Abstract
BACKGROUND
Dietary administration of 0.30% indole-3-carbinol (I3C) to Cyp1a1-Ren2 transgenic rats (TGRs) generates angiotensin II (ANG II)–dependent malignant hypertension (HTN) and increased renal vascular resistance. However, TGRs with HTN maintain a normal or slightly reduced glomerular filtration rate. We tested the hypothesis that maintenance of renal function in hypertensive Cyp1a1-Ren2 TGRs is due to preservation of the intrarenal nitric oxide (NO) and antioxidant systems.
METHODS
Kidney cortex, kidney medulla, aortic endothelial (e) and neuronal (n) nitric oxide synthase (NOS), superoxide dismutases (SODs), and p22phox (nicotinamide adenine dinucleotide phosphate-oxidase subunit) protein abundances were measured along with kidney cortex total antioxidant capacity (TAC) and NOx. TGRs were fed a normal diet that contained 0.3% I3C or 0.3% I3C + candesartan (AT1 receptor antagonist; 25mg/L in drinking water) (n = 5–6 per group) for 10 days.
RESULTS
Blood pressure increased and body weight decreased in I3C-induced TGRs, while candesartan blunted these responses. Abundances of NOS, SOD, and p22phox as well as TAC were maintained in the kidney cortex of I3C-induced TGRs with and without candesartan, while kidney cortex NOx production increased in both groups. Kidney medulla eNOS and extracellular (EC) SOD decreased and nNOS were unchanged in both groups of I3C-induced TGRs. In addition, a compensatory increase occurred in kidney medulla Mn SOD in I3C-induced TGRs + candesartan. Aortic eNOS and nNOS∝ fell and p22phox and Mn SOD increased in hypertensive I3C-induced TGRs; all changes were reversed with candesartan.
CONCLUSIONS
The preservation of renal cortical NO and antioxidant capacity is associated with preserved renal function in Cyp1a1-Ren2 TGRs with ANG II-dependent malignant HTN.
Keywords: angiotensin receptor blocker, aorta, blood pressure, candesartan, hypertension, kidney, superoxide dismutase, total antioxidant capacity.
Angiotensin II (ANG II)–dependent hypertension (HTN) and renal vasoconstriction are induced in Cyp1a1-Ren2 (strain name: TGR (Cyp1a1Ren2)) transgenic rats (TGR) by dietary administration of indole-3-carbinol (I3C).1 Some of the damaging cardiovascular actions of ANG II are mediated by reactive oxygen species.2–4 Studies have shown that administration of the superoxide dismutase mimetic, tempol, reduces ANG II–dependent HTN and protects tissues from oxidative stress.3,5–7 When ANG II–dependent malignant HTN is induced in Cyp1a1-Ren2 TGRs, acute systemic administration of tempol decreases the elevated blood pressure (BP) and renal vascular resistance (RVR) but produces no effect in normotensive rats.8 Chronic administration of the angiotensin type 1 (AT1) receptor blocker (ARB), candesartan, normalizes BP and restores the elevated urinary excretion of 8-isoprostane to normal, supporting a role for AT1-stimulated production of superoxide anion in contributing to the malignant HTN in Cyp1a1-Ren2 rats.8
Increased levels of the superoxide anion also reduce the bioavailability of nitric oxide (NO), which further contributes to elevations in BP and RVR.9–11 In hypertensive Cyp1a1-Ren2 TGRs treated with tempol, acute NO synthase (NOS) inhibition with nitro-L-arginine (NLA) attenuates the tempol-induced fall in BP, thus BP increases; this also occurs in the normotensive rat.8 In addition, NLA administration increases RVR in both normotensive and hypertensive Cyp1a1-Ren2 TGRs treated with tempol and also elicits decreases in glomerular filtration rate (GFR) and renal plasma flow (RPF) in hypertensive rats.8 Collectively, this suggests that a superoxide anion-mediated decrease in NO bioavailability does not fully contribute to the increased RVR in hypertensive Cyp1a1-Ren2 TGRs and that maintained intrarenal actions of NO preserve renal hemodynamics.8
The activity of renal endothelial (e)NOS and neuronal (n)NOS plays a critical role in regulating normal renal hemodynamics and sodium excretion. While eNOS is abundant throughout the renal vasculature and controls blood flow, nNOS is abundant in the macula densa and regulates blood flow via tubuloglomerular feedback.12–15 Tubular NO derived from both eNOS (mainly thick ascending limb) and nNOS (predominantly collecting duct) inhibits sodium reabsorption.15–19 In renal injury, nNOSα decreases and nNOSβ increases; although the locations and significance of these changes remain to be determined.12,14,20.
