Abstract
Objective: The efflux pump (EP) is one of the major mechanisms of antibiotic resistance in Klebsiella pneumoniae. However, there are few reports on the effect of the abuse of antibiotic use on the activity of EPs. To determine whether the use of low efficacy antibiotics has any effect on the activity of EPs and induces drug resistance in K. pneumoniae, we investigated the effect of ciprofloxacin on the activity of EPs in K. pneumoniae strains. Methods: Sixteen susceptible K. pneumoniae strains were isolated from patients and their minimum inhibitory concentrations (MICs) of ciprofloxacin were measured in the absence and presence of the pump inhibitor carbonyl cyanide m-chlorophenyl hydrazone (CCCP). The strains were then induced with a gradient of ciprofloxacin until the MICs of the strains showed no further increase, to obtain induced resistant strains. The EP activities of the strains before and after induction were compared using EP inhibition and ethidium bromide (EtBr) accumulation assays. Results: The MIC values of the strains were 16‒256 times higher after induction than before induction. In the presence of CCCP, the MIC values of 50% of the induced strains were 2‒4-fold lower than that in the absence of this inhibitor. The EtBr accumulation assay showed that the fluorescence of EtBr in the induced cells was lower than that in the cells before induction. Conclusions: EPs are widespread in susceptible and drug-resistant K. pneumoniae strains. Induction with ciprofloxacin may increase the activity of EPs in K. pneumoniae. The EtBr accumulation assay is more sensitive than the EP inhibition assay in evaluating the activity of EPs in K. pneumoniae.
Keywords: Klebsiella pneumoniae, Efflux pump, Ciprofloxacin, Antibiotic resistance
1. Introduction
The discovery and use of antibiotics were among the most significant scientific achievements of the 20th century. However, antibiotics have often been used inappropriately in humans and animals. The abusive use of antibiotics has produced multidrug-resistant pathogen strains. Klebsiella pneumoniae is one of the main conditional multidrug-resistant pathogens that cause nosocomial infection. The emergence of multidrug-resistant strains has become a major clinical problem (van der Donk et al., 2011). The main mechanisms of antibiotic-resistance in K. pneumoniae are: (1) releasing enzymes to inactivate antibiotics (Falagas and Karageorgopoulos, 2009); (2) modifying target site structures to prevent antibiotics from reaching their targets (Rodríguez-Martínez et al., 2011); (3) changing cell surface permeability to decrease the intracellular drug concentration (Nikaido, 2001); (4) increasing efflux to limit the intracellular concentration of antimicrobial agents (Pagès and Amaral, 2009). Among these mechanisms, increasing efflux by efflux pumps (EPs) is considered to be one of the most important contributors to bacterial antibiotic resistance (Li and Nikaido, 2009), and may be responsible for resistance to either one specific class of antibiotics or a large number of unrelated antimicrobial agents (van Bambeke et al., 2000; Lynch, 2006).
Ciprofloxacin (CIP), one of the fluoroquinolones, is a broad-spectrum antimicrobial that is effective in the treatment of a wide variety of clinical infections (Hopkins et al., 2005). The main causes of K. pneumoniae resistance to CIP are mutations occurring in the target enzymes of quinolones, namely DNA gyrase and topoisomerase IV, and EPs (Aathithan and French, 2011). In Gram-negative bacteria, the EPs belong to the resistance-nodulation-division (RND) superfamily, which is commonly responsible for multidrug resistance (MDR) (Kumar et al., 2008). The substrates of the RND superfamily are amphiphilic and charged, and include fluoroquinolones, β-lactams, and aminoglycosides (van Bambeke et al., 2000).
