Abstract
Statin use can be accompanied by a variety of musculoskeletal complaints. We describe the clinical characteristics of case subjects experiencing adverse statin-induced musculoskeletal symptoms within a large, population based cohort in Central Wisconsin. Case status was determined based upon elevated serum creatine kinase (CK) levels and the presence of at least one physician note reflecting an increased index of suspicion for statin intolerance. From the medical records of nearly 2 million unique patients, we identified more than 20,000 potential study subjects (∼1%) having CK data and at least one exposure to a statin drug. Manual screening was completed on 2,227 subjects with CK levels in the upper 10th percentile. Of those screened, 267 met inclusion criteria (12.0% eligibility), and 218 agreed to participate in a retrospective study characterizing the risk determinants of statin-induced muscle toxicity. Three categorical pain variables were graded retrospectively (distribution, location, and severity of pain). The presenting complaints of these case subjects were extremely heterogeneous. The number of subjects with a compelling pain syndrome (diffuse, proximal muscle pain of high intensity) increased at higher serum CK levels; the number of subjects with indeterminate pain variables decreased at higher serum CK levels. The lines reflecting these relationships cross at a CK level of approximately 1,175 U/l, approximately half the threshold level needed to make a clinical diagnosis of “myopathy” (i.e., CK > 10-fold upper limit).
Introduction
Musculoskeletal complaints related to clinical administration of an HMG CoA reductase inhibitor (i.e., statin use) can cause patients to alter their course of therapy1. Currently, there is great interest in characterizing the genetic architecture underlying statin-related adverse drug reactions (ADRs)2-5. The most extreme manifestation of statin-induced muscle toxicity is rhabdomyolysis 1, 6. In general, the incidence of rhabdomyolysis is extremely low7,8. In the context of monotherapy, statin-induced rhabdomyolysis occurs in less than 1 in every 100,000 patients exposed6, 9. With such a low event frequency, it may be difficult to obtain an adequate number of rhabdomyolysis cases to facilitate assessment of the underlying genetic predictors. Retrospective studies designed to identify these predictors would need to screen more than 10 million patients exposed in order to identify and recruit an adequate number of case subjects4.
Although international efforts are being made to coordinate case finding for rhabdomyolysis, the identification of subjects with mild muscle toxicity represents an approachable alternative (http://www.pharmgkb.org/network/members/parc.jsp). Since mild muscle toxicity occurs much more frequently than rhabdomyolysis, one promising investigative approach has been the identification of risk determinants associated with this more intermediate phenotype 2-5. To date, at least three intermediate myotoxicity phenotypes have been described in the clinical literature 1. These phenotypes, separated arbitrarily using serum creatine kinase (CK) levels as a marker of muscle damage severity, include (1) myalgias without elevated serum CK levels, (2) myalgias with mild to moderate elevation in serum CK levels (CK <10-fold upper limit of normal), and (3) myopathy (myalgias with CK >10-fold upper limit of normal). Elevated CK levels have also been observed in the absence of myalgias (“asymptomatic myopathy”) 10.
Historically, physicians have anticipated that the presentation of statin-related musculoskeletal pain would include diffuse myalgias in proximal muscle groups (i.e., particularly in the hip and shoulder girdle regions)1. However, patients using statins may complain of discomfort in a variety of anatomic locations, and in a variety of muscle groups related to both the axial skeleton and the appendicular skeleton11. Furthermore, patients may also report discomfort in their joints and/or periarticular tissues12. The clinical heterogeneity of this phenotype can therefore make statin-related musculoskeletal pain difficult to define, leading to challenges in the management of these patients.
To begin quantifying the clinical characteristics of statin-related musculoskeletal complaints specifically within a practice based setting, we screened the entire electronic medical record of a large multispecialty group practice serving patients in Central Wisconsin. This practice provides the majority of care for residents living within its geographic boundaries. As such, this setting provides an accurate estimate of the relative distribution of clinical variables related to intermediate statin-induced muscle toxicity.