In this study, we hypothesized that the maintenance of renal hemodynamic function in hypertensive Cyp1a1-Ren2 TGRs is due to the preservation of the intrarenal NO and antioxidant systems in this form of severe ANG II–dependent HTN. To test this, we investigated the intrarenal NOS and antioxidant/oxidant systems in the kidney and aorta of Cyp1a1-Ren2 TGRs with ANG II–dependent malignant HTN. The aorta was chosen as a comparison tissue to the kidney to reflect systemic vascular vs. intrarenal alterations in the NOS and antioxidant/oxidant systems in Cyp1a1-Ren2 TGRs with elevated BP.
METHODS
The experimental procedures in this study conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Tulane University Health Sciences Center. Experiments were performed on adult (aged 12–20 weeks) male Cyp1a1-Ren2 TGRs (TGR(Cyp1a1-Ren2)) with inducible expression of the mouse Ren2 renin gene.1 All transgenic rats were bred at Tulane University from stock animals (Harlan UK, Bicester, United Kingdom). The experimental animals were divided into three groups. Group 1 (noninduced; n = 6) control Cyp1a1-Ren2 rats were maintained on a normal rat diet (diet TD 99414; Harlan-Teklad, Madison, WI). Group 2 (0.3% I3C; n = 5) rats were fed a normal diet containing I3C (0.3% wt/wt; diet TD 05381; Harlan-Teklad) for 10 days to induce ANG II–dependent malignant HTN. Group 3 (0.3% I3C + ARB; n = 6) rats were fed a normal diet containing I3C (0.3%) for 10 days and simultaneously treated chronically with the AT1 receptor antagonist, candesartan (AstraZeneca R&D, Molndal, Sweden). Candesartan was administered chronically in the drinking water (25mg/L) in a dose that prevents development of ANG II–dependent malignant HTN in Cyp1a1-Ren2 TGRs.21
Systolic blood pressure (SBP) was measured in conscious rats using tail-cuff plethysmography (model 6R22931; IITC Life Science, Woodland Hills, CA). All rats were trained for 2 weeks before beginning the experiment to habituate them to this procedure. Blood pressures were measured every day throughout the study beginning 4 days prior to initiating dietary administration of I3C. Body weight was also measured every day throughout the study. At the completion of the experimental protocol, the rats were sacrificed, and sections of the thoracic aorta and left kidney were removed. The left kidney was separated into cortex and medulla. All tissues were shipped to the University of Florida College of Medicine for analysis.
Western blot
Kidney cortex, medulla, and thoracic aorta protein abundances were detected using Western blotting. Tissues were homogenized, and the protein concentrations were determined using the DC protein Assay (Bio-Rad Laboratories, Hercules, CA). Homogenate samples (200 μg of kidney cortex, 100 μg of medulla, and 200 μg of thoracic aorta) were loaded on 7.5% or 12% polyacrylamide gels and separated by electrophoresis. Membranes were incubated overnight with specific antibodies: mouse anti-eNOS antibody (1:250 dilution; BD Transduction Lab, San Jose, CA; Cat No: 610297), mouse anti-nNOSα antibody (1:50 dilution; Santa Cruz Biotechnology, Santa Cruz, CA; Cat No: sc-5302), rabbit anti-nNOSβ antibody (1:500 dilution; Thermo Scientific Pierce, Rockford, IL; Cat No: PA1-033), goat anti-p22phox antibody (1:50 dilution; Santa Cruz Biotechnology; Cat No: sc-11712), rabbit anti-ecSOD (1:250 dilution; Abcam, Cambridge, MA; Cat No: ab21974), rabbit anti-mnSOD (1:2000 dilution; Stressgen/Assay Designs, Plymouth Meeting, PA; Cat No: SOD-110), and rabbit anti-Cu/Zn SOD (1:2000 dilution; Stressgen/Assay Designs; Cat No: SOD-101). The membranes were then incubated with corresponding secondary antibodies: goat anti-mouse (1:2,000 and 1:3,000 dilution Bio-Rad; Cat No: 170–6515), donkey anti-goat antibody (1:2,000 dilution; Santa Cruz Biotechnology; Cat No: sc-2020), and goat anti-rabbit antibody (1:3,000 dilution; Bio-Rad; Cat No: sc-2004). Bands of interest were visualized using the enhanced chemiluminescence reagent and quantified by densitometry (VersaDoc imaging system and Quantity One Analysis software, Bio-Rad) as integrated optical density (IOD) after subtraction of background. IOD was factored for Ponceau (PON) Red staining (Sigma) to correct for any variations in total protein loading. Protein abundance is represented as IOD/PON. The antibody used to probe for the splice variant nNOSβ is a C-terminus specific antibody for mouse, rat, and human nNOS. This antibody will probe for several different splice variants of nNOS, including nNOSβ. A proteomics approach was taken in previous studies to identify the correct band probing for nNOSβ, which is ~140kDa.22 Antibodies probing for eNOS and nNOSα have no cross reactivity; a single band appears on the membrane, reflecting the molecular weights of ~140kDa and ~160kDa, respectively. The nNOSα antibody probes against a unique amino acid N-terminus sequence of mouse, rat, and human nNOS that is only present in the nNOSα variant on nNOS.