The function of an EP is to pump out toxic compounds (such as antibiotics) to limit the accumulation of antibiotics inside the cell. Hence, the bacteria become insensitive to antibiotics. Several methods have been used to measure EP system activity, such as an ethidium bromide (EtBr) accumulation assay (Paixão et al., 2009), an EP inhibition assay (Chollet et al., 2004), radiolabelled antibiotics (Hasdemir et al., 2004), and other methods (Daugelavičius et al., 2010). The most commonly used method is the EtBr accumulation assay, which is based on monitoring the accumulation of the fluorescent probe, EtBr (Paixão et al., 2009). The EP inhibition assay is based on the change in minimum inhibitory concentrations (MICs) when a pump inhibitor is added. Carbonyl cyanide m-chlorophenyl hydrazone (CCCP) interferes with the energy level of the bacterial membrane (Daugelavičius et al., 2010) and has been used to abolish completely the efflux of drugs. A reduction of more than two-fold in the MIC following the addition of CCCP indicates that an EP can extrude antibiotics.
The EtBr accumulation assay also demonstrates EP activity. The signal of EtBr inside cells can be detected and quantified by a quantitative fluorescent polymerase chain reaction (PCR) machine. This method evaluates accurately the activity of EPs in different strains before and after induction and in the presence or absence of CCCP. However, there have been no reports on comparisons between the EP inhibition and EtBr accumulation assays.
In this study, two different methods were used to evaluate the change in the activity of the EPs in susceptible strains before and after induction of resistance to CIP. Resistance of the induced strains to other antibiotics was also tested to evaluate the activity of the EPs in isolates.
2. Materials and methods
2.1. Bacterial strains and chemicals
Sixteen K. pneumoniae strains were isolated from various biological samples, including respiratory secretions, urine, and blood, which were collected from patients in Ningbo No. 2 Hospital, China. Species identification was confirmed by the French Merieux VITEK-2 microbiology analysis system and sensitivity analysis. The population analyzed was non-repetitive, all isolates were susceptible to CIP (MIC≤4 mg/L), and extended spectrum β-lactamases (ESBLs) were negative. The standard strain K. pneumoniae NCTC5056 was purchased from the China General Microbiological Culture Collection Center. MacConkey agar medium powder, Mueller-Hinton (MH) agar powder, nutrient broth powder, and CIP hydrochloride powder were purchased from Bio-Kont (China). EtBr and CCCP were purchased from Sigma-Aldrich Química SA (Madrid, Spain). Phosphate buffered solution (PBS) was purchased from Genom (China).
2.2. Antibiotic susceptibility test
Susceptibility testing was performed according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI, 2012) (M100-S22). The MICs for CIP were determined by the two-fold broth microdilution method in 96-well microtiter plates. Bacterial strains were incubated overnight on MacConkey agar broth at 37 °C. Bacterial cultures were diluted in sterilized distilled water to a McFarland 0.5 turbidity standard and then diluted 100 times with nutrient broth. Aliquots of 0.05 ml were transferred to each well of a 96-well plate that contained 0.05 ml of each compound at concentrations prepared in two-fold serial dilutions in nutrient broth medium. The plates were incubated at 37 °C and the MIC results registered after 16‒18 h. All assays were carried out three times.
2.3. EP inhibition assay
The EP inhibition assay and determination of the MICs were carried out simultaneously. The MIC testing of CCCP indicated that CCCP at a concentration of 20 mg/L had no detrimental effect on bacterial viability. This concentration was therefore used for all subsequent experiments using standard CLSI broth microdilution methods. The EP inhibitor CCCP was added to 96-well microtiter plates at a final concentration of 20 mg/L. The MICs of strains were tested both in the absence and presence of CCCP to evaluate the activity of the EP in the isolates.
2.4. Strain induction
Sixteen susceptible strains were recovered on MacConkey agar without CIP from strains stored at 37 °C. Then a single bacterial colony was transferred into 4 ml of nutrient broth medium containing CIP at a concentration of 1/2×MIC, followed by a shaking inoculation at 35 °C. A loopful of bacteria was placed on MacConkey agar broth with the same concentration of CIP and was incubated overnight at 37 °C to promote growth. After that, a single colony was chosen and put into nutrient broth with CIP at a concentration of 1×MIC, with shaking at 200 r/min at 35 °C overnight. In this way, the strains were induced by stepwise increases in the concentration of CIP until they could not grow at all, and were subcultured on MacConkey medium for two generations. The colony was enriched by liquid culture and preserved at −80 °C in a refrigerator.
