Abstract
Age-related macular degeneration (AMD) is still an incurable blinding eye disease because of complex pathogenic mechanisms and unusual diseased regions. With the use of chemical biology tools, great progress has been achieved in improving the understanding of AMD pathogenesis. The severity of AMD is, at least in part, linked to the non-degradable lipofuscin bis-retinoids in retinal pigment epithelial (RPE). This material is thought to result from the lifelong accumulation of lysosomal residual bodies containing the end products derived from the daily phagocytosis of rod outer segments by RPE cells. Here, we present previously recognized bis-retinoids with focus on structures and biosynthetic pathways. In addition to a brief discussion on the mutual conversion relationships of bis-retinoids, future perspectives and the medical relevance of such studies on these lipofuscin constituents are also highlighted.
Keywords: Age-related macular degeneration (AMD), Lipofuscin, Retinal pigment epithelial (RPE), Bis-retinoids, Structures, Biogenesis, Conversion relationships
1. Introduction
Time-dependent lipofuscin accumulation in retinal pigment epithelial (RPE) cells has been notoriously deemed as a major risk factor in association with age-related macular degeneration (AMD) (Bressler et al., 1988; Eldred and Katz, 1988; Eldred and Lasky, 1993; Kliffen et al., 1997; Sarks et al., 1999; Delori et al., 2000). AMD, the leading cause of blindness in elderly people, is a degenerative disease of the eye (Bressler et al., 1988; Mata et al., 2000; Telander, 2011), for which there is thus far no remedy. Increasing accumulation of lipofuscin granules in RPE is not a feature of aging RPE alone given that excessive amounts of these fluorophores are also remarkably detected in the RPE of juvenile patients with Stargardt’s disease (Eagle et al., 1980; Feeney-Burns et al., 1984; Rabb et al., 1986; Birnbach et al., 1994; Kennedy et al., 1995) caused by mutations in Abca4 gene (Delori et al., 1995; Shroyer et al., 1999). RPE lipofuscin is an enzymatically non-degradable heterogeneous mixture of numerous biomolecules (Ng et al., 2008), in which extensive attention has been drawn to the indigestible bis-retinoid constituents that contribute to the etiology of inherited and AMD (Broniec et al., 2005; Sparrow et al., 2010a).
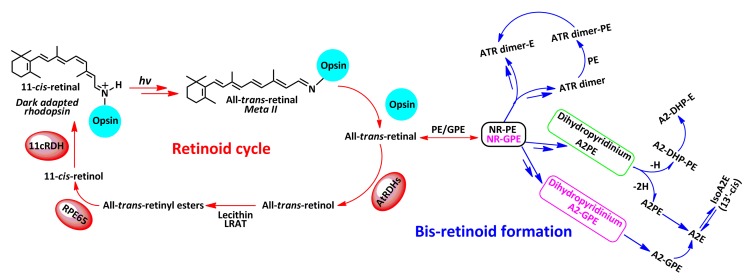
Vitamin A cycling leading to vision occurs in the retina, and is also regarded as the origin of adverse lipofuscin bis-retinoids (Fig. 1) (Borhan et al., 2000; Sparrow et al., 2010d). After capturing a photon, the inherent visual chromophore 11-cis-retinal bound to lysine296 of opsin, with a protonated covalent Schiff base bond, isomerizes to all-trans-retinal (Rando, 1996; Borhan et al., 2000). A sequence of conformational rearrangements causes the dark-adapted rhodopsin to undergo metarhodopsin II (Meta II) transition (Sakmar, 1997; Gelasco et al., 2000; Burns and Baylor, 2001; Okada et al., 2001; Isin et al., 2006). Given that Meta II comprises all-trans-retinal and opsin that bind together by a deprotonated Schiff base linkage, the absorbance of this light-activated intermediate is at 380 nm, reflecting around 120-nm blue shift relative to the maximal absorbance (λ max) value of native rhodopsin (500 nm) (Borhan et al., 2000; Liu et al., 2011). A hydrolysis of Meta II results in the release of a free all-trans-retinal, followed by a reduction in the latter to all-trans-retinol under the action of all-trans-retinol dehydrogenases (atRDHs). As an alternative, some all-trans-retinals react with either phosphatidylethanolamine (PE) or glycerophosphoethanolamine (GPE) distributed in the lipid bilayer to generate two covalent adducts, N-retinylidene-phosphatidylethanolamine (NR-PE) and N-retinylidene-glycerophosphoethanolamine (NR-GPE). After that, ABCA4 (ATP-binding cassette, sub-family A (ABC1), member 4) facilitates transfer of NR-PE/NR-GPE trapped inside the disk as a charged species out to the cytoplasmic surface (Fig. 2), where they hydrolyze to leave PE/GPE and all-trans-retinal (Molday et al., 2006). Subsequent reduction of the latter by atRDHs generates all-trans-retinol. Within the RPE cells, following the esterification of all-trans-retinol by the enzyme lecithin retinol acyl transferase (LRAT), RPE65, the visual cycle retinol isomerase, isomerizes the resulting ester from the all-trans configuration to the 11-cis-retinol. By 11-cis-retinol dehydrogenase (11cRDH), the gained alcohol is finally oxidized to 11-cis-retinal; this chromophore will bind opsin to regenerate the fresh rhodopsin (Jäger et al., 1996; Kim et al., 2004; Sparrow et al., 2010d). On the other hand, since some NR-PEs/NR-GPEs evade the degradation to create all-trans-retinal and PE, the pathway for formation of bis-retinoids starts with the condensation reaction of NR-PE/NR-GPE and a second molecule of all-trans-retinal. As depicted in Fig. 1, a multi-step pathway leads to the formation of dihydropyridinium N-retinylidene-N-retinyl-phosphatidylethanolamine (A2PE) and dihydropyridinium N-retinylidene-N-retinyl-glycerophosphoethanolamine (A2-GPE). Automatic oxidation of these two intermediates generates A2PE and A2-GPE, the immediate precursors of N-retinylidene-N-retinyl-ethanolamine (A2E). Loss of one hydrogen (–H) from dihydropyridinium A2PE generates N-retinylidene-N-retinyl-dihydropyridine-phosphatidylethanolamine (A2-DHP-PE); phosphate hydrolysis of the latter produces N-retinylidene-N-retinyl-dihydropyridine-ethanolamine (A2-DHP-E). Via an alternative path, all-trans-retinal dimer (ATR dimer) forms from the condensation of two molecules of all-trans-retinal. Reaction of ATR dimer with PE by formation of a protonated Schiff base linkage generates ATR-dimer-PE; phosphate hydrolysis of the latter yields ATR dimer-E. The toxic bis-retinoids form in rod outer segments that consist of nearly 1 500 thin disks (Fig. 2). Daily phagocytosis of spent photoreceptor outer segment debris by RPE cells is extremely critical for vision and, as a consequence, leads to the transfer of bis-retinoid pigments to the lysosomal bodies of RPE cells (Feeney-Burns, 1980; Feeney-Burns et al., 1984; Clancy et al., 2000; Sparrow and Boulton, 2005; Wu et al., 2011).