Tissue NOx and total antioxidant capacity
Tissue NOx levels were measured by the Griess assay.23 Total antioxidant capacity (TAC) was measured using the Antioxidant Assay Kit (Cayman Chemical Company, Ann Arbor, MI), according to the manufacturer’s instructions. This kit measures the ability of all aqueous and lipid-soluble antioxidants to inhibit the oxidation of 2,2′-azino-di-(3- ethylbenzthiazoline sulphonate) (ABTS) to ABTS+ by metmyoglobin by reading absorbance at 750nm. Five micrograms of protein from the kidney cortex was pipetted into 96-well plates. Sample antioxidant capacity was compared to trolox (a water-soluble tocopherol analogue) standards at 0, 0.045, 0.09, 0.135, 0.18, 0.225, and 0.330mM trolox. The TAC was quantified as millimolar trolox equivalents (mM Trolox/mg) at 750-nm wavelength.
Statistical analysis
Statistical analysis was performed using 1-way analysis of variance (ANOVA) followed by the Bonferoni post hoc test. If the analysis between groups failed the normality test, the Kruskal-Wallis 1-way ANOVA multiple comparison test was used followed by the Dunn post hoc test. All statistical analyses were performed using SigmaPlot for Windows (version 11, System Software Inc., San Jose, CA). Statistical significance was defined as P < 0.05. All data are expressed as mean ± SE.
RESULTS
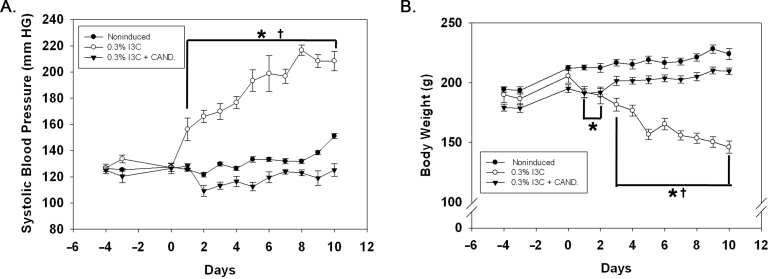
Chronic dietary administration of 0.3% I3C for 10 days resulted in the development of severe HTN (Figure 1A) in association with a marked decrease in body weight (Figure 1B). SBP remained unaltered in noninduced Cyp1a1-Ren2 rats and Cyp1a1-Ren2 rats induced with I3C and treated chronically with the ARB, candesartan (Figure 1A). Body weight increased over the 10-day observation period in both the noninduced rats and the Cyp1a1-Ren2 rats induced with I3C and treated chronically with the ARB (Figure 1B).
Figure 1.
Effects of 0.3% indole-3-carbinol (I3C) diet and 0.3% I3C diet + candesartan (CAND) treatment on (A) systolic blood pressure and (B) body weight in Cyp1a1-Ren2 transgenic rats. *P < 0.05 vs. noninduced and †P < 0.05 vs. 0.3% I3C diet + CAND.