2.5. Strain identification and determination of MICs
The identification of the species and the drug sensitivity of the induced strains were determined using the French Merieux VITEK-2 microbiology and sensitivity analysis system, respectively. The MIC of CIP and the level of EP activity of the induced strains were determined using the broth microdilution method and EP inhibition assay described above, respectively.
2.6. EtBr accumulation assay
The EtBr accumulation assay was employed using an ABI7500 real-time PCR machine (Applied Biosystems, USA). K. pneumoniae strains were grown in 4 ml of nutrient broth until they reached a mid-log phase, and then washed with PBS, to give an optical density at 600 nm (OD600) of 0.6 (ultraviolet spectrophotometer Lambda35, PerkinElmer, USA). Cells were then centrifuged at 11 000 r/min for 3 min. The pellet was washed twice with the same volume of 1× PBS and the OD600 of the cellular suspension was adjusted to 0.3. Glucose was then added to the cellular suspension to a final concentration of 4 g/L. Aliquots of 0.096 ml were added to 0.2 ml microtubes with EtBr to give a final concentration of 10 mg/L. To determine the effect of the EP inhibitor CCCP on efflux, CCCP was added to another suspension to a final concentration of 10 mg/L that did not exceed 1/2×MIC. All assays were performed at least three times, and the results were reproducible. The excitation and emission wavelengths selected for EtBr were 530 nm for the band-pass and 585 nm for the high-pass filters. Fluorescence was monitored in the ABI7500 over a period of 60 min. The conditions for testing were as follows: 36.5 °C for 1 min, 60 cycles of 36.5 °C for 15 s, and 37.5 °C for 45 s. Arbitrary units of fluorescence emitted by EtBr were recorded at the end of each cycle.
3. Results
3.1. Strain identification and CIP susceptibility
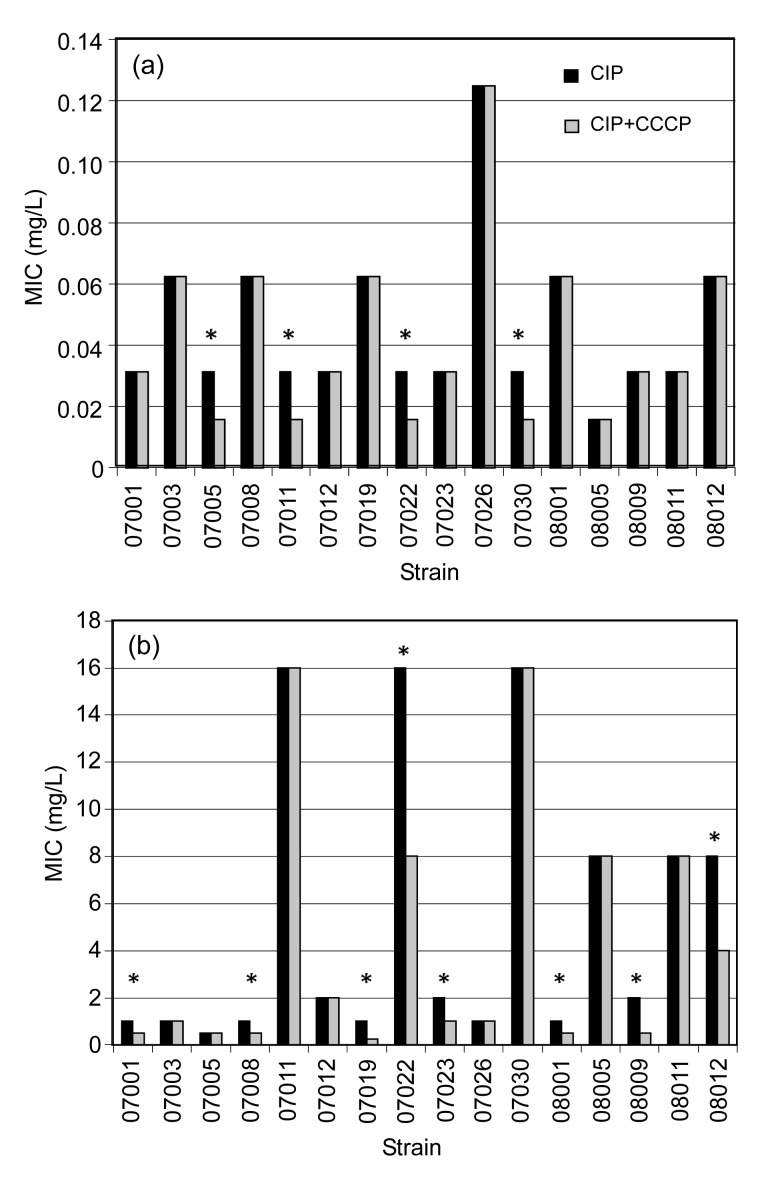
The induced strains were validated using a VITEK-2 microbiology analysis system. All sixteen strains were K. pneumoniae and ESBLs were negative. The MICs of CIP and the differences in the MIC values for each strain before and after induction, and with and without the inhibition of CCCP are shown in Fig. 1. The MIC values for strains after induction were 16‒256 times higher than those before induction. Six induced strains (MIC≥4 mg/L) became resistant and three (MIC=2 mg/L) showed intermediate resistance to CIP.
