Abstract
BACKGROUND
Elevated natriuretic peptide levels in asymptomatic individuals without heart failure are associated with increased risk of adverse cardiovascular outcomes and may reflect subclinical cardiac dysfunction.
METHODS
In a sample of 313 asymptomatic individuals (51% women, mean age 61 years) with hypertension and diastolic dysfunction, we examined the association of plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) with both conventional and advanced echocardiographic measures of systolic and diastolic function, including myocardial strain, using speckle-tracking–based analyses.
RESULTS
In univariate analyses, higher NT-proBNP was associated with greater left ventricular mass index (P = 0.003), left atrial volume index (P = 0.007), lateral E′ velocity (P < 0.0001), E/E′ ratio (P < 0.0001), peak global longitudinal systolic strain (P = 0.015), systolic strain rate (P = 0.021), and early diastolic strain rate (P < 0.0001). In multivariable analyses, NT-proBNP remained associated with measures of diastolic dysfunction, including lateral E′ velocity (P = 0.013) and the E/E′ ratio (P = 0.008). However, early diastolic strain rate was the echocardiographic parameter most strongly associated with NT-proBNP (P = 0.003).
CONCLUSIONS
In the setting of asymptomatic hypertensive heart disease and preserved ejection fraction, elevation in natriuretic peptide levels is predominantly associated with subclinical diastolic dysfunction.
Keywords: blood pressure, diastolic function, hypertension, imaging, natriuretic peptides, speckle-tracking echocardiography, strain.
Elevation in circulating natriuretic peptides is known to portend adverse outcomes in the setting of heart failure.1,2 Community-based studies have demonstrated that incremental elevations in B-type natriuretic peptide (BNP), even below current thresholds used to diagnose heart failure, are also associated with risk for incident heart failure in asymptomatic individuals.3,4 The physiology underlying this relationship between natriuretic peptides and the eventual development of heart failure in the general population remains unclear. However, it may be related to the cardioprotective effects of natriuretic peptides, such as vasodilation, natriuresis and diuresis, and inhibition of the renin– angiotensin–aldosterone axis. In addition, it may be related to antihypertrophic and antifibrotic properties mediated by activity at the guanylyl cyclase type A receptor and signaling pathways such as calcineurin/nuclear factor of activated T cells (NFAT), the sodium-hydrogen exchanger 1 (NHE-1), and transforming growth factor beta (TGF-β) pathways.5
Elevated BNP in apparently healthy individuals may represent an aggregate marker of subclinical abnormalities in cardiac structure and function that can develop during the progression from risk factors to heart failure. Natriuretic peptides have been shown to be associated with left ventricular (LV) hypertrophy and cardiac functional abnormalities in individuals with chronic kidney disease without overt heart failure,6 as well as LV systolic and diastolic dysfunction in coronary artery disease.7 Furthermore, prior studies have shown that BNP is increased in the setting of asymptomatic LV systolic dysfunction, defined as the presence of a reduced LV ejection fraction.8,9 However, the overall prevalence of reduced ejection fraction in the community is low, and the association between BNP and cardiac abnormalities in the setting of preserved ejection fraction is less clear.10,11
Conventional approaches to assessing cardiac dysfunction have limited sensitivity. However, the use of advanced methods for quantifying myocardial deformation may help to elucidate the relationship between BNP and LV function.12 Therefore, we examined the association between N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels and LV function using a combination of conventional and advanced echocardiographic measures of myocardial performance in a sample of asymptomatic hypertensive individuals with preserved ejection fraction (EF).
METHODS
Study sample
The Valsartan in Diastolic Dysfunction (VALIDD) study was a double-blind randomized trial designed to determine whether blood pressure lowering with the angiotensin receptor blocker (ARB) valsartan plus standard antihypertensive therapy for 38 weeks vs. placebo plus standard therapy improves diastolic function in patients with hypertension and diastolic dysfunction. The primary endpoint was change in early diastolic relaxation velocity of the mitral annulus (E′), a tissue Doppler parameter, between baseline and 38 weeks.13,14 The trial was conducted in 41 centers across the United States and Canada. Men and women aged ≥45 years with a history of stage 1 or 2 essential hypertension (mean systolic blood pressure [SBP] ≥140mm Hg or mean diastolic blood pressure [DBP] ≥90mm Hg) were screened by echocardiography for inclusion in the study if they had no history of hospital admission for heart failure in the past year and had not taken any renin–angiotensin–aldosterone inhibitors in the previous 3 months. Echocardiographic screening in eligible patients included tissue Doppler measurement of lateral E′ with enrollment determined by age-specific E′ thresholds: <10cm/s for individuals aged 45–54 years, <9cm/s for individuals aged 55–65 years, and <8cm/s for individuals aged >65 years. Patients were excluded if they had an LV ejection fraction (LVEF) ≤ 50%; contraindication to angiotensin-converting enzyme inhibitors or ARBs; stage 3 hypertension (SBP ≥180mm Hg or DBP ≥110mm Hg); history of stroke, transient ischemic attack, or myocardial infarction within 6 months of the first visit; a serum creatinine > 221 μmol/l; or uncontrolled diabetes mellitus, defined as a glycosylated hemoglobin >8.5%. Eligible patients were randomized to valsartan (160mg/day titrated up to 320mg/day) or matched placebo.