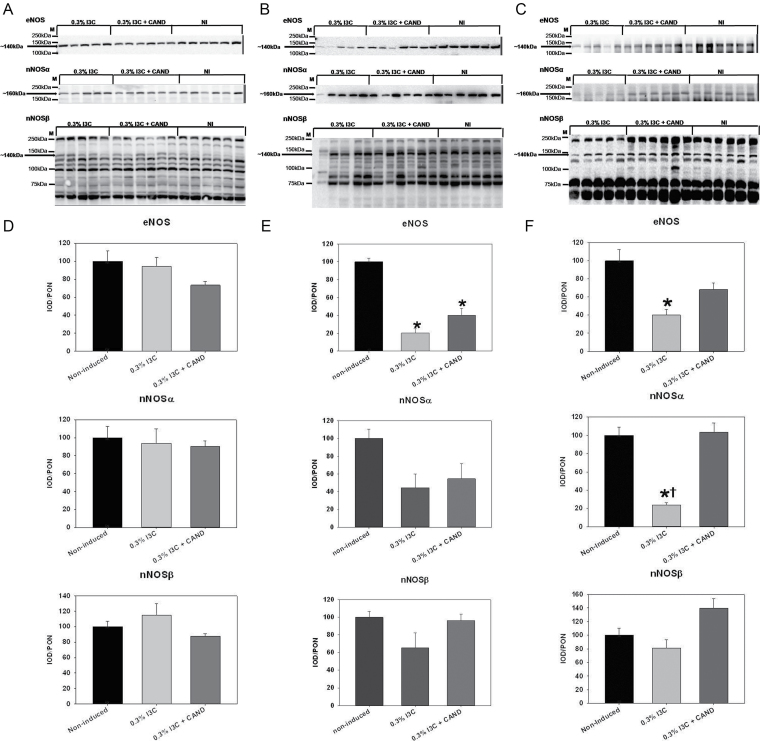
Kidney cortex eNOS, nNOSα, and nNOSβ protein abun dances were similar in noninduced (control) rats and rats induced with an I3C diet with and without candesartan (Figure 2A,D). Kidney cortex NOx content increased in both groups of rats fed the I3C diet, with and without candesartan and irrespective of BP (Figure 3A). In contrast, medulla eNOS protein abundance was lower in both induced groups vs. controls, while the nNOS isoforms were unchanged (Figure 2B,E). In aorta, both eNOS and nNOSα were reduced in the I3C-induced malignant hypertensives and were normalized by candesartan (Figure 2C,F). Lack of available tissue prevented measurement of renal medullary or aortic NOX.
Figure 2.
Effects of 0.3% indole-3-carbinol (I3C) diet and 0.3% I3C diet + candesartan (CAND) treatment on the protein abundance of endothelial nitric oxide synthase (eNOS), nNOSα, and nNOSβ in the (A) kidney cortex, (B) kidney medulla, and the (C) aorta of Cyp1a1-Ren2 transgenic rats. Each membrane blot contains molecular weight markers and an arrow pointing to the band of interest along with its molecular weight. Densitometric analysis of Western blots for these proteins in the kidney cortex, kidney medulla, and aorta are on panels (D), (E), and (F), respectively. The group order on the membrane image is different from the group order on the densitometric analysis from left to right. *P < 0.05 vs. noninduced and †P< 0 .05 vs. 0.3% I3C diet + CAND.
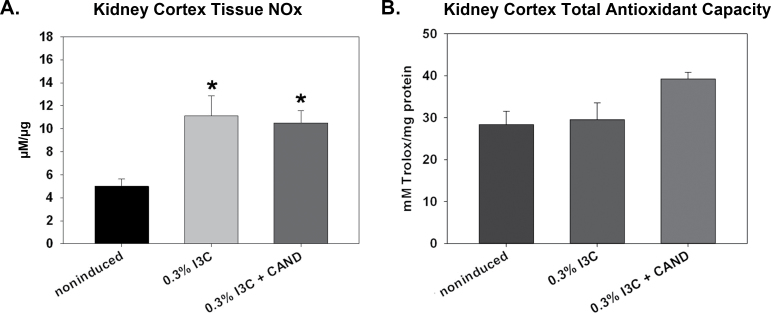
Figure 3.
Effects of 0.3% indole-3-carbinol (I3C) diet and 0.3% I3C diet + candesartan (CAND) treatment on kidney cortex (A) tissue NOx content and (B) total antioxidant capacity in Cyp1a1-Ren2 transgenic rats. *P < 0.05 vs. noninduced.
