Abstract
HIV infection and methamphetamine (Meth) abuse both may lead to oxidative stress. This study used HIV-1 transgenic (HIV-1Tg) rats to investigate the independent and combined effects of HIV viral protein expression and low dose repeated Meth exposure on the glutathione (GSH)-centered antioxidant system and oxidative stress in the brain. Total GSH content, gene expression and/or enzymatic activities of glutamylcysteine synthetase (GCS), gamma-glutamyltransferase (GGT), glutathione reductase (GR), glutathione peroxidase (GPx), glutaredoxin (Glrx), and glutathione-s-transferase (GST) were measured. The protein expression of cystine transporter (xCT) and oxidative stress marker 4-hydroxynonenal (HNE) were also analyzed. Brain regions studied include thalamus, frontal and remainder cortex, striatum, cerebellum and hippocampus. HIV-1Tg rats and Meth exposure showed highly regional specific responses. In the F344 rats, the thalamus had the highest baseline GSH concentration and potentially higher GSH recycle rate. HIV-1Tg rats showed high transcriptional responses to GSH depletion in the thalamus. Both HIV-1Tg and Meth resulted in decreased GR activity in thalamus, and decreased Glrx activity in frontal cortex. However, the increased GR and Glrx activities synergized with increased GSH concentration, which might have partially prevented Meth-induced oxidative stress in striatum. Interactive effects between Meth and HIV-1Tg were observed in thalamus on the activities of GCS and GGT, and in thalamus and frontal cortex on Glrx activity and xCT protein expression. Findings suggest that HIV viral protein and low dose repeated Meth exposure have separate and combined effects on the brain’s antioxidant capacity and the oxidative stress response that are regional specific.
Keywords: HIV, Methamphetamine, glutathione, lipid peroxidation, antioxidant, oxidative stress
INTRODUCTION
According to the World Health Organization (WHO), currently 34 million people are living with HIV infection. Methamphetamine (Meth) abuse is common in HIV-infected individuals and increases the risk for HIV infection (Farabee et al. 2002; Nemoto et al. 2002). Meth is often used for its stimulatory effects and as “self-medication” for the negative feelings by these HIV patients (Semple et al. 2002). Therefore, the co-morbidity of HIV infection and Meth abuse are exposing an increasing segment of the population to the consequences of both diseases, especially their neurotoxic effects, such as oxidative stress.
HIV induces oxidative stress through activation of microglia and astrocytes (Riddle 2007). HIV proteins that are released from infected glia also induce oxidative stress in neurons (Kruman et al. 1998; Mattson et al. 2005). In cerebrospinal fluid (CSF) or some brain regions of HIV-infected patients, levels of antioxidants such as glutathione (GSH) (Castagna et al. 1995), glutathione peroxidase (GPx), Cu/Zn superoxide dismutase (SOD) (Velazquez et al. 2009), ascorbic acid (Everall et al. 1997) and vitamin E (Bandaru et al. 2007) were reduced, while oxidants or oxidative stress markers such as 4-hydroxynonenal (HNE) (Bandaru et al. 2007; Sacktor et al. 2004), ceramide (Bandaru et al. 2007; Sacktor et al. 2004), sphingomyelin (Bandaru et al. 2007; Sacktor et al. 2004), and protein carbonyls (Turchan et al. 2003) were elevated. Similarly, in HIV viral protein-treated rats, lower levels of GSH and total SOD, but higher levels of hydroxyl radical and malondialdehyde (MDA), were found in the brain (Tang et al. 2009).
Meth also induces oxidative stress through auto-oxidation of dopamine, which results in hydrogen peroxide generation and lipid peroxidation. Increased oxidative stress markers, such as thiobarbituric acid reactive substances (TBARS) (Flora et al. 2002; Gluck et al. 2001), di-chlorofluorescein (DCF) (Wu et al. 2007; Zhang et al. 2009), and MDA (Yamamoto and Zhu 1998; Zhang et al. 2009), were consistently observed in rodents or cell culture models with Meth treatment. However, due to the differences in research models, and the variable amounts of Meth exposure (e.g., due to different routes, doses or duration of exposure), Meth-induced changes of antioxidants varied in previous studies (Acikgoz et al. 1998; Ajjimaporn et al. 2008; Dai et al. 2006; Flora et al. 2002; Harold et al. 2000; Zhang et al. 2009).
When HIV protein such as trans-activator of transcription (Tat) and Meth were combined, they synergistically increased oxidative stress in cultured neurons (Langford et al. 2004; Maragos et al. 2002) and neurotoxicity in rat striatum (Ajjimaporn et al. 2008; Langford et al. 2003a). Furthermore, post-mortem brain tissues from HIV patients who used Meth showed greater neuronal injury and dendritic loss in the frontal cortex compared to non-Meth users (Langford et al. 2003b). Such additive effects were also observed in HIV patients who used Meth by in vivo measurements of cerebral metabolite levels that reflect glial activation and neuronal injury (Chang et al. 2005) which might have resulted from additive effects on oxidative stress
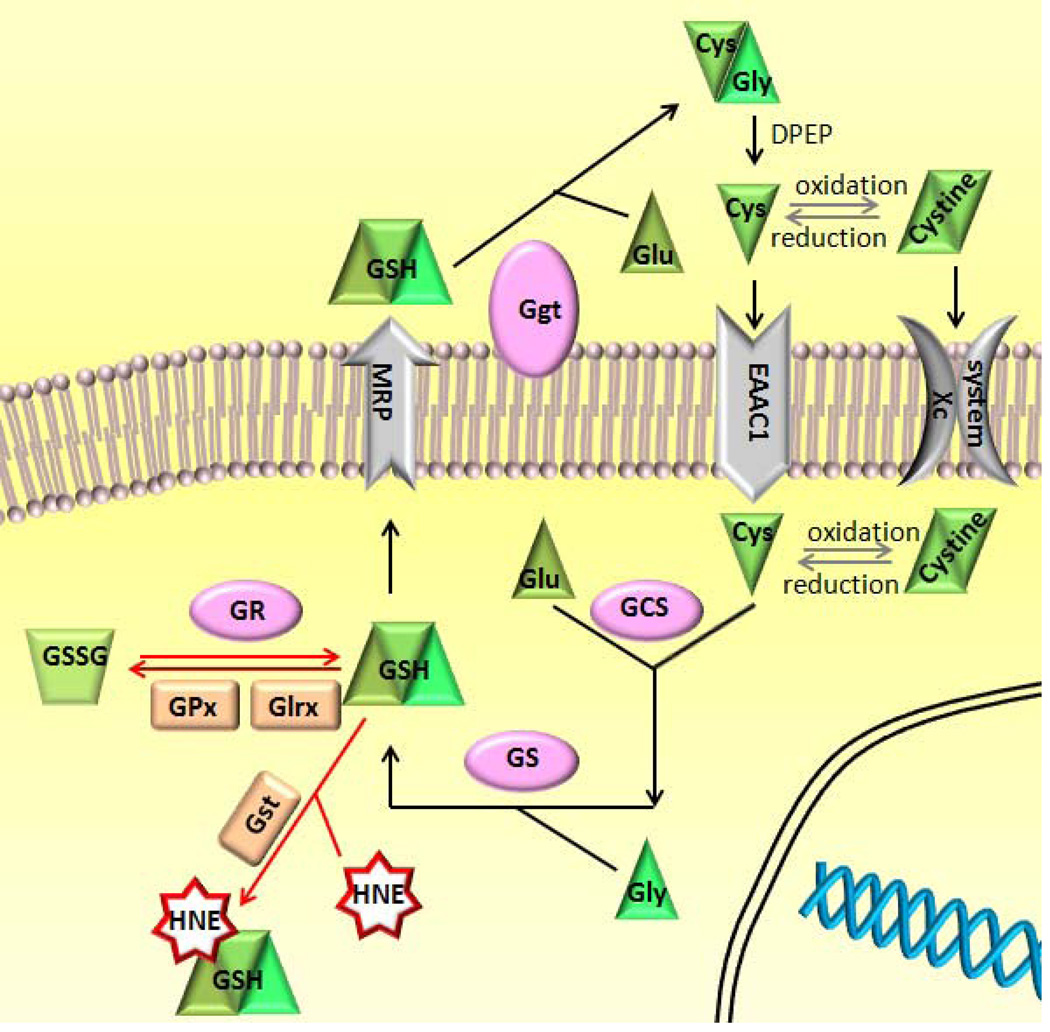
GSH is the most abundant endogenous antioxidant in the brain, counteracting oxidative stress by providing reducing power to antioxidant enzymes, such as GPx, glutathione-S-transferase (GST) and glutaredoxin (Glrx), or directly conjugating oxidants (Meister 1983). The oxidized GSH can be reduced and recycled by glutathione reductase (GR). GSH is synthesized in the cells and can be transported into the extracellular space where gamma-glutamyltransferase (GGT) catalyzes the rate-limiting step of GSH catabolism. Released cysteine is quickly oxidized to cystine and transported into the cells, where it can then be reduced to cysteine for next cycle of GSH synthesis (Bannai and Tateishi 1986). Transport of cystine occurs through the membrane protein cystine/glutamate antiporter system xc-, which consists of a light catalytic chain (xCT) and a heavy regulatory chain (4F2) (Lewerenz et al. 2012). In the central nervous system (CNS), xCT is considered as the determinant protein for the system xc-activity (Lewerenz et al. 2012). Increased expression of xCT and hence higher availability of cysteine for GSH biosynthesis has been regarded as a response to oxidative stress (Conrad and Sato 2011). Another limiting factor for GSH biosynthesis is the activity of glutamylcysteine synthetase (GCS), a holoenzyme consists of a catalytic heavy chain (GCS-HC) and a modulatory light chain (GCS-LC). This enzyme catalyzes the ligation of glutamate and cysteine, the first and rate-limiting step in the de novo synthesis of GSH (Griffith 1999). Oxidative stress has been shown to stimulate GCS activity via post-translational modification (Franklin et al. 2009). GSH synthesis is completed by glutathione synthetase (GS), which catalyzes ligation of glycine to gamma-glutamylcysteine. The GSH metabolic pathway and GSH-dependent antioxidants are summarized in Fig 1.
