Abstract
BACKGROUND
Recent reports have linked variability in visit-to-visit systolic blood pressure (SBP) to risk of mortality and stroke, independent of the effect of mean SBP level. This study aimed to evaluate whether variability in SBP is associated with all-cause mortality, incident myocardial infarction (MI), and incident stroke, independent of mean SBP or trends in SBP levels over time.
METHODS
The Cardiovascular Health Study is a longitudinal cohort study of vascular risk factors and disease in the elderly. Participants who attended their first 5 annual clinic visits and experienced no event before the 5th visit were eligible (n = 3,852). Primary analyses were restricted to participants not using antihypertensive medications throughout the first 5 clinic visits (n = 1,642). Intraindividual SBP variables were defined using each participant’s 5-visit blood pressure measures. Cox proportional hazards models estimated adjusted hazard ratios (HRs) per SD increase in intraindividual SBP variability, adjusted for intraindividual SBP mean and change over time.
RESULTS
Over a mean follow-up of 9.9 years, there were 844 deaths, 203 MIs, and 195 strokes. Intraindividual SBP variability was significantly associated with increased risk of mortality (HR = 1.13; 95% confidence interval (CI) = 1.05–1.21) and of incident MI (HR = 1.20; 95%CI = 1.06–1.36), independent of the effect from adjustment factors. Intraindividual SBP variability was not associated with risk of stroke (HR = 1.03; 95% CI = 0.89–1.21).
CONCLUSIONS
Long-term visit-to-visit SBP variability was independently associated with a higher risk of subsequent mortality and MI but not stroke. More research is needed to determine the relationship of BP variability with cardiovascular risk and the clinical implications.
Keywords: blood pressure, blood pressure variability, hypertension, mortality, myocardial infarction, stroke.
Hypertension, or high mean blood pressure (BP), is associated with increased risk of all-cause mortality, myocardial infarction (MI), and stroke.1–4 Recently, conventional approaches to understanding the biological and pathological effect of differences in BP have been reconsidered. In a recent series of articles, Rothwell and colleagues report that BP variability is a risk factor for stroke, independent of the mean BP, among high-risk patients. Yet, it is unclear whether variability in BP over multiple years among adults with lower risk is independently associated with excess risk of subsequent mortality, incident MI, or incident stroke.
Data from the Cardiovascular Health Study (CHS), a longitudinal cohort study of vascular risk factors and disease in the elderly, were used to address these questions. Our study sought to evaluate whether variability in annual clinic measures of BP is associated with all-cause mortality, incident MI, and incident stroke, independent of individual mean levels of BP or with individual changes or trends in BP levels over time. Further consideration was given to whether the use of antihypertensive medications modifies these associations.
METHODS
Study population
The study included participants from CHS, a population-based, longitudinal cohort study of cardiovascular risk factors in adults aged ≥65 years from 4 US metropolitan communities: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. The design and recruitment for CHS has been described previously.5 Briefly, the cohort is comprised of 5,888 participants; 5,201 participants were enrolled in 1989–1990 (original cohort), and an additional 687 black participants were enrolled in 1992–1993 (new cohort). Participants were excluded from CHS if they were institutionalized, wheelchair-bound, planning to move out of the area within 3 years, or undergoing treatment for a malignant condition. All participants attended a baseline clinic visit that included a physical examination and a detailed medical history review. Annual clinic visits and interim telephone interviews were conducted through 1999. Semiannual telephone interviews are ongoing to ascertain new events, changes in health status, and medication use. Each study center’s institutional review board approved the study, and all participants provided informed, written consent.
