Abstract
Background
Genetic deletion or antagonism of the neurokinin 1 receptor (NK1R) decreases alcohol intake, alcohol reward, and stress-induced alcohol relapse in rodents, while TACR1 variation is associated with alcoholism in humans.
Methods
We used L822429, a specific antagonist with high affinity for the rat NK1R, and examined whether sensitivity to NK1R blockade is altered in alcohol-preferring (P) rats. Operant alcohol self-administration and progressive ratio responding were analyzed in P-rats and their founder Wistar line. We also analyzed Tacr1 expression and binding and sequenced the Tacr1 promoter from both lines.
Results
Systemic L822429 decreased alcohol self-administration in P-rats but did not affect the lower rates of alcohol self-administration in Wistar rats. Tacr1 expression was elevated in the prefrontal cortex and the amygdala of P-rats. In central amygdala, elevated Tacr1 expression was accompanied by elevated NK1R binding. Central amygdala (but not prefrontal cortex) infusion of L822429 replicated the systemic antagonist effects on alcohol self-administration in P-rats. All P-rats, but only 18% of their founder Wistar population, were CC homozygous for a −1372G/C single nucleotide polymorphism. In silico analysis indicated that the Tacr1 −1372 genotype could modulate binding of the transcription factors GATA-2 and E2F-1. Electromobility shift and luciferase reporter assays suggested that the −1372C allele confers increased transcription factor binding and transcription.
Conclusions
Genetic variation at the Tacr1 locus may contribute to elevated rates of alcohol self-administration, while at the same time increasing sensitivity to NK1R antagonist treatment.
Keywords: Alcohol, amygdala, neurokinin, P-rat, self-administration, Substance P
The neurokinin 1 receptor (NK1R) and its cognate ligand substance P (SP) regulate stress- and anxiety-related behaviors (1,2) and play a role in opioid reward (3–6). Recent results suggest that the SP/NK1R system also regulates alcohol consumption. Neurokinin 1 receptor −/− mice do not exhibit conditioned place preference (a measure of drug reward) for alcohol and consume less alcohol than wild-type littermates (7,8). Accordingly, NK1R antagonists suppress voluntary alcohol consumption in mice and prevent stress-induced reinstatement of alcohol seeking in rats (8,9). These preclinical findings are paralleled by observations that NK1R antagonism decreases alcohol craving in alcohol-dependent patients and suppresses anterior insula activity in response to aversive visual stimuli in these subjects (7). Although these surrogate marker results suggest a potential for NK1R antagonism as a pharmacotherapy for alcoholism, it remains unknown whether the observed decrease in craving will translate to decreased alcohol consumption. It is further unclear whether particular patient characteristics might be predictive of a response to this therapeutic mechanism.
Recently, an association was reported between the human TACR1 locus that encodes the NK1R and a diagnosis of alcohol dependence (10). This finding, combined with preclinical data on modulation of alcohol consumption by NK1R inactivation or antagonism, suggests the possibility that functional TACR1 variation is a predisposing factor for excessive alcohol consumption, moderates sensitivity to NK1R antagonist treatment, or both. An implication of this hypothesis is that NK1R antagonists may have particular potential as clinical alcoholism treatments in genetically defined patient populations. Evaluation of this hypothesis may be aided by animal models that use genetic selection for high alcohol preference to enrich alleles contributing to excessive alcohol consumption (11–13). Perhaps the most widely used model in this category is the Indiana alcohol-preferring (P) rat, a line that has been selected over >100 generations for high alcohol preference from a founder Wistar population (11). A quantitative trait locus analysis of the preference phenotype in P-rats has been shown to show high linkage (logarithm of odds scores >9) to a region of chromosome 4 that harbors the Tacr1 gene (14). In the present study, we used the P-rat line, and applied an integrative behavioral, pharmacologic, and molecular approach to examine the role of the NK1R in escalated alcohol self-administration and response to NK1R antagonism.
Because neurokinin 1 receptors display considerable divergence between species (15), we used L822429, a highly selective brain penetrant NK1R antagonist with high affinity for the rat NK1R (1,16,17). In nondependent Wistar rats that had not been selected for high alcohol preference, we recently found that L822429 suppressed stress-induced reinstatement to alcohol seeking in a rat relapse model (18,19) but did not affect baseline rates of alcohol self-administration (9). Accordingly, another NK1R antagonist, ezlopitant, did not suppress alcohol self-administration in nonselected rats until doses were reached that also suppressed sucrose self-administration, indicating a nonspecific effect (20). Here, we examined the hypothesis that NK1R antagonism, while ineffective in suppressing baseline alcohol self-administration, would attenuate elevated alcohol self-administration in genetically predisposed rats. We used L822429 and P-rats to examine this hypothesis and to determine if the elevated rates of alcohol self-administration found in this line are, in part, due to genetic variation in the SP/NK1R system.
Methods and Materials
Details are provided in Supplement 1.
Drugs
L822429 was synthesized by Drs. Cheng and Rice. Systemic injections were in 2 mL/kg intraperitoneal. For intracranial injection, L822429 concentration was 15 μg/μL and injection volume .5 μL/side.
Subjects
Procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care & Use Committee. Male Wistar and P-rats were housed on a reversed light cycle and tested during the dark phase. Food and water were available ad libitum, except when stated.