Fig. 1.
MIC values of CIP and CIP plus CCCP for Klebsiella pneumoniae strains before (a) and after (b) induction
* Strains whose MICs changed in the presence or absence of CCCP
3.2. EP inhibition assay
CCCP had only a two-fold inhibition effect on the activity of the EPs in four strains before induction. CCCP inhibited EP activity by two- or four-fold in half of the strains after induction.
3.3. EtBr accumulation assay
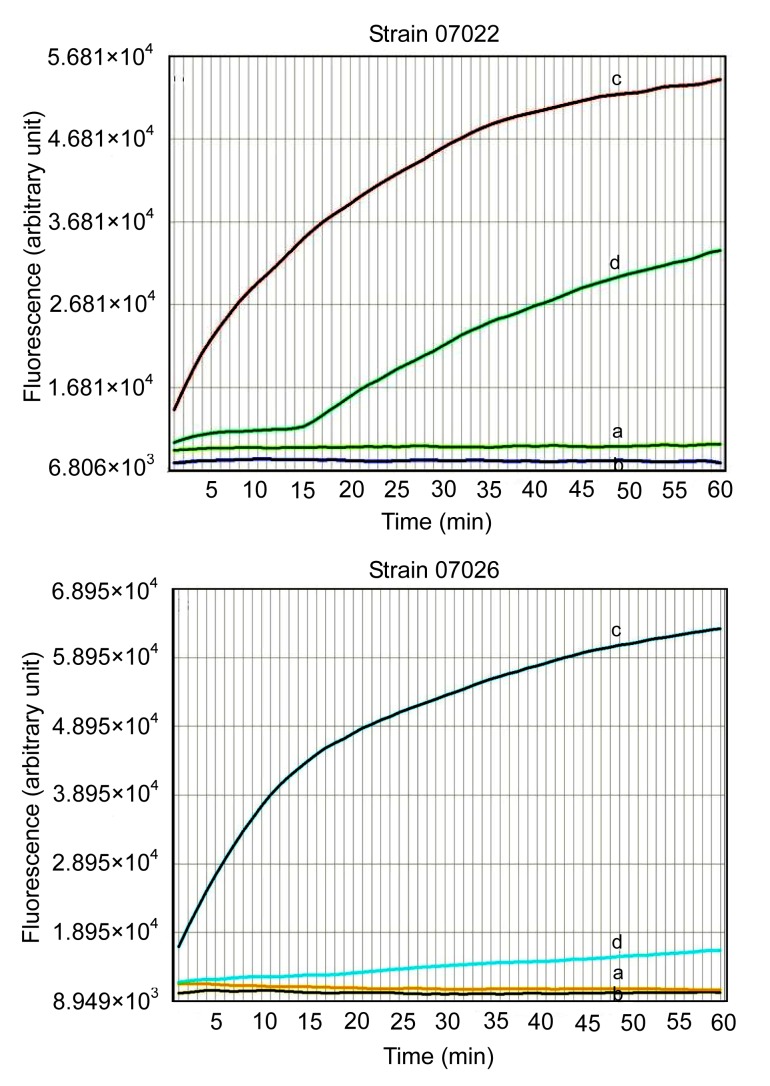
The results of the EtBr accumulation assay are shown in Fig. 2. The fluorescence value of cells in the presence of CCCP was significantly higher than that in the absence of CCCP both before and after induction (Fig. 2). Accumulation of EtBr inside the bacterial cells increased in the presence of CCCP. The fluorescence value of cells before induction was higher than that after induction, either with or without CCCP.