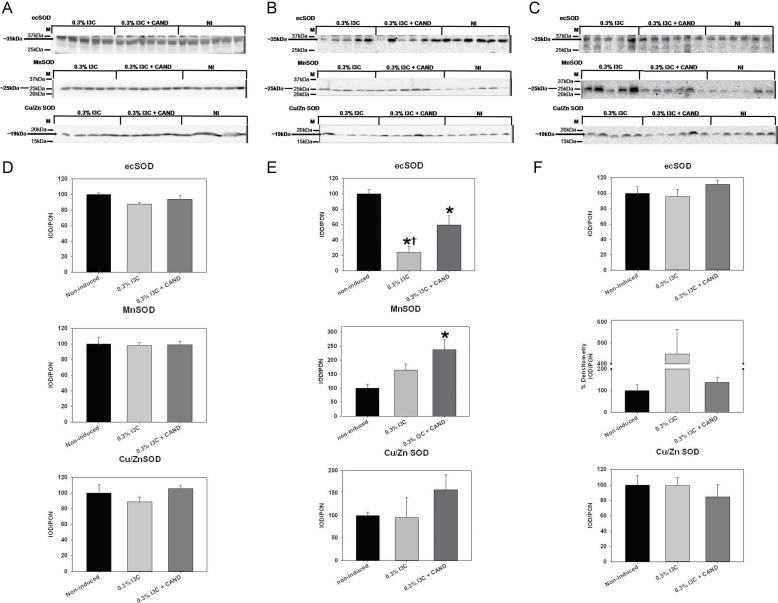
Kidney cortex abundance of the nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase subunit, p22phox, was similar in noninduced controls and in both groups of rats fed the I3C diet, with and without candesartan (data shown in Supplementary Figure 1). There were no differences in the relative protein abundances of EC SOD, Manganese (Mn) SOD, or Copper Zinc (CuZn) SOD in renal cortex of any of the 3 groups (Figure 4A,D). Furthermore, the TAC in the kidney cortex was not different among the 3 groups of rats (Figure 3B). In medulla, p22phox abundance was also similar among the 3 groups of Cyp1a1-Ren2 rats (data shown in Supplementary Figure 1), while EC SOD was reduced in medulla in I3C-fed rats with and without candesartan (Figure 5E). The CuZn SOD was similar in medulla in all 3 groups, while the Mn SOD was unchanged in the I3C-fed rats with malignant HTN and was elevated above control in the group receiving candesartan (Figure 4B,E). Hypertensive rats displayed a 45% ± 11% increase in aortic p22phox over noninduced control rats (Supplementary Figure 1). Aortic Mn SOD abundance (Figure 4C,F) was significantly higher in the hypertensive rats than in the noninduced controls rats. The lack of tissue prevented measurement of medullary and aortic TAC.
Figure 4.
Effects of 0.3% indole-3-carbinol (I3C) diet and 0.3% I3C diet + candesartan (CAND) treatment on the protein abundance of EC superoxide dismutases extracellular SOD, Mn SOD, and Cu/Zn SOD in the (A) kidney cortex, (B) kidney medulla, and the (C) aorta of Cyp1a1-Ren2 transgenic rats. Each membrane blot contains molecular weight markers and an arrow pointing to the band of interest along with its molecular weight. Densitometric analysis of Western blots for these proteins in the kidney cortex, kidney medulla, and aorta are on panels (D), (E), and (F), respectively. The group order on the membrane image is different from the group order on the densitometric analysis from left to right. *P < 0.05 vs. noninduced and †P < 0.05 vs. 0.3% I3C diet +CAND.
DISCUSSION
The major novel findings in the present study are that the renal cortex NOS protein abundance is maintained, while NO production is enhanced in I3C-induced Cyp1a1-Ren2 rats with ANG II–dependent malignant HTN. This effect is independent of BP since all indices of renal cortex NO are similar between the malignant hypertensives and the normotensive ARB-treated I3C-induced Cyp1a1-Ren2 rats. The enhanced renal cortical NO production is likely to be associated with maintenance of renal antioxidant mechanisms since abundance of all superoxide dismutases as well as TAC were similar in all three groups. This contrasts with the evidence of increased systemic oxidative stress from our earlier studies.8
As reported in an earlier study,8 SBP increased rapidly and progressively starting on day 1 on the I3C diet in association with a progressive loss of body weight, reflecting the development of malignant HTN. Co-administration of candesartan along with the I3C diet normalized both BP and body weight to noninduced levels in TGRs, reinforcing the importance of AT1 receptor activation in the pathogenesis of Cyp1a1-Ren2–induced ANG II–dependent malignant HTN.24
Plasma, urinary excretion, and intraadrenal and intrarenal aldosterone levels are elevated in Cyp1a1-Ren2 TGRs with malignant HTN.1,25 Administration of spironolactone, a mineralocorticoid receptor inhibitor that inhibits aldosterone actions, decreased proteinuria after 10 days of malignant HTN in Cyp1a1-Ren2 TGRs.25 However, spironolactone does not prevent the increase in SBP or the decrease in body weight due to malignant HTN, suggesting that the downstream activation of elevated aldosterone is not critical for the pathogenesis of malignant HTN.25
Renal cortex NO production was enhanced in I3C-induced Cyp1a1-Ren2 rats with ANG II–dependent malignant HTN and in normotensive I3C-induced Cyp1a1-Ren2 rats given candesartan treatment. The exact mechanism of increased NOx production in candesartan-treated normotensive rats is not clear. One possible explanation is an increase in expression, abundance, and/or activation of the angiotensin II type 2 receptors (AT2), which stimulate NO release.26 A study by Savoia et al. showed that chronic AT1 receptor blockade in the diabetic man led to increases in arterial AT2 expression and activity in humans independent of blood pressure.27 Therefore, the increase in renal cortex NOx production in normotensive Cyp1a1-Ren2 rats may result from increased AT2 receptor activation caused by candesartan-treated AT1 receptor blockade. Studies examining the AT2 receptor expression, protein abundance, and activity in the renal cortex of Cyp1a1-Ren2 rats given candesartan treatment will need to be conducted to test this possibility.