Fig. 1.
Retinoid cycle and bis-retinoid formation of lipofuscin in the eye
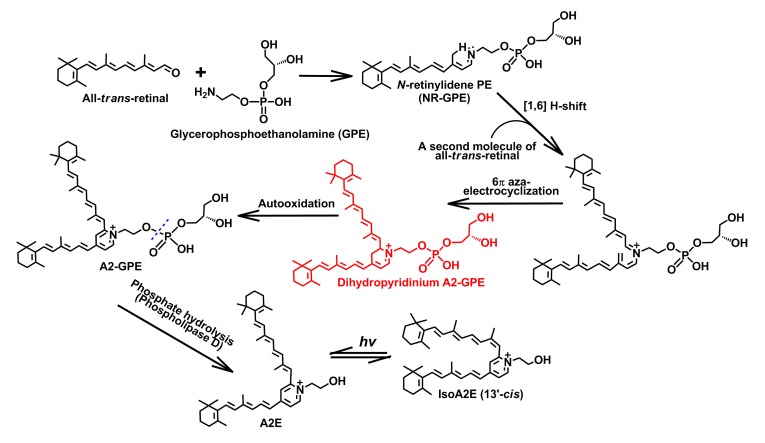
According to the proposed scheme that was published previously (Sparrow et al., 2010d), we here expand the schematic diagram regarding the formation of lipofuscin bis-retinoids; the leading feature is the formation of a novel bis-retinoid compound N-retinylidene-N-retinyl-glycerophosphoethanolamine (A2-GPE) (Yamamoto et al., 2011). Biogenesis of the latter starts with one molecule of all-trans-retinal incubated with GPE, followed by a [1,6]H-shift to give rise to N-retinylidene-glycerophosphoethanolamine (NR-GPE). Via a multi-step cascade, a proposed intermediate, dihydropyridinium A2-GPE is generated, and undergoes auto-oxidation to yield A2-GPE. Phosphate hydrolysis of the latter by phospholipase D (PLD) produces N-retinylidene-N-retinyl-ethanolamine (A2E), indicating that A2-GPE, like N-retinylidene-N-retinyl-phosphatidylethanolamine (A2PE), may also serve as the precursor of A2E
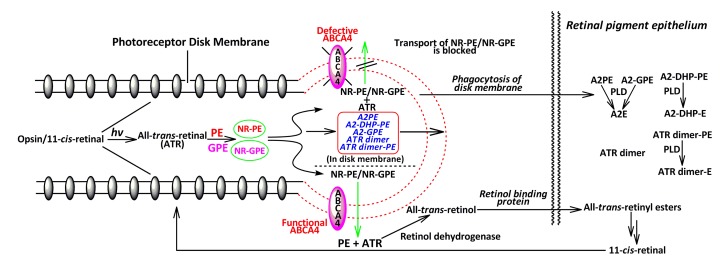
Fig. 2.
Correlation of the ABCA4 transporter with lipofuscin bis-retinoid biosynthesis
All-trans-retinal (ATR) comes from the isomerization of 11-cis-retinal that is released from light-activated rhodopsin in the retina. Incubation of ATR with phosphatidylethanolamine (PE)/glycerophosphoethanolamine (GPE) would give rise to N-retinylidene-phosphatidylethanolamine (NR-PE)/N-retinylidene-glycerophosphoethanolamine (NR-GPE) that can be delivered to the cytoplasmic side of disk membranes by ABCA4. When the activity of this transporter attenuates or becomes extinct, NR-PE and NR-GPE have no choice but to reside in the rod outer segments, and react with a second molecule of ATR to form various bis-retinoids including N-retinylidene-N-retinyl-phosphatidylethanolamine (A2PE), N-retinylidene-N-retinyl-dihydropyridine-phosphatidylethanolamine (A2-DHP-PE), N-retinylidene-N-retinyl-glycerophosphoethanolamine (A2-GPE), ATR dimer, and ATR dimer-PE via a multi-step cascade. Rod outer segments are routinely discarded on a daily basis, and subsequently are phagocytosed by RPE cells, resulting in the transfer of these di-retinal pigments to RPE where PLD, a lysosomal acid enzyme, can cleave A2PE, A2-GPE, A2-DHP-PE, and ATR dimer-PE to produce N-retinylidene-N-retinyl-ethanolamine (A2E), N-retinylidene-N-retinyl-dihydropyridine-ethanolamine (A2-DHP-E), and ATR dimer-E, respectively. However, ATR dimer remains intact due to the inability to be further digested by the lysosomal enzymes
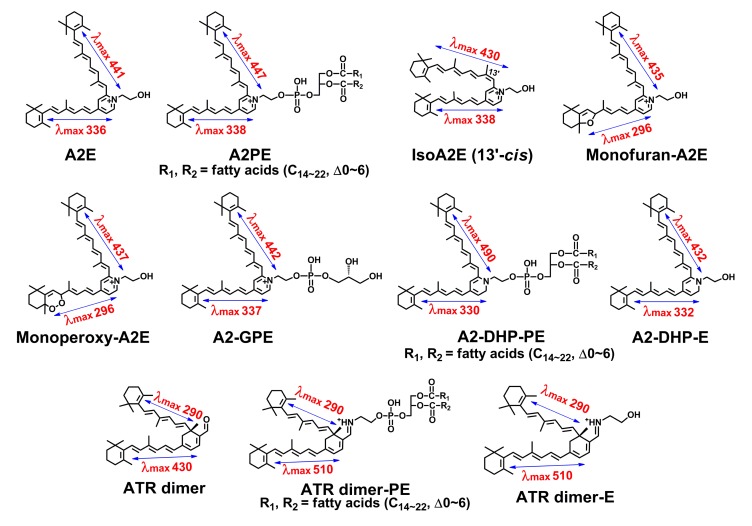
Up to the present time, at least eleven bis-retinoid pigments (Fig. 3) present in human RPE and mouse eyecups have been characterized structurally and chromatographically. Given significant implications of lipofuscin bis-retinoids in AMD, this review will mainly focus on the advances in both structure and biogenetic analysis of these inadvertent fluorophores.