Figure 1. Schematic illustration of the pathways of GSH biosynthesis and antioxidant function.
Abbreviations: Cys, cysteine; Cys-Gly, cysteinyglycine; DPEP, dipeptidase; EAAC, excitatory amino acid carrier; GCS, gamma-glutamyl-cysteine synthetase; GGT, gamma-glutamyl transferase; Glrx, glutaredoxin; Glu, glutamate; Glu-cys, gamma- glutamylcysteine; Gly, glycine; GPx, glutathione peroxidase; GR, glutathione reductase; GS, glutathione synthetase; GSH, glutathione; GSSG, glutathione disulfide; GST, glutathione S-transferase; HNE, 4-Hydroxynonenal; MRP, multidrug resistance proteins; X, electrophilic xenobiotic; xCT, cystine/glutamate transporter
The aims of this study were to characterize the changes in GSH and GSH-related enzymes, to assess oxidative stress caused by the combined and independent effects of HIV viral protein and low dose repeated Meth exposure in the brain, using a HIV-1 transgenic (HIV-1Tg) rat model. The HIV-1Tg rats express 7 out of 9 HIV-1 viral proteins: Vpr, Env, Nef, Vif, Vpu, Rev and Tat. This model recapitulates many key features of human AIDS, such as muscle wasting, respiratory difficulty and HIV-associated neurological abnormalities (Basselin et al. 2011; Hag et al. 2009; Kass et al. 2010; Reid et al. 2001; Vikulina et al. 2010). This animal model also demonstrates neuronal loss and reactive gliosis (Reid et al. 2001), age-dependent increase of viral protein gene expression in the brain (Peng et al. 2010), and impaired spatial learning ability (Vigorito et al. 2007). In this study, GSH content and total antioxidant capacities were first measured in the thalamus, frontal cortex, the remainder of cortex, striatum, hippocampus, and cerebellum. Thalamus, frontal cortex, and striatum were chosen as representative regions for additional analyses, which included gene expression and the activities of enzymes involved in GSH metabolism and antioxidant function, and lipid peroxidation.
MATERIALS AND METHODS
Animals
Eight (8) HIV-1 NL4-3 gag/pol transgenic rats (HIV-1Tg) and 8 genetic background control Fisher 344 (F344) rats with the same date of birth were purchased from Harlan Inc. (Indianapolis, IN) and housed at the Laboratory Animal Services facility of Seton Hall University, NJ. The rats were maintained on a 12-h light/dark schedule. Food and water were provided ad libitum throughout the experiment. All procedures were carried out in accordance with the guidance of the Seton Hall University Institutional Animal Care and Use Committee. The rats were separated into 4 groups with 4 rats in each group: (i) Control group, F344 rats without Meth exposure, but with a single intraperitoneal (i.p.) saline injection everyday for 6 consecutive days. This injection started when the rats were 13-week old, and the dose of the saline injection was 2 ml/kg body weight/day; (ii) Meth group, F344 rats treated with Meth through i.p. injection at 13 weeks. The dose of Meth was 2.5 mg in 2 ml saline/ kg body weight/day, one injection per day for 6 consecutive days; (iii) HIV group, HIV-1Tg rats with saline i.p. injection, same dose and schedule as the Control group; (iv) HIV+Meth group, HIV-1Tg rats with Meth i.p. injection, same dose and schedule as the Meth group. The relatively low dose of Meth used was intended only for behavior sensitization (Liu et al. 2009), to better emulate the recreational doses of Meth used by HIV infected individuals.. The rats were sacrificed by decapitation approximately 24 h following the last Meth administration.
Sample preparation
The brains were excised and immediately stored at −80°C. Prior to the assays, the brain samples were further dissected into 6 regions: cortex ("frontal" and the remainder which we called "other"), striatum, thalamus, hippocampus, and cerebellum. Soluble proteins/peptides were extracted by sonicating a suitable amount of tissue in cold PBS on ice. The samples were then centrifuged at 14,000 xg for 10 min at 4°C. The supernatants were collected, and the protein concentrations were measured using Bradford assay (Biorad, Hercules, CA). The processed samples were stored at −80°C.
Chemicals and instruments
All chemicals used in this study were purchased from Sigma (St. Louis, MO) unless otherwise noted. The spectrophotometers used in the colorimetric and fluorescent assays were SpectraMax 340 (for GSH, ABTS, FRAP, GCS, and HNE-His assays) or SpectraMax M3 (for GGT, GPx, Glrx, and GST assays) from Molecular Devices (Sunnyvale, CA). Protein electrophoresis system was from BioRad (Hercules, CA), and the western blot imaging was carried out using an Odyssey Infrared Imaging System, and Odyssey Application Software Version 3.0 (Li-Cor Biosciences, Lincoln, NE). Real-time PCR was carried out using a Lightcycler 480 II (Roche Applied Science, Indianapolis, IN).
Total GSH assay
This measurement quantitated a colorigenic reaction between GSH and 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) (Tietze 1970). Oxidized GSH (GSSG) was used to establish a standard curve, and the result was further normalized against sample protein concentration.
All assays in this study were carried out in triplicate unless noted otherwise.
ABTS assay
This assay measured the ability of the samples to scavenge the 2,2’-azino-bis (3-ethylbenzthiazoline- 6-sulfonic acid) (ABTS) radical as previously described (Panee et al. 2007). 6-hydroxy- 2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was used as a standard and the results were expressed as Trolox equivalent antioxidant capacity (TEAC). The result was further normalized against protein concentration.
FRAP assay
This assay measured the reducing ability of the samples to ferric ion at pH 3.6 (Chen et al. 2003). Trolox was used as a standard. The result was further normalized against protein concentration.
Real-time PCR
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA). The cDNA was synthesized using the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). The qRT-PCR primers were designed using an online tool provided by Roche Applied Science. The quantitative real-time PCR was performed in quadruplicate using Platinum SYBR Green qPCR SuperMix-UDG kit (Invitrogen). The primer sequences of the housekeeping genes were: GAPDH forward: tgggaagctggtcatcaac, reverse: gcatcaccccatttgatgtt; 28S forward: tgcagatcatgctgtttaagaac, reverse: ggacctgctccccagatt; HPRT forward: ctcctcagaccgcttttcc, reverse: tcataacctggttcatcatcactaa. The primer sequences of the target genes were: GCS-HC forward: cgatgttcttgaaactctgcaa, reverse: ctggtctccagagggttgg; GCS-LC forward: ctgactcacaatgacccaaaag, reverse: gatgctttcttgaagagcttcct; GS forward: gctggacaacgagcgagt, reverse: gctgcttctcatcctgcaa; GGT7 forward: tggcccaataggactgctaa, reverse: tccctggctgtaccgagtt; GR forward: ttcctcatgagaaccagatcc, reverse: tgaaagaacccatcactggtta; GPx1 forward: acagtccaccgtgtatgcctt, reverse: ctcttcattcttgccattctcctg; GPx4 forward: tctgtgtaaatggggacgatgc, reverse: tctctatcacctggggctcctc; Glrx3 forward: ccacagtgtgtacagatgaacg, reverse: agcttcggcttccagctt. The relative abundance of a target gene was normalized to a housekeeping gene using the ratio of 2 Cp housekeeping / 2 Cp target, where Cp is the value of crossing point.
GCS activity assay
This assay measured the amount of gamma-glutamylcysteine (GC) synthesized from L-glutamate and cysteine during a fix period of time, as indicated by a fluorescent signal derived from 2,3-naphthalenedicarboxaldehyde (NDA) and serine in the presence of thiols (White et al. 2003). The fluorescence intensities were measured at 472 ex/ 528 em.