Participants were eligible for this study if they attended all of their first 5 annual clinic visits (comprising the baseline period; Figure 1) and did not have an MI or stroke before their 5th clinic visit (n = 3,852). The new cohort was missing BP data for their 4th clinic visit, so the baseline period for these participants extended over 6 clinic visits to achieve consistency in the primary BP variable definitions. Different classes of antihypertensive medications may have different effects on BP variability; therefore, analyses were restricted to participants who did not use antihypertensive medications or who used the same antihypertensive medications over the entire baseline period.6 Primary analyses focused on the participants who were nonusers of these medications (n = 1,642), which included angiotensin-converting enzyme (ACE)-inhibitors, calcium-channel blockers, beta-blockers, diuretics, and vasodilators. Secondary analyses include results for participants using the same antihypertensive medication regimen the entire baseline period (n = 1,095). These results are summarized briefly in this article and detailed in the Supplementary Materials. Variable medication users were excluded because they comprised a mixture of those starting, stropping, and changing medication regimens during the exposure period.
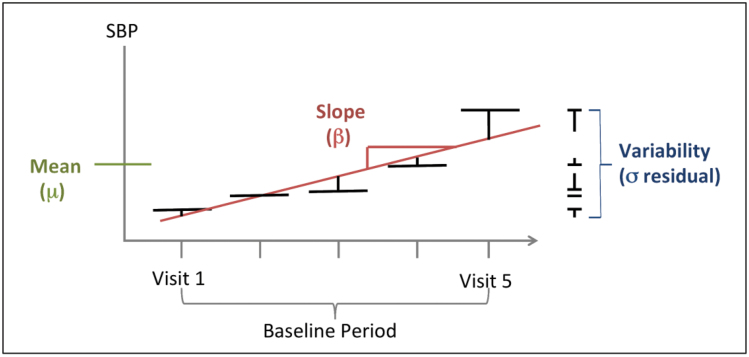
Figure 1.
Intraindividual components of systolic blood pressure (SBP). Intraindividual mean is defined as the mean of 5 SBP measures, 1 averaged measure per clinic visit. Intraindividual change over time, or slope, is defined as the beta coefficient for the linear regression of the 5 SBP measures. Intraindividual variability is defined as the SD of the residuals from the linear regression of the 5 SBP measures.
BP variability
BP was measured in the right arm using a standard mercury sphygmomanometer, except at the first visit, when the random zero method was used. Three seated systolic BP (SBP) readings were taken 5 minutes apart at each clinic visit, and the last 2 readings were used to calculate an average SBP for that visit. These 5 average SBP recordings for each participant’s 5 clinic visits comprise the set from which the intraindividual components of SBP were calculated (Figure 1). The use of 5 annual clinic visits was selected to provide a sufficient number of BP measures to create stable estimates of long-term variability while limiting the loss of participants to deaths, events, or changes in drug regimens during the course of the baseline period. During the first 5 years, 2,036 participants were excluded (from the “full” CHS cohort of 5,888) because of loss to follow-up, death, or missing a clinic visit; by the 6th visit, a similar exclusion would include 2,709, and by the 8th visit, the number would be 3,315 and include the entire “new” CHS cohort, which constituted most of the black participants in the study. Therefore, to limit the exclusions of subjects in the primary cohort while maximizing the number of visits available, which increases the stability of estimates of variability based on standard deviation, 5 visits was chosen.
Intraindividual SBP mean was calculated as the average of these 5 recorded SBPs and was included in primary analyses to account for variation in “usual” BP and its association with degree of variability. Intraindividual SBP change over the 5 visits, or slope, was calculated as the beta coefficient from a linear regression of these 5 SBP recordings, with the annual visit number serving as the independent variable. This measure of BP change over time was included in primary analyses to account for variability in time trends. Some subjects may increase or decrease over time, others may remain the same, and still others may fluctuate; such differences could confound or obscure an association between “true” variability and clinical outcomes. Finally, intraindividual SBP variability was calculated as the square root of the variance, or the residual mean square, from the 5 residuals from the participant-specific regressions. This final component measure served as the primary variable of interest for the main analyses. Similar sets of exposure measures were calculated for diastolic BP (DBP) and pulse pressure (PP) for the secondary analyses.