Fig. 2.
Fluorescence of EtBr accumulation of strains (07022 and 07026) before and after induction alone and in the presence of CCCP (final concentration, 10 mg/L)
The final concentration of EtBr was 10 mg/L. Curves a and b represent the fluorescence of strains before and after induction, respectively, in the absence of CCCP. Curves c and d represent the fluorescence of strains before and after induction, respectively, in the presence of CCCP
3.4. Susceptibility to other antibiotics
A sensitivity test of all strains to 14 other antibiotics was also carried out. It indicated that the induced strains had increased resistance not only to CIP, but also to other antibiotics, including ampicillin/sulbactam and nitrofurantoin. Before induction, 14 strains were sensitive and 2 strains were resistant to ampicillin/sulbactam. After induction, three of the sensitive strains became resistant and one showed intermediate resistance. In the course of induction, 9/16 of the intermediate strains were transformed into strains resistant to nitrofurantoin, and 3/16 of the susceptible strains were transformed into intermediate or resistant strains. Overall, 75% of the induced strains increased their resistance to nitrofurantoin.
4. Discussion
K. pneumoniae is an important nosocomial pathogen, which is highly resistant to clinically used antibiotics, causing a wide spectrum of infections and leading to substantial morbidity and mortality (Echeverri-Toro et al., 2012). EPs are one of the major mechanisms of antibiotic resistance in K. pneumoniae. To determine whether EPs are conferring resistance in drug-resistant K. pneumoniae, we compared the EPs of strains induced by stepwise increasing concentrations of CIP with those of susceptible clinical strains.
The results of the EP inhibition assay showed that the MIC values of the induced strains increased 16‒256 times compared to those of the same strains before induction. Moreover, while CCCP had no effect on strains 07001, 07008, 07019, 07023, 08001, 08009, and 08012 before induction, it had a two- or four-fold inhibition effect on their induced strains. This indicated that induction by CIP increased the activity of the EP, and that the activity of the pump can be inhibited by CCCP.
CCCP showed a two-fold inhibition effect on strains 07005, 07011, and 07030 before induction, but had no effect on their induced strains. This may be because CIP induction increased efflux activity and thus, CIP was pumped out from the cells. It may also have been because CCCP was not sufficient to inhibit the activity of the EP and so the MIC value was not affected. The results indicated that the inhibited EP assay was not the best method for evaluating the activity level of the EP.
To evaluate the activity of EPs, Paixão et al. (2009) chose EtBr as the substrate for efflux investigation. EtBr emits weak fluorescence, but when it enters cells and combines with DNA, it emits strong fluorescence. The EP on the cell membrane pumps EtBr out of the cell. The fluorescence of EtBr in strains before and after induction increased significantly in the presence of CCCP (Fig. 2). This indicated that the EP mediates the antibiotic mechanism widely found in susceptible and induced strains of K. pneumoniae.
For the induced strains, the MIC could be reduced two- or four-fold by CCCP in the EP inhibition assay. At the beginning of the curve, the fluorescent value of EtBr increased slowly (curve ‘d’ of strain 07022 in Fig. 2), and then increased dramatically. The reason for this phenomenon might be that EtBr was pumped out with adenosine triphosphate (ATP) when the energy inhibitor CCCP was not yet achieving complete inhibition, and EtBr accumulation increased quickly when ATP was consumed. For those induced strains whose MIC values were unchanged after CCCP was added, the fluorescent values increased smoothly (curve ‘d’ of strain 07026 in Fig. 2), suggesting that the level of EP activity was so high that EtBr was pumped out constantly and could not be inhibited by CCCP.
