gerontol. 1992;47:B48–51. doi: 10.1093/geronj/47.2.b48. [DOI] [PubMed] [Google Scholar]
- 83.Harlan RE, Gorski RA. Correlations between ovarian sensitivity, vaginal cyclicity and luteinizing hormone and prolactin secretion in lightly androgenized rats. Endocrinology. 1977;101:750–759. doi: 10.1210/endo-101-3-750. [DOI] [PubMed] [Google Scholar]
- 84.Mobbs CV, Kannegieter LS, Finch CE. Delayed anovulatory syndrome induced by estradiol in female C57BL/6J mice: age-like neuroendocrine, but not ovarian impairments. Biol Reprod. 1985;32:1010–1017. doi: 10.1095/biolreprod32.5.1010. [DOI] [PubMed] [Google Scholar]
- 85.Hoffman GE, Finch CE. LHRH neurons in the female C57BL/6J mouse brain during reproductive aging: no loss up to middle age. Neurobiol Aging. 1986;7:45–48. doi: 10.1016/0197-4580(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 86.Kohama SG, Brown SA, Finch CE, McNeill TH. Chronic estradiol administration did not cause loss of hypothalamic LHRH or TIDA neurons in young or middle-aged C57BL/6J mice. Brain Res. 1992;574:341–344. doi: 10.1016/0006-8993(92)90838-z. [DOI] [PubMed] [Google Scholar]
- 87.Gill S, Lavoie HB, Bo-Abbas Y, Hall JE. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab. 2002;87:2297–2302. doi: 10.1210/jcem.87.5.8510. [DOI] [PubMed] [Google Scholar]
- 88.Santoro N, Banwell T, Tortoriello D, Lieman H, Adel T, Skurnick J. Effects of aging and gonadal failure on the hypothalamic-pituitary axis in women. Am J Obstet Gynecol. 1998;178:732–741. doi: 10.1016/s0002-9378(98)70483-1. [DOI] [PubMed] [Google Scholar]
- 89.Keenan DM, Evans WS, Veldhuis JD. Control of LH secretory-burst frequency and interpulse-interval regularity in women. Am J Physiol Endocrinol Metab. 2003;285:E938–948. doi: 10.1152/ajpendo.00133.2003. [DOI] [PubMed] [Google Scholar]
- 90.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 91.Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84:2111–2118. doi: 10.1210/jcem.84.6.5689. [DOI] [PubMed] [Google Scholar]
- 92.Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, Dinov ID, Worthman CM, Sowell ER. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS One. 2012;7:e33850. doi: 10.1371/journal.pone.0033850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 94.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 95.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCarthy MM. How it’s made: organizational effects of hormones on the developing brain. J Neuroendocrinol. 2010;22:736–742. doi: 10.1111/j.1365-2826.2010.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McEwen BS. Steroid hormones: effect on brain development and function. Horm Res. 1992;37(Suppl):1–10. doi: 10.1159/000182393. [DOI] [PubMed] [Google Scholar]
- 98.Tam SP, Haché RJ, Deeley RG. Estrogen memory effect in human hepatocytes during repeated cell division without hormone. Science. 1986;234:1234–1237. doi: 10.1126/science.3022381. [DOI] [PubMed] [Google Scholar]
- 99.Arimoto J, Wong AM, Rozovsky I, Lin SW, Morgan TE, Finch CE. Age increase in astrocyte estrogen receptor alpha (ERα) in cortical astrocytes impairs neurotrophic support in male and female rats. Endocrinology. 2013 doi: 10.1210/en.2012-2046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lasley BL, Chen J, Stanczyk FZ, El Khoudary SR, Gee NA, Crawford S, McConnell DS. Androstenediol complements estrogenic bioactivity during the menopausal transition. Menopause. 2012;19:650–657. doi: 10.1097/gme.0b013e31823df577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lasley BL, Crawford S, McConnell DS. Adrenal androgens and the menopausal transition. Obstet Gynecol Clin North Am. 2011;38:467–475. doi: 10.1016/j.ogc.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod. 2011;85:431–441. doi: 10.1095/biolreprod.111.092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meffre D, Pianos A, Liere P, Eychenne B, Cambourg A, Schumacher M, Stein DG, Guennoun R. Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: analysis by gas chromatography/mass spectrometry. Endocrinology. 2007;148:2505–2517. doi: 10.1210/en.2006-1678. [DOI] [PubMed] [Google Scholar]
- 106.Moran FM, Chen J, Gee NA, Lohstroh PN, Lasley BL. Dehydroepiandrosterone sulfate levels reflect endogenous luteinizing hormone production and response to human chorionic gonadotropin challenge in older female macaque (Macaca fascicularis) Menopause. 2012 doi: 10.1097/GME.0b013e3182698f80. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baker ME. What are the physiological estrogens? Steroids. 2013;78:337–340. doi: 10.1016/j.steroids.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 108.Miller KK, Al-Rayyan N, Ivanova MM, Mattingly KA, Ripp SL, Klinge CM, Prough RA. DHEA metabolites activate estrogen receptors alpha and beta. Steroids. 2013;78:15–25. doi: 10.1016/j.steroids.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jones EC, Krohn PL. The relationships between age, numbers of oocytes and fertility in virgin and multiparous mice. J Endocrinol. 1961;21:469–495. doi: 10.1677/joe.0.0210469. [DOI] [PubMed] [Google Scholar]
- 110.Block E. Quantitative morphological examinations of the follicular system in women. Acta Anatomica. 1952;14:108–123. doi: 10.1159/000140595. [DOI] [PubMed] [Google Scholar]