Alcohol Self-Administration
Training
Alcohol (10% v/v) self-administration was as described (9,21), on fixed ratio 1 reinforcement for 14 days and fixed ratio 3 (FR3) (every third response reinforced) thereafter. Experiments were initiated after 14 days of FR3 self-administration, when response rates reached stability.
Systemic L822429 and Alcohol Self-Administration
Rats (n = 15–16/group) were injected with vehicle or L822429 (15 or 30 mg/kg), 60 minutes before 30-minute sessions. A counterbalanced within-subject design was used and self-administration was re-established without drug administration for at least three sessions between treatment days. Two-way analysis of variance (ANOVA) was used, with rat line (Wistar, P) as between-subjects and dose as within-subjects factors, followed by post hoc Newman-Keuls tests.
Progressive Ratio
Rats (n = 14–16/group) were injected with vehicle or 15 mg/kg L822429 60 minutes before self-administration sessions in which the response requirement to obtain an alcohol dose was progressively increased. A counterbalanced within-subjects design was used, with ≥3 days to reestablish FR3 self-administration without drug injections between testing. Mixed two-way ANOVA was carried out with rat line as between-subjects and treatment as within-subjects factors followed by post hoc Newman-Keuls tests.
Systemic L822429 and Locomotor Activity
Rats (n = 5–6/group) were injected with vehicle or L822429 (15 or 30 mg/kg) 60 minutes before 60-minute locomotor activity sessions, as described (9). A between-subjects design was used. Two-way ANOVA was carried out for total session activity with dose and rat line as factors, followed by post hoc Newman-Keuls tests.
Saccharin Self-Administration
Alcohol-preferring rats (n = 8) were trained to lever press for saccharin (.05% w/v). After 5 days of fixed ratio 1 responding, the response requirement was FR3 for 10 days, after which responding was stable. A counterbalanced within-subjects design was used, with ≥3 days to re-establish FR3 self-administration without drug injections between testing. Treatment conditions were compared using paired t tests.
Quantitative Real-Time Polymerase Chain Reaction
Rats (n = 8/group) were decapitated and tissue obtained from 2 mm sections. Total RNA was extracted, purified, and reverse transcribed. Reactions were in triplicate. FAM-labeled probes and forward and reverse primers for Tac1, Tacr1, and Gapdh were used (Applied Biosystems, Foster City, California). Reactions were normalized to Gapdh and compared using the 2ΔΔCt method (22).
Tacr1 Promoter Sequence analysis
Genomic DNA was isolated from 22 Wistar and 20 P-rats. Approximately 2 kilobase upstream of the Tacr1 transcriptional start site (TSS) was amplified, and the 2050 base pair polymerase chain reaction fragment was sequenced. To confirm genotypes and obtain reliable allele counts of Tacr1 −1372C/G variation, samples were genotyped using a Custom TaqMan SNP Genotyping Assay (Applied Biosystems). Allele frequencies were compared using Yates corrected χ2 test.
Electrophoretic Mobility Shift Assay
Nuclear extracts from amygdala or striatum were incubated with double-stranded oligonucleotides corresponding to the respective sequence labeled with [33P]-adenosine 5′-triphosphate. Competitor oligonucleotides were added, and samples were separated by electrophoresis. Bands were visualized on a PhosphorImager (Fujifilm, Tokyo, Japan). Each gel shift assay was performed at least three times.
Luciferase Assay
Neuro 2A cells were transfected with a custom GLuc-ON reporter construct (GeneCopoeia, Rockville, Maryland) containing the Tacr1 promoter with the respective sequence. As a negative control, a subset of cells was transfected with a GLuc-ON reporter construct (GeneCopoeia) without the Tacr1 promoter. Luminescence was measured, normalized, and compared using two-tailed Student t test.
Receptor Autoradiography
Rats (n =7–8/group) were decapitated, brains were removed and frozen, and 20 μm coronal cryosections were mounted on slides. Autoradiography was as described (23).
Intracranial L822429 and Alcohol Self-Administration
Alcohol-preferring rats (n = 24) were trained to self-administer 10% (vol/vol) alcohol on an FR3 schedule as above and implanted with 26-gauge guide cannulas directed at the prefrontal cortex (PFC) (n = 11) or central nucleus of the amygdala (CeA) (n = 13). Groups were matched for baseline responses. A separate group of Wistar rats was trained to self-administer alcohol and implanted with cannulas directed at the CeA (n = 11). Following recovery, self-administration was resumed for 10 days until stable response levels were reached.
Rats received intracranial infusions (vehicle or L822429, 7.5 μg in .5 μL/side) 5 to 10 minutes before self-administration sessions. A counterbalanced within-subject design was used, and self-administration was re-established without drug administration between treatment days. After behavioral experiments were completed, cannula placement was determined. Self-administration rates following infusion into the PFC (n = 8) or CeA (n = 12 for P-rat, n = 8 for Wistar) were compared between vehicle or L822429 using paired t tests.
Intracranial L822429 and Locomotor Activity
Alcohol-preferring rats received infusions (vehicle or L822429, 7.5 μg/side) into CeA (n = 6/group) or PFC (n = 3-5/group) before locomotor activity testing (n reflects the removal of misplaced cannulae, as indicated above). Rats were injected in a counterbalanced between-subjects design with vehicle or L822429 5 to 10 minutes before the start of 60-minute locomotor activity sessions. Total distance was compared between treatments within each brain region using unpaired t test.