Methods
The current study was approved by the Marshfield Clinic Institutional Review Board. The study population included residents living in Central and Northern Wisconsin, served by the Marshfield Clinic, a large multi-specialty group practice.
Medical Record
Electronic medical records have been utilized at the Marshfield Clinic since the 1960's, and the vast majority of patient records within this system have been electronic for over a decade. A variety of data are captured. One of the key features of the Marshfield Clinic electronic medical record is a Windows application called the combined medical record (CMR). CMR integrates data from all Marshfield Clinic facilities and cooperating hospitals, including Saint Joseph's Hospital (Marshfield). CMR includes clinical office notes and coded indices for all events and encounters that patients have experienced within the Marshfield Clinic system of care, and it can be used to access all textual documentation such as office notes, operative reports, and hospital discharge summaries. CMR also includes comprehensive patient problem lists, a summary of each clinic encounter (i.e., both diagnoses and procedures), a variety of medication alerts, and online access to over a decade of laboratory and radiology results.
Electronic Screening
We have previously utilized natural language processing (NLP) software to reconstruct retrospective drug use histories for patients served by this system of care13. We have also shown previously that these data are amenable to electronic extraction, and that they can be managed computationally to characterize lipid lowering therapy at the time of each patient's most recent clinical visit (e.g., 100% sensitive and 96% specific)14,15. In the current study, clinic records from all eligible subjects were re-interrogated electronically for text mention of any of the three most commonly used statins: atorvastatin, simvastatin, and pravastatin. This involved the application of NLP software entitled FreePharma® (Language & Computing; http://www.landc.be).
The medical records of approximately 2 million unique subjects were then further interrogated electronically to identify subject records with a text mention of any of the three most commonly used statins AND a serum creatine kinase (CK) level drawn within the last 20 years. These records were subsequently filtered to include only the upper 10th percentile for serum CK level, yielding 2,227 potential study subjects (CK range = 233 to 85,140 CK Units per liter). These subject records were then further reviewed manually.
Manual Screening
The medical history of each potential study subject was reviewed by a trained research coordinator to screen for inclusion and exclusion criteria. The inclusion criteria included (1) use of either atorvastatin, or simvastatin, or pravastatin, (2) elevated serum CK level during the time of statin intolerance, and (3) clinical notes indicating increased physician index of suspicion that the patient was experiencing statin intolerance (i.e., text evidence that the provider was in fact considering changing either the drug or the dose due to a suspected ADR). Exclusion criteria included (1) CK drawn for suspected acute coronary syndrome, (2) CK elevation secondary to trauma, (3) pre-existing kidney disease (defined as creatinine >2.0), (4) pre-existing liver disease (defined as AST or ALT > 200 prior to event), and (5) pre-existing neuromuscular disorder or inflammatory muscle disease (determined from clinical notes prior to the time of CK elevation).
After confirmation of eligibility, letters were sent to potential study subjects offering enrollment. An attempt was also made to contact these subjects at least once by phone. For patients choosing to enroll, informed consent was obtained during a personal appointment with the research coordinator, and blood was drawn for extraction of DNA.
Case Grading
After enrollment, the medical record of each participant was reviewed by a licensed practicing physician (RM, FM), and all cases were graded using categorical variables to further characterize each particpant's pain syndrome. The distribution of pain was graded either focal (one discrete anatomic area) or diffuse. The location of pain was graded either proximal (musculoskeletal pain related to the axial skeleton) or distal (related to the appendicular skeleton). The severity of pain was graded either low or high depending upon the original health care provider's description of the pain syndrome in the medical record. No effort was made to discriminate pain within the muscle body from arthralgia or periarticular pain. These syndromes are typically inter-related11.
Categorical variables were also assigned for potential contributory clinical factors. These included additional lipid lowering medication (i.e., gemfibrozil, fenofibrate, and niacin), relevant comorbidity (i.e., hypothyroidism), and environmental factors (i.e., vigorous physical exercise). For quality assurance, the records of 10% of participants were reviewed by a second physician (RW), to confirm accuracy of the case grading.