Clinical outcomes
The primary outcomes were death from any cause, incident MI, and incident stroke, and they were assessed starting after the last of 5 baseline clinic visits. Participants were censored when lost to follow-up or the end of the analysis period (June 30, 2008). Deaths were identified from national death records and semiannual contacts and were adjudicated by physicians.7 Stroke and MI events were identified by semiannual contacts or through linkage with Medicare hospitalization data and were confirmed by physician adjudication using medical and hospital records, in-person examinations, electrocardiograms, and laboratory or imaging studies, as previously described.7–9
Covariables
Covariable data were collected at different clinic visits over the 5-visit baseline period. For time-varying covariables, either a summary measure (for ever/never variables) or the most recent measure (for continuous variables) was used. Age, sex, clinic site, body mass index, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were measured during clinic visits. Smoking status was cumulative for the entire baseline period, based on ever having self-reported current or past smoking. New onset diabetes was based on a participant meeting any of the following criteria: use of insulin or oral hypoglycemic agents, fasting glucose ≥126mg/dl, or nonfasting glucose ≥200mg/dl. Carotid intima-media thickness (cIMT) was assessed by ultrasound and was calculated from the average of the left, right, near, and far wall thicknesses of the common carotid artery. Antihypertensive medications exposure was based on medication inventories and interviews conducted at each of the 5 clinic visits that comprised the baseline period.10
Statistical analyses
Pairwise correlation coefficients were calculated to examine the relationship among the intraindividual SBP components of mean, slope, and variability. Partial correlation coefficients were calculated to obtain adjusted estimates of the association between covariables of interest and intraindividual SBP mean, slope, and variability. Adjustment covariables for the primary analyses were specified a priori and included intraindividual SBP mean and slope, sex, age (years), race (white, black, other), clinic site, smoking (ever/never), body mass index (kg/m2), high-density lipoprotein and low-density lipoprotein cholesterol (mg/dl), and diabetes before baseline (yes/no). Carotid IMT (mm) was not included in primary analyses as an adjustment covariable because atherogenesis may lie in the causal pathway.
Cox proportional hazards models estimated adjusted hazard ratios (HRs) for the risk of all-cause mortality, incident MI, and incident stroke per SD in intraindividual SBP mean, slope, and variability.11 Analyses were stratified a priori based on antihypertensive medications use. Results for nonusers are reported herein, and detailed results for users of the same antihypertensive medications regimens over the 5 visits are included in the Supplementary Materials. Effect modification based on use or nonuse of antihypertensive medications was evaluated using a χ2 test. Users of changing medication regimens (n = 1,115) were not included in any of the time-to-event analyses. Proportionality of hazards was tested using plots of scaled Schoenfeld residuals over time. Secondary analyses included testing cIMT as a confounder or effect modifier, stratifying outcomes based on stroke subtypes or cause of death, and examining DBP and PP components as the primary exposure variables. Statistical analyses were conducted using Stata SE version 11 (StataCorp, Austin, TX).
RESULTS
Antihypertensive medications nonusers were generally similar to the rest of the eligible participants for most characteristics (Table 1), although nonusers were less likely to have diabetes. Medication nonusers also had a higher degree of intraindividual SBP slope compared with the rest of the eligible cohort, but lower intraindividual SBP mean and variability. Consistent medication users were slightly less likely than nonusers to be male or smokers and more likely to have diabetes; consistent users also had higher SBP mean and variability but lower change over time compared with nonusers. Subjects with inconsistent medication use were generally similar with respect to most demographic characteristics (Supplementary Table S1).
Table 1.