Results
Systemic NK1R Antagonism Selectively Suppresses Alcohol Self-Administration in P-Rats
Two-way ANOVA of alcohol deliveries obtained during 30-minute FR3 self-administration sessions showed a main effect of L822429 dose (F2,58 = 13.90, p < .0001) and a dose × line (P line, Wistar line) interaction (F2,58 = 3.55, p = .035; Figure 1A). Following vehicle injection, P-rats self-administered approximately twice as much alcohol as Wistar control rats (47.8 ± 7.9 vs. 27.2 ± 5.0 alcohol deliveries per 30 minutes, mean ± SEM; Newman-Keul post hoc test: p = .021). A moderate L822429 dose (15 mg/kg) suppressed alcohol self-administration in P-rats compared with vehicle treatment in this line (p = .041). At this dose, L822429 decreased P-rat self-administration rates to a level that did not differ from that of vehicle-treated Wistar rats (p = .81). At 30 mg/kg, an even greater suppression was observed (p < .001), but this dose also decreased locomotor activity in P-rats (see below).
Figure 1.
L822429 selectively decreases alcohol self-administration and reward in alcohol-preferring (P) rats. (A) L822429 selectively suppressed the elevated rates of alcohol self-administration in P-rats but did not affect self-administration in Wistar control rats. Data are shown as alcohol deliveries obtained (mean ± SEM) in the course of 30-minute sessions on fixed ratio 3 (three lever presses required to obtain an alcohol delivery). ***p < .001, *p < .05 versus P-rat group treated with vehicle, respectively. #p < .05 P-rats versus Wistar under vehicle treatment of both groups. (B) To assess motivation to obtain alcohol, progressive ratio sessions were carried out. Alcohol-preferring rats showed higher motivation to obtain alcohol, measured both as total number of responses emitted (left group of bars) and as break points, i.e., the highest ratio completed (right group of bars). **p < .01; *p < .05 versus Wistar. A dose of L822429 (15 mg/kg) that lacked sedative effects in other experiments selectively suppressed progressive ratio responding in P-rats, measured as the total number of responses emitted (left group of bars); +p < .05 versus P-rats receiving vehicle, with a similar trend for the break points that did not reach significance (right group of bars). For detailed statistics, see Methods and Materials and Results.
The ratio of active lever responses to alcohol deliveries obtained was approximately 3:1, as expected, and the effects of L822429 on active lever responses followed the same pattern as the effects on alcohol deliveries. Inactive lever responses were generally lower in P-rats relative to Wistars, as evidenced by a main effect of line (F1,58 = 9.69, p = .016; Figure S1 in Supplement 1). However, inactive lever response rates were unaffected by drug treatment (main effect: F2,58 = .91, p = .41), and there was not a line × drug interaction (F2,58 = 1.10, p = .34). Because there was a significant weight difference between age-matched P-rats and Wistar rats, the amount of alcohol consumed adjusted for body weight (g/kg) was also analyzed but yielded virtually identical results. Analysis of g/kg alcohol consumption during self-administration sessions revealed a main effect of dose (F2,58 = 13.80, p < .0001) and a line × dose interaction (F2,58 = 4.22, p = .019; Figure S2 in Supplement 1). The main effect of line reached a trend level of significance (F1,29 = 3.99, p = .055). Following vehicle treatment, P-rats consumed twice as much alcohol as Wistar control rats (.79 versus .39 g/kg, post hoc p value = .0043). L822429 treatment decreased alcohol consumption in P-rats only, at doses of 15 mg/kg (p = .024) and 30 mg/kg (p < .001). Neurokinin 1 receptor antagonism did not affect self-administration in Wistar rats at any dose tested, in agreement with our previous findings (9).
Systemic NK1R Antagonism Selectively Suppresses Motivation to Obtain Alcohol in P-Rats
Motivation to obtain alcohol was assessed using a progressive ratio schedule, in which increasing response demands are imposed with successive deliveries of the reinforcer, and the number of responses that an animal makes to obtain a reward is used as a measure of reward strength (24).
L822429 decreased progressive ratio responding in P-rats but not Wistar rats (Figure 1B). Two-way ANOVA on the number of active lever presses (left group of bars in figure) showed a main effect of line (F1,28 = 6.26, p = .018) and a dose × line interaction (F1,28 = 4.48, p = .043). Post hoc tests indicated a significant difference between P-rats treated with vehicle and all other groups (p < .05 for all comparisons). Analysis of break point values (the final ratio completed, right group of bars) showed a similar pattern, although some of the post hoc comparisons were only trend-level significant. These data indicate that the motivation to obtain alcohol is greater in P-rats compared with Wistar rats and that this heightened motivation is normalized by L822429 treatment, which was ineffective in Wistar rats.
