Abstract
Apoptosis and the subsequent clearance of these dying cells occur throughout development and adult life in many tissues. Failure to promptly clear apoptotic cells has been linked to many diseases1-3. ELMO1 is an evolutionarily conserved cytoplasmic engulfment protein that functions downstream of the phosphatidylserine receptor BAI1, and, along with Dock180 and Rac1, promotes internalization of the dying cells4-7. Here, we generated ELMO1-deficient mice, and unexpectedly found them to be viable and grossly normal. However, ELMO1-deficient mice had a striking testicular pathology, with disrupted seminiferous epithelium, multi-nucleated giant cells, uncleared apoptotic germ cells, and decreased sperm output. Subsequent in vitro and in vivo analyses revealed a crucial role for ELMO1 in the phagocytic clearance of apoptotic germ cells by Sertoli cells lining the seminiferous epithelium. The engulfment receptor BAI1 and the GTPase Rac (upstream and downstream of ELMO1, respectively) were also important for Sertoli cell-mediated engulfment. Collectively, these findings uncover a selective requirement for ELMO1 in Sertoli cell-mediated removal of apoptotic germ cells and make a compelling case for a relationship between engulfment and tissue homeostasis in vivo.
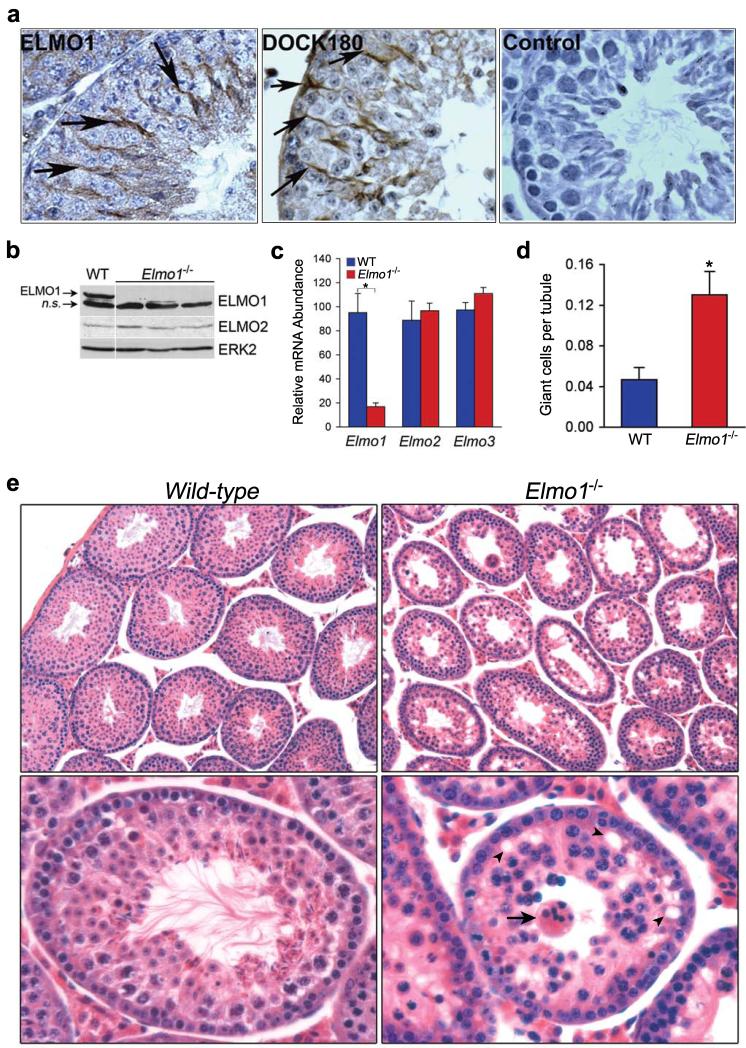
As part of several developmental processes throughout life, we generate excess or superfluous cells; only a small subset of these cells are deemed ‘fit’ for further maturation, while the rest undergo apoptosis and are quickly cleared. Examples of such developmental processes include hematopoiesis in the bone marrow and spermatogenesis in the testes. While several mammalian signaling proteins regulating engulfment have been recently identified based on cell culture studies8, 9, our understanding of signaling mechanisms that regulate clearance in vivo in mammals is limited. To better understand the in vivo requirement for the engulfment protein ELMO1, we generated mice with targeted deletion of Elmo1. Murine ELMO1 is a 727 amino acid protein encoded by 21 exons (ref. 6 and Supplementary Fig. S1c). We inserted loxP sites flanking exon 5 as it encodes a key functional region (an evolutionarily conserved Armadillo repeat10) (Supplementary Fig. S1c). Transgenic expression of ELMO1 lacking exon 5 (Δ-exon 5) failed to rescue the distal tip cell migration defects in a C. elegans strain mutant for ced-12, the Elmo1 orthologue (while wild-type ELMO1 was able to do so) (Supplementary Fig. S1b)4-7. Mice with ubiquitous deletion of Elmo1 exon 5 in all tissues (via EIIa-Cre), showed ~90% reduction in Elmo1 mRNA and a complete loss of ELMO1 protein (Fig. 1b, c and Supplementary Fig. S2d). Although we were expecting embryonic lethality due to the widespread expression of ELMO1 in many cell types/tissues6, surprisingly, Elmo1−/− mice were born at the predicted Mendelian ratios from Elmo1+/− matings, without gross abnormalities (not shown). Moreover, macrophages (both resident peritoneal and bone marrow-derived) and fibroblasts from Elmo1−/− mice engulfed apoptotic thymocytes comparable to controls (Supplementary Fig. S2a-c). This lack of an apparent defect due to loss of ELMO1 may be due in part to a compensatory upregulation of ELMO2 (Supplementary Fig S2d and data not shown). Intriguingly, Sertoli cells from Elmo1−/− mice failed to show a compensatory increase in ELMO2 levels (Fig. 1b, c), and the testes of these mice showed a severe defect, which we pursued further.