Of the 384 eligible patients who were randomized in the VALIDD trial, 330 had baseline NT-proBNP levels assayed prior to receiving valsartan or placebo. Of these individuals, 313 had echocardiographic images of adequate quality for speckle-tracking–based strain analyses. These individuals comprised the current study sample. Written informed consent was obtained for all patients before any study procedures were performed.
Clinical and echocardiographic assessment
Baseline blood samples were collected for measurement of NT-proBNP and sent to a central laboratory for analysis (Clinical Reference Laboratory, Inc., Lenexa, KS). Analysis was performed using the Elecsys proBNP sandwich immunoassay (Roche Diagnostics, Indianapolis, IN) with a measurable range of 5–35,000 pg/ml. Intraassay and interassay coefficients of variation were 2.7% and 3.2%, respectively, at 175 pg/ml; 2.4% and 2.9% at 355 pg/ml; 1.9% and 2.6% at 1,068 pg/ml; and 1.8% and 2.3% at 4,962 pg/ml.15 NT-proBNP reference ranges were reported as <125 pg/ml for individuals aged <75 years and <450 pg/mL for individuals aged ≥75 years.15 Blood pressure and heart rate measurements were performed using automatic cuffs (Omron model HEM-705, Omron, Inc., Song Jiang Lu, China). Clinical histories, including prior diagnoses of atrial fibrillation, coronary artery disease, diabetes mellitus, hyperlipidemia, myocardial infarction, peripheral vascular disease, and/or stroke, were obtained at screening and baseline visits.
All patients had standard echocardiograms at baseline. Echocardiograms were transferred digitally or by videotape to a core laboratory for analysis. LV volumes were derived using the modified biplane Simpsons rule. LVEF was calculated from LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV). LV wall thicknesses, dimensions, mass, and left atrial (LA) volume were also obtained according to guideline recommendations.16 LV mass and LA volume were additionally indexed to body surface area. Transmitral Doppler was used to obtain early diastolic (E) and late diastolic (A) flow velocities. Spectral pulsed-wave tissue Doppler imaging was used to record E′ and late diastolic relaxation velocity (A′) at both the septal and lateral mitral annuli from the apical four-chamber view. These measures were used to calculate E/E′, mitral deceleration time (DT), and isovolumetric relaxation time (IVRT).
Following review of suitability for strain-based measures, digital echocardiographic images were analyzed using an off-line speckle-tracking software (Velocity Vector Imaging [VVI], Siemens, Inc., Mountain View, CA) that has been previously validated with sonomicrometry and tagged magnetic resonance imaging in animals.17,18 Manual tracings were performed along the LV endocardial border from the septal mitral annulus to the lateral mitral annulus in apical four-chamber views and from the posterior mitral annulus to the anterior mitral annulus in apical two-chamber views at end-diastole. Images lacking distinct endocardial borders over ≥3 consecutive cardiac cycles were deemed poor quality and excluded from analyses. Software-generated regions of interest along the traced endocardium tracked frame-to-frame pixel movement to yield segmental quantitative strain (%) and strain rate (SR, s-1) data in systole and diastole. Global strain and SR measures were calculated by averaging apical four- and two-chamber segmental data.
Statistical analyses
Continuous variables were reported as mean ± SD, and categorical variables were reported as percentages unless otherwise noted. Statistically significant differences in baseline clinical and echocardiographic characteristics were reported across tertiles of NT-proBNP levels using an extension of the Wilcoxon rank sum test for trend for continuous variables19 and χ2 statistics for categorical variables. NT-proBNP was log-transformed (log NT-proBNP) due to its right-skewed distribution. Pearson coefficient of correlation was used to assess associations between echocardiographic parameters of diastolic function and log NT-proBNP. Clinical, echocardiographic, and strain measures were related to log NT-proBNP levels using univariate linear regression. Stepwise multivariable linear regression was used to examine the relation of clinical and echocardiographic parameters with log NT-proBNP. All P values were two-sided with P < 0.05 used to determine statistical significance. All statistical analyses were performed using STATA, version 11.2 (StataCorp., College Station, TX).
RESULTS
Our study sample included patients with a mean age of 61.0±9.2 years, and 51.4% were women. Patients with higher compared to lower NT-proBNP levels were older (P < 0.0001), tended to be female (P = 0.016), had histories significant for coronary artery disease (P = 0.005), had higher pulse pressures (P < 0.0001), and had lower estimated glomerular filtration rates (eGFR, P = 0.009; Table 1). There was no statistically significant trend in frequency of atrial fibrillation, diabetes mellitus, hyperlipidemia, myocardial infarction, or stroke across NT-proBNP tertiles.