Fig. 3.
Eleven bis-retinoids associated with formation of retinal pigment epithelial (RPE) lipofuscin
Structures, UV-visible absorbance (nm), and electronic transition assignments (↔) are shown. All of these fluorescent di-retinal pigments have dual conjugation systems located in two side-arms, conferring light-induced absorbance in both the ultraviolet and visible regions
2. Bis-retinoids with a pyridinium ring
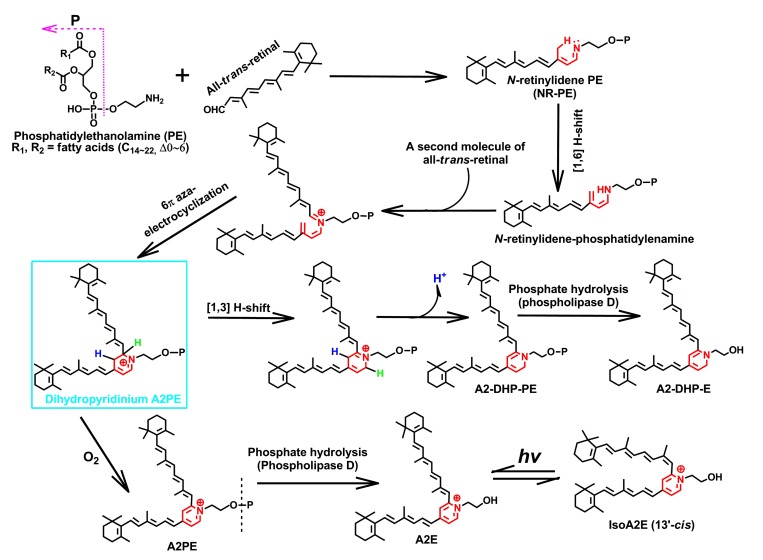
To date, the identified pyridinium bis-retinoids with a positive charge on the nitrogen atom residing in the six-membered heterocyclic ring include A2E, isoA2E (13′-cis), A2PE, oxidized derivatives of A2E and A2-GPE. A2E, an unprecedented pyridinium bis-retinoid was the most studied ocular age pigment, and was structurally characterized by Sakai et al. (1996). Ren et al. (1997) completed its first total synthesis. One year later, Parish et al. (1998) optimized in vitro A2E synthesis, which led to the one-step biomimetic preparation of this pigment in 49% yield. Given that A2E has two hydrophobic side-arms derived from all-trans-retinal, together with a hydrophilic pyridinium head group, this molecule is conferred with an unusual amphiphilic property (Sparrow et al., 2003a). Evidence from several fronts (Poincelot et al., 1969; Anderson and Maude, 1970; Mata et al., 2000; Sparrow et al., 2010a) denotes that the biosynthetic route of A2E (Fig. 4) begins in the photoreceptor outer segment membrane as a condensation reaction between PE and a single molecule of all-trans-retinal that generates a Schiff base conjugate NR-PE. NR-PE goes through a [1,6]-proton tautomerization to yield an N-retinylidene-phosphatidylenamine (Fig. 4). It is postulated that subsequent condensation of the latter with a second molecule of all-trans-retinal and 6π-aza-electrocyclization will produce an imperative phosphatidyl-dihydropyridinium bis-retinoid intermediate (dihydropyridinium A2PE) (Fig. 4) (Kim et al., 2007; Sparrow et al., 2012). Automatic oxidation of dihydropyridinium A2PE with loss of two hydrogens (−2H) yields A2PE, the immediate precursor of A2E and isoA2E (13′-cis). It is interesting to observe that A2E and isoA2E (13′-cis) would reach photo-equilibrium at a ratio of 4:1 when exposed to room light (Parish et al., 1998). The (430±20) nm blue light irradiation of A2E-laden ARPE-19 cells in a dark room generated two detectable types of oxidized A2E: monofuran-A2E and monoperoxy-A2E, and they also were found in aged human RPE and in eyecups of Abca4 −/− mice, an animal model of Stargardt’s disease (Jang et al., 2005).
Fig. 4.
Biosynthetic pathways of N-retinylidene-N-retinyl-ethanolamine (A2E) and N-retinylidene-N-retinyl-dihydropyridine-phosphatidylethanolamine (A2-DHP-PE)
These two bis-retinoids form from a proposed uniform intermediate, dihydropyridinium N-retinylidene-N-retinyl-phosphatidylethanolamine (A2PE). The latter is enclosed by a rectangle and is not detectable in the eye and biomimetic chemical reactions, probably resulting from the facile auto-oxidation of a unique dihydropyridinium ring, from which one or double particular hydrogen is eliminated with the electronic rearrangements. Hydrogens in blue and green represent the active atoms involved in the shifting and eliminating processes in the biosynthesis of these two heterocyclic fluorophores (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
Most recently, Yamamoto et al. (2011) mentioned that Sparrow and his colleagues illustrated a fresh bis-retinoid of retina, A2-GPE, to be more abundant than A2E in the eyecups of Abca4 −/− mice and to form from incubation of two molecules of all-trans-retinal and GPE. Due to the similarity in structure between PE and GPE, the biosynthesis of A2-GPE (Fig. 5), slightly different from that of A2E, starts with a condensation reaction between GPE and all-trans-retinal, which creates a Schiff base conjugate NR-GPE. Subsequently, a sequence of chemical processes including [1,6]-proton tautomerization, condensation reaction with a second molecule of all-trans-retinal and 6π-aza-electrocyclization, yields dihydropyridinium A2-GPE, the proposed intermediate in the biosynthetic pathway. With loss of two hydrogens (−2H) arising from the automatic oxidation, dihydropyridinium A2-GPE is converted into A2-GPE containing a pyridinium ring. An in vitro enzyme assay manifested that phospholipase D (PLD) activity can cleave A2-GPE to release A2E, thereby reflecting that A2-GPE, like A2PE, may also serve as a precursor of A2E (Sparrow et al., 2008). Like NR-PE acting as a key intermediate to form A2PE, NR-GPE also plays an important role in the biosynthetic pathway of A2-GPE. To corroborate A2-GPE biogenesis, it will be highly urgent to detect NR-GPE in mouse eyecups and human RPE. It is also worth noting that dihydropyridinium A2PE enclosed with a rounded rectangle (Fig. 4) and dihydropyridinium A2-GPE (Fig. 5) were not detectable in eyes and biomimetic chemical reactions on account of the transient auto-oxidation behavior.
Fig. 5.