Suppression of Alcohol Self-Administration in P-Rats by L822429 at 15 mg/kg Is Not Caused by Sedation
As a measure of potential sedative effects, locomotor activity was assessed following injections of vehicle or L822429 (15 or 30 mg/kg intraperitoneal; Table 1). Two-way ANOVA of total distance traveled during the 60-minute sessions showed a main effect of line (F1,25 = 10.65, p = .003) and dose (F2,25 = 8.08, p = .002), as well as a dose × line interaction (F2,25 = 4.30, p = .025). Post hoc tests indicated that the P-rat group injected with 30 mg/kg L822429 was significantly different from all other groups (p < .01 for all comparisons), while no other pairwise differences were present (all p values > .35). In particular, L822429 did not decrease locomotor activity in Wistar rats at any dose, as previously reported by our group (9), and did not influence locomotor activity in P-rats at 15 mg/kg.
Table 1. Locomotor Activity of P-Rats and Wistar Rats Following Systemic L822429.
| L822429 | |||
|---|---|---|---|
|
|
|||
| Line | Vehicle | 15 mg/kg | 30 mg/kg |
| Wistar | 2388 ± 307.3 | 2413 ± 389.1 | 2155 ± 195.0 |
| P-Rat | 2371 ± 122.2 | 1907 ± 159.7 | 745 ± 61.2a |
L822429 was administered 60 minutes before locomotor activity sessions, and total session activity was measured. L822429 did not affect locomotor activity of Wistar rats at any of the doses given and did not affect the activity of P-rats at 15 mg/kg. At 30 mg/kg, a significant suppression of locomotor activity was observed in P-rats. For detailed statistics, see Methods and Materials and Results.
P-Rat, alcohol-preferring rat.
p < .01.
Possible sedative effects of the 15 mg/kg L822429 dose were further evaluated in the P-rats using self-administration of .05% (wt/vol) saccharin on an FR3 reinforcement schedule identical to that used for alcohol self-administration. There was no difference between vehicle and 15 mg/kg treatment for the number of saccharin deliveries (mean ± SEM: 142.4 ± 13.2 vs. 150.8 ± 8.2, vehicle vs. L822429; t7 = .75, p = .48), active lever presses (512.4 ± 63.8 vs. 532 ± 28.9; t7 = .35, p = .74), or inactive lever presses (2.5 ± .7 vs. 6.0 ± 2.4; t7 = 1.8, p = .12).
Together, the locomotor activity and saccharin self-administration data indicate that the selective suppression of alcohol self-administration in P-rats by L822429 at 15 mg/kg is not due to sedative or other nonselective behavioral effects of the NK1R antagonist. At 30 mg/kg, a sedative effect of the antagonist is observed in the P-rats but not Wistar rats, further supporting an increased sensitivity to NK1R antagonism in the P-rats.
Tacr1 Expression Is Increased in the P-Rat Brain
Based on our behavioral findings, we hypothesized that the SP/NK1R system may be sensitized in P-rats. To assess this possibility, we first measured the expression of Tacr1, the gene encoding NK1R. Expression was assessed in the nucleus accumbens, caudate putamen, amygdala (AMG), ventral tegmental area, PFC, and hippocampus of alcohol-naïve Wistar and P-rats. Tacr1 levels were significantly increased in the PFC (t14 = 2.48, p = .03; 25% increase) and AMG (t14 = 3.03, p = .009; 37% increase) of P-rats. No significant differences were found in other brain regions examined (Table 2). Some changes in Tac1 (transcript for SP) expression were also observed. Specifically, in P-rats, there was a significant increase in Tac1 expression levels in the PFC (t14 = 2.36, p = .03; 11% increase) and caudate putamen (t14 = 5.12, p = .0002; 42% increase) and a trend level increase in expression in the AMG (t14 = 1.89, p = .08; 47% increase; Table S1 in Supplement 1).
Table 2. Elevated Tacr1 Expression in Amygdala and Prefrontal Cortex of P-Rats.
| Tacr1 | ||||
|---|---|---|---|---|
|
|
||||
| Region | ΔCT Wistar | ΔCT P-Rat | p Valuea | % Changeb |
| PFC | 10.93 ± .09 | 10.60 ± .09 | .03c | 25.40 |
| NAc | 8.13 ± .08 | 8.14 ± .06 | .92 | −.64 |
| CPU | 8.09 ± .05 | 8.08 ± .04 | .89 | .88 |
| AMG | 8.25 ± .13 | 7.79 ± .07 | .009d | 36.83 |
| Hipp | 9.97 ± .06 | 10.04 ± .10 | .50 | −5.35 |
| VTA | 9.86 ± .24 | 10.09 ± .21 | .48 | −14.75 |
Tacr1 expression was measured in punches of brain regions using realtime PCR. Significant Tacr1 upregulation was found in the PFC and AMG of P-rats compared with Wistars. For detailed statistics, see Methods and Materials and Results.
AMG, amygdala; CPU, caudate putamen; Hipp, hippocampus; NAc, nucleus accumbens; PCR, polymerase chain reaction; PFC, prefrontal cortex; P-Rat, alcohol-preferring rat; VTA, ventral tegmental area.
The respective p value was obtained by comparing ΔCt using unpaired t test.
Percent change was calculated using 2ΔΔCt method.
p < .05.
p < .01.