Table 1.
Clinical Characteristics
| Characteristic | NT-proBNP Tertile 1 (N = 105) | NT-proBNP Tertile 2 (N = 104) | NT-proBNP Tertile 3 (N = 104) | P-value |
|---|---|---|---|---|
| NT-proBNP levels, pg/mL | ||||
| Median | 28.2 | 67.8 | 174.9 | NA |
| Interquartile range | 17.7-38.1 | 55.7-82.6 | 121.3-269.0 | NA |
| Range | 5.9-46.1 | 46.8-95.8 | 96.6-1252 | NA |
| Age, years | 57.5±8.1 | 59.5±7.8 | 65.9±9.6 | <0.0001 |
| Sex (male), % | 60.0 | 42.3 | 43.2 | 0.016 |
| Race, % | ||||
| White | 77.1 | 78.9 | 83.7 | 0.48 |
| Non-white | 22.9 | 21.1 | 16.3 | |
| Height, m | 1.7±0.1 | 1.7±0.1 | 1.7±0.1 | 0.07 |
| BMI, kg/m² | 30.9±6.1 | 30.0±5.4 | 29.8±5.7 | 0.13 |
| Systolic blood pressure, mmHg | 145±16 | 148±16 | 150±17 | 0.09 |
| Diastolic blood pressure, mmHg | 86±12 | 86±11 | 82±12 | 0.002 |
| Pulse pressure, mmHg | 59±13 | 61±14 | 69±16 | <0.0001 |
| eGFR, mL/min | 94.0±28.8 | 83.8±31.1 | 84.3±27.1 | 0.009 |
| History, % | ||||
| Atrial fibrillation | 1.0 | 1.0 | 1.9 | 0.77 |
| Coronary artery disease | 3.8 | 9.6 | 17.3 | 0.005 |
| Diabetes mellitus | 8.6 | 11.5 | 15.4 | 0.31 |
| Hyperlipidemia | 54.3 | 50.0 | 51.0 | 0.81 |
| Myocardial infarction | 1.0 | 2.9 | 4.8 | 0.25 |
| Peripheral vascular disease | 0.0 | 2.9 | 2.9 | 0.21 |
| Stroke | 1.0 | 0.0 | 1.0 | 0.61 |
| Medications at baseline, % | ||||
| Alpha-blockers | 2.9 | 2.9 | 4.9 | 0.68 |
| Beta-blockers | 38.5 | 50.5 | 47.6 | 0.19 |
| Calcium channel blockers | 57.7 | 54.4 | 57.3 | 0.87 |
| Diuretics | 76.0 | 63.1 | 64.1 | 0.09 |
| Statins | 34.6 | 29.1 | 34.0 | 0.66 |
Abbreviation: BMI, body mass index.
Values are shown as means ± standard deviations or percentages unless otherwise indicated.
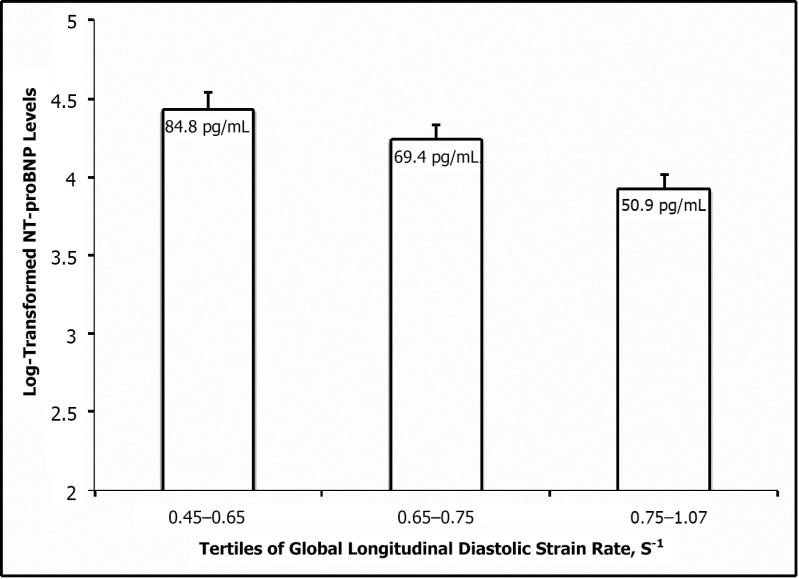
Mean LVEF was in the normal range, defined as 55% or greater,16 and did not differ across tertiles of NT-proBNP (P = 0.75; Table 2). LV mass index (LVMI) and LA volume index (LAVI) were slightly increased in the third tertile relative to the first and second tertiles (P = 0.034 and 0.022, respectively). Mean lateral E′ decreased with higher NT-proBNP levels (P < 0.0001) and E/E′ ratio increased across tertiles (P = 0.001). Mean global longitudinal systolic SR and early longitudinal diastolic SR both decreased across tertiles of NT-proBNP (P = 0.022 and 0.002, respectively). We observed lower mean NT-proBNP levels in individuals with higher diastolic strain rates (Figure 1). Pearson correlation coefficients with log NT-proBNP were comparable for diastolic strain rate (−0.220, P = 0.0001), lateral E′ velocity (−0.219, P = 0.0001), and E/E′ ratio (−0.239, P < 0.0001).