Biosynthetic pathway of N-retinylidene-N-retinyl-glycerophosphoethanolamine (A2-GPE)
N-retinylidene-glycerophosphoethanolamine (NR-GPE) and dihydropyridinium A2-GPE, like N-retinylidene-phosphatidylethanolamine (NR-PE) and dihydropyridinium N-retinylidene-N-retinyl-phosphatidylethanolamine (A2PE) in the N-retinylidene-N-retinyl-ethanolamine (A2E) biosynthetic route, ought to play important roles in the biogenesis of A2-GPE. Due to the susceptibility of the dihydropyridinium ring to auto-oxidation, it is concluded that dihydropyridinium A2-GPE, like dihydropyridinium A2PE, is not found in the eye and biomimetic chemical reactions, from which NR-GPE is, however, possible to be trapped by high-performance liquid chromatography (HPLC)
3. Bis-retinoids with a dihydropyridine ring
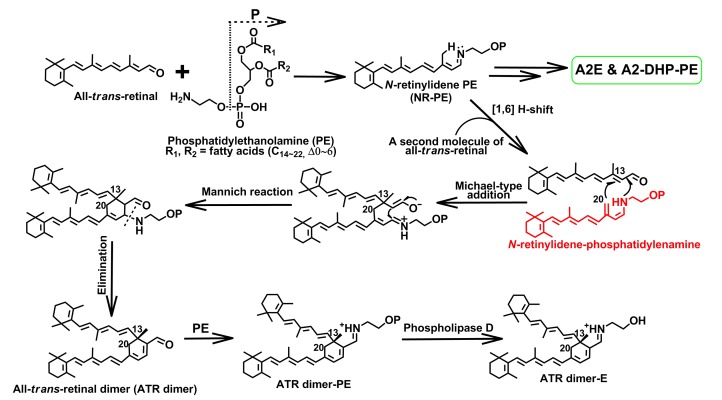
In addition to pyridinium bis-retinoids, Wu et al. (2009) identified two novel but correlated di-retinal pigments (A2-DHP-PE and A2-DHP-E) that were distinguished by containing a dihydropyridine ring rather than a pyridinium ring. As shown in Fig. 4, A2-DHP-PE biogenesis is closely associated with that of A2E. Following the generation of an estimated intermediate (dihydropyridinium A2PE), there is a bifurcation in the pathway. As mentioned above, one way gives rise to A2E and its isomers (Liu et al., 2000; Mata et al., 2000; Sparrow et al., 2010a). Conversely, another path to form A2-DHP-PE is involved with [1,3]-proton tautomerization and elimination of a hydrogen (Wu et al., 2009; Sparrow et al., 2010a). PLD-mediated hydrolysis of A2-DHP-PE yields A2-DHP-E, revealing that A2-DHP-PE may act as the precursor of A2-DHP-E (Wu et al., 2009).
4. Bis-retinoids with a cyclohexadiene ring
In comparison with the nitrogen-containing heterocyclic bis-retinoids, all-trans-retinal dimer series, including ATR dimer, ATR dimer-PE, and ATR dimer-E (Fishkin et al., 2005), are absolutely distinctive, given that their chemical structures contain a simpler cyclohexadiene ring in which no nitrogen atom resides. Cyclohexa-1,3-diene-carbaldehydes, incredibly similar to ATR dimer, could be released from a base-catalyzed condensation reaction between α,β-unsaturated aldehydes and a β-methyl substituent. Thus, the proposed pathway for ATR dimer biogenesis (Fig. 6) is rooted in the reported mechanism relevant to this type of transformation (Thomas and Guntzdubini, 1976; Duhamel et al., 1991). Like A2PE and A2-DHP-PE, ATR dimer is supposed to form in the photoreceptor cell outer segments (Fig. 2). All bis-retinoids appear to form from the same tautomer (N-retinylidene-phosphatidylenamine), probably arising from a [1,6] H-shift of NR-PE/NR-GPE (Liu et al., 2000; Mata et al., 2000; Wu et al., 2009; Sparrow et al., 2010a). Via a Michael-type addition reaction (Michael, 1887; Mather et al., 2006), the C20 of NR-PE/NR-GPE would tie with C13 of a second molecule of all-trans-retinal. After a Mannich reaction to close the ring (Mannich and Krösche, 1912), the elimination of amine group in PE/GPE yields ATR dimer, along with an electronic rearrangement in the six-membered ring (Fishkin et al., 2005). Apart from the nucleophilicity of C20 residing in N-retinylidene-phosphatidylenamine (Fig. 6), this carbon also possesses the ability to participate in the [1,4] conjugate addition. These analyses connote that ATR dimer ought to form in the rod outer segments rather than in RPE. Incubation of ATR dimer with PE produces ATR dimer-PE with creation of a protonated Schiff base linkage (Fishkin et al., 2005). ATR dimer-E is released from the phosphate hydrolysis of ATR dimer-PE by PLD (Fishkin et al., 2005). In addition to A2-DHP-PE, examination of the 7-d reaction mixture between all-trans-retinal and 1,2-diacyl-sn-glycero-3-phosphatidylethanolamine from egg yolk (egg-PE) by high-performance liquid chromatography (HPLC) also exhibited the formations of ATR dimer and ATR dimer-PE (Wu et al., 2009). Evidence gleaned from HPLC chromatograms of mouse eyecup extracts showed that ATR dimer-PE is more readily detectable than ATR dimer in the Abca4 −/− mice. These findings, together with evidence that an incubation of ATR dimer with PE produces ATR dimer-PE, corroborate that ATR dimer is obliged to form firstly and functions as a precursor of ATR dimer-PE (Figs. 1 and 6).
Fig. 6.
Biosynthetic pathways of all-trans-retinal (ATR) dimer, ATR dimer-E, and ATR dimer-PE
It is thought that ATR dimer series, N-retinylidene-N-retinyl-ethanolamine (A2E) and N-retinylidene-N-retinyl-dihydropyridine-phosphatidylethanolamine (A2-DHP-PE), form from the same intermediate N-retinylidene-phosphatidylenamine (see structure in red). Also, it is interesting to know that ATR dimer-E (λ max 290, 510 nm) and saturated ATR dimer-PE (λ max 290, 510 nm) exhibited a visible absorption maximum of about 80 nm red shifted from that of ATR dimer (λ max 290, 430 nm) because of a protonated Schiff base linkage formed in the position of aldehyde moiety (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
5. Structural features and properties
A common feature in structures of these bis-retinoids is two side-arms consisting of alternating single and double bonds that begin from an aromatic head group and extend into terminal β-ionone rings. The systems of conjugated double bonds in these bischromophores confer their own absorbance maxima in the visible region. It is noted that all of the bis-retinoids listed in Fig. 3 possess fluorescent properties and are the source of fundus autofluorescence (Sparrow et al., 2010b; 2010c). Moreover, it seems that these bis-retinoids form by the random condensation reactions of two molecules of all-trans-retinal, and are highly susceptible to photooxidation (Ben-Shabat et al., 2002; Sparrow et al., 2002; Dillon et al., 2004; Fishkin et al., 2005; Jang et al., 2005; Wang et al., 2006; Kim et al., 2008; Wu et al., 2010). RPE cells, different from other types of cells, are constantly exposed to sunlight strongest at 400‒700 nm. Given that the human eye is taking full advantage of the solar energy distribution, we envision that light-mediated actions on the malignant bis-retinoids accumulated in RPE with age, will of necessity influence RPE cells to some extent. Indeed, events initiated by the blue-light illumination of some bis-retinoids allow for the release of epoxides and cleavage products that have been verified to damage RPE cells (Sparrow et al., 2000; 2002; 2003b; Sparrow and Cai, 2001).