Figure 1. Disrupted seminiferous tubule architecture in ELMO1-deficient mice.
a, Immunolocalization of ELMO1 and DOCK180 in Sertoli cells (arrows) of adult C57BL/6 mice testes. Control, secondary antibody only. b, Immunoblotting of lysates from testes of adult mice with the indicated Elmo1 genotypes. A non-specific (n.s) band served as an internal loading control. c, mRNA levels of Elmo1, 2 and 3 in Sertoli cells relative to Gapdh and normalized to WT for each gene. *p=0.01, n=3 mice per group. d, Average number of giant cells per tubule in testes of 6-week old mice, *p=0.03, n=3 or 4 per group. e, H & E sections of testes of mice with the indicated Elmo1 genotypes. Magnification, 10× (upper panels); 25× (lower panels). Arrow, a giant cell; arrowhead, vacuolar spaces in the epithelium. Error bars indicate s.e.m.
Sertoli cells are large cells (>40 μm diameter at basal surface) that line the seminiferous tubules of the testes, playing a crucial support/nursing role throughout germ cell differentiation11-13. During spermatogenesis, non-viable or genetically compromised germ cells (comprising up to 75%), die by apoptosis and are cleared by Sertoli cells14, 15. However, signaling pathways regulating Sertoli cell-mediated clearance of apoptotic germ cells are not well understood. Expression of ELMO1 and DOCK180 proteins were detected in the Sertoli cells (but not germ cells) of normal mice (Fig. 1a and Supplementary Fig. S1a). ELMO1 protein was also detected in mouse Sertoli cell lines (TM4 and 15P-1) (Supplementary Fig. S1a, and not shown).
Histological examination of the testes from 6-8 week old Elmo1−/− mice revealed a striking disruption of the normal cellular organization of the seminiferous epithelium (Fig. 1e). There was also an increase in the number of multinucleated giant cells in the Elmo1−/− mice (Fig. 1d, e) and abundant vacuolar spaces (Fig. 1e). Giant cells arise from spermatacytes that abort meiosis and appear as a syncitium. Appearance of such giant cells is linked to defective spermatogenesis in genetically altered mice and in human pathologies linked to infertility16, 17. These observations suggested that ELMO1 is required for normal homeostasis of the seminiferous epithelium in mice.
Although ~75% of germ cells die by apoptosis, very few apoptotic cells are seen in the normal testes (Fig. 2a and reference 18). Elmo1−/− mice displayed a significantly higher number of apoptotic germ cell nuclei compared to littermate controls (Fig. 2a, b). The fraction of seminiferous tubules containing cell corpses was increased in Elmo1−/− mice (not shown), as well as the average number of corpses per tubule (Fig. 2b), with some tubules having >20 apoptotic cells (Fig. 2a). Thus, the loss of ELMO1 appeared to significantly increase the incidence and frequency of tubules containing uncleared apoptotic cells. Although we have previously established that the majority of the apoptotic cells occurs in the early and middle stages of spermatogenesis (stages I-VIII)19, due to the severe disruption of the seminiferous epithelium in the Elmo1−/− mice, we were unable to definitively determine the stages where the increased apoptotic nuclei are seen (Figs. 1e and 2a). It is noteworthy that there was no significant difference between the control and Elmo1−/− mice in the total numbers of Sertoli cells isolated, their morphology or the expression of Sertoli cell markers (Figs. 2c-e, and Supplementary Figure S4a). Thus, the development and maintenance of Sertoli cell numbers appear to occur normally in mice lacking ELMO1.
Figure 2. Requirement for ELMO1 in apoptotic cell clearance in vivo and in vitro.
a, Detection of apoptotic cells in the testes of wild-type and Elmo1−/− mice by IHC (apostain, brown) viewed under 10× (left) and 25× (right) magnification. Arrow, apoptotic cells. b, Average number of apoptotic nuclei per tubule. *p=0.04, n=3 or 5 mice per group. c, Total number of Sertoli cells isolated from testes of individual mice, n=4. d, e, Fluorescence microscopy of WT and Elmo1−/− Sertoli cells. d, Staining with antibodies against the Sertoli cell marker anti-Müllerian hormone (anti-Amh, green) or control IgG (inset). e, Sertoli cells were fed 2.1μm carboxylate beads (red) or apoptotic mouse spermatic germ cells labeled with CypHer 5 (red). Green, phalloidin staining. Arrows, cells with engulfed targets; arrowheads, individual engulfed targets. Blue, DAPI-stained nuclei. Scale bar, 50μm. f-h, In vitro phagocytosis by WT and Elmo1−/− Sertoli cells. Uptake of fluorescent 2.1μm carboxylate bead uptake (f) and CypHer 5-labeled apoptotic germ cells (h) was quantified by flow cytometry, n=4 mice per group, *p<0.005. g, Representative flow cytometry histograms of data in h.