Table 2.
Echocardiographic Characteristics
| Parameter | NT-proBNP Tertile 1 (N = 105) | NT-proBNP Tertile 2 (N = 104) | NT-proBNP Tertile 3 (N = 104) | P-value |
|---|---|---|---|---|
| LV wall thickness, cm | 0.89±0.08 | 0.86±0.10 | 0.90±0.10 | 0.57 |
| LVEDD, cm | 4.59±0.12 | 4.56±0.13 | 4.57±0.13 | 0.26 |
| Relative wall thickness | 0.39±0.04 | 0.38±0.04 | 0.40±0.05 | 0.55 |
| LV mass, g | 138.0±18.0 | 131.7±23.6 | 140.2±21.6 | 0.75 |
| LV mass index, g/m2 | 69.4±9.4 | 68.4±10.2 | 73.2±12.4 | 0.034 |
| LA volume index, mL/m2 | 31.1±4.5 | 30.4±4.1 | 32.5±5.0 | 0.022 |
| LV end-diastolic volume, mL | 91.3±13.4 | 87.3±16.5 | 88.7±13.2 | 0.14 |
| LV end-systolic volume, mL | 39.4±7.6 | 38.0±8.8 | 38.0±7.2 | 0.20 |
| LVEF, % | 57.0±2.9 | 56.7±3.3 | 57.2±3.2 | 0.75 |
| Lateral E’ velocity, cm/s | 7.74±1.18 | 7.77±1.08 | 7.08±1.27 | <0.0001 |
| E/E’ ratio | 9.29±2.37 | 9.93±2.76 | 10.67±2.86 | 0.001 |
| IVRT, msec | 104±18 | 101±18 | 103±17 | 0.73 |
| Deceleration time, msec | 220±44 | 211±45 | 225±51 | 0.77 |
| Myocardial performance index | 0.49±0.12 | 0.46±0.11 | 0.47±0.10 | 0.58 |
| GLSS, % | -14.6±1.9 | -14.2±2.1 | -14.0±2.2 | 0.07 |
| GLSSR, s-1 | -0.76±0.11 | -0.74±0.09 | -0.72±0.11 | 0.022 |
| GLDSR, s-1 | 0.73±0.12 | 0.71±0.11 | 0.68±0.12 | 0.002 |
Abbreviations: LVEDD, LV end-diastolic diameter; Deceleration time, E wave deceleration time; GLSS, peak global longitudinal systolic strain; GLSSR, peak global longitudinal systolic strain rate; GLDSR, peak global early diastolic strain rate.
Values are shown as means ± standard deviations.
Figure 1.
Mean N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels across tertiles of peak global longitudinal early diastolic strain rate. Mean (± standard error of the mean) of NT-proBNP levels are shown across tertiles of peak global longitudinal early diastolic strain rate (P trend = 0.001). NT-proBNP levels tend to be higher as diastolic strain rates decrease.
In unadjusted regression models, age (P < 0.0001), female sex (P = 0.016), DBP (P < 0.0001), history of coronary artery disease (P < 0.0001), eGFR (P = 0.013), and age-specific entry criteria (P < 0.0001) showed relations with log-transformed NT-proBNP (Table 3). Among the echocardiographic parameters, LVMI (P = 0.003), LAVI (P = 0.007), lateral E′ (P < 0.0001), E/E′ ratio (P < 0.0001), longitudinal strain (P = 0.015), systolic strain rate (P = 0.021), and diastolic strain rate (P < 0.0001) were significantly associated with log NT-proBNP.
Table 3.