Functional studies with the nonselective NOS inhibitor NLA suggest that maintenance of renal NO contributes to the protection of renal function in hypertensive Cyp1a1-Ren2 TGRs.8 Subsequent studies using the nNOS selective inhibitor s-methyl thiocitrulline suggest that renal nNOS-derived NO contributes to the control of renal hemodynamics in this strain.28 Our present observations support a functional role for NOS-derived NO in the control of renal hemodynamics in Cyp1a1-Ren2 TGRs with malignant HTN due to the preservation of both nNOS and eNOS protein abundances in the renal cortex and enhanced renal cortex tissue NOx production, along with maintenance of the GFR. Although we did not conduct renal function studies, previous studies by our group have demonstrated relative maintance of GFR in the presence of malignant HTN.8,21
A number of studies have implicated increased oxidative stress in the pathogenesis of ANG II infusion–induced HTN in the rat and mouse.2–7 We previously reported that oxidative stress plays a role in the genesis of malignant HTN in the I3C-induced Cyp1a1-Ren2 TGR since tempol blunts the increased BP.8 We should point out, however, that in heterozygous TGR(mRen2)27 rats, where HTN develops spontaneously and early in development, the severe HTN seen in the young and adult rats is not tempol responsive,29 although end-organ injury and inflammation were protected.29,30
Despite playing an important role in the increased total peripheral vascular resistance and BP of I3C-induced Cyp1a1-Ren2 TGRs, increased superoxide anion activity does not seem to contribute to renal vasoconstriction. As we reported earlier, nonselective NOS inhibition during tempol administration to I3C-induced Cyp1a1-Ren2 TGRs elicits decreases in GFR and RPF, suggesting that the intrarenal NO system is independent of superoxide.8 The data in the present study are consistent with this view since we observed no loss of renal cortical SODs or TAC, nor any increase in the NADPH-oxidase subunit, p22phox, in these malignant hypertensives. Together, preservation of the renal cortex NOS and antioxidant systems in early malignant HTN in I3C-induced Cyp1a1-Ren2 TGRs supports a role for intrarenal NO in preventing excessive decreases in renal hemodynamic function in ANG II–dependent malignant HTN.
In contrast to the stability of the renal cortical NOS and antioxidant systems in this model of malignant HTN, we observed significant reductions in renal medullary and aortic NOS protein abundance, a fall in medullary EC SOD, and an increased p22phox abundance in the aorta. The protein profiles observed in the renal medulla and aorta suggest a decrease in NO bioavailability, which puts these tissues at greater risk of oxidative stress damage. NO in the kidney medulla plays a key role in the regulation of blood flow and, most importantly, sodium excretion by inhibiting sodium uptake in the thick ascending limb of the loop of Henle and collecting duct of the nephron.15 A lack of NO bioavailability in the medulla can lead to long-term alterations in fluid and electrolyte homeostasis, resulting in net sodium retention and increased BP.16,18,31,32 The regulation of NO is complex and can involve posttranslational modifications of the NOS and antioxidant enzymes. The lack of aortic and renal medullary tissue availability prevented measurements of NOx content and the TAC in these tissues. However, data regarding the renal cortex reflect enhanced or maintained NO bioavailability, despite elevated systemic oxidative stress, ANG II, and intrarenal ANG II.8,24
Earlier work by Kantachuvesiri et al. showed that the kidney was protected from structural damage at 7 days after I3C-induced malignant HTN in Cyp1a1-Ren2 TGRs.1 In contrast, the heart and mesenteric arteries displayed fibrinoid necrosis and endarteritis. At day 14, the kidneys displayed small amounts of damage (such as endarteritis) with no fibrinoid necrosis, while both heart and mesenteric arteries displayed marked injury with fibrinoid necrosis, endarteritis, microinfarcts, inflammatory infiltration, and fibroblast proliferation.1 Together with the maintained kidney function, the protection from end-organ damage may be derived from the preserved NO bioavailability.
Maintenance of NO production and protein abundance in the kidney cortex of rats with malignant HTN in our study are consistent with observations by Zuckerman et al. who examined regional NO production in Wistar Kyoto rats (WKY) and stroke-prone spontaneously hypertensive rats (SHRSP).33 They observed no difference in NO production and NOS protein abundance in the cortex between WKY and SHRSP on a regular diet despite a higher mean arterial pressure (MAP) in the SHRSP.33 In the SHRSP given a high salt/stroke-prone diet, there was no further increase in MAP vs. those on a regular diet after 16 weeks.33 However, they showed a marked increase in NO production in all regions of the kidney. Results from this study along with our study results suggest that renal NO deficiency is not associated with elevated BP in these two models of HTN. Rather, renal NO production is elevated and may protect both renal function (i.e., GFR in our studies) and renal structure (i.e., malignant nephrosclerosis in Zuckerman et al.’s studies).