GGT activity assay
This assay measured the rate of transferring the glutamyl residue from L-gamma-glutamyl-p-nitroanilide (GPNA) to glycine-glycine (Meister et al. 1981). One unit of activity was defined as consumption of 1 µmol of GPNA per minute calculated from the expression (Vmax * Vt/Vs)/ (0.0088* D), where Vt is the total volume of reaction solution in microliters, Vs is the sample volume in microliters, 0.0088 µM−1cm−1 is the extinction coefficient for GPNA at 410 nm, and D is the reaction solution depth in wells in centimeters.
GR activity assay
GR was measured using a commercial kit from Cayman Chemical (Ann Arbor, MI, Catalog number 703202). The rate of absorbance decrease of NADPH at 340nm was used as an indicator for GR activity.
Glrx activity assay
This assay measured the disulfide reduction in bis(2-hydroxyethyl) disulfide (HED) catalyzed by the samples in the presence of GSH and NADPH (Diotte et al. 2009). One unit of activity was defined as a consumption of 1 µmol of NADPH per minute calculated from the expression (Vmax * Vt/Vs)/(0.0062* D), where 0.0062 µM−1cm−1 is the extinction coefficient for NADPH at 340 nm.
GST activity assay
This assay measured the conjugation rate of GSH to 1-chloro-2,4-dinitrobenzene (CDNB) (Gonzalez et al. 1989). One unit of activity was defined as a consumption of 1 µmol of CDNB per minute calculated from the expression (Vmax * Vt/Vs)/(0.0096* D), where 0.0096 µM−1cm−1 is the extinction coefficient for glutathione-dinitrobenzene conjugate (GS-DNB) at 340 nm.
GPx activity assay
This assay measured the reduction rate of tert-butyl hydroperoxide catalyzed by the samples upon the oxidation of GSH and NADPH (Panee et al. 2007). One unit of enzyme activity was defined as a consumption of 1 µmol of NADPH per minute calculated from the expression (Vmax * Vt/Vs)/ (0.0062* D), where 0.0062 µM−1cm−1 is the extinction coefficient for NADPH at 340 nm.
xCT Western Blot
Proteins in the tissue lysates were separated electrophoretically in 4–20% SDS-PAGE gels (Bio-Rad, 567–1094), and transferred overnight to Immobilon PVDF transfer membranes (Millipore, Billerica, MA, IPFL00010). The membranes were blocked with blocking buffer (Li-Cor, 927–40000) for at least 1 hour before incubated in primary antibody solutions (0.3% PBST and blocking buffer, 1:1). After washing 3 times with 0.3% 1x PBST (10 min each wash), the membranes were incubated in secondary antibody solutions and washed 3 times with PBST before scanning. The primary antibodies were rabbit anti-xCT (1:500 dilution, Abcam ab93030) and mouse anti-beta-actin (1:20,000 dilution, Li-Cor, 926–42212), and the secondary antibodies were anti-rabbit secondary antibody (1:10,000 dilution, Li-Cor, 926–32211) and anti-mouse secondary antibody (1:20,000 dilution, Li-Cor 926–68020). The membranes were scanned using an Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE) and the densitometric analysis was performed using Odyssey Application Software Version 3.0.
Lipid peroxidation assay
Histidine-conjugated HNE (HNE-His) is a major HNE conjugates (Doorn and Petersen 2002). Its content was measured using an ELISA kit from Cell Biolabs (San Diego, CA).
Statistical analysis
Prism 5.0 (GraphPad Software Inc., La Jolla, CA) was used for statistical analyses. Two-way ANOVA was used to evaluate the effects of HIV transgenesis, Meth injection, and their possible interaction. Differences among the means were analyzed using (1) one-way ANOVA followed by Tukey’s post-hoc test, or (2) Student t-test. Non-parametric Mann Whitney test was used to compare the means of ratios in Table 3 and Fig 2. - P < 0.05- was considered statistically significant in all tests.
Table 3. Regulatory effects of HIV transgenesis and methamphetamine treatment on gene expression of enzymes involved in GSH metabolism and function, and viral protein Tat and gp120 in the thalamus and the striatum.
The results were calculated using HPRT as a housekeeping gene. Mean (SD), n=4/group, unit = relative abundance compared to HPRT.
| F344+Saline (Control) |
F344+Meth (Meth) |
HIV-1Tg +Saline (HIV-1Tg) |
HIV-1Tg +Meth | Two-way ANOVA | |||
|---|---|---|---|---|---|---|---|
| HIV-1Tg Effect |
Meth Effect | HIV-1Tg×Met Interaction |
|||||
| Thalamus | |||||||
| Enzymes involved in GSH metabolism | |||||||
| GCS-HC | 0.22 (0.093) | 0.11 (0.052) | 0.32 (0.17) | 0.38 (0.13)b | P=0.014 | n.s | n.s |
| GCS-LC | 0.24 (0.031) | 0.12 (0.041) | 0.19 (0.053) | 0.37 (0.25)b | n.s | n.s | n.s |
| GS | 0.055 (0.045) | 0.034 (0.018) | 0.11 (0.039) | 0.17 (0.14)b | P=0.049 | n.s | n.s |
| GGT7 | 0.48 (0.31) | 0.20 (0.11) | 1.17 (0.66) | 0.88 (0.27) | P=0.0091 | n.s | n.s |
| Enzymes that use or recycle GSH | |||||||
| GR | 0.036 (0.01) | 0.032 (0.006) | 0.030 (0.009) | 0.041(0.019) | n.s | n.s | n.s |
| GPx1 | 0.061 (0.015) | 0.047 (0.013) | 0.040 (0.02) | 0.042 (0.018) | n.s | n.s | n.s |
| GPx4 | 1.36 (0.18) | 1.09 (0.18) | 1.37 (0.57) | 1.06 (0.3) | n.s | n.s | n.s |
| Glrx3 | 0.88 (0.21) | 0.70 (0.39) | 1.0 (0.24) | 0.79 (0.54) | n.s | n.s | n.s |
| HIV proteins | |||||||
| Tat | – | – | 0.032 (0.01) | 0.026 (0.017) | |||
| gp120 | – | – | 0.051 (0.015) | 0.042 (0.033) | |||
| Striatum | |||||||
| Enzymes involved in GSH metabolism | |||||||
| GCS-HC | 0.32 (0.06) | 0.29 (0.035) | 0.27 (0.035) | 0.35 (0.09) | n.s | n.s | n.s |
| GCS-LC | 0.26 (0.07) | 0.24 (0.046) | 0.24 (0.019) | 0.25 (0.051) | n.s | n.s | n.s |
| GS | 0.071 (0.016) | 0.07 (0.014) | 0.057 (0.011) | 0.063 (0.027) | n.s | n.s | n.s |
| GGT7 | 0.61 (0.10) | 0.50 (0.026) | 0.57 (0.01)b | 0.5 (0.22) | n.s | n.s | n.s |
| Enzymes that use or recycle GSH | |||||||
| GR | 0.025 (0.015) | 0.047 (0.023) | 0.046 (0.029) | 0.041 (0.008) | n.s | n.s | n.s |
| GPx1 | 0.005 (0.002) | 0.011 (0.008) | 0.005 (0.002) | 0.006 (0.003) | n.s | n.s | n.s |
| GPx4 | 2.30 (0.92) | 2.10 (0.87) | 2.11 (0.52) | 2.51 (1.23) | n.s | n.s | n.s |
| Glrx3 | 0.92 (0.16) | 0.68 (0.19) | 0.88 (0.23) | 0.85 (0.37) | n.s | n.s | n.s |
| HIV proteins | |||||||
| Tat | – | – | 0.028 (0.021) | 0.04 (0.026) | |||
| gp120 | – | – | 0.044 (0.043) | 0.054 (0.03) | |||
compared to Control
compared to Meth
P<0.05, one-way ANOVA post hoc or Mann Whitney Test. n.s., non-significant.
