Abstract
Background
Immune reconstitution inflammatory syndrome (IRIS) is a phenomenon initially described in patients with human immunodeficiency virus. Upon initiation of combination antiretroviral therapy, recovery of cellular immunity triggers inflammation to a preexisting infection or antigen that causes paradoxical worsening of clinical disease. A similar phenomenon can occur in human immunodeficiency virus–negative patients, including pregnant women, neutropenic hosts, solidorgan or stem cell transplant recipients, and patients receiving tumor necrosis factor inhibitors.
Observations
We report a case of leprosy unmasking and downgrading reaction after stem cell transplantation that highlights some of the challenges inherent to the diagnosis of IRIS, especially in patients without human immunodeficiency virus infection, as well as review the spectrum of previously reported cases of IRIS reactions in this population.
Conclusions
The mechanism of immune reconstitution reactions is complex and variable, depending on the underlying antigen and the mechanism of immunosuppression or shift in immune status. Use of the term IRIS can aid our recognition of an important phenomenon that occurs in the setting of immunosuppression or shifts in immunity but should not deter us from thinking critically about the distinct processes that underlie this heterogeneous group of conditions.
IMMUNE RECONSTITUTION INFLAMmatory syndrome (IRIS) is a clinical phenomenon first characterized in patients with human immunodeficiency virus (HIV) with initiation of combination antiretroviral therapy (cART). In IRIS, recovery of antigen-specific cellular immunity is associated with inflammation of a preexisting antigen or infection that causes paradoxical clinical deterioration. More than half of HIV-associated IRIS cases present with cutaneous manifestations.1 These include worsening or unmasking of common skin conditions, such as seborrheic dermatitis, herpes virus infection, or molluscum contagiosum, as well as more rare dermatologic entities, such as Kaposi’s sarcoma, leishmaniasis, and leprosy.2,3
The concept of IRIS occurring in HIV-negative patients has also been reported4; a similar phenomenon, termed a paradoxical response, was first described in otherwise healthy patients undergoing treatment for tuberculosis. More recently, however, many individual case reports5,6 have described similar reactions in a wide array of immunosuppressed patients, including pregnant women, neutropenic hosts, solid-organ or stem cell transplant recipients, and patients receiving tumor necrosis factor inhibitors. The spectrum of IRIS-related conditions seen in these populations is broad but dominated by deep fungal and mycobacterial infections.
Here we present a case of leprosy-downgrading reaction in a patient following engraftment after a stem cell transplant (SCT). This case shares many features with IRIS in leprosy-infected HIV-positive patients, but there are important differences. We discuss this case in the context of a brief review of the types of reactions seen in other HIV-negative patients and the applicability of the term IRIS to reactions seen in the HIV-negative population.
REPORT OF A CASE
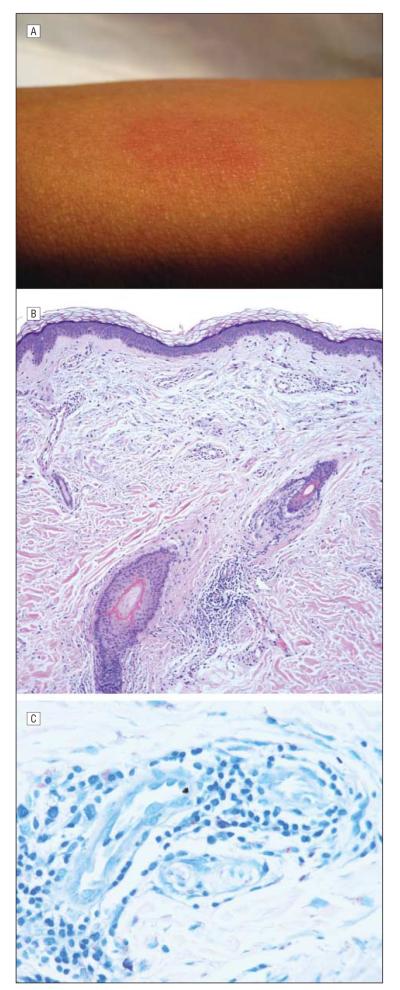
A 50-year-old Filipino man was admitted for allogeneic SCT to treat idiopathic myelofibrosis in which medical management had failed. His history was notable for 2 pruritic plaques on the left arm and right cheek that had been present for 6 months. He underwent induction chemotherapy with fludarabine phosphate and busulfan, followed by a matched sibling donor allogeneic SCT. On day 5 after transplant, he developed neutropenia and fever. On day 6, the dermatology service was consulted to evaluate a new skin lesion on the patient’s left forearm. Examination revealed a small, ill-defined, indurated, erythematous plaque on the left forearm (Figure 1A) and an annular erythematous plaque with central clearing and fine scale measuring several centimeters on the left bicep. A biopsy specimen from the left forearm showed a sparse, superficial and deep, perivascular and periadnexal infiltrate for which a specific diagnosis could not be rendered (Figure 1B). Results of tissue cultures for bacteria, fungus, and mycobacteria were negative.