6. Relations between ABCA4 transporter and bis-retinoid formation
ABCA4 (formerly known as ATP-binding cassette transporter, retina-specific (ABCR)) is a member of the ABCA subfamily of adenosine triphosphate (ATP) binding cassette (ABC) transporters expressed in vertebrate rod and cone cells, and is localized to the outer segment disk edges. Allikmets et al. (1997) first cloned and characterized Abca4 gene. Mutations in this gene have been verified to account for all cases of early- and late-onset autosomal recessive Stargardt macular degeneration and the major part of autosomal recessive cone-rod dystrophies (Gerber et al., 1998; Maugeri et al., 2000; Briggs et al., 2001; Ducroq et al., 2002) as well as some exceptional cases of retinitis pigmentosa (Martinez-Mir et al., 1998; Rozet et al., 1999; Fukui et al., 2002). These hereditarily degenerative diseases would cause severe vision loss in affected individuals with age and reflect a continuum of disease phenotype associated with severity of gene mutations. As a critical intermediate to form bis-retinoids, NR-PE is the putative ligand translocated from the lumen to the cytoplasmic side of the disk membrane by the ABCA4 acting as a flippase (Fig. 2) (Sun et al., 1999; Weng et al., 1999). According to a recent finding of A2-GPE by the Sparrow group (Yamamoto et al., 2011), we here propose its biosynthetic pathway (Fig. 5), in which a newly estimated intermediate named NR-GPE is considered to form. Due to the abundance of A2-GPE in the eyecups of ABCA4 null mutant mice, ABCA4 is also considered to be the carrier of NR-GPE. Following the hydrolysis of NR-PE and NR-GPE, all-trans-retinal is released and reduced to all-trans-retinol. The retinol binding proteins carry vitamin A to RPE where it is isomerized and re-oxidized to 11-cis-retinal. This chromophore is subsequently reincorporated into opsin, yielding ground state rhodopsin (Fig. 2). When ABCA4 activity is reduced or absent, NR-PE and NR-GPE abundantly accumulate in the disk membrane, and trigger further reaction with an additional molecule of all-trans-retinal to generate A2PE, A2-DHP-PE, A2-GPE, ATR dimer, and ATR dimer-PE (Fig. 2). Daily phagocytosis of the shed outer segment membrane by RPE cells would take these bis-retinoid pigments to RPE lysosomal bodies where the PLD, an acidic hydrolase in the lysosomes of RPE cells, serves as a blade to cleave to A2PE, A2-GPE, A2-DHP-PE and ATR dimer-PE to give A2E, A2-DHP-E, and ATR dimer-E, respectively (Fig. 2).
7. Medical relevance and future perspective
With regard to treatment for AMD, some attempts at mediating the retinoid cycling rate have been made by utilizing the exogenous small-molecule inhibitors of 11cRDH (Radu et al., 2003), RPE65 (Maiti et al., 2006; Golczak et al., 2008), retinol binding protein and transthyretin (Radu et al., 2005). Indeed, these compounds slow lipofuscin accumulation in RPE cells of the eye. Thus, it will be a promising strategy to screen a large body of low-molecular-weight antagonists limiting the visual cycle with an eventual aim of developing clinical medicines in favor of alleviating AMD. In contrast, no remedies can reverse malicious accumulation of lipofuscin bis-retinoids refractory to lysosomal enzyme degradation, once it has already occurred. In view of this fact, it would be beneficial if there are some exogenous enzymes that could be delivered to RPE cells and directly degrade these aging pigments. A promising finding by Wu et al. (2011) attested to the ability of horseradish peroxidase (HRP) to degrade A2E loaded in RPE cells.
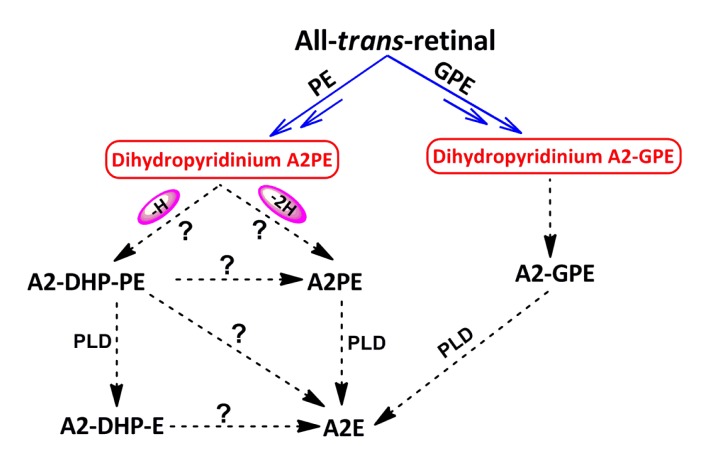
To contribute towards a better understanding of biosynthetic pathways of toxic bis-retinoids, a mutual conversion relationship among the bis-retinoids is expected to be clarified. As illustrated in Fig. 7, a future objective will be to establish whether either A2-DHP-PE or A2-DHP-E can be converted into A2E and to investigate whether A2-DHP-PE can undergo further transformation to form A2PE under certain conditions. On the other hand, continuing research and advances in this field should be directed towards characterizing those unrecognized compositions of RPE lipofuscin, understanding the properties of each lipofuscin constituent, and clarifying in vivo mechanisms by which they form and the adverse effects of these pigments on retina. Characterization of bis-retinoid constituents in RPE lipofuscin is essential for providing insights into the burden placed on RPE cells by the life-long deposition of these vitamin A aldehyde-derived compounds. An understanding of bis-retinoid biogenesis is fundamental to scientific efforts aimed at blocking the formation of these aggressive pigments.