Results
Process Efficiency
Initial electronic screening of the medical records from nearly 2 million subjects yielded more than 20,000 unique subject records with a creatine kinase (CK) level drawn in the last 20 years and a clinical note mentioning one of our three drugs of interest: atorvastatin, simvastatin, or pravastatin. Within this system of care, atorvastatin was clearly the most frequently prescribed statin. Of the patients with mention of any statin in their medical record, 46% had been exposed to atorvastatin, 26% had been exposed to simvastatin, and 8% had been exposed to pravastatin.
The medical records of 2,227 potential study subjects with a CK level at or above the 90th percentile were then reviewed by a certified research coordinator, to screen for inclusion and exclusion criteria. As shown in Table 1, 88.0% (1,960) of these subjects were excluded from further consideration because they did not meet these criteria. Of the 267 subjects determined eligible, 98.9% (264 subjects) were sent letters for enrollment. An attempt was also made to speak with each one of these potential study subjects personally over the phone. Contact was unsuccessful in 8.3% (22 subjects) and 9.5% (25 subjects) declined to enroll in the study. Two-hundred eighteen subjects (83% of those who received letters) chose to enroll in the study. Four of these subjects were subsequently removed from the dataset, during retrospective chart review by a licensed practicing physician, due to equivocal exclusion criteria: two subjects were ineligible due to prior muscle disease, one subject was ineligible due to an acute systemic inflammatory disease, and one subject was ineligible due to recent trauma.
Table I.
Reason for subject ineligibility. Based upon exclusion criteria assigned by a research coordinator during manual screening, 1,960 of 2,227 patients were ineligible.
| REASON NOT ELIGIBLE | Total | |
|---|---|---|
| N | % | |
| Acute coronary syndrome at time of elevated CK | 836 | 42.7 |
| Acute pulmonary disease at time of elevated CK | 31 | 1.6 |