Relations of NT-proBNP with Clinical and Echocardiographic Parameters
| Variable | Unadjusted | Multivariable-Adjusted* | |||
|---|---|---|---|---|---|
| Coefficient (SE) | P value | Coefficient (SE) | P value | Partial R2 | |
| Age, years | 0.043 (0.006) | <0.0001 | 0.042 (0.007) | <0.0001 | 0.114 |
| GLDSR, s-1 | -1.900 (0.479) | <0.0001 | -1.488 (0.500) | 0.003 | 0.034 |
| E/E’ ratio | 0.087 (0.021) | <0.0001 | 0.067 (0.025) | 0.008 | 0.030 |
| History of coronary artery disease | 0.725 (0.182) | <0.0001 | 0.522 (0.179) | 0.004 | 0.028 |
| Lateral E’ velocity, cm/s | -0.180 (0.045) | <0.0001 | 0.175 (0.070) | 0.013 | 0.027 |
| Female sex | 0.270 (0.112) | 0.016 | — | — | — |
| Non-white race | -0.275 (0.140) | 0.051 | — | — | — |
| BMI, kg/m2 | -0.019 (0.010) | 0.052 | — | — | — |
| Systolic blood pressure, mmHg | 0.006 (0.003) | 0.07 | — | — | — |
| Diastolic blood pressure, mmHg | -0.016 (0.005) | 0.001 | — | — | — |
| eGFR, mL/min | -0.005 (0.002) | 0.013 | — | — | — |
| LV mass index, g/m2 | 0.015 (0.005) | 0.003 | — | — | — |
| LA volume index, mL/m2 | 0.033 (0.012) | 0.007 | — | — | — |
| LV end-diastolic volume, mL | -0.005 (0.004) | 0.16 | — | — | — |
| LV ejection fraction, % | 0.016 (0.018) | 0.39 | — | — | — |
| IVRT, msec | 0.801 (3.309) | 0.81 | — | — | — |
| Deceleration time, msec | 0.586 (1.224) | 0.63 | — | — | — |
| GLSS, % | 0.066 (0.027) | 0.015 | — | — | — |
| GLSSR, s-1 | 1.292 (0.555) | 0.021 | — | — | — |
*Stepwise multivariable regression model included all covariates shown, in addition to age-specific entry criteria (P = 0.29 in the multivariable model).
In stepwise multivariable analyses, age (P < 0.0001), history of coronary artery disease (P = 0.005), lateral E′ velocity (P = 0.013), E/E′ ratio (P = 0.008), and diastolic strain rate (P = 0.003) remained significantly associated with increased NT-proBNP. Notably, increased diastolic strain rate was the most prominent echocardiographic measure associated with higher log NT-proBNP levels. SBP and DBP were not significantly associated with NT-proBNP in multivariable analyses, adjusting for these echocardiographic traits. In secondary analyses, we observed no effect modification by age or sex on the association between diastolic strain rate and log NT-proBNP (P > 0.25).
DISCUSSION
In a sample of asymptomatic individuals with hypertension and diastolic dysfunction, we observed significant associations of NT-proBNP with age, coronary artery disease, and conventional echocardiographic measures of diastolic dysfunction. Systolic function as measured by myocardial strain parameters was also reduced in relation to NT-proBNP levels. However, diastolic dysfunction was more prominently associated with NT-proBNP than systolic dysfunction. Furthermore, we observed that among echocardiographic measures studied, reduction in early longitudinal diastolic strain rate demonstrated the strongest association with increased NT-proBNP.
Population-based investigations have reported age-related increases in BNP levels in individuals without cardiovascular, renal or pulmonary disease or diabetes as well as normal LV systolic and diastolic function.20,21 Several explanations have been offered for this association, including a higher prevalence of subclinical cardiac dysfunction in the elderly,22 decreased clearance of natriuretic peptides in elderly patients without underlying renal dysfunction,23 and less effective nonrenal clearance mechanisms such as platelet-associated clearance receptors.24 Additionally, animal studies suggest an age-related increase in expression of genes coding for natriuretic peptides.25
Previous studies of asymptomatic individuals have consistently reported increased natriuretic peptide levels in relation to abnormalities in LV structure, including alterations in LV dimensions, volumes, and mass. However, studies of the association between natriuretic peptide levels and measures of LV function, assessed using conventional echocardiographic methods, have been mixed.9,26,27 Consistent with the results from prior studies, we observed an unadjusted association between NT-proBNP and increased LV mass. However, this association became nonsignificant in multivariable models. As mean values of LV mass and wall thickness in our sample were within normal ranges, individuals included in the present analyses may represent patients at an earlier stage of cardiac dysfunction in the progression from hypertensive heart disease to heart failure.13,28,29 We observed associations of elevated NT-proBNP with both systolic and diastolic dysfunction, particularly as reflected by sensitive strain-based measures of myocardial performance. While previous studies of the association of natriuretic peptides with myocardial strain have focused on patients with a history of or concomitant symptoms consistent with heart failure,10,12 our analysis extends these findings to individuals with subclinical disease, suggesting a possible role for natriuretic peptide levels in identifying the presence of subclinical cardiac dysfunction among hypertensive individuals without a history of heart failure.
While the cross-sectional design of the present study precludes an ability to draw conclusions about causality, our findings are consistent with those of previous studies regarding the role of LV diastolic wall stress on natriuretic peptide levels9 and suggest that impaired myocardial relaxation in early diastole may also contribute to peptide elevations in hypertensive individuals with preserved ejection fraction. Conventional echocardiographic measures of diastolic function, such as IVRT and deceleration time, are abnormally elevated across all tertiles30 as expected per VALIDD inclusion criteria. However, these measures were not associated with NT-proBNP in the present study. E′ velocity and E/E′ ratio were associated with NT-proBNP levels, but peak early diastolic strain rate displayed the strongest association with peptide levels. Thus, increases in natriuretic peptides may represent an aggregate marker of increased LV filling pressures and intrinsic myocardial dysfunction. Additionally, the stronger association between parameters of diastolic function and NT-proBNP levels suggests that subclinical diastolic dysfunction in individuals with hypertension may be among the major contributors to peptide elevations within the normal range. The association of systolic function, as measured by strain parameters, with natriuretic peptides may be more prominent with progressive dysfunction or perhaps may reflect the mechanical coupling of systole and diastole.