We next asked whether the loss of ELMO1 affected the ability of Sertoli cells to engulf apoptotic germ cells. A technical issue in performing engulfment assays using germ cells is that Sertoli cells are also nurse cells and therefore can bind to both live and apoptotic germ cells. To distinguish bound and internalized germ cells, the targets were labeled with a pH-dependent fluorescent dye (CypHer5) that displays significantly enhanced fluorescence upon uptake and entry of apoptotic cells into the acidic environment of the phagolysosome (Fig. 2g and Supplementary Fig. S4c). Sertoli cells from Elmo1−/− mice showed significant reduction in the internalization of apoptotic germ cells as determined by fluorescence microscopy and flow cytometry (Figs. 2e, g-h, and Supplementary Fig. S4c). Sertoli cells from Elmo1−/− mice were also significantly impaired in their ability to engulf negatively charged 2.1 μm carboxylate-modified beads, which can serve as surrogate apoptotic targets7 (Fig 2e, f and Supplementary Fig. S4b). These data suggested that the defective engulfment of apoptotic germ cells by Sertoli cells in the absence of ELMO1 could have contributed to the phenotype of the Elmo1−/− mice.
To directly test the requirement for ELMO1 in Sertoli cells, we crossed mice carrying a floxed Elmo1 allele (Elmo1F, Supplementary Fig. S1c) with Amh-Cre transgenic mice, where the Cre recombinase is expressed under control of the anti-Müllerian hormone promoter specific for Sertoli cells20. Sertoli-specific deletion in this system was confirmed using a YFP reporter strain crossed with the Amh-Cre transgenic mice (Supplementary Fig. S5a, b). Moreover, Elmo1 mRNA was specifically reduced in the Sertoli cells in the Amh-Cre/Elmo1F/F mice (Fig. 3b). Analysis of the seminiferous epithelium of the Amh-Cre/Elmo1F/F mice showed a significant increase in the number of uncleared apoptotic nuclei compared to control littermates (3.6-fold, p=0.04) (Fig. 3a, c). We also noted that the disruption of the seminiferous epithelium observed in Elmo1−/− mice was not readily detected in the Amh-Cre/Elmo1F/F mice, possibly due to residual ELMO1 expression (Fig. 3b). This less severe phenotype in the Amh-Cre/Elmo1F/F context allowed us to stage the seminiferous tubules with respect to spermatogenesis; 90% of the detectable apoptotic nuclei in the Amh-Cre/Elmo1F/F mice were restricted to the early and middle stages of spermatogenesis (Supplementary Fig. S5c), essentially similar to the littermate controls. To determine if loss of ELMO1 affected spermatogenesis in these mice, we quantified sperm production in the Amh-Cre/Elmo1FF mice. Compared to littermate controls, these mice displayed a reduction in mature sperm at 8 weeks of age (n=6-8 testis per group, p=0.007) (Fig. 3d). Taken together, these data suggested a specific requirement for ELMO1 in the Sertoli cell-dependent homeostatic clearance of apoptotic germ cells in vivo, with consequences for sperm production.
Figure 3. ELMO1 functions in Sertoli cell-mediated apoptotic germ cell clearance.
a, Detection of apoptotic cells (apostain, brown) in testes of 6-8 week Amh-Cre/Elmo1F/W and Amh-Cre/Elmo1F/F mice (arrow, apoptotic cell). b, qPCR of Elmo1 and Elmo2 mRNA levels in Sertoli cells from Amh-Cre+ mice with the indicated genotypes. c, Average number of apoptotic cells per tubule. *p=0.04, 4-6 mice per group. d, Total sperm output in 8 week Amh-Cre+ Elmo1F/F mice with littermate controls. *p=0.007, n=6-8 testis per group. e, Phagocytosis by TM4 Sertoli cells expressing GFP, GFP-tagged wild-type or dominant-negative ELMO1 (T625) expression constructs. f, RAC1-GTP levels in TM4 Sertoli cells transfected with the indicated constructs. RAC1-GTP relative to actin was quantified by densitometry. g, Uptake of 2.1μm carboxylated targets by TM4 Sertoli cells following siRNA knockdown of RAC1. Inset, immunoblot of lysates after RAC1 knockdown. DOCK180, loading control.