| Traumatic injury at time of elevated CK | 346 | 17.7 |
| Chronic kidney disease prior to CK elevation | 70 | 3.6 |
| Chronic muscle disease prior to CK elevation | 75 | 3.8 |
| Patient not on statin at time of elevated CK | 252 | 12.9 |
| Insufficient data | 350 | 17.9 |
| Total | 1960 | 100.0 |
Case Characteristics
Of the 214 validated myotoxicity case subjects enrolled in this study, 106 were associated with atorvastatin use, 64 were associated with simvastatin use, and 44 were associated with pravastatin use (Table 2). Within the entire case cohort, the observed mean CK elevation was 1993 Units per liter (min = 233 U/l; 25th percentile = 460 U/l; median = 570 U/l; 75th percentile = 838 U/l; max = 85,140 U/l).
Table II.
Myotoxicity. Observed combinations of 3 discrete pain variables.
| Statin drug taken at time of highest CK elevation | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Atorvastatin | Pravastatin | Simvastatin | ||||||||
| N | % | N | % | N | % | N | % | |||
| Severity | Distribution | Location | 22 | 20.8 | 2 | 4.5 | 3 | 4.7 | 27 | 12.6 |
| High | Diffuse | Proximal | ||||||||
| High | Diffuse | Distal | 2 | 1.9 | 1 | 2.3 | 4 | 6.3 | 7 | 3.3 |
| High | Diffuse | CD | 3 | 2.8 | 0 | 0.0 | 0 | 0.0 | 3 | 1.4 |
| High | Focal | Proximal | 6 | 5.7 | 0 | 0.0 | 2 | 3.1 | 8 | 3.7 |
| High | Focal | Distal | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 |
| High | CD | CD | 1 | 0.9 | 0 | 0.0 | 1 | 1.6 | 2 | 0.9 |
| Low | Diffuse | Proximal | 11 | 10.4 | 2 | 4.5 | 8 | 12.5 | 21 | 9.8 |
| Low | Diffuse | Distal | 18 | 17.0 | 0 | 0.0 | 5 | 7.8 | 23 | 10.7 |
| Low | Diffuse | CD | 5 | 4.7 | 4 | 9.1 | 9 | 14.1 | 18 | 8.4 |
| Low | Focal | Proximal | 3 | 2.8 | 0 | 0.0 | 6 | 9.4 | 9 | 4.2 |
| Low | Focal | Distal | 3 | 2.8 | 2 | 4.5 | 4 | 6.3 | 9 | 4.2 |
| Low | Focal | CD | 0 | 0.0 | 1 | 2.3 | 1 | 1.6 | 2 | 0.9 |
| Low | CD | CD | 3 | 2.8 | 2 | 4.5 | 5 | 7.8 | 10 | 4.7 |
| CD | Diffuse | Proximal | 1 | 0.9 | 0 | 0.0 | 1 | 1.6 | 2 | 0.9 |