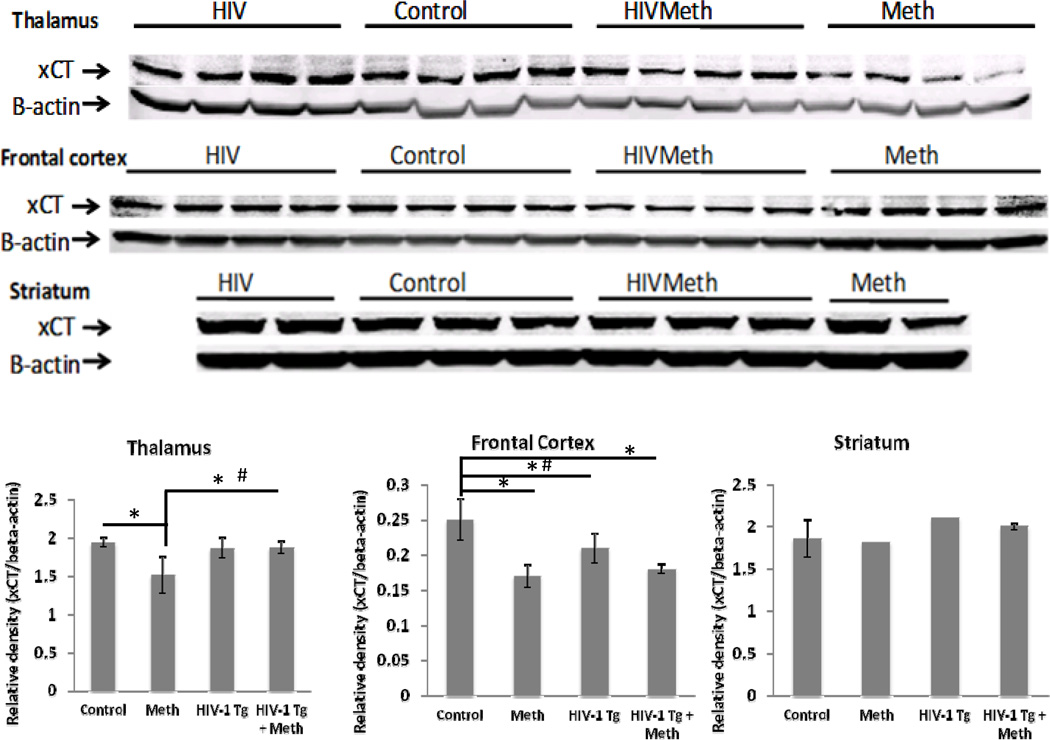
Figure 2. Image and quantification of xCT western blotting in thalamus, frontal cortex, and striatum.
Average (SD). * P<0.05, Mann-Whitney test; # P<0.05, Bonferroni post hoc test. Note: only 2 striatum samples were available for western blotting in the HIV-1Tg and Meth groups. No statistical analysis was performed on these samples due to the small sample size
RESULTS
Profile of total antioxidant capacities in six brain regions
To assess antioxidant distribution throughout the brain, we measured total antioxidant capacities using both ABTS and FRAP methods in six brain regions of the 4 groups of rats (Table 1). The baseline ABTS reading was found 1–2 folds higher in the thalamus than in the other regions in the Control group. Two-way ANOVA showed that HIV-1Tg resulted in a significant decrease of the ABTS value in the hippocampus (PHIV-1Tg =0.013). A significant difference in the cerebellum between the Meth group and the HIV-1Tg+Meth group was also detected.
Table 1. Effects of HIV-1 transgenesis and methamphetamine treatment on total antioxidant capacities measured by the ABTS and FRAP methods in six brain regions.
Mean (SD), n=4 rats/ group, unit = µmol trolox equivalent antioxidant capacity per gram protein.
| ABTS | F344 + Saline (Control) |
F344
+ Meth (Meth) |
HIV-1Tg
+ Saline (HIV-1Tg) |
HIV-1Tg +Meth | Two-way ANOVA | ||
|---|---|---|---|---|---|---|---|
| HIV-1Tg Effect |
Meth Effect |
HIV-1Tg×Meth Interaction |
|||||
| Thalamus | 422.12 (145.23)* | 474.36 (248.22) | 494.03 (121.12) | 504.00 (174.09) | n.s. | n.s. | n.s. |
| Striatum | 133.09 (14.61) | 127.85 (35.82) | 126.61 (16.84) | 132.74 (33.44) | n.s. | n.s. | n.s. |
| Cortex (frontal) | 140.80 (12.55) | 155.76 (21.03) | 136.86 (2.80) | 133.71 (11.24) | n.s. | n.s. | n.s. |
| Cortex (other) | 225.95 (48.60) | 214.77 (62.04) | 193.01 (31.14) | 205.48 (51.83) | n.s. | n.s. | n.s. |
| Hippocampus | 151.43 (52.76) | 178.05 (29.23) | 112.10 (31.51)b | 114.48 (19.07)b | P=0.013 | n.s. | n.s. |
| Cerebellum | 217.01 (56.26) | 194.71 (20.65) | 238.14 (29.64) | 234.39 (22.41)b | n.s. | n.s. | n.s. |
| FRAP | F344 + Saline (Control) |
F344
+ Meth (Meth) |
HIV-1Tg
+ Saline (HIV-1Tg) |
HIV-1Tg +Meth | Two-way ANOVA | ||
|---|---|---|---|---|---|---|---|
| HIV-1Tg Effect |
Meth Effect |
HIV-1Tg×Meth Interaction |
|||||
| Thalamus | 13.84 (3.20) | 12.27 (3.70) | 16.45 (2.97) | 11.00 (4.58) | n.s. | n.s. | n.s. |
| Striatum | 16.18 (2.61) | 10.17 (3.40)a | 12.39 (2.63) | 13.28 (4.83) | n.s. | n.s. | n.s. |
| Cortex (frontal) | 17.52 (3.40) | 22.54 (1.92)a | 20.63 (2.36) | 16.80 (2.08)b | n.s. | n.s. | P=0.0041 |
| Cortex (other) | 31.50 (10.58)* | 24.07 (3.70) | 31.45 (3.71)b | 32.13 (4.11)b | n.s. | n.s. | n.s. |
| Hippocampus | 12.18 (2.49) | 16.99 (4.41) | 15.37 (3.73) | 13.93 (2.59) | n.s. | n.s. | n.s. |
| Cerebellum | 33.97 (4.40)* | 70.06 (30.03) | 30.67 (12.74) | 36.17 (21.05) | n.s. | n.s. | n.s. |
compared to the same region of Control F344
compared to the same region of Meth-treated F344
compared to the same region of HIV-1Tg;
compared to all the other regions of Control F344;
P<0.05, one-way ANOVA post hoc or Student t-test. n.s., non-significant.
The baseline readings of FRAP were ~2 folds higher in the cortex (other) and the cerebellum than the other regions in the Control group. F344 rats exposed to Meth had increased FRAP in the frontal cortex (+29%, Meth vs. Control), but the HIV-1Tg rats exposed to Meth paradoxically had decreased levels (−19%, HIV-1Tg+Meth vs. HIV-1Tg, P=0.0506), which led to an interactive effect between HIV-1Tg and Meth on FRAP in the frontal cortex (two-way ANOVA, Pinteraction=0.0041). FRAP levels tended to be increased in Meth exposed control rats in the cerebellum (+106%, Meth vs. Control, P=0.055), but decreased in the striatum (−37%, Meth vs. Control).
The two methods employed in this section of the study depicted antioxidant potential of different categories of molecules. i.e. the ABTS method measures the radical scavenging ability of peptides, proteins and other small molecules at a physiological pH value (7.4), while FRAP measures the reducing ability of non-protein small molecules (such as ascorbic acid) at pH 3.6. It is interesting to observe that HIV-1Tg affected the ABTS values in the hippocampus, while Meth affected the FRAP readings in the frontal cortex, cerebellum, and striatum.
Regional variations of total brain GSH in the 4 groups of rats
Similar to the ABTS result in table 1, the baseline concentration of total GSH was over 1–2 folds higher in the thalamus than in the other regions of the Control F344 rats (Table 2). In the thalamus, HIV-1Tg rats had significantly lower GSH levels than the F344 rats (2-way ANOVA, PHIV=0.0011), 31% lower in HIV-Tg and 46% lower in HIV-1Tg+Meth than in controls. Similarly, HIV-1Tg rats, regardless of Meth exposure status, had a trend for lower GSH levels in the striatum (2-way ANOVA-PHIV=0.045). Conversely, Meth exposed animals had a trend for higher GSH contents in the striatum (2-way ANOVA-PMeth=0.019). For example, the average GSH concentration in the striatum of Meth-treated F344 rats was 37% higher than that in saline-treated F344 rats. The GSH contents in the cortex (frontal and other), hippocampus, and cerebellum did not show significant differences among the four groups.
Table 2. Effects of HIV-1 transgenesis and methamphetamine on total glutathione content, Mean (SD), in six brain regions (n=4 / group).
Unit=µmol per g protein.
| F344
+ Saline (Control) |
F344
+ Meth (Meth) |
HIV-1Tg
+ Saline (HIV-1Tg) |
HIV-1Tg +Meth |
Two-way ANOVA |
|||
|---|---|---|---|---|---|---|---|
| HIV-1Tg Effect |
Meth Effect |
HIV-1Tg×Meth Interaction |
|||||
| Thalamus | 21.62 (5.40)* | 25.76 (4.58) | 14.92 (4.35)b | 14.01 (2.44)b | P=0.001 | n.s. | n.s. |
| Striatum | 8.65 (1.72) | 11.89 (2.03)a | 7.24 (1.75)b | 9.10 (1.97) | P=0.045 | P=0.019 | n.s. |
| Cortex (frontal) | 8.76 (1.64) | 10.07 (2.20) | 9.77 (2.36) | 8.37 (1.20) | n.s. | n.s. | n.s. |
| Cortex (other) | 6.82 (1.03) | 5.65 (1.36) | 6.36 (1.28) | 6.34 (1.39) | n.s. | n.s. | n.s. |
| Hippocampus | 6.20 (2.09) | 5.17 (1.16) | 4.81 (0.76) | 4.39 (0.79) | n.s. | n.s. | n.s. |
| Cerebellum | 5.89 (0.41) | 6.48 (0.70) | 6.15 (0.94) | 6.41 (0.43) | n.s. | n.s. | n.s. |
compared to the same region of Control F344
compared to the same region of Meth-treated
compared to all the other regions of F344 Control
P<0.05, one-way ANOVA post hoc or Student t-test.