We also tested the ELMO1 signaling pathway in Sertoli cell lines in vitro. Overexpression of ELMO1 in the TM4 Sertoli cell line promoted phagocytosis, while expression of a previously known dominant-negative mutant of ELMO1 (T625)21 inhibited uptake of apoptotic targets (Fig. 3e). ELMO1 in Sertoli cells also appeared to be enriched around targets during engulfment (Supplementary Fig. S4d). Previously, ELMO1 along with DOCK180 has been shown to function as a guanine nucleotide exchange factor (GEF) for the GTPase RAC1 and thereby promote cytoskeletal rearrangement during engulfment6, 21. Overexpression of wild-type ELMO1 consistently enhanced RAC-GTP levels, while the ELMO1-T625 mutant failed to enhance RAC-GTP (Fig. 3f). Furthermore, siRNA-mediated depletion of RAC1 in TM4 cells reduced the phagocytic capacity by >75% (Fig. 3g), indicating that RAC1 is necessary for Sertoli cell engulfment. Thus, ELMO1 appears to promote downstream activation of RAC1 during phagocytosis by Sertoli cells.
We next sought to gain a better understanding of how Sertoli cells recognize apoptotic germ cells and the role of known upstream engulfment receptors in signaling to ELMO1. Uptake of apoptotic targets by Sertoli cells was phosphatidylserine dependent as determined by addition of annexin V, which masks the recognition of PtdSer on targets (data not shown). Furthermore, Sertoli cells could mediate the specific recognition and internalization of fluorescently labeled phosphatidylserine-containing bubbles, but not control bubbles containing only phosphatidlycholine (Fig. 4a). We then assessed the relative expression of three recently identified PtdSer recognition receptors Bai1, Tim4, and Stabilin2 in primary murine Sertoli cells7, 22, 23. Remarkably, Bai1 was expressed the highest in Sertoli cells, with Stabilin2 and Tim4 at or below the limits of detection (Fig. 4b). Although several TIM family members (TIM-1, TIM-3 and TIM-4) have been linked to PtdSer recognition, none of these appeared to be expressed in the testes (Mammalian Reproductive Genetics database). BAI1 (brain angiogenesis inhibitor 1) is a seven-transmembrane protein belonging to the type II adhesion GPCR family, with an extended extracellular domain that can recognize PtdSer via thrombospondin type 1 repeats (TSR). BAI1 also interacts via its cytoplasmic tail with ELMO1, and can activate RAC through the ELMO1/DOCK180 complex thereby promoting apoptotic cell phagocytosis7. Transfection of TM4 Sertoli cells with BAI1 siRNA led to a significant reduction in engulfment (Fig. 4c, 41.3% engulfment in control siRNA treated compared to 31% in Bai1 siRNA, n=3, p<0.05).
Figure 4. BAI1 participates as an engulfment receptor in Sertoli cell-mediated engulfment.
a, Phosphatidylcholine (PC, blue) or phosphatidylserine (PtdSer, red) microbubble uptake by TM4 Sertoli cells. *p < 0.05, n=4. b, Levels of PtdSer recognition receptors by qPCR from the indicated murine tissues. # indicates mRNA below detectable level. c, Phagocytosis by TM4 Sertoli cells transfected with control or BAI1 siRNA. *p<0.05. d, Left, schematic of microinjection into rete testis. Rete testis and efferent ducts prior to (top right) and 24 h after injection of fluorescent microspheres under light (middle) and fluorescence (lower) microscopy of whole mounted seminiferous. Dashed lines, tubule wall borders. Arrow, cluster of microspheres internalized by Sertoli cells. e, 15P-1 Sertoli cells on Matrigel incubated with fluorescent targets along with GST or BAI1-TSR protein. f-g, Intra-testicular injection of GST or BAI1-TSR into 6-8 week old C57BL/6 mice. f, Apoptotic cells detected by IHC (apostain, brown) after 24 hr. Magnification, 12.5× (upper panels) and 25× (lower panels). Arrow, apoptotic cells. g, Sperm counts normalized to testis weight. n=3, *p<0.05.
Prior to addressing the potential role of BAI1 in engulfment in vivo, we designed an in vivo model, wherein apoptotic targets can be injected into the rete testes of mice by in vivo micropuncture, providing a retrograde filling of the seminiferous tubules (Fig. 4d). We injected surrogate apoptotic targets (fluorescently labeled 2.1 μm carboxylate modified beads) into the rete testis of mice, and 24-hours later prepared cryosections or squash preparations of the testes. Introduction of targets via this approach filled the seminiferous tubules on the luminal side of the testes and the beads specifically clustered/ingested along the lining of the tubule where Sertoli cells were present (Fig. 4d). We also established conditions for introducing soluble proteins/peptides into the interstitium of the testes by intratesticular injection. To address the potential role of BAI1 in engulfment by Sertoli cells in vivo, we used a soluble fragment containing the TSR region of BAI1 (denoted GST-BAI1-TSR)7 that strongly blocked the in vitro binding/internalization of apoptotic targets to murine Sertoli cells (Fig. 4e). We microinjected soluble BAI-TSR peptide into the intestitium of one testes and the control protein GST in the second testis of the same animal (3 mice/group). After 8 days, we analyzed the testes and made three striking observations. First, there was a marked increase in apoptotic germ cell nuclei, analogous to the genetic disruption of Elmo1 (Fig. 1e and 4f). Second, we also saw a marked disruption of the seminiferous epithelium similar to the Elmo1−/− mice (Fig. 1d and 4f). Third, injection of BAI1-TSR also led to reduced levels of mature sperm in the testes (Fig. 4g). Thus, the acute disruption of apoptotic cell recognition through BAI1-TSR confirms and extends the phenotype seen in mice with genetic deletion of Elmo1 in Sertoli cells.