This research assessed the levels of EP activity among strains before and after induction by adopting EP inhibition and EtBr accumulation assays. The EP inhibition assay showed that MIC values increased after induction, and that in half of the strains EP activity could be inhibited by CCCP. The EtBr accumulation assay showed that EtBr accumulation was greater in cells after induction, whether CCCP was added or not. These results show that EPs play a major role in drug-resistance of induced strains. It was clear that the EtBr accumulation assay was more sensitive than the EP inhibition assay. Although the EP inhibition assay is widely used in investigations of resistance mechanisms, we found that the EtBr accumulation assay could reflect the status of the EP more accurately.
In this study, the resistance of strains to CIP increased after induction. To determine whether resistance to other antibiotics was enhanced after induction, we also tested their susceptibility to 14 other antibiotics. The resistance of the induced strains to most antibiotics was unchanged after induction. Before induction, 14 K. pneumoniae strains were intrinsically resistant to ampicillin (Schito et al., 2009), but sensitive to ampicillin/sulbactam. Their ESBLs were negative. This may be because other β-lactamases exist in bacteria that were inhibited by sulbactam. Some of the induced strains showed increased resistance to ampicillin/sulbactam, and most showed increased resistance to nitrofurantoin. Therefore, EPs are probably involved in the resistance to these two antibiotics. These antibiotics may share some structural or chemical properties with CIP, making their extrusion by EPs easier than that of other drugs.
Our results support the importance of the role played by EPs in this bacterium’s physiology. Our investigations also revealed that drug-resistant strains could be induced by long-term excessive use of CIP.
The inappropriate use of antibiotics leading to changes in bacterial resistance reinforces the necessity and significance of the rational use of antibiotics. As EPs contribute to antibacterial resistance, they could be an attractive target for the development of new drugs. In our study, CCCP reduced MIC values and increased the cellular accumulation of EtBr. Hence, together with antibiotics, EP inhibitors might be critical for reducing invasiveness, in addition to their established role in determining the MIC. Regarding the involvement of multidrug-resistant bacteria in treatment failures and the re-emergence of infectious diseases, the activities of some compounds (in the presence of CCCP) could be very promising.
The genes and proteins involved in EP functions will be investigated to identify the causes of differences in the molecular structures of EPs in the strains before and after induction. The availability of molecular methods and a more profound understanding of the function and regulation of efflux systems will facilitate the exploration of pumps as new drug targets.
Acknowledgments
We thank the Microbiology Laboratory of Ningbo No. 2 Hospital, China and relevant personnel.
Footnotes
Project supported by the Programme of Zhejiang Scientific Research Fund in Traditional Chinese Medicine, China (No. 2011ZA094) and the Zhejiang Provincial Natural Science Foundation of China (No. LY13H190008)
Compliance with ethics guidelines: Hai-qin ZHONG, Shun ZHANG, Hong PAN, and Ting CAI declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Aathithan S, French GL. Prevalence and role of efflux pump activity in ciprofloxacin resistance in clinical isolates of Klebsiella pneumoniae . Eur J Clin Microbiol Infect Dis. 2011;30(6):745–752. doi: 10.1007/s10096-010-1147-0. [DOI] [PubMed] [Google Scholar]
- 2.Chollet R, Chevalier J, Bryskier A, Pagès JM. The AcrAB-TolC pump is involved in macrolide resistance but not in telithromycin efflux in Enterobacter aerogenes and Escherichia coli. Antimicrob . Agents Chemother. 2004;48(9):3621–3624. doi: 10.1128/AAC.48.9.3621-3624.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CLSI (Clinical and Laboratory Standards Institute) ISO M100-S22:2012. America: Clinical and Laboratory Standards Institute; 2012. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-second Informational Supplement. [Google Scholar]