Transcriptional responses of GSH-related genes and viral protein genes in the thalamus and striatum
To further evaluate the enzymes related to changes of GSH contents in the thalamus and striatum, the mRNA levels of genes involved in GSH biosynthesis (GCS-HC, GCS-LC, GS), catabolism (GGT7), recycling (GR), and antioxidant function (GPx1, GPx4, Glrx3) were measured. The gene expression levels of multiple isoforms of GGT and Glrx have been screened in rat brain tissues in a pilot study, the mRNA levels of GGT7 and Glrx3 were found to be the highest in each family of the enzymes (data not shown), and therefore these two isoforms were selected in this part of the study. In order to assess the potential impact of Meth exposure on HIV-1 viral protein gene expression, the mRNA levels of Tat and gp120, the two viral proteins most commonly recognized as causative factors of oxidative stress, were measured in the thalamus and striatum. Three housekeeping genes (HPRT, GAPDH, and 28S) were tested first, and the expression levels of HPRT were found to be unchanged by HIV-1Tg and Meth;therefore, it was used to normalize the relative expression levels of the target genes.
As shown in Table 3, in the thalamus, HIV-1Tg resulted in significant upregulation of the genes involved in GSH metabolism, such as GCS-HC, GS, and GGT7 (two-way ANOVA). Interestingly, Meth exposure of F344 rats seemed to decrease the expression of the genes in this category, while Meth exposure of HIV-1Tg rats appeared to have the opposite effect (except for GGT7). Consequently, the mRNA levels of these genes were 2–4 folds higher in the HIV-1Tg+Meth group compared to the Meth group. However, in the striatum, neither HIV-1Tg nor Meth injections caused any significant changes of the expression of these genes.
Quantification of the mRNA levels of Tat and gp120 in the HIV-1Tg and HIV-1Tg+Meth groups indicated Meth had no significant regulatory effect on the expression of these 2 viral protein genes in thalamus and striatum.
Activities of GSH-related enzymes
In addition to gene transcription, the activities of the aforementioned enzymes were also measured in thalamus and striatum. For comparison, we also included frontal cortex in this part of the study as a brain region in which the GSH content did not change in response to HIV-1Tg or Meth. As shown in Table 4, HIV-1Tg did not affect the activity of GCS and GGT in any of the 3 brain regions, which is in contrast to the upregulated gene expression of GCS-HC and GGT7 in the thalamus. Meth was associated with marked elevation of the GCS activity in the F344 rats (+88% Meth vs. Control) and even greater elevation in the HIV-1Tg rats (+224% HIV-1Tg+Meth vs. HIV-1Tg), leading to an interactive effect between HIV-1Tg and Meth (two-way ANOVA: Pmeth<0.0001; Pinteraction=0.02). Conversely, Meth injections resulted in decreased GCS activities in both striatum (Two-way ANOVA: Pmeth=0.0002) and frontal cortex (Two-way ANOVA: Pmeth<0.0001). In the frontal cortex, Meth increased the activity of GGT (Two-way ANOVA: Pmeth=0.044), while in thalamus, an interactive effect was found for GGT activity, which was elevated in the HIV-1Tg rats (+36%, vs. Control) but normalized in the HIV-1Tg+Meth rats (PInteraction=0.014)
Table 4. Activities of enzymes involved in GSH metabolism, use, and recycling in the thalamus, striatum and frontal cortex of F344 and HIV-1Tg rats with and without methamphetamine (Meth).
Mean (SD), N=4/group. Units: GCS, fluorescent intensity; the others, U per g protein.
| F344+Saline (Control) |
F344+Meth (Meth) |
HIV-1
Tg +Saline (HIV-1Tg) |
HIV-1Tg +Meth |
Two-way ANOVA | |||
|---|---|---|---|---|---|---|---|
| HIV-1Tg Effect |
Meth Effect |
HIV-1Tg×Meth Interaction |
|||||
| Thalamus | |||||||
| Enzymes involved in GSH metabolism | |||||||
| GCS | 1.64 (0.66) | 3.09 (0.81)a | 1.37 (0.33)b | 4.44 (0.51)a,b,c | n.s. | P<0.0001 | P=0.020 |
| GGT | 68.20 (9.68) | 75.40 (10.24) | 92.80 (14.12)a | 68.25 (9.16)c | n.s. | n.s. | P=0.014 |
| Enzymes that use or recycle GSH | |||||||
| GR | 0.49 (0.10) | 0.39 (0.03) | 0.36 (0.06) | 0.30 (0.03)a,c | P=0.042 | P=0.011 | n.s. |
| GPx | 3.48 (0.91) | 2.30 (1.23) | 2.92 (1.21) | 2.12 (0.60) | n.s. | n.s. | n.s. |
| Glrx | 9.04 (1.29) | 12.98 (0.58)a | 10.21 (1.53)b | 8.63 (1.16)b | P=0.020 | n.s. | P=0.0006 |
| GST | 12.19 (1.82) | 9.98 (2.26) | 11.13 (1.51) | 11.13 (1.25) | n.s. | n.s. | n.s. |
| Striatum | |||||||
| Enzymes involved in GSH metabolism | |||||||
| GCS | 8.12 (0.63) | 5.14 (2.10) | 9.73 (0.61)a,b | 4.46 (1.09)a,c | n.s. | P=0.0002 | n.s. |
| GGT | 30.14 (2.99) | 29.50 (2.75) | 29.28 (4.24) | 29.80 (10.48) | n.s. | n.s. | n.s. |
| Enzymes that use or recycle GSH | |||||||
| GR | 0.066 (0.010) | 0.144 (0.019)a | 0.060 (0.031)b | 0.090 (0.045) | n.s. | P=0.009 | n.s. |
| GPx | 2.31 (0.37) | 1.73 (0.22)a | 2.13 (0.23) | 1.44 (0.19)a,c | n.s. | P=0.001 | n.s. |
| Glrx | 5.05 (0.14) | 9.54 (0.70)a | 5.28 (0.60)b | 9.61 (0.09)a,c | n.s. | P<0.0001 | n.s. |
| GST | 5.47 (0.49) | 5.61 (0.65) | 4.56 (0.31) | 4.49 (0.90) | P=0.016 | n.s. | n.s. |
| Frontal Cortex | |||||||
| Enzymes involved in GSH metabolism | |||||||
| GCS | 1.83 (0.10) | 0.53 (0.50)a | 1.77 (0.19)b | 0.54 (0.16)a,c | n.s. | P<0.0001 | n.s. |
| GGT | 26.16 (6.10) | 37.49 (10.75) | 22.93 (3.82)b | 28.16 (7.01) | n.s. | P=0.044 | n.s. |
| Enzymes that use or recycle GSH | |||||||
| GR | 0.16 (0.016) | 0.20 (0.055) | 0.14 (0.023) | 0.17 (0.053) | n.s. | n.s. | n.s. |
| GPx | 10.54 (1.08) | 10.02 (0.64) | 9.44 (0.68) | 9.81 (1.92) | n.s. | n.s. | n.s. |
| Glrx | 10.79 (0.73) | 8.32 (0.60)a | 6.71 (0.33)a,b | 7.56 (0.07)a,b,c | P<0.0001 | P=0.007 | P<0.0001 |
| GST | 8.10 (0.87) | 8.21 (0.90) | 8.03 (0.55) | 8.21 (0.54) | n.s. | n.s. | n.s. |
compared to Control F344
compared to Meth-Treated
compared to HIV-1 Tg
P<0.05, one-way ANOVA post hoc or Student t-test.
As a key enzyme catalyzing GSH recycling, the activity of GR was decreased by both HIV-1Tg and Meth in the thalamus (Two-way ANOVA PHIV=0.042, Pmeth=0.011), but was increased by Meth in the striatum (Two-way ANOVA Pmeth=0.0002). No significant change of GR activity was found in the frontal cortex.