| CD | Diffuse | CD | 0 | 0.0 | 6 | 13.6 | 2 | 3.1 | 8 | 3.7 |
| CD | Focal | Distal | 1 | 0.9 | 1 | 2.3 | 0 | 0.0 | 2 | 0.9 |
| CD | CD | CD | 26 | 24.5 | 23 | 52.3 | 13 | 20.3 | 62 | 29.0 |
| Total | 106 | 100.0 | 44 | 100.0 | 64 | 100.0 | 214 | 100.0 | ||
CD = Cannot determine
Clinical notes were reviewed for all 214 case records, and each subject's pain syndrome was graded according to three categorical pain variables: distribution of pain, anatomic location of pain, and severity of pain. As shown in Table 2, we commonly observed that one or more variables could not be graded due to insufficient data within the original clinical note (24.5% for atorvastatin; 20.3% for simvastatin; and 52.3% for pravastatin). For those subject records containing sufficient documentation to assign pain variables, the most frequent pain syndrome for atorvastatin was severe, diffuse pain within proximal muscles (20.8%, Table 2), and the most frequent pain syndrome for simvastatin was mild, diffuse pain within proximal muscles (12.5%, Table 2). No combination of pain variables was observed at frequency >10% for pravastatin (Table 2).
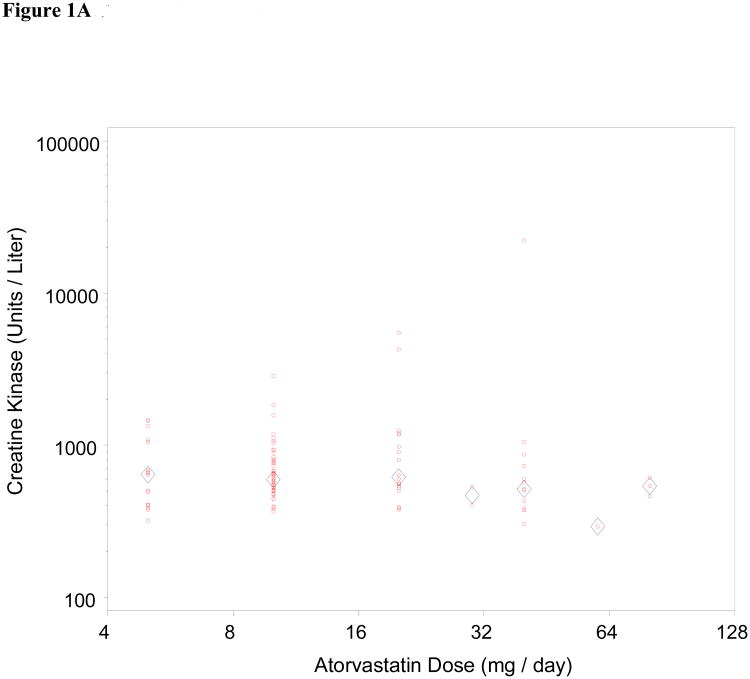
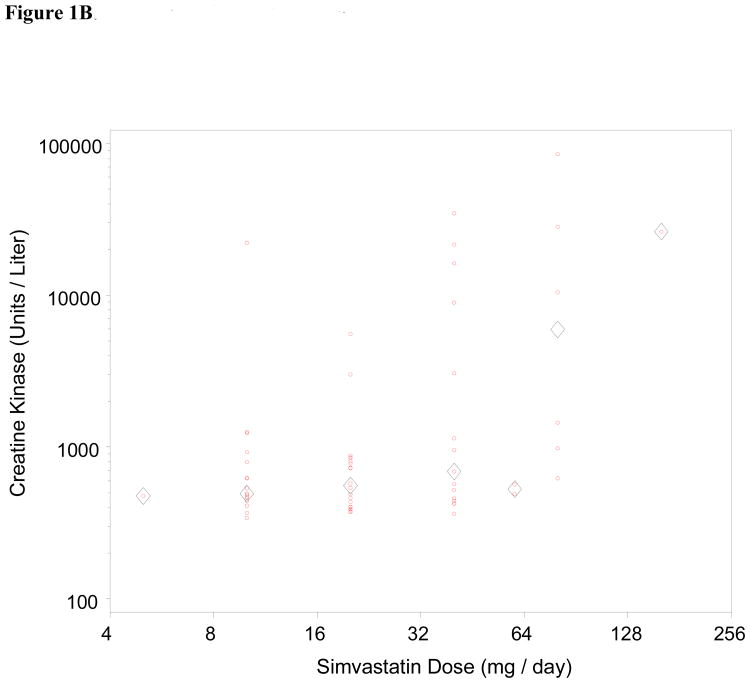
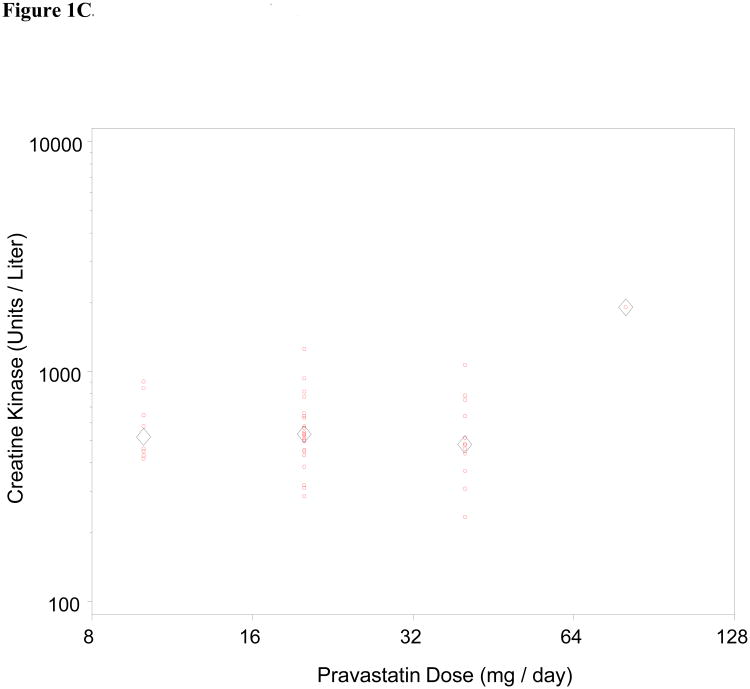
We also assessed dose-dependence of the relationship between statin exposure and CK level for all three drugs (Figure 1). Statin myotoxicity was only noted to be dose-dependent for simvastatin (p = 0.005; Spearman correlation 0.35). We also note a disproportionate relationship between simvastatin and the most severe form of myotoxicity, rhabdomyolysis. Of the 9 patients identified with CK greater than 10,000 Units per liter (roughly 50-fold ULN), 1 was on atorvastatin and 8 were on simvastatin. A number of factors could have contributed to this disproportionate representation with respect to a single drug, including biological factors (pharmacodynamic and pharmacokinetic differences) as well as clinical factors (changes in practice pattern within this dynamic population-based cohort over time).
Figure 1.