The major and novel conclusions that are derived from this study are that preservation of antioxidant- and NO-generating capacity in the renal cortex of Cyp1a1-Ren2 rats with ANG II–dependent malignant HTN are consistent with an important role of renal NO in preventing the elevated ANG II levels from eliciting excessive reductions in renal hemodynamic function in Cyp1a1-Ren2 TGRs with ANG II–dependent malignant HTN.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dale M. Seth and Porcha D. Davis for excellent technical assistance. This work was funded by the Center of Biomedical Research Excellence (COBRE) in Hypertension and Renal Biology National Center for Research Resources (NCRR) 2P20RR017659, National Heart, Lung and Blood Institute (NHLBI) grant HL26371 (K.D.M., C.J.M.), and RO1 DK 56843 (C.B.). Funding for M.W.C is provided by the National Institutes of Health T32DK751825, for C.A.W. by the National Institutes of Health T32 HL083810, and for J.M.S.by the American Heart Association 11SDG6910000.
REFERENCES
- 1. Kantachuvesiri S, Fleming S, Peters J, Peters B, Brooker G, Lammie AG, McGath I, Kotelevtsev Y, Mullins JJ. Controlled hypertension, a transgenic toggle switch reveals differential mechanism underlying vascular disease. J Biol Chem 2001; 276:36727–36733 [DOI] [PubMed] [Google Scholar]
- 2. Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 2000; 86:494–501 [DOI] [PubMed] [Google Scholar]
- 3. Kopkan L, Castillo A, Navar LG, Majid DS. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. AM J Physiol Renal Physiol 2006; 290:F80–F86 [DOI] [PubMed] [Google Scholar]
- 4. Nishiyama A, Fukui T, Fujisawa Y, Rahman M, Tian RX, Kimura S, Abe Y. Systemic and regional hemodynamic responses to tempol in angiotensin II-infused hypertensive rats. Hypertension 2001; 37:77–83 [DOI] [PubMed] [Google Scholar]
- 5. Samuni A, Godinger D, Aronovitch J, Russo A, Mitchell JB. Nitroxide blocks DNA scission and protect cells from oxidative damage. Biochemistry 1991; 30:555–561 [DOI] [PubMed] [Google Scholar]
- 6. Schnackenberg CG, Wilcox CS. The SOD mimetic tempol restores vasodilation in afferent arterioles of experimental diabetes. Kidney Int 2001; 59:1859–1864 [DOI] [PubMed] [Google Scholar]
- 7. Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 1998; 32:59–64 [DOI] [PubMed] [Google Scholar]
- 8. Patterson ME, Mouton CR, Mullins JJ, Mitchell KD. Interactive effects of superoxide anion and nitric oxide on blood pressure and renal hemodynamics in transgenic rats with inducible malignant hypertension. Am J Physiol Renal Physiol 2005; 289:F754–F759 [DOI] [PubMed] [Google Scholar]
- 9. Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 1986; 320:454–456 [DOI] [PubMed] [Google Scholar]
- 10. Schnackenberg CG. Oxygen radicals in cardiovascular-renal disease. Curr Opin Pharmacol 2002; 2:121–125 [DOI] [PubMed] [Google Scholar]
- 11. Wilcox CS, Welch WJ. Interaction between nitric oxide and oxygen radicals in regulation of tubuloglomerular feedback. Acta Physiol Scand 2000; 168:119–124 [DOI] [PubMed] [Google Scholar]
- 12. Baylis C. Nitric oxide deficiency in chronic kidney disease. Gottschalk Lecture 2007. Am J Physiol Renal 2008; 294:F1–F9 [DOI] [PubMed] [Google Scholar]
- 13. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 2001; 357:593–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szabo AJ, Wagner L, Erdely A, Lau K, Baylis C. Renal neuronal nitric oxide synthase protein expression as a marker of renal injury. Kidney Int 2003; 64:1765–1771 [DOI] [PubMed] [Google Scholar]
- 15. Mattson DL, Wu F. Nitric oxide synthase activity and isoforms in rat renal vasculature. Hypertension 2000; 35:337–341 [DOI] [PubMed] [Google Scholar]
- 16. Ortiz PA, Garvin JL. Trafficking and activation of eNOS in epithelial cells. Acta Physiol Scand 2003; 179:107–114 [DOI] [PubMed] [Google Scholar]