Among the GSH-dependent antioxidant enzymes, the activity of Glrx was found significantly influenced by HIV-1Tg, Meth, or both in the 3 brain regions. In the striatum, the Glrx activity was upregulated by Meth (+89%, Meth vs. Control; +82% HIV-1Tg+Meth vs. HIV-1Tg, two-way ANOVA PMeth<0.0001). In thalamus and frontal cortex, Meth and HIV-1Tg interactively regulated Glrx activities. In the frontal cortex, both HIV-1Tg and Meth rats had decreased Glrx activity (−24%, Meth vs. Control, −38%, HIV-1Tg vs. Control), but converging of the 2 factors did not result in further activity reduction (−30%, HIV-1Tg+Meth vs. Control); in the thalamus, Meth increased the Glrx activity (+44%, Meth vs. Control), but the concurrence of HIV-1Tg and Meth normalized the activity level. Changes in GPx and GST activities were only found in striatum; Meth decreased GPx activity and HIV-1Tg decreased GST activity (two-way ANOVA: PMeth=0.001, PHIV-1Tg=0.016).
Protein expression of xCT
Another rate-limiting factor in GSH de novo biosynthesis is the supply of cysteine, which can be derived from dipeptide cystine that is transported into the cells through system xc-. The activity of system xc- is determined by the catalytic chain xCT. Therefore the protein expression of xCT was measured using western blotting (Fig 2). In the thalamus, Meth-treated F344 rats showed significantly lower amount of xCT protein compared to the control group (−22%) and the HIV-1Tg+Meth group (−19%), and the difference showed a trend for significance compared to the HIV-1Tg group (−19%, P=0.057). In the frontal cortex, the amount of xCT protein decreased in all 3 experimental groups compared to the Control group, and two-way ANOVA showed that Meth exposure contributed to some of these decreases (PMeth=0.0002). Interestingly, interactions between HIV-1Tg and Meth were found in both thalamus and frontal cortex (thalamus, Pinteraction=0.0114; frontal cortex, Pinteraction=0.018). In the striatum, no significant difference was found between Control and HIV-1Tg+Meth rats; however, comparisons with the Meth and HIV-1Tg rats were not performed in this region due to the fewer sample sizes available.
Lipid peroxidation
HNE-His concentration was measured in the thalamus, striatum, and frontal cortex (Table 5). In both thalamus and frontal cortex, HNE-His levels were higher in the Meth-treated rats than in the Control (thalamus: +33%, p=0.005; frontal cortex: +52%, p=0.003) and in HIV-1Tg rats (thalamus: +44%, p=0.016; frontal cortex: +60%, p=0.01). Since Meth exposure also led to a strong trend for higher HNE-His levels in the HIV-1Tg+Meth rats (+45% vs. HIV-1Tg, p=0.024) in the frontal cortex, a stronger Meth effect was observed in this brain region (Two-way ANOVA Pmeth=0.0002) than in thalamus (Two-way ANOVA Pmeth=0.0122). No significant changes in the HNE-His level were found in the striatum.
Table 5. Effects of HIV-1 transgenesis and methamphetamine on lipid peroxidation as indicated by 4-hydroxynonenal content.
Mean (SD), N=4/group. Unit = fluorescent intensity/µg protein.
| F344+Saline (Control) |
F344+Meth (Meth) |
HIV-1Tg + Saline (HIV-1Tg) |
HIV-1 Tg+Meth |
Two-way ANOVA | |||
|---|---|---|---|---|---|---|---|
| HIV-1 Tg Effect |
Meth Effect |
HIV-1Tg×Meth Interaction |
|||||
| Thalamus Striatum Frontal cortex | 0.27 (0.025) | 0.36 (0.027)a | 0.25(0.046)b | 0.31 (0.074) 0.30 (0.056) | n.s. | P=0.0122 | n.s. |
| Striatum | 0.34(0.050) | 0.27 (0.059) | 0.27 (0.017) | 0.30 (0.056) | n.s. | n.s. | n.s. |
| Frontal cortex | 0.33 (0.033) | 0.50 (0.061)a | 0.31 (0.083)b | 0.45 (0.032)a,ca | n.s. | P=0.0002 | n.s. |
compared to Controls
compared to Meth
compared to HIV-1 Tg
P<0.05, one-way ANOVA post hoc or Student t-test
DISCUSSION
This study focused on the intrinsic changes of GSH-centered antioxidants and oxidative stress caused by low doses of Meth exposure and HIV-1Tg in the rat brain. The main findings included: HIV-1Tg rats showed decreased GSH content in both thalamus and striatum suggesting decreased antioxidant defense. Their thalamus showed upregulation of the gene expression of enzymes involved in GSH metabolism, but not the activity levels of these enzymes, indicating the lack of compensation. In contrast, rats exposed to low doses of Meth showed compensatory increased GSH concentration and altered activities of GSH-centered enzymes only in the striatum. Furthermore, the low dose Meth exposure also regulated the activities of GSH-related enzymes, inhibited xCT protein expression, and increased lipid peroxidation in the thalamus and frontal cortex.
In this study, the highest GSH content and the ABTS radical scavenging ability were found in thalamus, which were 1–2 folds higher than the other five brain regions. These findings suggest that the thalamus has the highest antioxidant capacity. Similarly, an HPLC study on human brain tissues also reported the highest GSH level in the thalamus among 7 brain regions (Bronowicka-Adamska et al. 2011), but the differences were marginal, which contrasts with the several-fold differences in our study. Contrary to our findings, a NMR study reported that GSH content in the thalamus of Sprague–Dawley rats (5–6 months) was comparable to the levels in 4 other brain regions (Lee et al. 2012). These variable findings of GSH levels in the thalamus relative to the other brain regions may be due to differences in species, strain, age of the study subjects, or a combination of these factors.
The lower GSH levels in both thalamus and striatum of the HIV-1Tg rats may be due to oxidative stress caused by the neurotoxic viral proteins. Similarly, intravenous injections of Tat and gp120 resulted in GSH depletion in the whole brain in mice (Banerjee et al. 2010). In the thalamus, this GSH depletion was associated with upregulation of the genes for enzymes involved in GSH metabolism, such as GCS-HC, GS, and GGT7, which reflects an ongoing compensatory response to the depletion in this brain region. However, similar decrease of GSH in the striatum did not cause any transcriptional changes in these genes. This difference may implicate the relative importance of the GSH system in different regions of the brain. Additionally, in HIV-infected human macrophages, GSH along with the mRNA levels of GCS-HC, GCS-LC, GS, and GGT1 were found to be decreased (Morris et al. 2012), suggesting a different regulatory mechanism in these peripheral cells in response to HIV-1 mediated GSH depletion.
In a previous study, the mRNA levels of Tat, gp120, nef, and vif were measured in 6 brain regions (hypothalamus, striatum, hippocampus, cerebellum, prefrontal cortex, and the remainder parts of cortex) of 2–3 months old HIV-1Tg rats, and the gene expression levels varied across these regions (Peng et al. 2010). Tat and gp120, the two most commonly known pro-oxidant viral proteins, had the highest expression level in the cerebellum, but a moderate expression level in the striatum. Table 2 shows that in response to HIV-1Tg, the GSH content was normal in the cerebellum, but markedly reduced in the striatum. Taken together, these findings show that the regulation of GSH metabolism is region specific in the context of HIV-1Tg, but is not related to the expression levels of the viral protein genes.
HIV-1Tg rats also showed decreased activities of GR (in the thalamus), GST (striatum), and Glrx (frontal cortex), but no changes in lipid peroxidation as assessed by HNE-His. The apparent lack of oxidative stress in these brain regions of HIV-1Tg rats contrasts with prior reports of oxidative stress caused by HIV infection or viral protein treatment (Bandaru et al. 2007; Banerjee et al. 2010; Sacktor et al. 2004; Turchan et al. 2003). This discrepancy indicates that viral infection, endogenous viral protein expression, and exogenous viral protein injection may lead to different pathophysiology. Moreover, since the HIV-1Tg rats were born with viral protein expression, systemic and partial adaptation to the chronic exposure of these neurotoxic proteins may have been developed. The compromised GSH-centered antioxidant system might be compensated by the upregulation of other antioxidants, such as SOD, catalase, or non-protein small molecules.
Although the low dose and repeated Meth administration in this study did not affect the gene expression of GCS-HC and GCS-LC, it significantly increased the activity of GCS in the thalamus, and decreased it in striatum and frontal cortex, suggesting that Meth regulates GCS activity at translational and/or post-translational levels. Meth also affected Glrx activities in these three brain regions. Elevated Glrx in thalamus and striatum of Meth rats suggests that this small redox enzyme may have an important role in neuroprotection during Meth exposure, potentially through maintaining mitochondrial integrity or activating NFkappaB survival pathway (Daily et al. 2001; Kenchappa and Ravindranath 2003; Saeed et al. 2008). However, the activity of Glrx in the frontal cortex was decreased by both HIV-1Tg and Meth, which suggest that the frontal brain region have less capacity to respond and may be more vulnerable to the additive effects of oxidative stress.