Characteristics of eligible Cardiovascular Health Study participants and medications nonusers
| All | Nonusersa | Consistent usersa | |
|---|---|---|---|
| (n = 3,852) | (n = 1,642) | (n = 1,095) | |
| Age, mean (SD) | 72 (5.0) | 72 (4.9) | 72 (4.9) |
| Male, no. (%) | 1578 (41.0) | 698 (42.5) | 411 (37.5) |
| White, no. (%) | 3357 (87.2) | 1508 (91.8) | 928 (84.8) |
| Clinic site | |||
| Bowman Gray, no. (%) | 930 (24.1) | 406 (24.7) | 260 (23.7) |
| Davis, no. (%) | 1068 (27.7) | 485 (29.5) | 239 (21.8) |
| Hopkins, no. (%) | 846 (22.0) | 334 (20.3) | 304 (27.8) |
| Pittsburgh, no. (%) | 1008 (26.2) | 417 (25.4) | 292 (26.7) |
| BMI, mean (SD)b | 26.6 (4.5) | 25.6 (4.0) | 27.6 (4.9) |
| Smoking ever, no. (%) | 2302 (59.8) | 991 (60.4) | 628 (57.4) |
| HDL cholesterol, mg/dl, mean (SD)b | 55 (15.6) | 56 (15.8) | 52 (15.2) |
| LDL cholesterol, mg/dl, mean (SD)b | 130.3 (35.1) | 130.8 (33.6) | 129.4 (35.3) |
| Diabetes, no. (%) | 628 (16.3) | 158 (9.6) | 244 (22.3) |
| Common cIMT, mean (SD) | 1.00 (0.20) | 0.97 (0.19) | 1.01 (0.21) |
| Intraindividual mean SBP | 134 (17) | 129 (15) | 136 (17) |
| Intraindividual slope SBP | 0.076 (4.6) | 0.507 (3.7) | 0.135 (4.9) |
| Intraindividual SD SBP | 9 (5) | 8 (4) | 10 (5) |
Abbreviations: BMI, body mass index; cIMT, carotid intima-media thickness; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
aBased on medications use records for the first 5 annual clinic visits.
bSubject counts lower for some variables: BMI: n = 3,840; HDL cholesterol: n = 3,821; LDL cholesterol: n = 3,784.
Evaluation of the primary exposure variables among the medication nonusers (n = 1,642) indicates that intraindividual SBP mean and variability are strongly and significantly correlated with each other (Table 2). R2 was calculated for each subject-specific linear regression to evaluate the within-subject linear trend term; on average, intraindividual slope accounted for 29% of the variance around the subject-specific means (R2 interquartile range = 5%–49%).
Table 2.
Predictors of intraindividual characteristics in systolic blood pressure (SBP) among all eligible study participants (n = 3,852)a
| Correlations with intraindividual SD SBP | Correlations with intraindividual mean SBP | |||
|---|---|---|---|---|
| Partial CC | P value | Partial CC | P value | |
| Slope SBP (unadjusted, pairwise CC) | −0.0573 | <0.0001 | −0.0356 | 0.03 |
| Mean SBP (unadjusted, pairwise CC) | 0.3702 | <0.0001 | — | — |
| Mean | 0.3249 | <0.001 | — | — |
| Male sex | −0.0498 | 0.01 | −0.0506 | 0.002 |
| Age | 0.0498 | 0.002 | 0.1254 | <0.001 |
| White race | 0.0314 | 0.06 | 0.0108 | 0.51 |
| Smoking | 0.0370 | 0.02 | −0.0404 | 0.01 |
| BMI | −0.0376 | 0.02 | 0.0849 | <0.001 |
| Type 2 diabetes | 0.0353 | 0.03 | 0.0642 | <0.001 |
| HDL cholesterol | 0.0002 | 0.99 | 0.0223 | 0.17 |
| LDL cholesterol | −0.002 | 0.90 | 0.0045 | 0.79 |
| Common cIMT | 0.0715 | <0.001 | 0.1654 | <0.001 |
Abbreviations: CC, correlation coefficient; cIMT, carotid intima-media thickiness; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
aCorrelations adjusted for SD, slope, mean, age, sex, race, clinic site, BMI, diabetes, smoking, HDL cholesterol, LDL cholesterol, and common cIMT.
Further evaluation of covariable associations with the primary exposure variables suggests that age, smoking, and cIMT were marginally or significantly associated with intraindividual SBP variability, independent of the associations with intraindividual SBP mean and slope. Similar associations were observed for intraindividual SBP mean, except that body mass index, new onset diabetes, and high-density lipoprotein cholesterol were also significantly associated, independent of associations with intraindividual SBP slope and variability. Smoking and diabetes were significantly associated with increases in SBP variability, whereas body mass index was inversely correlated.