Collectively, these studies define a mammalian engulfment signaling pathway required for efficient clearance of apoptotic germ cells and identify a non-redundant role for the cytoplasmic engulfment protein ELMO1 and its upstream engulfment receptor BAI1 in this process6, 7. Our findings also provide a formal genetic link between ELMO1 and one of the key functions of Sertoli cells, i.e. the clearance of apoptotic germ cells.
Although it has long been proposed that clearance of dying cells is a key component of organogenesis in mammals, genetic models demonstrating this have been minimal. The data presented here using conditional Elmo1 knockout mice provides such an example. Still, our work does not exclude contribution from other signaling pathways that may operate concurrently for Sertoli cell-mediated clearance of apoptotic germ cells. Other corpse recognition mechanisms may also be operational in Sertoli-cell mediated clearance of apoptotic germ cells, and testes homeostasis, including scavenger receptors (SRB1, CD36)24, 25 and MER/Axl/Tyro3 family members26, 27, although signaling downstream of these receptors are not well defined. Additional PtdSer recognition/bridging molecules (such as MFG-E8) and integrins expressed in the testes may provide further redundancy in corpse clearance28, 29, and whether ELMO1 may participate signaling downstream of these additional recognition mechanisms in Sertoli cells is unclear. Due to the less pronounced phenotype in the Amh-Cre/Elmo1F/F mice compared to straight Elmo1−/− mice, it is possible that ELMO1 may also play a role in other cell types in the testes. Finally, this work reveals a novel connection between efficient corpse clearance and spermatogenesis that may open up newer avenues of investigations into immune privilege in the testes and removal of non-viable germ cells. Since the failure to promptly recognize and clear dying cells has deleterious consequences (such as autoimmunity)28, 30, defining the engulfment steps during germ cell clearance could be important for disease states such as orchitis, and loss of tolerance to testicular antigens.
Methods
Antibodies and reagents
The anti-ELMO1 antibody has been described previously21. Anti-ELMO2 was generated by immunizing rabbits with GST-tagged full-length mouse ELMO2. Anti-ELMO1 antibody (for immunohistochemistry), Erk2, Dock180, Anti-Müllerian hormone and CathepsinL antibodies were purchased from Santa Cruz Biotechnology Inc. Other antibodies were purchased from the following sources: RAC1 (Upstate Biotechnology), F7-26 anti-ssDNA (Apostain; Alexis Biochemicals), β-actin (Sigma-Aldrich), and GFP (Invitrogen). DNA oligonucleotides were obtained from Integrated DNA Technologies. Chemicals used for isolation and routine culture of Sertoli cells were purchased from Sigma-Aldrich.
Elmo1 conditional knockout mice
The Elmo1 targeting vector was generated using an 11.5 kb BamHI-XhoI fragment of Elmo1 genomic DNA containing exon 5 isolated from a Sv/129 mouse strain BAC library (Incyte Genomics). The 5′ and 3′ homology arms were inserted into the pK11-frt-PgkNeo-frt-loxP plasmid32 using standard subcloning procedures. For the 5′ homology arm, a 3.0 kb BglII to ApaI fragment was inserted 5′ to the frt-flanked neomycin resistance cassette. The 4.3 kb ApaI to AvrII fragment containing exon 5 was inserted 3′ of the neomycin cassette. A loxP site was introduced into the ApaI site 3′ of the second frt sequence. A second loxP site was inserted into the KpnI site 2.3 kb downstream of the first loxP site to flank exon 5 to allow conditional Cre-mediated deletion. The entire Elmo1 region of the targeting construct was sequenced to ensure fidelity. IB10 ES cells were electroporated with 20 μg of SacI-linearized vector and selected with 200 mg/mL Geneticin (Invitrogen) on mitomycin C-treated MEF cells. Of 224 neomycin-resistant ES clones screened, two were positive for homologous recombination as determined by PCR and Southern blotting using multiple restriction enzymes and probes (Fig. S1d and data not shown). After blastocyst injections, chimeras were bred to C57BL/6 mice. The offspring were then crossed to β-actin/Flp mice (Jackson) to delete the neomycin cassette, yielding mice carrying a “floxed” Elmo1 exon 5 allele (Elmo1F, Fig. S1d). For the straight Elmo1 knockout, mice with floxed Elmo1 allele were crossed to EIIa-Cre mice (Jackson) expressing Cre from the two cell stage of embryonic development to delete Elmo1 exon 5 in all tissues33. These mice were then crossed with C57BL/6 to remove Flp and Cre from background. Resulting mice, lacking Flp and Cre, were then intercrossed to obtain the straight knockout mice used for this study.
For Sertoli cell-specific targeting, mice expressing Cre under control of the anti-Müllerian hormone promoter (Amh-Cre) were obtained from Jackson Laboratory20. Specificity of Cre-mediated deletion in Sertoli cells was tested by crossing Amh-Cre+ males to female Rosa26-YFP mice expressing EYFP in the Rosa26 locus downstream of a loxP-flanked STOP cassette34. The testes of mice positive for YFP and Amh-Cre were tested for Sertoli-specific deletion of the STOP cassette (by YFP expression) by immunohistochemistry and confocal fluorescent imaging of whole-mounted seminiferous tubules (Fig. S5). For targeted deletion of Elmo1 in Sertoli cells, Elmo1F/F females were crossed with Amh-Cre+/Elmo1F/W males to obtain the Amh-Cre+/Elmo1F/F mice used in this study.