In addition to the high GSH content, thalamus also showed high GGT and GR activities in comparison to striatum and frontal cortex (Table 4), which are essential in supporting GSH recycling through extracellular catabolism and intracellular reduction. Notably that both HIV-1Tg and Meth caused decreases in GR activity in thalamus, suggesting that lowered rates of GSH recycling may limit the utilization of GSH in thalamus in the context of HIV −1 Tg and Meth, and the two factors also seem to have an additive effect in inhibiting thalamus GR activity.
Meth-mediated neurotoxicity (Langford et al. 2003a) and oxidative stress (Moszczynska and Yamamoto 2011) in striatum have been reported previously. In the present study, while the thalamus and frontal cortex showed increased lipid peroxidation, the striatum showed no sign of oxidative stress after the relatively low dose and repetitive Meth administration. Instead, the striatum of Meth rats showed compensatory increases of GSH concentration, and GR and Glrx activities, implicating the importance of GSH-centered antioxidants in this brain region. Similarly, when male C57BL/6 mice were treated with a single intraperitoneal dose (10 mg/kg body weight) of Meth, the GSH content in the striatum increased in 3 h, but normalized to baseline level in 24 h (Flora et al. 2002). Furthermore, when the same dose of Meth was administered to adult male Sprague-Dawley rats over an 8 h period at 2-h intervals, the GSH content in the striatum increased in 2 h and normalized in 24 h (Wu et al. 2007). The brain samples in the present study were harvested approximately 24 h after the last Meth injection, but the low dose chronic treatment (2.5 mg/kg body weight/day for 6 days) differs from the high dose acute treatment in previous reports. Future studies with multiple time point sampling will be needed to fully elucidate the temporal profile of repetitive low dose Meth-induced GSH changes in the striatum, and to understand if the decreased GCS activity observed in our study reflects a negative feedback response to the elevated GSH (Franklin et al. 2009).
xCT protein expression was downregulated by Meth in both frontal cortex and thalamus. Although the expression of xCT subunit is critical to the system xc- activity (Lewerenz et al. 2012), the cellular uptake of cystine is collectively determined by several factors. For example, as a membrane protein, the trafficking of xCT to the cell membrane determines the efficiency of cystine transport (Aoyama et al. 2008). Moreover, the gradient between the high intracellular and low extracellular glutamate concentrations is the driving force for cystine import (Lewerenz et al. 2012). Increased extracellular glutamate, such as that caused by HIV-mediated blockage of glutamate reuptake, can lead to system xc- dysfunction, deplete intracellular glutathione and result in nerve cell death (Albrecht et al. 2010). The function of system xc- is also associated with the excitatory amino acid transporters (EAAT), which clean up extracellular glutamate and help maintain the intra- and extra-cellular glutamate gradient (Lewerenz et al. 2012). In fact, cysteine can be directly transported into neurons by excitatory amino acid carrier 1 (EAAC1, a member of the EAAT), and this process is competed by extracellular glutamate (Watabe et al. 2008). The function of EAAT may be inhibited by HIV or gp120 (Ernst et al. 2010). Meth-treatment also may increase extracellular glutamate concentration (Flora et al. 2002; Harold et al. 2000), and how this affects GSH metabolism has not been studied.
Glutathione metabolism in the brain is a complex network involving interactions between astrocytes and neurons (Rice and Russo-Menna 1998). The investigation methods used in the present study did not distinguish cell types in the brain tissues. Future work employing immunohistochemical staining to localize and quantify target proteins may lead to a better understanding of the cellular mechanisms at play.
Lastly, since this study focused on the GSH-related antioxidant system, changes in other antioxidants, such as SOD and catalase, were not investigated. In a nonhuman primate study, the combination of SIV and Meth showed increased protein concentrations of both GST and extracellular SOD in plasma compared to SIV treatment alone (Pendyala et al. 2011). The alterations of the non-GSH-related enzymes may have significant roles in preventing HIV- and Meth-induced oxidative stress.
In conclusion, this study demonstrated region-specific responses of GSH-centered antioxidants and oxidative stress in the rat brain to endogenous HIV viral protein expression and Meth exposure. In the F344 rats, the thalamus has the highest baseline GSH concentrations, potentially higher GSH recycling rates, and transcriptional responses to GSH depletion in HIV-1Tg. Both HIV-1Tg and low doses of Meth resulted in decreased GR activity in thalamus, and decreased Glrx activity in frontal cortex. Increased GR and Glrx activities synergized with increased GSH concentration might have partially prevented Meth-induced oxidative stress in striatum. Interactive effects between Meth and HIV-1Tg were observed in thalamus on the activities of GCS and GGT, and in thalamus and frontal cortex on Glrx activity and xCT protein expression.
ACKNOWLEDGMENTS
This study was made possible by grant numbers U54NS56883 (SNRP), 5P20RR016467-11 and 8P20GMl03466-11 (INBRE II), 5G12RR003061 and 8G12MD007601 (RCMI/BRIDGES), 2K24DA016170 (to LC) and 2K02016149 (to SLC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
REFERENCES
- Acikgoz O, Gonenc S, Kayatekin BM, Uysal N, Pekcetin C, et al. Methamphetamine causes lipid peroxidation and an increase in superoxide dismutase activity in the rat striatum. Brain Res. 1998;813:200–202. doi: 10.1016/s0006-8993(98)01020-8. [DOI] [PubMed] [Google Scholar]
- Ajjimaporn A, Shavali S, Ebadi M, Govitrapong P. Zinc rescues dopaminergic SK-N-SH cell lines from methamphetamine-induced toxicity. Brain Res Bull. 2008;77:361–366. doi: 10.1016/j.brainresbull.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Albrecht P, Lewerenz J, Dittmer S, Noack R, Maher P, Methner A. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target. CNS Neurol Disord Drug Targets. 2010;9:373–382. doi: 10.2174/187152710791292567. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- Bandaru VV, McArthur JC, Sacktor N, Cutler RG, Knapp EL, et al. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68:1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Zhang X, Manda KR, Banks WA, Ercal N. HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: potential role of the thiol antioxidant N-acetylcysteine amide. Free radical biology & medicine. 2010;48:1388–1398. doi: 10.1016/j.freeradbiomed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S, Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. J Membr Biol. 1986;89:1–8. doi: 10.1007/BF01870891. [DOI] [PubMed] [Google Scholar]
- Basselin M, Ramadan E, Igarashi M, Chang L, Chen M, et al. Imaging upregulated brain arachidonic acid metabolism in HIV-1 transgenic rats. J Cereb Blood Flow Metab. 2011;31:486–493. doi: 10.1038/jcbfm.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bronowicka-Adamska P, Zagajewski J, Czubak J, Wróbel M. RP-HPLC method for quantitative determination of cystathionine, cysteine and glutathione: An application for the study of the metabolism of cysteine in human brain. J Chromatogr B Analyt Technol Biomed Life Sci. 2011 doi: 10.1016/j.jchromb.2011.05.026. Epub May 23. [DOI] [PubMed] [Google Scholar]
- Castagna A, Le Grazie C, Accordini A, Giulidori P, Cavalli G, et al. Cerebrospinal fluid S-adenosylmethionine (SAMe) and glutathione concentrations in HIV infection: effect of parenteral treatment with SAMe. Neurology. 1995;45:1678–1683. doi: 10.1212/wnl.45.9.1678. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lindmark-Mansson H, Gorton L, Akesson B. Total antioxidant capacity of bovine milk as assayed by spectrophotometric and amperometric methods. International Dairy Journal. 2003;13:927–935. [Google Scholar]
- Conrad M, Sato H. The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (−) : cystine supplier and beyond. Amino Acids Epub ahead of print. 2011 doi: 10.1007/s00726-011-0867-5. [DOI] [PubMed] [Google Scholar]
- Dai F, Yang JY, Gu PF, Hou Y, Wu CF. Effect of drug-induced ascorbic acid release in the striatum and the nucleus accumbens in hippocampus-lesioned rats. Brain Res. 2006;1125:163–170. doi: 10.1016/j.brainres.2006.09.098. [DOI] [PubMed] [Google Scholar]
- Daily D, Vlamis-Gardikas A, Offen D, Mittelman L, Melamed E, et al. Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by activating NF-kappa B via Ref-1. The Journal of biological chemistry. 2001;276:1335–1344. doi: 10.1074/jbc.M008121200. [DOI] [PubMed] [Google Scholar]
- Diotte NM, Xiong Y, Gao J, Chua BH, Ho YS. Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim Biophys Acta. 2009;1793:427–438. doi: 10.1016/j.bbamcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Doorn JA, Petersen DR. Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxy-2-nonenal and 4-oxo-2-nonenal. Chem Res Toxicol. 2002;15:1445–1450. doi: 10.1021/tx025590o. [DOI] [PubMed] [Google Scholar]
- Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging. 2010;32:1045–1053. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Hudson L, Kerwin RW. Decreased absolute levels of ascorbic acid and unaltered vasoactive intestinal polypeptide receptor binding in the frontal cortex in acquired immunodeficiency syndrome. Neurosci Lett. 1997;224:119F–122F. doi: 10.1016/s0304-3940(97)13473-5. [DOI] [PubMed] [Google Scholar]
- Farabee D, Prendergast M, Cartier J. Methamphetamine use and HIV risk among substance-abusing offenders in California. J Psychoactive Drugs. 2002;34:295–300. doi: 10.1080/02791072.2002.10399966. [DOI] [PubMed] [Google Scholar]
- Flora G, Lee YW, Nath A, Maragos W, Hennig B, Toborek M. Methamphetamine-induced TNF-alpha gene expression and activation of AP-1 in discrete regions of mouse brain: potential role of reactive oxygen intermediates and lipid peroxidation. Neuromolecular Med. 2002;2:71–85. doi: 10.1385/NMM:2:1:71. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck MR, Moy LY, Jayatilleke E, Hogan KA, Manzino L, Sonsalla PK. Parallel increases in lipid and protein oxidative markers in several mouse brain regions after methamphetamine treatment. J Neurochem. 2001;79:152–160. doi: 10.1046/j.1471-4159.2001.00549.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez P, Tunon MJ, Manrique V, Garcia-Pardo LA, Gonzalez J. Changes in hepatic cytosolic glutathione S-transferase enzymes induced by clotrimazole treatment in rats. Clin Exp Pharmacol Physiol. 1989;16:867–871. doi: 10.1111/j.1440-1681.1989.tb01526.x. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Hag AM, Kristoffersen US, Pedersen SF, Gutte H, Lebech AM, Kjaer A. Regional gene expression of LOX-1, VCAM-1, and ICAM-1 in aorta of HIV-1 transgenic rats. PLoS One. 2009;4:e8170. doi: 10.1371/journal.pone.0008170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold C, Wallace T, Friedman R, Gudelsky G, Yamamoto B. Methamphetamine selectively alters brain glutathione. Eur J Pharmacol. 2000;400:99–102. doi: 10.1016/s0014-2999(00)00392-7. [DOI] [PubMed] [Google Scholar]
- Kass MD, Liu X, Vigorito M, Chang L, Chang SL. Methamphetamine-induced behavioral and physiological effects in adolescent and adult HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2010;5:566–573. doi: 10.1007/s11481-010-9221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchappa RS, Ravindranath V. Glutaredoxin is essential for maintenance of brain mitochondrial complex I: studies with MPTP. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:717–719. doi: 10.1096/fj.02-0771fje. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, et al. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr. 2003a;34:467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, et al. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr. 2003b;34:467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- Langford D, Grigorian A, Hurford R, Adame A, Crews L, Masliah E. The role of mitochondrial alterations in the combined toxic effects of human immunodeficiency virus Tat protein and methamphetamine on calbindin positive-neurons. J Neurovirol. 2004;10:327–337. doi: 10.1080/13550280490520961. [DOI] [PubMed] [Google Scholar]
- Lee DW, Kim SY, Lee T, Nam YK, Ju A, et al. Ex vivo detection for chronic ethanol consumption-induced neurochemical changes in rats. Brain Res. 2012;1429:134–144. doi: 10.1016/j.brainres.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Maher P, Methner A. Regulation of xCT expression and system x (c) (−) function in neuronal cells. Amino Acids. 2012;42:171–179. doi: 10.1007/s00726-011-0862-x. [DOI] [PubMed] [Google Scholar]
- Liu X, Chang L, Vigorito M, Kass M, Li H, Chang SL. Methamphetamine-induced behavioral sensitization is enhanced in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2009;4:309–316. doi: 10.1007/s11481-009-9160-8. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, et al. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem. 2002;83:955–963. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- Marchan R, Hammond CL, Ballatori N. Multidrug resistance-associated protein 1 as a major mediator of basal and apoptotic glutathione release. Biochim Biophys Acta. 2008;1778:2413–2420. doi: 10.1016/j.bbamem.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983;220:472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Meister A, Tate SS, Griffith OW. Gamma-glutamyl transpeptidase. Methods Enzymol. 1981;77:237–253. doi: 10.1016/s0076-6879(81)77032-0. [DOI] [PubMed] [Google Scholar]
- Morris D, Guerra C, Donohue C, Oh H, Khurasany M, Venketaraman V. Unveiling the mechanisms for decreased glutathione in individuals with HIV infection. Clin Dev Immunol. 2012;2012:734125. doi: 10.1155/2012/734125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moszczynska A, Yamamoto BK. Methamphetamine oxidatively damages parkin and decreases the activity of 26S proteasome in vivo. J Neurochem. 2011;116:1005–1017. doi: 10.1111/j.1471-4159.2010.07147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto T, Operario D, Soma T. Risk behaviors of Filipino methamphetamine users in San Francisco: implications for prevention and treatment of drug use and HIV. Public Health Rep 117 Suppl. 2002;1:S30–S38. [PMC free article] [PubMed] [Google Scholar]
- Panee J, Stoytcheva ZR, Liu W, Berry MJ. Selenoprotein H is a redox-sensing high mobility group family DNA-binding protein that up-regulates genes involved in glutathione synthesis and phase II detoxification. J Biol Chem. 2007;282:23759–23765. doi: 10.1074/jbc.M702267200. [DOI] [PubMed] [Google Scholar]
- Pendyala G, Trauger SA, Siuzdak G, Fox HS. Short communication: quantitative proteomic plasma profiling reveals activation of host defense to oxidative stress in chronic SIV and methamphetamine comorbidity. AIDS research and human retroviruses. 2011;27:179–182. doi: 10.1089/aid.2010.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr., et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- Riddle DR. Brain Aging: Models, Methods, and Mechanisms. 2007 [PubMed] [Google Scholar]
- Sacktor N, Haughey N, Cutler R, Tamara A, Turchan J, et al. Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol. 2004;157:176–184. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Saeed U, Durgadoss L, Valli RK, Joshi DC, Joshi PG, Ravindranath V. Knockdown of cytosolic glutaredoxin 1 leads to loss of mitochondrial membrane potential: implication in neurodegenerative diseases. PLoS One. 2008;3:e2459. doi: 10.1371/journal.pone.0002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I. Motivations associated with methamphetamine use among HIV+ men who have sex with men. Journal of Substance Abuse Treatment. 2002;22:149–156. doi: 10.1016/s0740-5472(02)00223-4. [DOI] [PubMed] [Google Scholar]
- Tang H, Lu D, Pan R, Qin X, Xiong H, Dong J. Curcumin improves spatial memory impairment induced by human immunodeficiency virus type 1 glycoprotein 120 V3 loop peptide in rats. Life Sci. 2009;85:1–10. doi: 10.1016/j.lfs.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Tietze F. Disulfide reduction in rat liver. I. Evidence for the presence of nonspecific nucleotide-dependent disulfide reductase and GSH-disulfide transhydrogenase activities in the high-speed supernatant fraction. Arch Biochem Biophys. 1970;138:177–188. doi: 10.1016/0003-9861(70)90297-3. [DOI] [PubMed] [Google Scholar]
- Turchan J, Pocernich CB, Gairola C, Chauhan A, Schifitto G, et al. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60:307–314. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- Velazquez I, Plaud M, Wojna V, Skolasky R, Laspiur JP, Melendez LM. Antioxidant enzyme dysfunction in monocytes and CSF of Hispanic women with HIV-associated cognitive impairment. Journal of neuroimmunology. 2009;206:106–111. doi: 10.1016/j.jneuroim.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2007;2:319–328. doi: 10.1007/s11481-007-9078-y. [DOI] [PubMed] [Google Scholar]
- Vikulina T, Fan X, Yamaguchi M, Roser-Page S, Zayzafoon M, et al. Alterations in the immuno-skeletal interface drive bone destruction in HIV-1 transgenic rats. Proc Natl Acad Sci U S A. 2010;107:13848–13853. doi: 10.1073/pnas.1003020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe M, Aoyama K, Nakaki T. A dominant role of GTRAP3–18 in neuronal glutathione synthesis. J Neurosci. 2008;28:9404–9413. doi: 10.1523/JNEUROSCI.3351-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CC, Viernes H, Krejsa CM, Botta D, Kavanagh TJ. Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal Biochem. 2003;318:175–180. doi: 10.1016/s0003-2697(03)00143-x. [DOI] [PubMed] [Google Scholar]
- Wu CW, Ping YH, Yen JC, Chang CY, Wang SF, et al. Enhanced oxidative stress and aberrant mitochondrial biogenesis in human neuroblastoma SH-SY5Y cells during methamphetamine induced apoptosis. Toxicol Appl Pharmacol. 2007;220:243–251. doi: 10.1016/j.taap.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther. 1998;287:107–114. [PubMed] [Google Scholar]
- Zhang X, Banerjee A, Banks WA, Ercal N. N-Acetylcysteine amide protects against methamphetamine-induced oxidative stress and neurotoxicity in immortalized human brain endothelial cells. Brain Res. 2009;1275:87–95. doi: 10.1016/j.brainres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]