Of the 1,642 antihypertensive medications nonusers, 844 individuals died, 203 had an MI, and 195 had a stroke over a mean follow-up of 9.9 years. Intraindividual SBP mean was significantly associated with increased risk of mortality, incident MI, and incident stroke, independent of the effects from intraindividual SBP slope, intraindividual SBP variability, and adjustment covariables (Table 3). Intraindividual SBP slope was not independently associated with any of these outcomes.
Table 3.
Hazard ratios (HRs) for selected outcomes, per 1 SD increase in intraindividual measures of systolic blood pressure (SBP)a
| Mortality | MI | Stroke | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | HR | (95% CI) | P value | HR | (95% CI) | P value | HR | (95% CI) | P value |
| Medications nonusers | |||||||||
| Intraindividual mean SBP | 1.10 | (1.02–1.18) | 0.01 | 1.39 | (1.20–1.62) | <0.001 | 1.38 | (1.19–1.61) | <0.001 |
| Intraindividual slope SBP | 0.99 | (0.92–1.08) | 0.88 | 1.04 | (0.89–1.23) | 0.61 | 0.97 | (0.83–1.14) | 0.74 |
| Intraindividual variability in SBP | 1.13 | (1.05–1.21) | <0.001 | 1.20 | (1.06–1.36) | 0.005 | 1.03 | (0.89–1.21) | 0.67 |
| Consistent medications users | |||||||||
| Intraindividual mean SBP | 1.05 | (0.97–1.13) | 0.27 | 1.17 | (0.98–1.40) | 0.08 | 1.32 | (1.11–1.56) | 0.001 |
| Intraindividual slope SBP | 1.01 | (0.95–1.09) | 0.72 | 1.22 | (1.06–1.41) | 0.005 | 1.06 | (0.92–1.23) | 0.40 |
| Intraindividual variability in SBP | 1.09 | (1.02–1.17) | 0.01 | 1.11 | (0.95–1.28) | 0.19 | 1.00 | (0.86–1.17) | 0.10 |
Abbreviations: CI, confidence interval; MI, myocardial infarction.
aAll estimates adjusted for age, sex, white race, clinics, body mass index, type 2 diabetes, ever smoking, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol (as of 5th clinic visit).
Intraindividual SBP variability was significantly associated with increased risk of mortality (HR = 1.13; 95% confidence interval (CI) = 1.05–1.21) and incident MI (HR = 1.20; 95% CI = 1.06–1.36), independent of the effect from adjustment factors (Table 3). Intraindividual SBP variability was not associated with increased risk of stroke (HR = 1.03; 95% CI = 0.89–1.21). Associations were similar when evaluated separately among consistent antihypertensive medications users, with the exception that the associations with MI were not statistically significant (Table 3). There was inadequate evidence to support the conclusion that consistent antihypertensive medication use compared with nonuse was an effect modifier of the associations between long-term SBP variability and risk of mortality, MI, or stroke (Supplementary Table S2).
Sensitivity analyses indicated that inclusion of cIMT as an adjustment factor did not materially change the associations between intraindividual SBP variability and mortality, MI, or stroke, and evidence that cIMT was an effect modifier was lacking (Supplementary Table S2).
Sensitivity analyses to replicate previously reported analyses (e.g., without the “slope” or trend variable included in the models) detected significant associations for SBP variability with mortality and MI among medications nonusers and for mortality among users; stroke was not significantly associated with SBP variability in either subgroup (Supplementary Materials). There was evidence of interaction between SBP variability and medication use for the MI outcome (P = 0.02). However this finding should be interpreted with caution because the effect of underlying BP variability is inextricably mixed with the variability attributable to changes in medication use over time, which may include adding, dropping, or changing use of specific medication classes (Supplementary Table S3).