Several possible biological mechanisms may be responsible for the observed relation between subclinical myocardial dysfunction and elevated natriuretic peptide levels in the setting of hypertension. In particular, natriuretic peptides may be markers of the pathologic processes associated with hypertension, such as increased interstitial fibrosis and/or abnormalities in myocardial calcium homeostasis.31,32 Alternatively, BNP may be directly involved in underlying changes in cardiac mechanics. BNP stimulates its downstream effects through a cyclic guanosine monophosphate- dependent (cGMP) signaling cascade after binding natriuretic peptide receptor A (NPR-A).33 Altered cGMP levels from natriuretic peptide activity offers one viable mechanism, although data from current literature are conflicting. In vivo studies in experimentally hypertensive canines suggest titin-based cardiomyocyte stiffness decreases in the setting of increased cGMP activity following BNP supplementation after administration of a phosphodiesterase-5A inhibitor, thus improving LV diastolic distensibility.34 In vitro studies of rabbit ventricular cardiomyocytes suggest that increased cGMP levels reduce cardiomyocyte shortening as well as the rate of relaxation.35 The effect of BNP on ventricular contractility and distensibility may also be concentration dependent.36
Alternatively, BNP levels may mediate cardioprotective responses to hypertension, as shown by investigation of the association between common variants at the natriuretic peptide precursor A and natriuretic peptide precursor B with increased natriuretic peptides levels that also demonstrated lower SBP and DBP and a 15% reduction in odds of hypertension.37 Individuals with lower circulating concentrations of natriuretic peptides exhibited elevated blood pressures and risk for hypertension. Similarly, increased cardiac rat proBNP expression via single injection of a myocardium-tropic adeno-associated virus serotype 9 vector in spontaneously hypertensive rats improved blood pressure and conventional measures of cardiac structure and function, including LV wall thickness, LV mass, and ejection fraction.38 These results lend further support for a cardioprotective effect of natriuretic peptides in cardiac dysfunction and remodeling related to early hypertension. Elevated NT-proBNP levels in individuals with hypertensive heart disease may be indicative of a compensatory mechanism. However, persistent increases of NT-proBNP to thresholds diagnostic of heart failure may reflect desensitization of the NPR-A, possibly via a calcineurin-mediated pathway.39 In this setting, the cardioprotective effect of natriuretic peptides may not be a long-term phenomenon.
Our overall findings suggest that among individuals with hypertension, incremental elevations in natriuretic peptide levels may indicate the presence of subclinical cardiac dysfunction, particularly diastolic dysfunction that may manifest as a reduction in early diastolic strain rate. The extent to which elevated natriuretic peptides in this specific setting portends the eventual development of heart failure warrants further investigation. Additional studies are also needed to clarify the mechanisms by which natriuretic peptides may be associated with cardiac dysfunction, even at a very early stage in the progression from risk factors to heart failure.
Limitations
Several limitations of this study deserve consideration. Patients in VALIDD were generally young, only mildly hypertensive, and without LV hypertrophy or heart failure, which may limit the generalizability of our findings to other populations. Silent ischemia cannot be ruled out in this cohort given the statistically significant trend in coronary artery disease among the tertiles. The number of medications taken by study participants at time of entry into VALIDD and dosing was not reported. Right ventricular systolic pressure was not measured due to inadequate tricuspid regurgitant jet velocity profiles in a majority of individuals. Strain analyses were conducted on images converted and digitized from videotape-based cassette, resulting in lower frame rates (approximately 30 frames per second) and increased probability of peak strain and strain rate values falling between image frames. However, any resulting reduction in measurement precision would have biased our results to the null. Additionally, previous comparison of peak strain rate and time to peak strain rate between video and digital images to test the effect of frame rate suggest a good correlation between the formats and support use of videotape-based images when digital images are unavailable.40 Long-term clinical outcomes were not collected in VALIDD, precluding any analyses of the predictive value of NT-proBNP and/or myocardial function parameters in this sample.
Conclusions
In a sample of asymptomatic individuals with hypertensive heart disease, we observed that impairment in diastolic, more so than systolic, myocardial function was prominently associated with increased NT-proBNP. Thus, elevations of NT-proBNP levels, even within the normal range, may indicate subclinical cardiac dysfunction despite a preserved EF. Further research is needed to investigate the biological mechanisms underlying the relation of increased natriuretic peptides with abnormalities in diastolic function and its possible implications for prognostication and stratification with respect to risk for future HF.