- 4.Daugelavičius R, Buivydas A, Senčilo A, Bamford DH. Assessment of the activity of RND-type multidrug efflux pumps in Pseudomonasaeruginosa using tetraphenylphosphonium ions. Int J Antimicrob Agents. 2010;36(3):234–238. doi: 10.1016/j.ijantimicag.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 5.Echeverri-Toro LM, Rueda ZV, Maya W, Agudelo Y, Ospina S. Multidrug-resistant Klebsiella pneumoniae, predisposing factors and associated mortality in a tertiary-care hospital in Colombia. Rev Chilena Infectol. 2012;29(2):175–182. doi: 10.4067/S0716-10182012000200009. [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Karageorgopoulos DE. Extended-spectrum β-lactamase-producing organisms. J Hosp Infect. 2009;73(4):345–354. doi: 10.1016/j.jhin.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Hasdemir UO, Chevalier J, Nordmann P, Pagès JM. Detection and prevalence of active drug efflux mechanism in various multidrug-resistant Klebsiella pneumoniae strains from Turkey. J Clin Microbiol. 2004;42(6):2701–2706. doi: 10.1128/JCM.42.6.2701-2706.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopkins KL, Davies RH, Threlfall EJ. Mechanisms of quinolone resistance in Escherichia coli and Salmonella: recent developments. Int J Antimicrob Agents. 2005;25(5):358–373. doi: 10.1016/j.ijantimicag.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Mayo M, Trunck LA, Cheng AC, Currie BJ, Schweizer HP. Expression of resistance-nodulation-cell-division efflux pumps in commonly used Burkholderia pseudomallei strains and clinical isolates from northern Australia. Trans R Soc Trop Med Hyg. 2008;102:S145–S151. doi: 10.1016/S0035-9203(08)70032-4. [DOI] [PubMed] [Google Scholar]
- 10.Li XZ, Nikaido H. Efflux-mediated drug resistance in bacteria: an update. Drugs. 2009;69(12):1555–1623. doi: 10.2165/11317030-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch AS. Efflux systems in bacterial pathogens: an opportunity for therapeutic intervention? An industry view. Biochem Pharmacol. 2006;71(7):949–956. doi: 10.1016/j.bcp.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Nikaido H. Preventing drug access to targets: cell surface permeability barriers and active efflux in bacteria. Semin Cell Dev Biol. 2001;12(3):215–223. doi: 10.1006/scdb.2000.0247. [DOI] [PubMed] [Google Scholar]
- 13.Pagès JM, Amaral L. Mechanisms of drug efflux and strategies to combat them: challenging the efflux pump of Gram-negative bacteria. Biochim Biophys Acta. 2009;1794(5):826–833. doi: 10.1016/j.bbapap.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Paixão L, Rodrigues L, Couto I, Martins M, Fernandes P, de Carvalho CC, Monteiro GA, Sansonetty F, Amaral L, Viveiros M. Fluorometric determination of ethidium bromide efflux kinetics in Escherichia coli . J Biol Eng. 2009;3(1):18. doi: 10.1186/1754-1611-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez-Martínez JM, Cano ME, Velasco C, Martínez-Martínez L, Pascual A. Plamid-mediated quinolone resistance: an update. J Infect Chemother. 2011;17(2):149–182. doi: 10.1007/s10156-010-0120-2. [DOI] [PubMed] [Google Scholar]
- 16.Schito GC, Naber KG, Botto H, Palou J, Mazzei T, Gualco L, Marchese A. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents. 2009;34(5):407–413. doi: 10.1016/j.ijantimicag.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 17.van Bambeke F, Balzi E, Tulkens PM. Antibiotic efflux pumps. Biochem Pharmacol. 2000;60(4):457–470. doi: 10.1016/S0006-2952(00)00291-4. [DOI] [PubMed] [Google Scholar]
- 18.van der Donk CF, Beisser PS, Hoogkamp-Korstanje JA, Bruggeman CA, Stobberingh EE Antibotic Resistance Surveillance Group. A 12-year (1998‒2009) antibiotic resistance surveillance of Klebsiella pneumoniae collected from intensive care and urology patients in 14 Dutch hospitals. J Antimicrob Chemother. 2011;66(4):855–858. doi: 10.1093/jac/dkq538. [DOI] [PubMed] [Google Scholar]