Mouse genotyping
Genotyping by Southern blot was carried out using probes generated by PCR from Elmo1 BAC template DNA. Two probes, one 5′ (SBP2) and another 3′ (SBP5) of the targeting sequence were generated using the following primers: SBP2F: 5′-tct cct cct gct ttc tca ttc cgt, SBP2R: 5′-ggc ctg tta cag tta gag aga acc ca, SBP5F: 5′-tct ccc ctt tgc caa tcc cac atc, SBP5R: 5′-cag ctg cgg agg gac ttt aag gaa. Southern blotting of BglI digested of genomic DNA yielded the predicted fragments of 10 kb (WT), 4.9 kb (targeted) and 8.1 kb (Elmo1Δ5) for both probes. Also, NheI and SacI double digestion of DNA produced the predicted fragments of 10.6 kb (WT) and 8.6 kb (Elmo1D5) mice by Southern blotting with SBP2.
Routine genotyping of mice was done by PCR using the following primers: Elmo1 WT (F): acc cat atc agg ctg ccc aca taa, Elmo1 WT (R): atg caa tgt gag cag cat tcc ctc, Elmo1Δ5 (F): gct tcc tga cgg tgg atg caa tgt, Elmo1Δ5 (R): ggc cgc gtc gac gaa gtt cct ata. For detection of WT versus floxed Elmo1 alleles, Elmo1 WT primers were used and yielded amplicons of 302 bp (WT) and 342 bp (floxed). For detection of WT versus exon5 deleted alleles, all four primers were used in a single PCR reaction and yielded bands of 302 bp (WT) and 338 bp (Δ5). PCR screening for Amh-Cre was done using the PCR primers and conditions recommended by the provider.
Sertoli cell isolation and tissue culture
For isolation of primary murine Sertoli cells according to previously published methods35, 10-12 day old animals were euthanized and the testes removed and decapsulated in HBSS. The seminiferous tubules were washed once in HBSS and then gently dispersed in HBSS/0.25% trypsin/10 μg per mL DNase using fine tweezers. To this, 0.25% final soybean trypsin inhibitor (SBTI) was added and the supernatant containing interstitial cells was discarded. Dispersed tubules were then resuspended in HBSS/1 M glycine/2 mM EDTA/0.01% SBTI/10 μg per mL DNase and incubated at room temperature for 10 min and centrifuged at 1000xg for 2 min. The tubules were resuspended in HBSS/1mg per mL hyaluronidase/10 μg per mL DNase, minced with a razor and incubated for 30 min in a 37°C shaking water bath. Resulting fragments were allowed to sediment in a tube for 10 min at room temperature and the suspended material removed and Sertoli cell-enriched fragments were washed in HBSS by centrifugation. The fragments were then incubated with HBSS/1mg per mL hyaluronidase/10 μg per mL DNase and incubated for 30 min in a 37°C shaking water bath to release Sertoli cells. Cells were then washed once in HBSS and resuspended in complete Sertoli medium (F12/DMEM medium with 10% HIFBS, 1% Na pyruvate, 15 mM HEPES, 5 μg/mL transferrin, 2.5 ng/mL EGF, 10 μg/mL insulin, 1% Pen-Strep). Cell were plated on 10 cm tissue culture dishes and incubated at 34°C/5% CO2. After 2 days, non-adherent cells were removed by washing with complete medium. Fresh complete medium was added and the adherent Sertoli cells were incubated at 34°C/5% CO2 for 4 more days. Cells were then collected by trypsinization, and the total number of Sertoli cells were counted by Trypan exclusion on a hemacytometer (Figure 2c) and replated as indicated. This population was found to be >90% positive for Sertoli markers Cathepsin L and anti-Müllerian hormone by immunofluorescence microscopy (see Fig. 2d and Supplementary Fig. S4a).
For apoptotic germ cell preparation, testes of 8-14 week old C57BL/6 mice were prepared as above except that the non-adherent cells were collected after 18-24 hrs. This population was found to be 40-50% apoptotic by flow cytometry (Annexin V+/propidium iodide-, data not shown). TM4 and 15-P1 Sertoli cells were routinely cultured using the same conditions as primary Sertoli cells.
Phagocytosis assays (beads, cells, bubbles)
Sertoli phagocytosis assays were carried out in either complete Sertoli medium for primary Sertoli or in simplified medium (F12/DMEM, 10% HIFBS, Pen-Strep) for TM4 and 15P-1 cells. For bead engulfment, 6 μL of red fluorescent 2.1 μm carboxylate beads (Invitrogen) in 500 μL medium per well were incubated for 2 hrs at 34°C/5% CO2. For germ cell engulfment, apoptotic germ cells were labeled with 5 μM CypHer5 dye (GE Life Sciences) in HBSS for 30 min at 37°C/5% CO2. After washing, 5×105 germ cells in 500 μL engulfment medium per well were added to the Sertoli cells, centrifuged at 50xg/30 sec and incubated at 34°C/5% CO2 for 2 hrs. Microbubble uptake was performed as previously described for a total of 30 min7. For analysis of all phagocytosis assays, wells were washed 5 times with cold HBSS, trypsinized, and brought to 400 μL final volume with engulfment medium. Samples were analyzed by flow cytometry.