FUNDING
The original clinical trial was funded by Novartis Pharmaceuticals.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
I.U. was a research fellow supported by the Sarnoff Cardiovascular Research Foundation. S.C. is supported by the Ellison Foundation and National Heart, Lung, and Blood Institute (grantK99Hl107642).
REFERENCES
- 1. Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AHB, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002; 347:161–167 [DOI] [PubMed] [Google Scholar]
- 2. Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, Pfisterer M, Perruchoud AP. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med 2004; 350:647–654 [DOI] [PubMed] [Google Scholar]
- 3. Wang T, Larson M, Levy D, Benjamin E, Leip E, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. New Engl J Med 2004; 350:655–663 [DOI] [PubMed] [Google Scholar]
- 4. Kurl S, Ala-Kopsala M, Ruskoaho H, Mäkikallio T, Nyyssönen K, Vuolteenaho O, Sivenius J, Salonen JT, Laukkanen JA. Plasma N-terminal fragments of natriuretic peptides predict the risk of stroke and atrial fibrillation in men. Heart 2009; 95:1067–1071 [DOI] [PubMed] [Google Scholar]
- 5. Calvieri C, Rubattu S, Volpe M. Molecular mechanisms underlying cardiac antihypertrophic and antifibrotic effects of natriuretic peptides. J Mol Med 2012; 90:5–13 [DOI] [PubMed] [Google Scholar]
- 6. Mishra RK, Li Y, Ricardo AC, Yang W, Keane M, Cuevas M, Christenson R, DeFilippi C, Chen J, He J, Kallem RR, Raj DS, Schelling JR, Wright J, Go AS, Shlipak MG. Association of N-Terminal pro-B-type natriuretic peptide with left ventricular structure and function in chronic kidney disease (from the Chronic Renal Insufficiency Cohort [CRIC]). Am J Cardiol 2013; 111:432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sonoda H, Ohte N, Goto T, Wakami K, Fukuta H, Kikuchi S, Tani T, Kimura G. Plasma N-terminal pro-brain natriuretic peptide levels identifying left ventricular diastolic dysfunction in patients with preserved ejection fraction. Circ J 2012; 76:2599–2605 [DOI] [PubMed] [Google Scholar]
- 8. McDonagh T, Robb S, Murdoch D, Morton J, Ford I, Morrison C, Tunstall-Pedoe H, McMurray JJV, Dargie HJ. Biochemical detection of left-ventricular systolic dysfunction. Lancet 1998; 351:9–13 [DOI] [PubMed] [Google Scholar]
- 9. Troughton RW, Richards AM. B-type natriuretic peptides and echocardiographic measures of cardiac structure and function. JACC: Cardiovascular Imaging 2009; 2:216–225 [DOI] [PubMed] [Google Scholar]
- 10. Mottram P, Leano R, Marwick T. Usefulness of B-type natriuretic peptide in hypertensive patients with exertional dyspnea and normal left ventricular ejection fraction and correlation with new echocardiographic indexes of systolic and diastolic function. Am J Cardiol 2003; 92:1434–1438 [DOI] [PubMed] [Google Scholar]
- 11. Knebel F, Eddicks S, Schimke I, Bierbaum M, Schattke S, Beling M, Raab V, Baumann G, Borges AC. Myocardial tissue Doppler echocardiography and N-terminal B-type natriuretic peptide (NT-proBNP) in diastolic and systolic heart failure. Cardiovasc Ultrasound 2008; 6:45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoneyama A, Koyama J, Tomita T, Kumazaki S, Tsutsui H, Watanabe N, Kinoshita O, Ikeda U. Relationship of plasma brain-type natriuretic peptide levels to left ventricular longitudinal function in patients with congestive heart failure assessed by strain Doppler imaging. Int J Cardiol 2008; 130:56–63 [DOI] [PubMed] [Google Scholar]
- 13. Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourcière Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JMO, Thomas JD, Zile MR, Aurigemma GP. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet 2007; 369:2079–2087 [DOI] [PubMed] [Google Scholar]
- 14. Janardhanan R, Daley W, Naqvi T, Mulvagh S, Aurigemma G, Zile M, Arnold JMO, Artis E, Purkayastha D, Thomas JD, Solomon SD. Rationale and design: The VALsartan In Diastolic Dysfunction (VALIDD) Trial: Evolving the management of diastolic dysfunction in hypertension. Am Heart J 2006; 152:246–252 [DOI] [PubMed] [Google Scholar]
- 15. Roche Diagnostics Elecsys proBNP package insert. 2003;1–4
- 16. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, St John Sutton M, Stewart WJ. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the Euro pean Society of Cardiology. J Am Soc Echocardiogr 2005; 18:1440–1463 [DOI] [PubMed] [Google Scholar]