A Tacr1 Promoter Variant Is Associated with Differential Tacr1 Expression in P-Rats
Sequencing 2 kilobase upstream of TSS in Wistar and P-rats identified a G-C single nucleotide polymorphism (SNP) at position −1372 relative to the TSS. All 20 P-rats genotyped, but only 18% of 22 Wistar rats were homozygous for the C-allele at this position, and allele frequencies differed significantly between P-rats and Wistar rats (Yates corrected χ21 = 29.70, p < .0001). Transcriptional Element Search System (University of Pennsylvania, Philadelphia, Pennsylvania) analysis predicted that two transcription factors, GATA-2 and E2F-1, bind with increased affinity to position −1372 when a C nucleotide is present. These data suggest that genetic variation at the −1372 position may result in greater transcriptional activation of Tacr1 expression by one or more of these factors in P-rats compared with their founder Wistar line.
Electrophoretic Mobility Shift Assay and Luciferase Assays
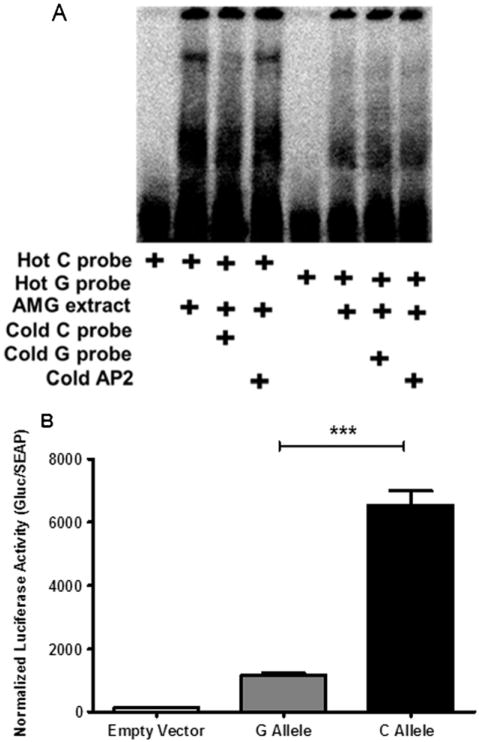
To assess transcription factor binding potential in the two alleles of the Tacr1 promoter, we used electrophoretic mobility shift assay with radioactive probes containing either a C or G nucleotide at position −1372. In these assays, we observed an increased shift with radioactive probes containing cytosine compared with those containing guanine when using nuclear extracts from the AMG (Figure 2A). Interestingly, we did not observe a shift with probes containing either nucleotide when using nuclear extracts from the striatum (Figure S3 in Supplement 1). Luciferase assays were used to determine the functional effect of this G-C SNP in the Tacr1 promoter. We found that when cytosine was present at this position, there was a significantly greater expression of the luciferase reporter when compared with the G allele (t16 = 11.94, p < .0001; Figure 2B).
Figure 2.
Electromobility shift (electrophoretic mobility shift assay) and luciferase reporter assays for −1372 single nucleotide polymorphism function. (A) Electromobility shift, indicating transcription factor binding, was observed when oligonucleotide probes corresponding to the −1372C Tacr1 promoter sequence were incubated with nuclear extracts from rat amygdala (AMG) (lane 2). Specificity of binding was demonstrated by elimination of the shift in presence of cold competitor (lane 3) and maintained shift in presence of excess cold unrelated sequence (AP2) probe (lane 4). No shift was observed with −1372G probe (lanes 5–8). (B) −1372C Tacr1 promoter sequence cloned into a luciferase reporter construct drives markedly increased expression compared with − 1372G construct (***p < .001; for detailed statistics, see Results). AP2, activator protein 2; Gluc, gaussia luciferase; SEAP, secreted alkaline phosphatase.
NK1R Binding Is Increased in the CeA of P-Rats
We next determined if increased NK1R messenger RNA levels are associated with increased receptor availability. 125I-substance P autoradiography was used to measure NK1R density in Wistar and P-rats in the PFC and three subregions of the AMG (CeA, basolateral nucleus, and medial nucleus; Figure 3A). 125I-substance P binding was increased in the CeA (t13 = 2.23, p = .04; Figure 3B). There was no difference in 125I-SP binding in the basolateral nucleus (t13 = .57, p = .58; Figure 3C), medial nucleus (t13 = .88, p = .39; Figure 3D), or the PFC (t13 = 1.48, p = .16; Figure 3E).
Figure 3.
Neurokinin 1 receptor binding is increased in the central nucleus of the amygdala (CeA) of alcohol-preferring (P) rat. To determine neurokinin 1 receptor binding in regions where increased Tacr1 expression had been detected, autoradiography was carried out using [125I]substance P in alcoholnaïve Wistar and P-rats. (A) shows representative autoradiograms from P-rat and Wistar brains at the level of the amygdala, a corresponding schematic that delineates the anatomy and nonspecific binding obtained in the presence of excess unlabelled substance P. [125I]substance P binding was increased in the CeA of P-rats (B) but not in the other regions examined (C–E). Data are shown as mean ± SEM. *p < .05. For details, see Methods and Materials and Results. BLA, basolateral nucleus; MeA, medial nucleus; PFC, prefrontal cortex.