Fig. 7.
Diagram of mutual conversion relationships among some bis-retinoids
The symbols (?) represent the potential conversion relationships amongst dihydropyridinium N-retinylidene-N-retinyl-phosphatidylethanolamine (A2PE), N-retinylidene-N-retinyl-dihydropyridine-phosphatidylethanolamine (A2-DHP-PE), A2PE, N-retinylidene-N-retinyl-dihydropyridine-ethanolamine (A2-DHP-E), and N-retinylidene-N-retinyl-ethanolamine (A2E), for which more evidence is needed
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 21202146 and 81271018), the Fundamental Research Funds for the Central Universities, China, and the Zhejiang Key Laboratory Fund of China (No. 2011E10006)
Compliance with ethics guidelines: Ya-lin WU, Jie LI, and Ke YAO declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15(3):236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RE, Maude MB. Phospholipids of bovine outer segments. Biochemistry. 1970;9(18):3624–3628. doi: 10.1021/bi00820a019. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Shabat S, Itagaki Y, Jockusch S, Sparrow JR, Turro NJ, Nakanishi K. Formation of a nonaoxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew Chem Int Ed. 2002;41(5):814–817. doi: 10.1002/1521-3773(20020301)41:5<814::AID-ANIE814>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Birnbach CD, Jarvelainen M, Possin DE, Milam AH. Histopathology and immunocytochemistry of the neurosensory retina in fundus flavimaculatus. Ophthalmology. 1994;101(7):1211–1219. doi: 10.1016/s0161-6420(13)31725-4. [DOI] [PubMed] [Google Scholar]
- 5.Borhan B, Souto ML, Imai H, Shichida Y, Nakanishi K. Movement of retinal along the visual transducin path. Science. 2000;288(5474):2209–2212. doi: 10.1126/science.288.5474.2209. [DOI] [PubMed] [Google Scholar]
- 6.Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32(6):375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- 7.Briggs CE, Rucinski D, Rosenfeld PJ, Hirose T, Berson EL, Dryja TP. Mutations in ABCR (ABCA4) in patients with Stargardt macular degeneration or cone-rod degeneration. Invest Ophthalmol Vis Sci. 2001;42(10):2229–2236. [PubMed] [Google Scholar]
- 8.Broniec A, Pawlak A, Sarna T, Wielgus A, Roberts J, Land E, Truscott T, Edge R, Navaratnam S. Spectroscopic properties and reactivity of free radical forms of A2E. Free Radic Biol Med. 2005;38(8):1037–1046. doi: 10.1016/j.freeradbiomed.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci. 2001;24(1):779–805. doi: 10.1146/annurev.neuro.24.1.779. [DOI] [PubMed] [Google Scholar]
- 10.Clancy C, Krogmeier JR, Pawlak A, Rozanowska M, Sarna T, Dunn RC, Simon JD. Atomic force microscopy and near-field scanning optical microscopy measurements of single human retinal lipofuscin granules. J Phys Chem B. 2000;104(51):12098–12101. doi: 10.1021/jp0030544. [DOI] [Google Scholar]
- 11.Delori FC, Staurenghi G, Arend O, Dorey CK, Goger DG, Weiter JJ. In vivo measurement of lipofuscin in Stargardt’s disease-fundus flavimaculatus. Invest Ophthalmol Vis Sci. 1995;36(11):2327–2331. [PubMed] [Google Scholar]
- 12.Delori FC, Fleckner MR, Goger DG, Weiter JJ, Dorey CK. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41(2):496–504. [PubMed] [Google Scholar]
- 13.Dillon J, Wang Z, Avalle LB, Gaillard ER. The photochemical oxidation of A2E results in the formation of a 5,8,5′,8′-bis-furanoid oxide. Exp Eye Res. 2004;79(4):537–542. doi: 10.1016/j.exer.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Ducroq D, Rozet J, Gerber S, Perrault I, Barbet F, Hanein S, Hakiki S, Dufier J, Munnich A, Hamel C, et al. The ABCA4 gene in autosomal recessive cone-rod dystrophies. Am J Hum Genet. 2002;71(6):1480–1482. doi: 10.1086/344829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duhamel L, Guillemont J, Poirier JM, Chabardes P. A new prenylation method using the lithium enolate of prenal. Reaction with aldehydes and α,β-unsaturated aldehydes. Tetrahedron Lett. 1991;32(35):4495–4498. doi: 10.1016/0040-4039(91)80021-W. [DOI] [Google Scholar]
- 16.Eagle RC, Jr, Lucier AC, Bernardino VB, Yanoff M. Retinal pigment epithelial abnormalities in fundus flavimaculatus: a light and electron microscopic study. Ophthalmology. 1980;87(12):1189–1200. doi: 10.1016/s0161-6420(80)35106-3. [DOI] [PubMed] [Google Scholar]
- 17.Eldred GE, Katz ML. Fluorophores of the human retinal pigment opithelium: separation and spectral characterization. Exp Eye Res. 1988;47(1):71–86. doi: 10.1016/0014-4835(88)90025-5. [DOI] [PubMed] [Google Scholar]
- 18.Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotrophic detergents. Nature. 1993;361(6414):724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- 19.Feeney-Burns L. The pigments of the retinal pigment epithelium. Curr Top Eye Res. 1980;2:119–178. [PubMed] [Google Scholar]
- 20.Feeney-Burns L, Hilderbrand ES, Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984;25(2):195–200. [PubMed] [Google Scholar]
- 21.Fishkin NE, Sparrow JR, Allikmets R, Nakanishi K. Isolation and characterization of a retinal pigment epithelial cell fluorophore: an all-trans-retinal dimer conjugate. PNAS. 2005;102(20):7091–7096. doi: 10.1073/pnas.0501266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukui T, Yamamoto S, Nakano K, Tsujikawa M, Morimura H, Nishida K, Ohguro N, Fujikado T, Irifune M, Kuniyoshi K, et al. ABCA4 gene mutations in Japanese patients with Stargardt disease and retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002;43(9):2819–2824. [PubMed] [Google Scholar]
- 23.Gelasco A, Crouch RK, Knapp DR. Intrahelical arrangement in the integral membrane protein rhodopsin investigated by site-specific chemical cleavage and mass spectrometry. Biochemistry. 2000;39(16):4907–4914. doi: 10.1021/bi992736i. [DOI] [PubMed] [Google Scholar]
- 24.Gerber S, Rozet JM, van de Pol T, Hoyng CB, Munnich A, Blankenagel A, Kaplan J, Cremers FP. Complete exon-intron structure of the retina-specific ATP binding transporter gene (ABCR) allows the identification of novel mutations underlying Stargardt disease. Genomics. 1998;48(1):139–142. doi: 10.1006/geno.1997.5164. [DOI] [PubMed] [Google Scholar]