Secondary analyses examining associations with the intraindividual BP components based on DBP or PP were similar to those for SBP (Supplementary Table S4). Intraindividual DBP variability was independently associated with increased risk of all-cause mortality (HR = 1.17; 95% CI = 1.10–1.24). Associations for MI and stroke were similar to those of mortality, but not statistically significant for stroke (HR = 1.13; 95% CI = 0.98–1.30). Intraindividual PP variability was not strongly or significantly related to risk of mortality, MI, or stroke.
Secondary analyses examining the associations with SBP, DBP, or PP variability among inconsistent medication users were generally nonsignificant, although these results are hard to interpret, given the mixed patterns of medications exposures in this group (Supplementary Table S5). Secondary analyses for specific causes of death, for stroke subtypes, and among users of specific drug classes were conducted but were not substantially different from the main results or were not adequately powered to provide meaningful risk estimates (data not shown).
Based on the scaled Schoenfeld plots, the assumption of proportional hazards may not have been met for some of the covariables. Models including interaction terms for these covariables with the baseline hazard function or allowing the HRs for these covariables to change over time indicated that the primary results and conclusions were not substantially changed (data not shown).
Correction for multiple testing was not formally done as part of the primary analyses because the associations with the three outcomes were conceived separately and hypotheses were constructed a priori. However, a conservative Bonferroni correction for 3 hypothesis tests yields a P value for the significance threshold at 0.02 for an alpha level of 0.05. All discussed “significant” tests surpassed this threshold; therefore it is unlikely that the results reported herein are statistically significant due to chance alone.
DISCUSSION
In this study, although intraindividual SBP mean over approximately 5 years was independently associated with higher risk of subsequent all-cause mortality, incident MI, and incident stroke, intraindividual SBP variability was independently associated with risk of mortality and MI but not stroke. Few studies to date have examined whether long-term BP variability is related to increased risk of death or vascular events, and reported estimates have been varied. Rothwell and colleagues observed a positive association between intraindividual SBP variability and risk of fatal or nonfatal MI.12 Another recent analysis of patients with mild to moderate hypertension found no association between long-term SBP variability and cIMT or cardiovascular events.13 Prior observational studies had detected associations of SBP variability with mortality, coronary heart disease, stroke, and white matter disease.14–19
Our study results are not consistent with a prior report indicating an association between intraindividual SBP variability and stroke; however differences between study populations and analytic approaches may account for this discrepancy: sensitivity analyses indicated that changes in the statistical analyses were unlikely to fully account for the differences in results with prior reports. Rothwell and colleagues found that variability in 2–10 measures of BP over approximately 2 years is a risk factor for stroke independent of the mean BP, using data from 4 randomized controlled trials of patients with hypertension, prior stroke, or prior transient ischemic attack.12 , 20 In contrast, our study used a model that accounted for intraindividual change over time to obtain an independent estimate of risk for intraindividaul BP variability outside of the effects of change over time. Second, our study used a population-based cohort of older US adults, who were predominantly white, with consistent antihypertensive medications use patterns during the baseline period; these results may not be generalizable to other populations.
This study may have been underpowered to detect associations with stroke; however, the number of stroke events and MI events were similar, and the estimates of association for stroke were consistently close to the null. It is possible that the relationship between intraindividual BP variability and stroke operates at a different stage in the disease process than is detectable using the current cohort population. Because previous reports had focused on subjects with hypertension or prior events, BP variability may be a determinant of stroke risk in a subpopulation with an established higher risk of stroke. However, the possiblity that low power limited the strength of observable associations cannot be ruled out.
Estimates of association for DBP components, although not statistically significant, were closer in effect size to the findings for SBP with mortality and MI and may have been simply underpowered; therefore, it is not possible from these models to draw conclusions regarding whether associations in the primary analyses on SBP and the secondary analyses on DBP are similar or different.