CeA Infusion of L822429 Suppresses Alcohol Self-Administration in P-Rats
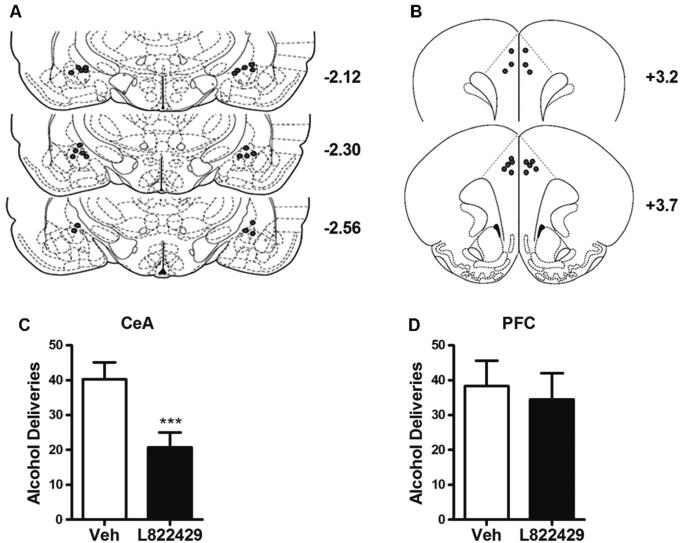
If increased expression and availability of NK1Rs in the CeA causally contributes to the higher rates of alcohol self-administration in P-rats, then injection of L822429 into this structure would be predicted to suppress self-administration in this line. To examine this, we injected L822429 (7.5 μg/side) or vehicle in the CeA of P-rats, in a counterbalanced, randomized within-subjects design (placements from P-rats included in the analysis are shown in Figure 4A,B). In agreement with our prediction, CeA injections of L822429 decreased the number of alcohol deliveries obtained (t11 = 5.26, p = .0003; Figure 4C). The ratio of active lever responses to alcohol deliveries obtained was approximately 3:1, as expected, and the effect of L822429 on active lever responses was the same as on alcohol deliveries (t11 = 4.3, p = .001). Inactive lever presses were unaffected (t11 = .52, p = .61; Figure S4A in Supplement 1). Importantly, CeA injections of the same L822429 dose (n = 6/group) had no effect on locomotor activity, as measured by the total distance traveled during a 60-minute locomotor activity test after these injections (mean ± SEM: 2611 ± 213 cm vs. 2513 ± 277 cm, vehicle vs. L822429; t10 = .28, p = .79). In contrast, PFC injections of the same L822429 dose had no effect on alcohol deliveries obtained (t7 = .56, p = .60; Figure 4D), active lever presses (t7 = .62, p = .56), or inactive lever presses (t7 = .69, p = .51; Figure S4B in Supplement 1). However, intra-PFC L822429 did suppress locomotor activity (n = 3-5/group; mean ± SEM: 3738 ± 332 cm vs. 2591 ± 128, vehicle vs. L822429; t6 = 2.54, p = .044). To further address the specificity of this effect for the P-rat, we also infused L822429 into the CeA of Wistar rats (placements for rats included in the analysis are included in Figure S5A in Supplement 1) and found that NK1R antagonism in this region did not affect alcohol deliveries obtained (t7 = 1.5, p = .17; Figure S5B in Supplement 1), active lever responses (t7 = 1.572, p = .16; Figure S5C in Supplement 1), or inactive lever responses (t7 = 1.572, p = .16; Figure S5D in Supplement 1).
Figure 4.
Intra-cranial infusions of L822429 into the central nucleus of the amygdala (CeA) attenuates alcohol self-administration in alcohol-preferring (P) rat. To examine the potential contribution of CeA for the high rates of alcohol self-administration found in P-rats, local infusions of L822429 (7.5 μg/side) were given into this structure, as well as the prefrontal cortex (PFC). Injection sites are shown in panels (A, B). L822429 robustly suppressed alcohol self-administration in P-rats following administration into the CeA (C) but had no effect after PFC infusion (D). ***p < .001. For details, see Methods and Materials and Results. Veh, vehicle. A and B reprinted from Paxinos G, Watson C (2005): The Rat Brain in Stereotaxic Coordinates. 5th ed. Amsterdam: Elsevier Academic Press, with permission.
Discussion
We report that elevated alcohol self-administration in alcoholpreferring rats is selectively sensitive to NK1R blockade. Increased sensitivity of P-rats to NK1R antagonism was associated with increased Tacr1 expression in the AMG and PFC. In the CeA of P-rats, upregulated Tacr1 expression was also accompanied by upregulated NK1R radioligand binding. A functional relevance of this upregulation was supported by microinjections of L822429 into CeA, which potently suppressed alcohol self-administration of P-rats, while PFC injections did not. Sequencing of the rat Tacr1 promoter identified an SNP in position −1372 from the TSS. In silico analysis predicted enhanced binding of the transcription factors GATA-2 and E2F-1 to the C-allele at this locus, for which all P-rats, but only 18% of Wistar rats, were homozygous, potentially driving increased Tacr1 expression in P-rats. Reporter assays showed that the −1372C allele drives increased transcription, while electrophoretic mobility shift assay showed that it alters transcription factor binding specifically in amygdalar nuclear extracts. Collectively, these findings suggest that the SP/NK1R system contributes to the innate alcohol preference of P-rats and influences their sensitivity to NK1R antagonists. With the exception of our prior corticotropin-releasing hormone (CRH) 1 receptor data (25), we are unaware of findings linking a specific polymorphism in a receptor gene with the ability of a medication to suppress alcohol self-administration.