- 25.Golczak M, Maeda A, Bereta G, Maeda T, Kiser PD, Hunzelmann S, Lintig J, Blaner WS, Palczewski K. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J Biol Chem. 2008;283(15):9543–9554. doi: 10.1074/jbc.M708982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isin B, Rader AJ, Dhiman HK, Klein-Seetharaman J, Bahar I. Predisposition of the dark state of rhodopsin to functional changes in structure. Proteins. 2006;65(4):970–983. doi: 10.1002/prot.21158. [DOI] [PubMed] [Google Scholar]
- 27.Jäger S, Palczewski K, Hofmann KP. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry. 1996;35(9):2901–2908. doi: 10.1021/bi9524068. [DOI] [PubMed] [Google Scholar]
- 28.Jang YP, Matsuda H, Itagaki Y, Nakanishi K, Sparrow JR. Characterization of peroxy-A2E and furan-A2E photooxidation products and detection in human and mouse retinal pigment epithelial cell lipofuscin. J Biol Chem. 2005;280(48):39732–39739. doi: 10.1074/jbc.M504933200. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy CJ, Rakoczy PE, Constable IJ. Lipofuscin of the retinal pigment epithelium: a review. Eye. 1995;9(6):763–771. doi: 10.1038/eye.1995.192. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Jockusch S, Itagaki Y, Turro NJ, Sparrow JR. Mechanisms involved in A2E oxidation. Exp Eye Res. 2008;86(6):975–982. doi: 10.1016/j.exer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SR, Fishkin N, Kong J, Nakanishi K, Allikmets R, Sparrow JR. Rpe65 Leu450Met variants is associated with reduced levels of the retinal pigment epithelium lipofuscin fluorophore A2E and iso-A2E. PNAS. 2004;101(32):11668–11672. doi: 10.1073/pnas.0403499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SR, He J, Yanase E, Jang Y, Berova N, Sparrow JR, Nakanishi K. Characterization of dihydro-A2PE: an intermediate in the A2E biosynthetic pathway. Biochemistry. 2007;46(35):10122–10129. doi: 10.1021/bi7009635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kliffen M, van der Schaft T, Mooy CM, de Jong PT. Morphologic changes in age-related maculopathy. Microsc Res Tech. 1997;36(2):106–122. doi: 10.1002/(SICI)1097-0029(19970115)36:2<106::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Itagaki Y, Ben-Shabat S, Nakanishi K, Sparrow J R. The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J Biol Chem. 2000;275(38):29354–29360. doi: 10.1074/jbc.M910191199. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Liu MY, Nguyen JB, Bhagat A, Mooney V, Yan EC. Thermal properties of rhodopsin: insight into the molecular mechanism of dim-light vision. J Biol Chem. 2011;286(31):27622–27629. doi: 10.1074/jbc.M111.233312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maiti P, Kong J, Kim SR, Sparrow JR, Allikmets R, Rando RR. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochemistry. 2006;45(3):852–860. doi: 10.1021/bi0518545. [DOI] [PubMed] [Google Scholar]
- 37.Mannich C, Krösche W. Ueber ein kondensationsprodukt aus formaldehyd, ammoniak und antipyrin. Archiv der Pharmazie. 1912;250(1):647–667. doi: 10.1002/ardp.19122500151. (in German) [DOI] [Google Scholar]
- 38.Martinez-Mir A, Paloma E, Allikmets R, Ayuso C, del Rio T, Dean M, Vilageliu L, Gonzàlez-Duarte R, Balcells S. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR . Nat Genet. 1998;18(1):11–12. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- 39.Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophorein mice and humans with ABCR-mediated retinal and macular degeneration. PNAS. 2000;97(13):7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mather B, Viswanathan K, Miller K, Long T. Michael addition reactions in macromolecular design for emerging technologies. Prog Polym Sci. 2006;31(5):487–531. doi: 10.1016/j.progpolymsci.2006.03.001. [DOI] [Google Scholar]
- 41.Maugeri A, Klevering BJ, Rohrschneider K, Blankenagel A, Brunner HG, Deutman AF, Hoyng CB, Cremers FP. Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am J Hum Genet. 2000;67(4):960–966. doi: 10.1086/303079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michael A. On the addition of sodium acetacetic ether and analogous sodium compounds to unsaturated organic ethers. Am Chem J. 1887;9:115. [Google Scholar]
- 43.Molday RS, Beharry S, Ahn J, Zhong M. Binding of N-retinylidene-PE to ABCA4 and a model for its transport across membranes. Adv Exp Med Biol. 2006;572:465–470. doi: 10.1007/0-387-32442-9_64. [DOI] [PubMed] [Google Scholar]
- 44.Ng KP, Gugiu B, Crabb JW. Retinal pigment epithelium lipofuscin proteomics. Mol Cell Proteomics. 2008;7(7):1397–1405. doi: 10.1074/mcp.M700525-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: new insights from structural and biochemical studies. Trends Biochem Sci. 2001;26(5):318–324. doi: 10.1016/S0968-0004(01)01799-6. [DOI] [PubMed] [Google Scholar]
- 46.Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. PNAS. 1998;95(25):14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poincelot RP, Millar PG, Kimbel RL, Abramson EW. Lipid to protein chromophore transfer in the photolysis of visual pigments. Nature. 1969;221(5177):256–257. doi: 10.1038/221256a0. [DOI] [PubMed] [Google Scholar]
- 48.Rabb MF, Tso MO, Fishman GA. Cone-rod dystrophy. A clinical and histopathologic report. Ophthalmology. 1986;93(11):1443–1451. doi: 10.1016/s0161-6420(86)33547-4. [DOI] [PubMed] [Google Scholar]
- 49.Radu RA, Mata NL, Nusinowitz S, Liu X, Sieving PA, Travis GH. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt’s macular degeneration. PNAS. 2003;100(8):4742–4747. doi: 10.1073/pnas.0737855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radu RA, Han Y, Bui TV, Nusinowitz S, Bok D, Lichter J, Widder K, Travis GH, Mata NL. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46(12):4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 51.Rando RR. Polyenes and vision. Chem Biol. 1996;3(4):255–262. doi: 10.1016/S1074-5521(96)90105-2. [DOI] [PubMed] [Google Scholar]