Figure 1.
Unmasking of occult leprosy. A, Ill-defined erythematous forearm plaque noted 6 days after transplant. B, Biopsy specimen revealed a perivascular and periadnexal lymphocytic infiltrate (hematoxylin-eosin, original magnification ×200). C, A retrospective Fite stain subsequently demonstrated many acid-fast bacilli consistent with the diagnosis of leprosy (original magnification ×400).
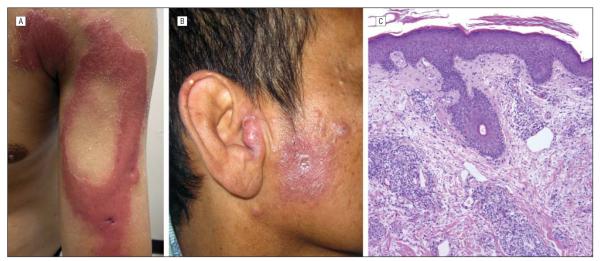
The patient was discharged and presented to the outpatient clinic 4 months later with marked induration of the previously noted areas plus new erythematous to violaceous papules and plaques on the right deltoid, cheek, and tragus (Figure 2A and 2B). Examination of a second biopsy specimen revealed clusters of epithelioid and foamy macrophages (Figure 2C) shown by Fite stain to contain many acid-fast bacilli. There were also numerous lymphocytes, consistent with a clinical diagnosis of multibacillary leprosy undergoing type 1 reaction. Acidfast bacilli were seen on a biopsy touch preparation but were not recovered in the tissue culture. A retrospective Fite stain performed on the initial skin biopsy specimen taken 6 days after transplant demonstrated acid-fast bacilli in the perivascular and perineural dermis in a microscopic pattern consistent with an occult undefined leprosy (Figure 1C). Antimicrobial treatment was started, but the patient developed chronic graft-vs-host disease requiring ongoing immunosuppression. He died of non-mycobacterial pneumonia and sepsis 2 years after the transplant.
Figure 2.
Leprosy type 1 reaction. A, Annular plaque on the arm. B, Indurated papules and plaques on the tragus. C, Biopsy specimen demonstrated a perivascular and periadnexal infiltrate of lymphocytes and foamy histiocytes (hematoxylin-eosin, original magnification ×200). A Fite stain (not shown) demonstrated many acid-fast bacilli.
COMMENT
Immune reconstitution inflammatory syndrome describes the clinical phenomenon of paradoxical worsening of disease resulting from a proinflammatory response to a preexisting antigen or infection; however, a universal set of diagnostic criteria is lacking.7,8 When inflammation is seen in the setting of immune recovery, it is tempting to indiscriminately ascribe it to IRIS, but are we always correct in doing so? In the case presented herein, we questioned whether the observed phenomena represented IRIS or rather just the natural progression of leprosy. Here we explore potential explanations for our patient’s clinical course and discuss more broadly the concept of IRIS outside the setting of HIV.
This case demonstrates the evolution of leprosy infection in response to multiple immunologic shifts from a state of terminal myelodysplasia, through induction chemotherapy, and finally toward a reconstituted immune state following SCT. Our patient was likely infected with Mycobacterium leprae years earlier in the Philippines. The plaques on his left arm and right cheek preceding transplant probably represented early lepromatous lesions but were never examined by a dermatologist, tested for anesthesia, or biopsied, making firm classification difficult. After the myeloablative conditioning regimen for his SCT, our patient experienced rapid progression toward a multibacillary lepromatous phenotype evidenced by many acid-fast bacilli in the biopsy specimen 1 week after transplant and the appearance of new lesions. Several months after transplant, he developed marked clinical and microscopic inflammation of existing and new skin lesions consistent with a type 1 reaction.9
Our patient’s case details a series of events that could be consistent with IRIS. In patients coinfected with HIV and M leprae, initiation of cART has been associated with 2 IRIS phenomena: either unmasking of previously unrecognized leprosy or type 1 upgrading reactions with inflammation in preexisting skin lesions.10-12 Our case demonstrated unmasking of previously occult leprosy, but this occurred in the setting of worsening immunosuppression just after preconditioning chemotherapy and SCT and so cannot be considered along the IRIS spectrum. Conversely, several months after transplant and following engraftment, there was clinical and histopathologic evidence of inflammation corresponding to a type 1 leprosy reaction. Because this reaction occurred in the window of immune recovery, it could fit with the definition of IRIS in the preceding paragraph. An analogous reaction to leprosy in an SCT recipient has been reported.13 In that case, borderline leprosy was diagnosed before SCT, with treatment resulting in regression of skin lesions. Months after SCT, despite ongoing antimycobacterial therapy, the patient developed recurrent skin lesions and new inflammation. The authors postulated that regeneration of CD4+ cells during this period triggered a type 1 upgrading reaction. Similarly, we suspect that gradual recovery of our patient’s cell-mediated immunity may have led to increased clinical recognition and inflammation of his multibacillary disease, which could reasonably be termed IRIS.