In this study, intraindividual BP variability has been associated with increased risk of mortality and cardiovascular events among a general population of older adults. The individual biologic mechanisms by which long-term variability may affect risk of mortality or cardiovascular disease are yet unclear and may act through several pathways. Short-term, diurnal variability is correlated with end-organ damage, including left ventricular mass and arterial stiffness.21–23 Long-term BP variability may similarly affect the underlying health of vascular tissues, thereby affecting the development or severity of atherosclerosis. Disturbed hemodynamics and inconsistent shear stress patterns contribute to damage to the endothelium and the development of atherosclerotic lesions based on specific, focal patterns of branching in the arterial tree.24 Possibly, inconsistent vessel pressure patterns affect the formation of plaques in a similar manner. In this study, extent of atherosclerosis—as reflected by cIMT—was strongly associated cross-sectionally with long-term SBP variability. However, adjustment for cIMT did not materially change our estimates, suggesting that additional mechanisms may be at work. High long-term BP variability may also reflect vessel sclerosis, as stiff vessels may contribute to variability in BP over multiple longitudinal measures.25 , 26
This study has several strengths, including use of a population-based cohort with rich information on potential confounders and adjudicated outcomes. This study was able to adjust for trends in BP over time, which most prior studies have failed to do. In addition, this study provides longer follow-up than previous reports.
This study also has some limitations. The analyses were restricted to participants taking no antihypertensive medications throughout their baseline period of 5–6 years (primary results) or to stable antihypertensive medication users (Supplementary Materials), and this restriction may influence the generalizability of these results to other groups. However, users and nonusers did not appear different from individuals who initiated, changed, or stopped antihypertensives, with respect to the measured covariables included in these analyses. Also, because users and nonusers were not explicitly compared with one another, confounding by indication and healthy user bias are not expected to have a marked effect on these results. Prior studies suggest that different classes of antihypertensive medications may have differing effects on intraindividual SBP variability and consequent cardiovascular risk. Unfortunately, this study was not adequately powered to address this question of effect modification by antihypertensive drug class. In addition, changes to participants’ drug use were not taken into account after the baseline period, which may influence their future risk and therefore affect the ability to characterize differences in risk between users and nonusers. This also results in a selected subject population that is healthier than the CHS cohort as a whole. Individuals were required to survive, event-free, over the course of their first 5 clinic visits without initiating, changing, or stopping antihypertensive medication use. Finally, residual confounding may persist if covariables were measured with substantial error or if important confounders were not considered.
In summary, this study provides evidence that long-term, visit-to-visit SBP variability in older adults may be an important indicator of the underlying state of vascular health that is linked to all-cause mortality and incident MI but may not be an indictor of incident stroke. Subgroups may exist where intraindividual SBP variability is associated with stroke, but these are yet to be defined. More research is needed to elucidate etiologic pathways and to establish if these findings carry any clinical implications for individual patients.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
B.M.P. serves on the Data Safety Monitoring Board for a clinical trial of a device funded by the manufacturer (2011 LifeCor) and serves on the steering committee of the Yale Open Data Access Project funded by Medtronic. All other authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
A.M.S.-D. and E.R.W. contributed equally to this study in conception, development, analysis, and writing. They are supported by the NHLBI Cardiovascular Disease Training Grant, NIH I-T32-HL07902. The manuscript and all related analyses were developed with support from the CHS Neurology working group. The research reported in this article was supported by contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www. chs-nhlbi.org/pi.htm.