Immunohistochemistry
Testes were fixed in Bouin’s solution, paraffin-embedded and 8 μm cross-sections prepared. Immunodetection was carried out as previously described using the indicated antibodies18. For enumeration of apoptotic cells, Apostain labeled sections were examined under 12.5× magnification and the total number of tubules and apoptotic cells were counted from at least two sections per testis per mouse.
Giant cell analysis
Testes were fixed in Bouin’s solution and cross sections prepared and stained with hematoxylin and eosin. Giant cells were identified by their characteristic features of having multiple (≥2) round spermatid nuclei in a syncytial body present in the lumen. For each section of testis, the total number of giant cells along with the total number of seminiferous tubules were counted and the average number of giant cells per tubule determined (Figure 1d).
Immunofluorescence microscopy
Primary murine Sertoli cells were plated in culture medium on LabTek II chamber slides (Nunc) at 3-5×104 cells/well and cultured overnight. Cells were washed and fixed with 3.2% paraformaldehyde in PBS for 10 min at room temperature and permeabilized in 0.2% Triton X-100 in PBS for 2 min at room temperature. After blocking with 1% BSA/PBS, cells were stained with the indicated antibody (1:100) or phalloidin-Alexa488 (Invitrogen) at 1:50 for 1 h at room temperature. After washing, cells were incubated with fluorochrome-labeled secondary antibodies for 1 hr at room temperature, washed and mounted with ProLong Gold + DAPI (Invitrogen). Microscopy was performed using the Axio Imager 2 (Zeiss).
qPCR
DNAase I-treated RNA was obtained by RNeasy (Qiagen), and 50-100 ng of RNA was used as template to generate cDNA using Superscript III SuperMix (Invitrogen). qPCR was performed using gene-specific TaqMan probes on a StepOne Plus Real-Time PCR instrument (Applied Biosystems). TaqMan probes used are as follows: mElmo1 (Mm00519109_m1), mElmo2 (Mm01248046_m1), mElmo3 (Mm00555221_g1), mBai1 (Mm00558144_m1), mTim4 (Mm00724709_m1), mStabilin2 (Mm00454684_m1), mGapdh (4352339E).
Rac-GTP assay
The amount of endogenous active (GTP-bound) Rac was assayed by precipitation of cell lysates using GST-CRIB beads as previously described36. Briefly, 24 hrs after plasmid transfection TM4 cells were washed twice and lysed. The cell lysates were incubated with 30 μg of GST-CRIB immobilized on beads for 1 h at 4 °C. The precipitation of RAC by GST-CRIB was detected by immunoblotting with anti-RAC1.
Transfections
For knockdown experiments, TM4 Sertoli cells were transfected with 1 μg of SmartPool siRNA (Dharmacon) using the Nucleofector Kit L with the T-30 program according to manufacturers’ instructions (Amaxa). Forty-eight hours post-transfection, cells were trypsinized and replated at 5×104 cells/well of a 24-well plate in triplicate and cultured overnight prior to phagocytosis assay. For overexpression experiments, TM4 cells were transfected as above using 5-15 μg of previously described ELMO1 and GFP plasmid DNA7 and used in assays at 24 hours post-transfection.
Supplementary Material
Acknowledgements
We thank members of the Ravichandran and Lysiak groups for helpful advice in the preparation of this manuscript. We also thank Lisa Haney, Annie-Carole Tosello-Trampont, Jungeun Kim and Sarah Klugston for expert assistance. This work was supported by funding from the National Institutes of Health (K.S.R. and J.J.L.) and the American Cancer Society (M.R.E.).
Footnotes
Author Contributions
The project was conceived and the experiments planned by J.J.L., K.S.R and M.R.E. M.R.E. generated the Elmo1 knockout mice and performed and analysed most of the experiments in this study. S.Z. carried out the experiments using ELMO-GFP constructs. R.I.W. performed all of the IHC, Sertoli cell extraction and sperm counts. M.A.R. performed the microbubble uptake experiments. I.J.J. performed the phagocytosis assays on non-Sertoli cells. D.P. performed phagocytosis assays with Bai1 siRNA and synthesized and characterized Bai1-TSR. C. elegans mismigration analysis using Elmo1 transgenes were conducted by J.M.K. J.Z. assisted with immunoblotting and qPCR studies of Sertoli cells. J.J.L. performed intratesticular injections. K.S.R., M.R.E and J.J.L. wrote the manuscript with input from the co-authors.