- 17. Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce dieresis and natriuresis via NOS1. Am J Physiol Renal Physiol 2008; 294:F1205–F1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pollock JS, Pollock DM. Endothelin and NOS1/nitric oxide signaling and regulation of sodium homeostasis. Curr Opin Nephrol Hypertens 2008; 17:70–75 [DOI] [PubMed] [Google Scholar]
- 19. Wu F, Park F, Cowley AW, Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segment of rat kidney. Am J Physiol Renal Physiol 1999; 276:F874–F881 [DOI] [PubMed] [Google Scholar]
- 20. Smith C, Merchant M, Fekete A, Nyugen HL, Oh P, Tain YL, Klein JB, Baylis C. Splice variants of neuronal nitric oxide synthase are present in rat kidney. Nephrol Dial Transplant 2009; 24:1422–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mitchell KD, Bagatell SJ, Miller CS, Mouton CR, Seth DM, Mullins JJ. Genetic clamping of rennin gene expression induces hypertension and elevation of intrarenal Ang II levels of graded severity in Cyp1a1-Ren2 transgenic rats. J Renin Angiotensin Aldosterone Syst 2006; 7:74–86 [DOI] [PubMed] [Google Scholar]
- 22. Smith C, Merchant M, Fekete A, Nyugen HL, Oh P, Tain YL, Klein JB, Baylis C. Splice variants of neuronal nitric oxide synthase are present in rat kidney. Nephrol Dial Transplant 2009; 24:1422–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suto T, Losonczy G, Qiu C, Hill C, Samsell L, Ruby J, Charon N, Venuto R, Baylis C. Acute changes in urinary excretion of nitrite + nitrate do not necessarily predict renal vascular NO production. Kidney Int 1995; 48:1272–1277 [DOI] [PubMed] [Google Scholar]
- 24. Vanourkova Z, Kramer HJ, Huskova Z, Vaneckova I, Opocensky M, Chabova VC, Tesar V, Skaroupkova P, Thumovia M, Dohnalova M, Mullins JJ, Cervenka L. AT1 receptor blockade is superior to conventional triple therapy in protecting against end-organ damage in Cyp1a1-Ren-2 transgenic rats with inducible hypertension. J Hypertens 2006; 24:2465–2472 [DOI] [PubMed] [Google Scholar]
- 25. Ortiz RM, Graciano ML, Mullins JJ, Mitchell KD. Aldosterone receptor antagonism alleviates proteinuria, but not malignant HTN, IN cyp1a1-Rens transgenic rats. Am J Physiol Renal Physiol 2007; 293:F1584–F1591 [DOI] [PubMed] [Google Scholar]
- 26. Hernandez Schulman I, Zhou MS, Raij L. Cross-talk between angiotensin II receptor types 1 and 2: potential role in vascular remodeling in humans. Hypertension 2007; 49:270–271 [DOI] [PubMed] [Google Scholar]
- 27. Savoia C, Touyz RM, Volpe M, Schiffrin EL. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension 2007; 49:341–346 [DOI] [PubMed] [Google Scholar]
- 28. Patterson ME, Mullins JJ, Mitchell KD. Renoprotective effects of neuronal NOS-derived nitric oxide and cyclooxygenase-2 metabolites in transgenic rats with inducible malignant hypertension. Am J Physiol Renal Physiol 2008; 294:F205–F211 [DOI] [PubMed] [Google Scholar]
- 29. Kopkan L, Huskova Z, Vanourkova Z, Thumova M, Skaroupkove P, Maly J, Kramer HJ, Dvorak P, Cervenka L. Reduction of oxidative stress does not attenuate the development of angiotensin II-dependent hypertension in Ren-2 transgenic rats. Vascul Pharmacol 2009; 51:175–181 [DOI] [PubMed] [Google Scholar]
- 30. Wei Y, Clark SE, Morris EM, Thyfault JP, Uptergrove GM, Whaley-Connell AT, Ferrario CM, Sowers JR, Ibdah JA. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG(mRen2)27(Ren2) rats. J Hepatol 2008; 49:417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mattson DL. Importance of renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 2003; 284:R13–R27 [DOI] [PubMed] [Google Scholar]
- 32. Pallone TL, Mattson DL. Role of nitric oxide in regulation of the renal medulla in normal and hypertensive kidneys. Curr Opin Nephrol Hypertens 2002; 11:93–98 [DOI] [PubMed] [Google Scholar]
- 33. Zuckerman A, Chander PN, Zeballos GA, Stier CT., Jr Regional renal nitric oxide release in stroke-prone spontaneously hypertensive rats. Hypertension 1997; 30:1479–1486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.