The suppression of alcohol self-administration by L822429 in Prats seems to be behaviorally specific. At 15 mg/kg, L822429 decreased alcohol self-administration and progressive ratio responding of P-rats to the level of Wistar rats but was without effects on locomotor activity or saccharin self-administration. Similarly, intra-CeA injections of L822429 suppressed alcohol self-administration of P-rats but had no effect on locomotor activity. This demonstrates that NK1R antagonism can suppress alcohol self-administration in the absence of measurable sedative or performance-impairing effects. At a higher systemic dose, 30 mg/kg, alcohol self-administration was further suppressed in the P-rats, but this dose also suppressed locomotor activity and therefore likely represents a contribution of sedative drug effects. L822429 influenced neither self-administration nor locomotor activity of Wistar rats at any of the doses examined, suggesting that P-rats are more sensitive to NK1R antagonism with regard to both of these behaviors. The microinjection data further indicate that NK1R antagonist effects on alcohol self-administration and sedation in P-rats can be anatomically dissociated: CeA injections produced the former but not the latter effect, while the reverse was true of PFC injections. Intra-CeA injection of the antagonist in Wistar rats had no effect on alcohol self-administration.
Neurokinin 1 receptors are expressed in brain areas associated with positively as well as negatively reinforcing alcohol actions, and their blockade might influence alcohol self-administration through either mechanism (26). Increased anxiety-like behavior has been found in P-rats (27), suggesting that this line may be particularly sensitive to therapeutics that influence negative reinforcement. In prior experiments with nondependent Wistar rats, L822429 decreased stress-induced reinstatement of alcohol seeking but left cue-induced reinstatement and basal alcohol self-administration unaffected (9). Our present findings suggest that P-rats are made sensitive to suppression of alcohol self-administration by NK1R antagonism due to genetic factors that influence their SP/NK1R system. This parallels prior findings with neuropeptide Y, where expression in P-rats is lower within the AMG and cortex (28), presumably due to genetic factors (29) rendering them sensitive to suppression of alcohol self-administration by neuropeptide Y (30). In another alcohol-preferring rat line, the Marchigian-Sardinian Preferring rat, expression of CRH1 receptors is genetically upregulated, and alcohol self-administration in this line is highly sensitive to CRH1 blockade (25,31). Together, these findings illustrate that multiple loci contribute to heritable phenotypes of preference and elevated self-administration and that different constellations of these factors may be at play in different individuals. Our findings provide preclinical evidence that some of these genetic factors may also determine therapeutic response to alcoholism treatments (32). The published association of human TACR1 allelic variation with alcoholism (10) may therefore be relevant not only as a susceptibility factor but also as a pharmacogenetic predictor of response to NK1R antagonists as treatments for excessive alcohol use. This suggests that the design of clinical efficacy studies of NK1R antagonists in alcoholism may need to consider TACR1 variation.
A possible limitation of our data is that random fixation occurs during selection over multiple generations. We, therefore, cannot exclude that the association of Tacr1 −1372CC genotype with high alcohol preference and L822429 response in P-rats is causally unrelated to our behavioral observations and merely reflects fixation. We believe that several observations make this possibility less likely. First, Tacr1 maps to a chromosomal location that shows high linkage with the preference phenotype of P-rats (14). Second, dysregulated NK1Rs were found in the P-rat specifically within the CeA, a brain area important for regulation of negatively reinforced alcohol consumption (33). Third, the alcohol-preferring phenotype of P-rats was rescued by systemic NK1R antagonist administration, and this was replicated by CeA injections. Together, we believe that these findings make a causal contribution of Tacr1 variation plausible. Finally, we note that the present data may appear inconsistent with our prior findings of reduced alcohol consumption and reward following Tacr1 deletion or NK1R antagonist administration in mice that had not been selected for alcohol preference (7,8). This inconsistency is, however, only apparent. Our mouse data were obtained in C57BL/6 mice, the line that has the highest spontaneous alcohol consumption and preference among inbred mice. Although not specifically selected for high alcohol preference, C57BL/6 mice are, in fact, alcohol preferring. They are likely to have accumulated alleles within the SP/NK1R system, or systems interacting with it, that contribute to their high preference and render them sensitive to NK1R antagonism.
In conclusion, we provide evidence that genetic factors affecting the SP/NK1R system contribute to escalated alcohol self-administration and reward present in the P-rat line as a result of selective breeding for elevated alcohol preference. The elevated alcohol self-administration and reward of P-rats is accompanied by enhanced sensitivity to NK1R antagonism. Both of these traits are likely related to NK1R upregulation within the CeA, potentially driven by an SNP at position −1372 in the Tacr1 promoter. An implication of these findings is that NK1R antagonists may hold particular promise as alcoholism pharmacotherapy in genetically defined patient populations.
Supplementary Material
Acknowledgments
We thank the Indiana University for providing the alcohol-preferring rats for these experiments. The generation of these animals is supported by the R24 Alcohol Research Resource Award Grant (R24 AA015512) from the National Institute on Alcohol Abuse and Alcoholism. We thank Dr. Yavin Shaham for valuable discussion and comments on the manuscript and Dr. Jennifer Bossert for training on procedures.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2012.12.027.