References
- 1.Henson PM. Dampening inflammation. Nat Immunol. 2005;6:1179–1181. doi: 10.1038/ni1205-1179. [DOI] [PubMed] [Google Scholar]
- 2.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Gregory CD, Pound JD. Results of Defective Clearance of Apoptotic Cells: Lessons from Knock-out Mouse Models. In: Krysko DV, Vandenabeele P, editors. Phagocytosis of Dying Cells. Vol. 1. Springer Science; 2009. pp. 271–298. [Google Scholar]
- 4.Zhou Z, Caron E, Hartwieg E, Hall A, Horvitz HR. The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev Cell. 2001;1:477–489. doi: 10.1016/s1534-5807(01)00058-2. [DOI] [PubMed] [Google Scholar]
- 5.Wu YC, Tsai MC, Cheng LC, Chou CJ, Weng NY. C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev Cell. 2001;1:491–502. doi: 10.1016/s1534-5807(01)00056-9. [DOI] [PubMed] [Google Scholar]
- 6.Gumienny TL, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 7.Park D, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 8.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z, Mangahas PM, Yu X. The genetics of hiding the corpse: engulfment and degradation of apoptotic cells in C. elegans and D. melanogaster. Curr Top Dev Biol. 2004;63:91–143. doi: 10.1016/S0070-2153(04)63004-3. [DOI] [PubMed] [Google Scholar]
- 10.deBakker CD, et al. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr Biol. 2004;14:2208–2216. doi: 10.1016/j.cub.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Cool J, Capel B. Mixed signals: development of the testis. Semin Reprod Med. 2009;27:5–13. doi: 10.1055/s-0028-1108005. [DOI] [PubMed] [Google Scholar]
- 12.Loveland KL, et al. Drivers of germ cell maturation. Ann N Y Acad Sci. 2005;1061:173–182. doi: 10.1196/annals.1336.018. [DOI] [PubMed] [Google Scholar]
- 13.Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 14.Bailey RW, Aronow B, Harmony JA, Griswold MD. Heat shock-initiated apoptosis is accelerated and removal of damaged cells is delayed in the testis of clusterin/ApoJ knock-out mice. Biol Reprod. 2002;66:1042–1053. doi: 10.1095/biolreprod66.4.1042. [DOI] [PubMed] [Google Scholar]
- 15.Braun RE. Every sperm is sacred--or is it? Nat Genet. 1998;18:202–204. doi: 10.1038/ng0398-202. [DOI] [PubMed] [Google Scholar]
- 16.Rotter V, et al. Mice with reduced levels of p53 protein exhibit the testicular giant-cell degenerative syndrome. Proc Natl Acad Sci U S A. 1993;90:9075–9079. doi: 10.1073/pnas.90.19.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holstein AF, Eckmann C. Multinucleated spermatocytes and spermatids in human seminiferous tubules. Andrologia. 1986;18:5–16. doi: 10.1111/j.1439-0272.1986.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 18.Lysiak JJ, Zheng S, Woodson R, Turner TT. Caspase-9-dependent pathway to murine germ cell apoptosis: mediation by oxidative stress, BAX, and caspase 2. Cell Tissue Res. 2007;328:411–419. doi: 10.1007/s00441-006-0341-y. [DOI] [PubMed] [Google Scholar]
- 19.Lysiak JJ, Turner SD, Turner TT. Molecular pathway of germ cell apoptosis following ischemia/reperfusion of the rat testis. Biol Reprod. 2000;63:1465–1472. doi: 10.1095/biolreprod63.5.1465. [DOI] [PubMed] [Google Scholar]
- 20.Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 21.Brugnera E, et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 2002;4:574–582. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- 22.Miyanishi M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 23.Park SY, et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2007 doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 24.Shiratsuchi A, Kawasaki Y, Ikemoto M, Arai H, Nakanishi Y. Role of class B scavenger receptor type I in phagocytosis of apoptotic rat spermatogenic cells by Sertoli cells. J Biol Chem. 1999;274:5901–5908. doi: 10.1074/jbc.274.9.5901. [DOI] [PubMed] [Google Scholar]
- 25.Gillot I, et al. Germ cells and fatty acids induce translocation of CD36 scavenger receptor to the plasma membrane of Sertoli cells. J Cell Sci. 2005;118:3027–3035. doi: 10.1242/jcs.02430. [DOI] [PubMed] [Google Scholar]
- 26.Lu Q, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, et al. Functions of TAM RTKs in regulating spermatogenesis and male fertility in mice. Reproduction. 2009;138:655–666. doi: 10.1530/REP-09-0101. [DOI] [PubMed] [Google Scholar]
- 28.Hanayama R, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 29.Akakura S, et al. The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp Cell Res. 2004;292:403–416. doi: 10.1016/j.yexcr.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 30.A-Gonzalez N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lysiak JJ, et al. Essential role of neutrophils in germ cell-specific apoptosis following ischemia/reperfusion injury of the mouse testis. Biol Reprod. 2001;65:718–725. doi: 10.1095/biolreprod65.3.718. [DOI] [PubMed] [Google Scholar]
- 32.Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- 33.Lakso M, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong W, et al. Gas6 and the Tyro 3 receptor tyrosine kinase subfamily regulate the phagocytic function of Sertoli cells. Reproduction. 2008;135:77–87. doi: 10.1530/REP-07-0287. [DOI] [PubMed] [Google Scholar]
- 36.Lu M, et al. PH domain of ELMO functions in trans to regulate Rac activation via Dock180. Nat Struct Mol Biol. 2004;11:756–762. doi: 10.1038/nsmb800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.