An alternative explanation for these same events is that our patient experienced a natural progression of his leprosy infection and that the observed inflammation was not a result of immune reconstitution. For most antigens or infections, the immune response is relatively uniform across a population and constant over time. However, leprosy gives rise to a broad clinical spectrum of disease, even in immunocompetent individuals.14 Likewise, the disease course is characteristically dynamic, with type 1 leprosy reactions occurring spontaneously or after initiation of leprosy treatment.15 Our understanding of these phenomena remains limited. The traditional schema of tuberculoid paucibacillary disease representing strong cell-mediated immunity to M leprae and lepromatous multibacillary disease representing weak cell-mediated immunity has obvious limitations as we learn more about the role of various T-cell subsets (eg, helper T cell type 17 [TH17] and regulatory T cells) and innate immunity (eg, pattern recognition receptors) in disease pathogenesis.16,17 The effects of immunosuppression on leprosy are likewise complicated and poorly understood, as evidenced by the surprising observation that paucibacillary disease still predominates in HIV-infected patients with low CD4+ counts.18 We believe that the shifts in our patient’s immune system caused by his chemotherapy and subsequent SCT are likely to have influenced the course of his disease; however, proof is lacking. One plausible explanation is that the inflammation seen months after transplant was a delayed manifestation of an initial downgrading reaction triggered by the SCT preconditioning regimen and not related to immune reconstitution. Unfortunately, distinguishing between these 2 possibilities is difficult, if not impossible.
This case highlights not only the complex immunopathophysiologic characteristics of leprosy but also those of IRIS. Immune reconstitution inflammatory syndrome is classically thought of in HIV-positive patients after initiation of cART and has been best studied in this setting. The recovery of CD4+ T cells following cART is accompanied by a shift from a TH2- toward a TH1- and TH17-dominant profile, as well as deficient expansion or delayed functional recovery of the regulatory T-cell population. In patients who develop IRIS, these factors may lead to a dysregulated proinflammatory state causing unchecked inflammation driven by an occult infection or residual organism or antigenic debris from a prior infection.19 Other less well-characterized immune shifts may accompany cART, such as those affecting CD8+ T cells or innate immunity. The relative importance of these various immunologic events in IRIS is unclear and likely differs on a case-by-case basis, with the underlying antigen being a key differentiating factor. Thus, even with HIV, it is probably best to conceptualize IRIS as a clinical phenomenon that can result from a wide variety of immunologic pathways.20
Immune reconstitution reactions in HIV-negative patients are similarly defined by a robust inflammatory response to a preexisting antigen or infection that occurs with recovery of host immune function.6 The mechanism of these reactions has not been studied in detail but likely reflects an even broader spectrum of immunologic events that differ on the basis of not only the underlying antigenic trigger but also the specific nature of the immunosuppressive state. For solid-organ transplant recipients, medications used to prevent graft rejection suppress TH1 and TH17 subsets preferentially.21 Withdrawal or tapering of these agents is often seen as a trigger for the IRIS reaction. In pregnancy, maternal hormones modulate the immune system by enhancing TH2 and regulatory T-cell responses to promote tolerance to fetal antigens. Rebound toward a TH1-dominant response in the postpartum period may theoretically increase the inflammatory response to an underlying infection or exacerbate autoimmune disease.22 Rapid recovery of neutrophils in neutropenic patients has been associated with clinical worsening of pulmonary aspergillosis and disseminated candidiasis.23,24 Tumor necrosis factor inhibitors suppress TH1-type inflammation and are well known to increase the risk for tuberculosis. In patients who develop tuberculosis while receiving tumor necrosis factor inhibitors, there are reports25 of paradoxical clinical deterioration after withdrawal of the medication despite initiation of tuberculosis therapy. Leprosy unmasking has also been reported26 during treatment with tumor necrosis factor inhibitors; patients experienced accelerated progression of infection while receiving infliximab and subsequent type 1 upgrading reaction on its withdrawal. For SCT recipients, inflammation may stem from recovery of host (and/or donor) cellular immunity following chemotherapy and engraftment, withdrawal of immunosuppression, and other factors.27,28
Such wide variety in both clinical presentation and underlying immunopathophysiologic factors makes confident diagnosis of immune reconstitution reactions a unique challenge for the health care provider. This challenge is even more apparent when considering IRIS in other forms of immunosuppression in contrast to IRIS seen in the setting of HIV. Using the common term IRIS across these populations risks oversimplification but highlights the key unifying principle: IRIS represents a clinical phenomenon of immune-mediated inflammation associated with infection or antigens that occurs in an array of immunosuppressive states. Recognizing cases in which worsening symptoms are attributable to immune reconstitution rather than a new infection or an adverse drug reaction is clinically important, since treatment often requires counterintuitive measures, such as increased immunosuppression. Use of a common term should not deter us, however, from seeking to understand the mechanisms on a case-by-case basis, defining diagnostic criteria, or considering alternative explanations for what is observed. Instead, continuing to compare and contrast similar phenomena between HIV-positive and HIV-negative patients may shed additional light on the subject.