- 52.Ren R, Sakai N, Nakanishi K. Total synthesis of the ocular age pigment A2-E: a convergent pathway. J Am Chem Soc. 1997;119(15):3619–3620. doi: 10.1021/ja9700414. [DOI] [Google Scholar]
- 53.Rozet JM, Gerber S, Ghazi I, Perrault I, Ducroq D, Souied E, Cabot A, Dufier JL, Munnich A, Kaplan J. Mutations of the retinal specific ATP binding transporter gene (ABCR) in a single family segregating both autosomal recessive retinitis pigmentosa RP19 and Stargardt disease: evidence of clinical heterogeneity at this locus. J Med Genet. 1999;36(6):447–451. [PMC free article] [PubMed] [Google Scholar]
- 54.Sakai N, Decatur J, Nakanishi K. Ocular age pigment “A2-E”: an unprecedented pyridinium bisretinoid. J Am Chem Soc. 1996;118(6):1559–1560. doi: 10.1021/ja953480g. [DOI] [Google Scholar]
- 55.Sakmar TP. Rhodopsin: a prototypical G protein-coupled receptor. Prog Nucleic Acid Res Mol Biol. 1997;59:1–34. doi: 10.1016/S0079-6603(08)61027-2. [DOI] [PubMed] [Google Scholar]
- 56.Sarks SH, Arnold JJ, Killingsworth MC, Sarks JP. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. Br J Ophthalmol. 1999;83(3):358–368. doi: 10.1136/bjo.83.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shroyer NF, Lewis RA, Allikmets R, Singh N, Dean M, Leppert M, Lupski JR. The rod photoreceptor ATP-binding cassette transporter gene, ABCR, and retinal disease: from monogenic to multifactorial. Vision Res. 1999;39(15):2537–2544. doi: 10.1016/S0042-6989(99)00037-1. [DOI] [PubMed] [Google Scholar]
- 58.Sparrow JR, Cai B. Blue light-induced apoptosis of A2E-containing RPE: involvement of caspase-3 and protection by Bcl2. Invest Ophthalmol Vis Sci. 2001;42(6):1356–1362. [PubMed] [Google Scholar]
- 59.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal photobiology. Exp Eye Res. 2005;80(5):595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41(7):1981–1989. [PubMed] [Google Scholar]
- 61.Sparrow JR, Zhou J, Ben-Shabat S, Vollmer HR, Itagaki Y, Nakanishi K. Involvement of oxidative mechanism in blue light induced damage to A2E-laden RPE. Invest Ophthalmol Vis Sci. 2002;43(4):1222–1227. [PubMed] [Google Scholar]
- 62.Sparrow JR, Fishkin N, Zhou J, Cai B, Jang Y, Krane S, Itagaki Y, Nakanishi K. A2E, a byproduct of the visual cycle. Vision Res. 2003;43(28):2983–2990. doi: 10.1016/S0042-6989(03)00475-9. [DOI] [PubMed] [Google Scholar]
- 63.Sparrow JR, Vollmer HR, Zhou J, Jang YP, Jockusch S, Itagaki Y, Nakanishi K. A2E-epoxides damage DNA in retinal pigment epithelial cells. J Biol Chem. 2003;278(20):18207–18213. doi: 10.1074/jbc.M300457200. [DOI] [PubMed] [Google Scholar]
- 64.Sparrow JR, Kim SR, Cuervo AM, Bandhyopadhyayand U. A2E, a pigment of RPE lipofuscin is generated from the precursor A2PE by a lysosomal enzyme activity. Adv Exp Med Biol. 2008;613:393–398. doi: 10.1007/978-0-387-74904-4_46. [DOI] [PubMed] [Google Scholar]
- 65.Sparrow JR, Kim SR, Wu Y. Experimental approaches to the study of A2E, a bisretinoid constituent of the lipofuscin of retinal pigment epithelium. Methods Mol Biol. 2010;652:315–327. doi: 10.1007/978-1-60327-325-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sparrow JR, Wu Y, Takayuki N, Yoon K, Yamamoto K, Zhou J. Fundus autofluorescence and the bisretinoids of retina. Photochem Photobiol Sci. 2010;9(11):1480–1489. doi: 10.1039/c0pp00207k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sparrow JR, Yoon K, Wu Y, Yamamoto K. Interpretations of fundus autofluorescence from studies of the bisretinoids of retina. Invest Ophthalmol Vis Sci. 2010;51(9):4351–4357. doi: 10.1167/iovs.10-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sparrow JR, Wu Y, Kim CY, Zhou J. Phospholipid meets all-trans-retinal: the making of RPE bisretinoids. J Lipid Res. 2010;51(2):247–261. doi: 10.1194/jlr.R000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sparrow JR, Gregory-Roberts E, Yamamoto K, Blonska A, Ghosh SK, Ueda K, Zhou J. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012;31(2):121–135. doi: 10.1016/j.preteyeres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274(12):8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- 71.Telander DG. Inflammation and age-related macular degeneration (AMD) Semin Ophthalmol. 2011;26(3):192–197. doi: 10.3109/08820538.2011.570849. [DOI] [PubMed] [Google Scholar]
- 72.Thomas AF, Guntzdubini R. The ‘aldol condensation’ of citral and related reactions. Helv Chim Acta. 1976;59(6):2261–2267. doi: 10.1002/hlca.19760590642. [DOI] [Google Scholar]
- 73.Wang Z, Keller LM, Dillon J, Gaillard ER. Oxidation of A2E results in the formation of highly reactive aldehydes and ketones. Photochem Photobiol. 2006;82(5):1251–1257. doi: 10.1562/2006-04-01-RA-864. [DOI] [PubMed] [Google Scholar]
- 74.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98(1):13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 75.Wu Y, Fishkin NE, Pande A, Pande J, Sparrow JR. Novel lipofuscin bisretinoids prominent in human retina and in a model of recessive Stargardt disease. J Biol Chem. 2009;284(30):20155–20166. doi: 10.1074/jbc.M109.021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Y, Yanase E, Feng X, Siegel M, Sparrow J. Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. PNAS. 2010;107(16):7275–7280. doi: 10.1073/pnas.0913112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Y, Zhou J, Fishkin NE, Rittman BE, Sparrow JR. Enzymatic degradation of A2E, an RPE lipofuscin pigment. J Am Chem Soc. 2011;133(4):849–857. doi: 10.1021/ja107195u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamamoto K, Yoon KD, Ueda K, Hashimoto M, Sparrow JR. A novel bisretinoid of retina is an adduct on glycerophosphoethanolamine. Invest Ophthalmol Vis Sci. 2011;52(12):9084–9090. doi: 10.1167/iovs.11-8632. [DOI] [PMC free article] [PubMed] [Google Scholar]