- 17. Pirat B, Khoury DS, Hartley CJ, Tiller L, Rao L, Schulz DG, Nagueh SF, Zoghbi WA. A novel feature-tracking echocardiographic method for the quantitation of regional myocardial function: validation in an animal model of ischemia-reperfusion. J Am Coll Cardiol 2008; 51:651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Støylen A, Ihlen H, Lima JAC, Smiseth OA, Slørdahl SA. Noninvasive myocardial strain measurement by speckle tracking echocardiography. J Am Coll Cardiol 2006; 47:789–793 [DOI] [PubMed] [Google Scholar]
- 19. Cuzick J. A Wilcoxon-type test for trend. Stat Med 1985; 4:87–90 [DOI] [PubMed] [Google Scholar]
- 20. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002; 40:976–982 [DOI] [PubMed] [Google Scholar]
- 21. Wang T, Larson M, Levy D, Leip E, Benjamin E, Wilson P, Sutherland P, Omland T, Vasan RS. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002; 90:254–258 [DOI] [PubMed] [Google Scholar]
- 22. Sayama H, Nakamura Y, Saito N, Kinoshita M. Why is the concentration of plasma brain natriuretic peptide in elderly inpatients greater than normal? Coron Artery Dis 1999; 10:537–540 [DOI] [PubMed] [Google Scholar]
- 23. Clark BA, Elahi D, Shannon RP, Wei JY, Epstein FH. Influence of age and dose on the end-organ responses to atrial natriuretic peptide in humans. Am J Hypertens 1991; 4:500–507 [DOI] [PubMed] [Google Scholar]
- 24. Giannessi D, Andreassi MG, Del Ry S, Clerico A, Colombo MG, Dini N. Possibility of age regulation of the natriuretic peptide C-receptor in human platelets. J Endocrinol Invest 2001; 24:8–16 [DOI] [PubMed] [Google Scholar]
- 25. Raizada V, Thakore K, Luo W, McGuire PG. Cardiac chamber-specific alterations of ANP and BNP expression with advancing age and with systemic hypertension. Mol Cell Biochem 2001; 216:137–140 [DOI] [PubMed] [Google Scholar]
- 26. de Lemos JA, McGuire DK, Khera A, Das SR, Murphy SA, Omland T, Drazner MH. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: Results from the Dallas Heart Study. Am Heart J 2009; 157:746–753.e2 [DOI] [PubMed] [Google Scholar]
- 27. Vasan RS, Benjamin EJ, Larson MG, Leip EP, Wang TJ, Wilson PWF, Levy D. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. J Am Med Assoc 2002; 288:1252–1259 [DOI] [PubMed] [Google Scholar]
- 28. Verma A, Solomon SD. Diastolic dysfunction as a link between hypertension and heart failure. Med Clin North Am 2009; 93:647–664 [DOI] [PubMed] [Google Scholar]
- 29. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. J Am Med Assoc 1996; 275:1557–1562 [PubMed] [Google Scholar]
- 30. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009; 10 165–193 [DOI] [PubMed] [Google Scholar]
- 31. Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83:1849–1865 [DOI] [PubMed] [Google Scholar]
- 32. Solomon SD, Verma A, Desai A, Hassanein A, Izzo J, Oparil S, Lacourciere Y, Lee J, Seifu Y, Hilkert RJ, Rocha R, Pitt B. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension 2010; 55:241–248 [DOI] [PubMed] [Google Scholar]
- 33. Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 1998; 339:321–328 [DOI] [PubMed] [Google Scholar]
- 34. Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC, Linke WA, Redfield MM. Sildenafil and B-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation 2011; 124:2882–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Q, Moalem J, Tse J, Scholz PM, Weiss HR. Effects of natriuretic peptides on ventricular myocyte contraction and role of cyclic GMP signaling. Eur J Pharmacol 2005; 510:209–215 [DOI] [PubMed] [Google Scholar]
- 36. D’Souza SP, Davis M, Baxter GF. Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther 2004; 101:113–129 [DOI] [PubMed] [Google Scholar]
- 37. Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet 2009; 41:348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cataliotti A, Tonne JM, Bellavia D, Martin FL, Oehler EA, Harders GE, Campbell JM, Peng KW, Russell SJ, Malatino LS, Burnett JC, Ikeda Y. Long-term cardiac pro-B-type natriuretic peptide gene delivery prevents the development of hypertensive heart disease in spontaneously hypertensive rats. Circulation 2011; 123:1297–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fortin Y, De Léan A. Role of cyclic GMP and calcineurin in homologous and heterologous desensitization of natriuretic peptide receptor-A. Can J Physiol Pharmacol 2006; 84:539–546 [DOI] [PubMed] [Google Scholar]
- 40. Shin SH, Hung CL, Uno H, Hassanein AH, Verma A, Bourgoun M, Køber L, Ghali JK, Velazquez EJ, Califf RM, Pfeffer MA, Solomon SD. Mechanical dyssynchrony after myocardial infarction in patients with left ventricular dysfunction, heart failure, or both. Circulation 2010; 121:1096–1103 [DOI] [PubMed] [Google Scholar]