Dose dependence of statin myotoxicity. The CK level has been plotted against daily dose for atorvastatin (Fig 1A), simvastatin (Fig 1B), and pravastatin (Fig 1C). Circles represent individual patients; diamonds represent mean CK for each dose. The Spearman correlation of CK with dose is significant for the simvastatin cases (r = 0.35, p = 0.005). The correlations are not significant for the atorvastatin cases (r = -0.08, p = 0.41) or the pravastatin cases (r = -0.02, p = 0.90).
Co-morbid diseases and environmental factors could also have influenced myotoxicity risk disproportionately. Nine percent of all myotoxicity cases (20 subjects) had co-morbid hypothyroidism, and 14% (31 subjects) had evidence of recent vigorous physical exercise within their record. Further, 9% of all myotoxicity cases (20 subjects) were on gemfibrozil at the time of CK elevation, whereas 2% (4 subjects) were on fenofibrate (not shown). It is well established that concomitant gemfibrozil uniquely places patients at higher risk of developing statin myotoxicity16. We therefore specifically looked at the frequency of gemfibrozil use, statin-by-statin. Eleven of the simvastatin myotoxicity subjects were on gemfibrozil at the time of CK elevation (not shown).
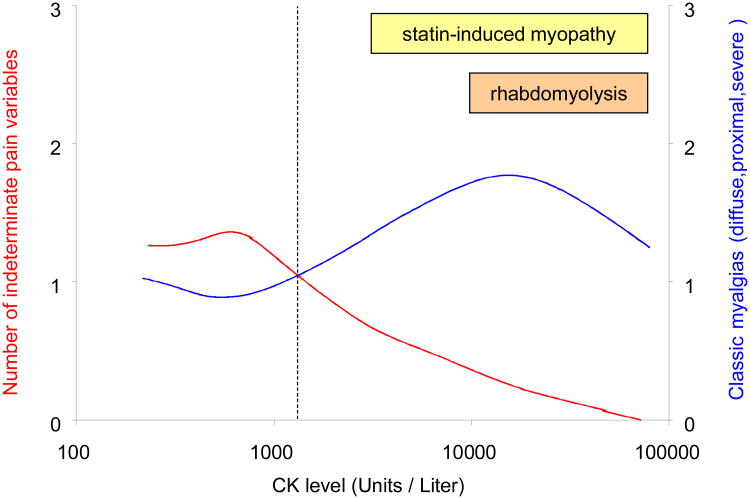
Lastly, to describe the relationship between severity of myotoxicity and documentation of the pain syndrome within each subject record, we plotted frequency of a commonly anticipated pain syndrome (diffuse distribution and proximal location and high severity) as a function of CK level in Figure 2. We also plotted the frequency of the indeterminate pain syndrome on the same x-axis. This revealed a confidence threshold (i.e., point at which indeterminate clinical notes become less frequent than the anticpated pain syndrome) at a CK level of approximately 1,175 units/liter. This level is roughly equal to half of the current diagnostic threshold for “myopathy” (CK >10-fold ULN)1.
Figure 2.
Correlation between serum CK level and pain variables in combination. Y-axis on left side (red) represents pain variables graded as “cannot determine”; Y-axis on right side (blue) represents one typical pain syndrome; X-axis represents the CK level. The number of subjects with an indeterminate pain syndrome decreases according to CK level (curved red line), whereas the number of subjects with the most commonly anticipated statin-induced myalgias (severe, diffuse pain within proximal muscles) increases by CK level (curved blue line). Each curved line represents a cubic spline created in SAS Graph (registered trademark of SAS Institute Inc.), using the penalized least squares method. The character of each line reflects a parameter which varies from 0 to 100. In the absence of graphic smoothing, each line would vary discontinuously between the bottom and the top. Conversely, full graphic smoothing would produce a straight line. We display values between these extremes, to identify trends in the data. The two lines shown here intersect at approximately 1,175 CK units per liter. This level is roughly equivalent to half of the current diagnostic threshold for “myopathy” (CK >10-fold ULN or approximately 2,000 CK units per liter).