Acknowledgments
Funding/Support: Dr Scharschmidt is supported by a grant from the Dermatology Foundation, Dr Amerson is supported by a grant from the National Cancer Institute, Dr Rosenberg is supported by grants from the Sandler Family Foundation and from the National Institute of Allergy and Infectious Diseases, and Dr Shinkai is supported by a grant from the Dermatology Foundation.
Footnotes
Author Contributions: Drs Scharschmidt, Amerson, and Shinkai had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Scharschmidt, Amerson, and Shinkai. Acquisition of data: Scharschmidt, Rosenberg, Jacobs, McCalmont, and Shinkai. Analysis and interpretation of data: Scharschmidt, Amerson, Rosenberg, and Shinkai. Drafting of the manuscript: Scharschmidt and Shinkai. Critical revision of the manuscript for important intellectual content: Rosenberg, Amerson, Jacobs, McCalmont, and Shinkai. Study supervision: Amerson, McCalmont, and Shinkai.
Conflict of Interest Disclosures: Dr McCalmont receives an honorarium as editor of the Journal of Clinical Pathology.
Additional Contributions: James P. Harnisch, MD, provided critical review of this manuscript.
REFERENCES
- 1.Osei-Sekyere B, Karstaedt AS. Immune reconstitution inflammatory syndrome involving the skin. Clin Exp Dermatol. 2010;35(5):477–481. doi: 10.1111/j.1365-2230.2009.03620.x. [DOI] [PubMed] [Google Scholar]
- 2.Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M, IeDEA Southern and Central Africa Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(4):251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shinkai K, Fox LP. What is your diagnosis? inflamed molluscum contagiosum as a manifestation of immune reconstitution inflammatory syndrome. Cutis. 2012;89(5):212, 219–220. [PubMed] [Google Scholar]
- 4.Smith H. Paradoxical responses during the chemotherapy of tuberculosis. J Infect. 1987;15(1):1–3. doi: 10.1016/s0163-4453(87)91276-x. [DOI] [PubMed] [Google Scholar]
- 5.Cheng VC, Yuen KY, Wong SSY, et al. Immunorestitution diseases in patients not infected with HIV. Eur J Clin Microbiol Infect Dis. 2001;20(6):402–406. doi: 10.1007/s100960100507. [DOI] [PubMed] [Google Scholar]
- 6.Sun HY, Singh N. Immune reconstitution inflammatory syndrome in non-HIV immunocompromised patients. Curr Opin Infect Dis. 2009;22(4):394–402. doi: 10.1097/QCO.0b013e32832d7aff. [DOI] [PubMed] [Google Scholar]
- 7.Shelburne SA, Montes M, Hamill RJ. Immune reconstitution inflammatory syndrome: more answers, more questions. J Antimicrob Chemother. 2006;57(2):167–170. doi: 10.1093/jac/dki444. [DOI] [PubMed] [Google Scholar]
- 8.General IRIS case definition [Accessed October 7, 2011];International Association for the Study of HIV-Associated IRIS (INSHI) website. http://www.inshi.umn.edu/definitions/General_IRIS/home.html.