REFERENCES
- 1. Staessen JA, Gasowski J, Wang JG, Thijs L, Den Hond E, Boissel JP, Coope J, Ekbom T, Gueyffier F, Liu L, Kerlikowske K, Pocock S, Fagard RH. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355: 865–872 [DOI] [PubMed] [Google Scholar]
- 2. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913 [DOI] [PubMed] [Google Scholar]
- 3. Pastor-Barriuso R, Banegas JR, Damian J, Appel LJ, Guallar E. Systolic blood pressure, diastolic blood pressure, and pulse pressure: an evaluation of their joint effect on mortality. Ann Intern Med 2003; 139: 731–739 [DOI] [PubMed] [Google Scholar]
- 4. Psaty BM, Furberg CD, Kuller LH, Borhani NO, Rautaharju PM, O’Leary DH, Bild DE, Robbins J, Fried LP, Reid C. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the Cardiovascular Health Study. JAMA 1992; 268: 1287–1291 [PubMed] [Google Scholar]
- 5. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1: 263–276 [DOI] [PubMed] [Google Scholar]
- 6. Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010; 375: 906–915 [DOI] [PubMed] [Google Scholar]
- 7. Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 1995; 5: 278–285 [DOI] [PubMed] [Google Scholar]
- 8. Longstreth WT, Jr, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, Furberg CD. Frequency and predictors of stroke death in 5,888 participants in the cardiovascular health study. Neurology 2001; 56: 368–375 [DOI] [PubMed] [Google Scholar]
- 9. Mittelmark MB, Psaty BM, Rautaharju PM, Fried LP, Borhani NO, Tracy RP, Gardin JM, O’Leary DH. Prevalence of cardiovascular diseases among older adults. The Cardiovascular Health Study. Am J Epidemiol 1993; 137: 311–317 [DOI] [PubMed] [Google Scholar]
- 10. Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study collaborative research group. J Clin Epidemiol 1992; 45: 683–692 [DOI] [PubMed] [Google Scholar]
- 11. Cox DR. Regression models and life-tables. J Roy Stat Soc B 1972; 34: 187–220 [Google Scholar]
- 12. Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010; 375: 895–905 [DOI] [PubMed] [Google Scholar]
- 13. Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability, carotid atherosclerosis and cardiovascular events in the European Lacidipine Study on Atherosclerosis. Circulation,2012; 126(5): 569–578 [DOI] [PubMed] [Google Scholar]
- 14. Hata Y, Kimura Y, Muratani H, Fukiyama K, Kawano Y, Ashida T, Yokouchi M, Imai Y, Ozawa T, Fujii J, Omae T. Office blood pressure variability as a predictor of brain infarction in elderly hypertensive patients. Hypertens Res 2000; 23: 553–560 [DOI] [PubMed] [Google Scholar]
- 15. Hata Y, Muratani H, Kimura Y, Fukiyama K, Kawano Y, Ashida T, Yokouchi M, Imai Y, Ozawa T, Fujii J, Omae T. Office blood pressure variability as a predictor of acute myocardial infarction in elderly patients receiving antihypertensive therapy. J Hum Hypertens 2002; 16: 141–146 [DOI] [PubMed] [Google Scholar]
- 16. Grove JS, Reed DM, Yano K, Hwang LJ. Variability in systolic blood pressure—a risk factor for coronary heart disease? Am J Epidemiol 1997; 145: 771–776 [DOI] [PubMed] [Google Scholar]
- 17. Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: The Honolulu-Asia Aging Study. Stroke 2002; 33: 26–30 [DOI] [PubMed] [Google Scholar]
- 18. Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol 2010; 67: 564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension 2011; 57: 160–166 [DOI] [PubMed] [Google Scholar]
- 20. Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 2010; 375: 938–948 [DOI] [PubMed] [Google Scholar]
- 21. Sander D, Klingelhofer J. Diurnal systolic blood pressure variability is the strongest predictor of early carotid atherosclerosis. Neurology 1996; 47: 500–507 [DOI] [PubMed] [Google Scholar]
- 22. Mancia G, Giannattasio C, Failla M, Sega R, Parati G. Systolic blood pressure and pulse pressure: role of 24-h mean values and variability in the determination of organ damage. J Hypertens 1999; 17: S55–S61 [PubMed] [Google Scholar]
- 23. Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schillaci G, De Caterina R. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension 2007; 50: 325–332 [DOI] [PubMed] [Google Scholar]
- 24. Davies PF, Shi C, Depaola N, Helmke BP, Polacek DC. Hemodynamics and the focal origin of atherosclerosis: a spatial approach to endothelial structure, gene expression, and function. Ann N Y Acad Sci 2001; 947: 7–16; discussion 16–17 [PubMed] [Google Scholar]
- 25. Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res 2010; 107: 615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karwowski W, Naumnik B, Szczepanski M, Mysliwiec M. The mechanism of vascular calcification—a systematic review. Med Sci Monit 2012; 18: RA1–RA11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.