Discussion
Statin induced muscle toxicity is a heterogenous phenotype. Circulating levels of creatine kinase (CK) are often used clinically as a marker of severity for statin-induced muscle damage 1,2,5. However, the presence of statin-induced muscle damage may or may not be accompanied by a robust leakage of enzymes into the blood1, 17. We now report the clinical characteristics of this phenotype for 214 case subjects recruited for genetic association studies from a large practice-based cohort in Central Wisconsin. Serum CK level ranged from 233 to 85,140 units/liter, and the clinical presentation of these subjects (musculoskeletal pain) was extremely variable.
In our retrospective assessment of statin-induced musculoskeletal pain, we observed a clinical confidence threshold (i.e., a point at which indeterminate clinical notes become less frequent than the clinically anticipated pain syndrome) at a CK level of approximately 1,175 units/liter. This level is roughly equivalent to half of the level currently required to make a clinical diagnosis of “myopathy” (i.e., CK level > 10-fold ULN, or approximately 2000 units/liter)1,5. Since our data indicate that the clinical heterogeneity of the myotoxicity phenotype is greatly reduced at CK levels above 1,175 units / liter (Figure 2), future genetic association studies focusing on myotoxicity cases above this threshold would be most likely to reduce misclassification bias.
Since mild to moderate muscle toxicity occurs more often than rhabdomyolysis6, 7, one promising investigative approach has been to identify genetic risk determinants associated with the more intermediate phenotype2,5. This strategy has been successful for subjects using statin monotherapy5 as well as for subjects using statin in combination with niacin or a fibric acid derivative2. It remains unclear, however, whether inclusion of a description for the corresponding pain syndrome will increase statistical power. As noted earlier, there is precedent for an intermediate myotoxicity phenotype presenting as elevated CK level in the absence of pain 18; and conversely, patients may experience statin-related pain with no elevation in CK level15, 19, 20. While the current study included case subjects with the former phenotype (elevated CK level in the absence of pain; Table 2) we did not evaluate subjects with the latter phenotype (pain without CK elevation).
Intermediate myotoxicity accompanied by a pain syndrome can be clinically relevant21. If pain disrupts a patient's activities of daily living, and the patient perceives this pain to be secondary to a statin, the patient may opt to discontinue the drug 1, 5. Since statin therapy is associated with a marked reduction in cardiovascular disease risk, interruption of statin therapy, due to intermediate phenotypes, will have obvious public health implications. Genetic markers that predict the onset of myotoxicity, and its rate of progression, are therefore likely to have diagnostic value. Rigorous characterization of phenotype will reduce misclassification in future genetic association studies and facilitate an improved understanding of the genetic architecture underlying statin-induced ADRs.
Acknowledgments
This work was funded by U01HL069757 (RMK).
References
- 1.Thompson PD, Clarkson PM, Rosenson RS. An Assessment of Statin Safety by Muscle Experts. The American Journal of Cardiology. 2006;97(8, Supplement 1):S69–S76. doi: 10.1016/j.amjcard.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Wilke RA, Moore JH, Burmester JK. Relative impact of CYP3A genotype and concomitant medication on the severity of atorvastatin-induced muscle damage. Pharmacogenet Genomics. 2005;15(6):415–21. doi: 10.1097/01213011-200506000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Wilke RA, Reif DM, Moore JH. Combinatorial Pharmacogenetics. Nature Reviews Drug Discovery. 2005;4(11):911–8. doi: 10.1038/nrd1874. [DOI] [PubMed] [Google Scholar]