- 9.Ridley DS, Radia KB. The histological course of reactions in borderline leprosy and their outcome. Int J Lepr Other Mycobact Dis. 1981;49(4):383–392. [PubMed] [Google Scholar]
- 10.Deps PD, Lockwood DN. Leprosy occurring as immune reconstitution syndrome. Trans R Soc Trop Med Hyg. 2008;102(10):966–968. doi: 10.1016/j.trstmh.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Amerson EH, Maurer TA. Immune reconstitution inflammatory syndrome and tropical dermatoses. Dermatol Clin. 2011;29(1):39–43. doi: 10.1016/j.det.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Menezes VM, Sales AM, Illarramendi X, et al. Leprosy reaction as a manifestation of immune reconstitution inflammatory syndrome: a case series of a Brazilian cohort. AIDS. 2009;23(5):641–643. doi: 10.1097/QAD.0b013e3283291405. [DOI] [PubMed] [Google Scholar]
- 13.Pieroni F, Stracieri AB, Moraes DA, et al. Six cases of leprosy associated with allogeneic hematopoietic SCT. Bone Marrow Transplant. 2007;40(9):859–863. doi: 10.1038/sj.bmt.1705824. [DOI] [PubMed] [Google Scholar]
- 14.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19(2):338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker SL, Lockwood DN. Leprosy type 1 (reversal) reactions and their management. Lepr Rev. 2008;79(4):372–386. [PubMed] [Google Scholar]
- 16.Berrington WR, Macdonald M, Khadge S, et al. Common polymorphisms in the NOD2 gene region are associated with leprosy and its reactive states. J Infect Dis. 2010;201(9):1422–1435. doi: 10.1086/651559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misch EA, Macdonald M, Ranjit C, et al. Human TLR1 deficiency is associated with impaired mycobacterial signaling and protection from leprosy reversal reaction. PLoS Negl Trop Dis. 2008;2(5):e231. doi: 10.1371/journal.pntd.0000231. doi:10.1371/journal.pntd.0000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lockwood DN, Lambert SM. Human immunodeficiency virus and leprosy: an update. Dermatol Clin. 2011;29(1):125–128. doi: 10.1016/j.det.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Gupta AO, Singh N. Immune reconstitution syndrome and fungal infections. Curr Opin Infect Dis. 2011;24(6):527–533. doi: 10.1097/QCO.0b013e32834ab20a. [DOI] [PubMed] [Google Scholar]
- 20.Price P, Murdoch DM, Agarwal U, Lewin SR, Elliott JH, French MA. Immune restoration diseases reflect diverse immunopathological mechanisms. Clin Microbiol Rev. 2009;22(4):651–663. doi: 10.1128/CMR.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh N. Novel immune regulatory pathways and their role in immune reconstitution syndrome in organ transplant recipients with invasive mycoses. Eur J Clin Microbiol Infect Dis. 2008;27(6):403–408. doi: 10.1007/s10096-008-0461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis. 2007;45(9):1192–1199. doi: 10.1086/522182. [DOI] [PubMed] [Google Scholar]
- 23.Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001;19(1):253–259. doi: 10.1200/JCO.2001.19.1.253. [DOI] [PubMed] [Google Scholar]
- 24.Legrand F, Lecuit M, Dupont B, et al. Adjuvant corticosteroid therapy for chronic disseminated candidiasis. Clin Infect Dis. 2008;46(5):696–702. doi: 10.1086/527390. [DOI] [PubMed] [Google Scholar]
- 25.Garcia Vidal C, Rodríguez Fernández S, Martínez Lacasa J, et al. Paradoxical response to antituberculous therapy in infliximab-treated patients with disseminated tuberculosis. Clin Infect Dis. 2005;40(5):756–759. doi: 10.1086/427941. [DOI] [PubMed] [Google Scholar]
- 26.Scollard DM, Joyce MP, Gillis TP. Development of leprosy and type 1 leprosy reactions after treatment with infliximab: a report of 2 cases. Clin Infect Dis. 2006;43(2):e19–e22. doi: 10.1086/505222. doi:10.1086/505222. [DOI] [PubMed] [Google Scholar]
- 27.Antinori S, Corbellino M, Necchi A, et al. Immune reconstitution inflammatory syndrome associated with Aspergillus terreus pulmonary infection in an autologous stem cell transplant recipient. Transpl Infect Dis. 2010;12(1):64–68. doi: 10.1111/j.1399-3062.2009.00460.x. [DOI] [PubMed] [Google Scholar]
- 28.Airas L, Päivärinta M, Röyttä M, et al. Central nervous system immune reconstitution inflammatory syndrome (IRIS) after hematopoietic SCT. Bone Marrow Transplant. 2010;45(3):593–596. doi: 10.1038/bmt.2009.186. [DOI] [PubMed] [Google Scholar]