References
- 1.Ebner K, Rupniak NM, Saria A, Singewald N. Substance P in the medial amygdala: Emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc Natl Acad Sci USA. 2004;101:4280–4285. doi: 10.1073/pnas.0400794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31:251–272. doi: 10.1007/s00726-006-0335-9. [DOI] [PubMed] [Google Scholar]
- 3.Commons KG. Neuronal pathways linking substance P to drug addiction and stress. Brain Res. 2010;1314:175–182. doi: 10.1016/j.brainres.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murtra P, Sheasby AM, Hunt SP, De Felipe C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature. 2000;405:180–183. doi: 10.1038/35012069. [DOI] [PubMed] [Google Scholar]
- 5.Ripley TL, Gadd CA, De Felipe C, Hunt SP, Stephens DN. Lack of self-administration and behavioural sensitisation to morphine, but not cocaine, in mice lacking NK1 receptors. Neuropharmacology. 2002;43:1258–1268. doi: 10.1016/s0028-3908(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 6.Gadd CA, Murtra P, De Felipe C, Hunt SP. Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. J Neurosci. 2003;23:8271–8280. doi: 10.1523/JNEUROSCI.23-23-08271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, et al. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- 8.Thorsell A, Schank JR, Singley E, Hunt SP, Heilig M. Neurokinin-1 receptors (NK1R:s), alcohol consumption, and alcohol reward in mice. Psychopharmacology (Berl) 2010;209:103–111. doi: 10.1007/s00213-010-1775-1. [DOI] [PubMed] [Google Scholar]
- 9.Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, et al. Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl) 2011;218:111–119. doi: 10.1007/s00213-011-2201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seneviratne C, Ait-Daoud N, Ma JZ, Chen G, Johnson BA, Li MD. Susceptibility locus in neurokinin-1 receptor gene associated with alcohol dependence. Neuropsychopharmacology. 2009;34:2442–2449. doi: 10.1038/npp.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 12.Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, et al. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: An animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommer W, Hyytia P, Kiianmaa K. The alcohol-preferring AA and alcohol-avoiding ANA rats: Neurobiology of the regulation of alcohol drinking. Addict Biol. 2006;11:289–309. doi: 10.1111/j.1369-1600.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- 14.Bice P, Foroud T, Bo RH, Castelluccio P, Lumeng L, Li TK, Carr LG. Genomic screen for QTLs underlying alcohol consumption in the P and NP rat lines. Mamm Genome. 1998;9:949–955. doi: 10.1007/s003359900905. [DOI] [PubMed] [Google Scholar]
- 15.Leffler A, Ahlstedt I, Engberg S, Svensson A, Billger M, Oberg L, et al. Characterization of species-related differences in the pharmacology of tachykinin NK receptors 1, 2 and 3. Biochem Pharmacol. 2009;77:1522–1530. doi: 10.1016/j.bcp.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Ebner K, Muigg P, Singewald G, Singewald N. Substance P in stress and anxiety: NK-1 receptor antagonism interacts with key brain areas of the stress circuitry. Ann N Y Acad Sci. 2008;1144:61–73. doi: 10.1196/annals.1418.018. [DOI] [PubMed] [Google Scholar]
- 17.Singewald N, Chicchi GG, Thurner CC, Tsao KL, Spetea M, Schmidhammer H, et al. Modulation of basal and stress-induced amygdaloid substance P release by the potent and selective NK1 receptor antagonist L-822429. J Neurochem. 2008;106:2476–2488. doi: 10.1111/j.1471-4159.2008.05596.x. [DOI] [PubMed] [Google Scholar]
- 18.Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- 19.Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- 20.Steensland P, Simms JA, Nielsen CK, Holgate J, Bito-Onon JJ, Bartlett SE. The neurokinin 1 receptor antagonist, ezlopitant, reduces appetitive responding for sucrose and ethanol. Plos One. 2010;5:e12527. doi: 10.1371/journal.pone.0012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cippitelli A, Karlsson C, Shaw JL, Thorsell A, Gehlert DR, Heilig M. Suppression of alcohol self-administration and reinstatement of alcohol seeking by melanin-concentrating hormone receptor 1 (MCH1-R) antagonism in Wistar rats. Psychopharmacology (Berl) 2010;211:367–375. doi: 10.1007/s00213-010-1891-y. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta] CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Geraghty DP, Maguire CM. Reduced [125I]-Bolton Hunter substance P binding (NK1 receptors) in the basal forebrain nuclei of aged rats. Clin Exp Pharmacol Physiol. 2002;29:1112–1115. doi: 10.1046/j.1440-1681.2002.03781.x. [DOI] [PubMed] [Google Scholar]
- 24.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 25.Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M. Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron. 2012;76:192–208. doi: 10.1016/j.neuron.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- 28.Ehlers CL, Li TK, Lumeng L, Hwang BH, Somes C, Jimenez P, Mathe AA. Neuropeptide Y levels in ethanol-naive alcohol-preferring and nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res. 1998;22:1778–1782. [PubMed] [Google Scholar]
- 29.Carr LG, Foroud T, Bice P, Gobbett T, Ivashina J, Edenberg H, et al. A quantitative trait locus for alcohol consumption in selectively bred rat lines. Alcohol Clin Exp Res. 1998;22:884–887. [PubMed] [Google Scholar]
- 30.Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li TK. Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and -nonpreferring (NP) rats. Alcohol Clin Exp Res. 2001;25:386–390. [PubMed] [Google Scholar]
- 31.Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: A novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Möller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.