- 4.Wilke RA, Lin DW, Roden DM, et al. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nature Reviews Drug Discovery. 2007;6(11):904–16. doi: 10.1038/nrd2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SEARCH Collaborative Group. Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy-a genomewide study. N Engl J Med. 2008;359(8):789–99. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 6.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final Conclusions and Recommendations of the National Lipid Association Statin Safety Assessment Task Force. The American Journal of Cardiology. 2006;97(8, Supplement 1):S89–S94. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Graham DJ, Staffa JA, Shatin D, et al. Incidence of Hospitalized Rhabdomyolysis in Patients Treated With Lipid-Lowering Drugs. JAMA. 2004;292(21):2585–90. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 8.Davidson MH. Rosuvastatin safety: lessons from the FDA review and post-approval surveillance. Expert Opinion on Drug Safety. 2004;3(6):547–57. doi: 10.1517/14740338.3.6.547. [DOI] [PubMed] [Google Scholar]
- 9.McClure DL, Valuck RJ, Glanz M, Murphy JR, Hokanson JE. Statin and statin-fibrate use was significantly associated with increased myositis risk in a managed care population. Journal of Clinical Epidemiology. 2007;60(8):812–8. doi: 10.1016/j.jclinepi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Schindler C, Thoms M, Matschke K, Tugtekin SM, Kirch W. Asymptomatic statin-induced rhabdomyolysis after long-term therapy with the hydrophilic drug pravastatin. Clinical Therapeutics. 2007;29(1):172–6. doi: 10.1016/j.clinthera.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Asbach P, Paetsch I, Stawowy P, Sander B, Fleck E. Statin-associated focal myositis. International Journal of Cardiology. doi: 10.1016/j.ijcard.2007.08.109. In Press. [DOI] [PubMed] [Google Scholar]
- 12.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Circulation. 2002;106(8):1024–8. doi: 10.1161/01.cir.0000032466.44170.44. [DOI] [PubMed] [Google Scholar]
- 13.Sirohi E, Peissig P. Study of effect of drug lexicons on medication extraction from electronic medical records. Pac Symp Biocomput. 2005:308–18. doi: 10.1142/9789812702456_0029. [DOI] [PubMed] [Google Scholar]
- 14.Peissig P, Sirohi E, Berg RL, Brown-Switzer C, Ghebranious N, McCarty CA, Wilke RA. Construction of atorvastatin dose-response relationships using data from a large population-based DNA Biobank. Basic Clin Pharmacol Toxicol. 2007;100:286–8. doi: 10.1111/j.1742-7843.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilke RA, Berg RL, Linneman JG, Zhao CF, McCarty CA, Krauss RM. Characterization of LDL-cholesterol lowering efficacy for atorvastatin in a population-based DNA Biorepository. Basic Clin Pharmacol Toxicol. 2008 doi: 10.1111/j.1742-7843.2008.00291.x. In press. [DOI] [PubMed] [Google Scholar]
- 16.Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate plus statin versus gemfibrozil plus any statin. Am J Cardiol. 2005;95(1):120–2. doi: 10.1016/j.amjcard.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 17.Phillips PS, Haas RH, Bannykh S, et al. Statin-Associated Myopathy with Normal Creatine Kinase Levels. Ann Intern Med. 2002;137(7):581–5. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- 18.Draeger KM, Mohaupt M, Hoppeler H, Savolainen H, Allemann C, Babiychuk EB. Statin therapy induces ultrastructural damage in skeletal muscle in patients without myalgia. The Journal of Pathology. 2006;210(1):94–102. doi: 10.1002/path.2018. [DOI] [PubMed] [Google Scholar]
- 19.Troseid M, Henriksen OA, Lindahl S. Statin-associated myopathy with normal creatine kinase levels. Case report from a Norwegian family. APMIS. 2005;113(9):635–7. doi: 10.1111/j.1600-0463.2005.apm_270.x. [DOI] [PubMed] [Google Scholar]
- 20.Ruaño G, Thompson PD, Windemuth A, Seip RL, Dande A, Sorokin A, Kocherla M, Smith A, Holford TR, Wu AH. Physiogenomic association of statin-related myalgia to serotonin receptors. Muscle Nerve. 2007;36(3):329–35. doi: 10.1002/mus.20871. [DOI] [PubMed] [Google Scholar]
- 21.Thompson PD, Clarkson P, Karas RH. Statin-Associated Myopathy. JAMA. 2003;289(13):1681–90. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]