Abstract
The CCAAT/enhancer binding protein β (C/EBPβ) is implicated in the regulation of many different molecular and physiological processes. Mice with a germline deletion of C/EBPβ (C/EBPβ−/−) display phenotypes in a multitude of cell types and organ systems, including skin where C/EBPβ−/− mice exhibit increased apoptosis in epidermal keratinocytes in response to carcinogen treatment and are completely resistant to carcinogen-induced skin tumorigenesis. To determine the contribution of systemic versus cell autonomous functions of C/EBPβ to specific phenotypes, mice with a conditional ‘floxed’ C/EBPβ null allele were generated. Epidermal-specific deletion of C/EBPβ was achieved by Cre recombinase expression from a keratin 5 (K5) promoter. Similar to C/EBPβ−/− mice, K5-Cre;C/EBPβfl/fl mice were completely refractory to 7,12 dimethylbenz[a]anthracene (DMBA)-induced skin tumorigenesis and these mice displayed increased DMBA-induced apoptosis in epidermal keratinocytes compared to wild-type mice. In contrast, mice lacking the related gene, C/EBPδ, were not resistant to DMBA-induced skin tumorigenesis, indicating a unique role of C/EBPβ in skin tumor development. Our findings demonstrate that C/EBPβ exerts an essential, keratinocyte-intrinsic role in cell survival in response to carcinogen treatment and the elimination of C/EBPβ in keratinocytes is sufficient to confer complete resistance of the skin to chemical carcinogenesis.
Keywords: C/EBP, apoptosis, carcinogenesis
The CCAAT/enhancer binding proteins (C/EBPs) are members of the basic region – leucine zipper (bZIP) class of transcription factors and play important roles in fundamental cellular processes including proliferation, growth arrest, and differentiation in a cell-type specific manner. Mice deficient in any of the six members of the C/EBP family (C/EBPα, C/EBPβ, C/EBPγ, C/EBPδ, C/EBPε, and C/EBPζ) each exhibit unique phenotypes. Of these, the null mutation of C/EBPβ confers by far the most phenotypes by affecting diverse cell types and a multitude of functions in many different organs. For example, C/EBPβ is involved in innate and acquired immunity, and differentiation of mammary epithelial cells, adipocytes, ovarian granulosa cells, and epidermal keratinocytes (Ramji and Foka, 2002).
C/EBPβ is implicated in cell transformation/tumor formation in several cell types/tissues. For example, C/EBPβ can cooperate with Ras to transform NIH 3T3 cells in a phosphorylation-dependent manner (Zhu et al., 2002; Shuman et al., 2004). The C/EBPβ gene expresses three different isoforms by virtue of alternative translation initiation or regulated proteolysis (Ramji and Foka, 2002). Human C/EBPβ-2 (comparable to the major form expressed in mouse) expression is increased in breast cancer cell lines and primary breast tumors (Bundy and Sealy, 2003). Moreover, overexpression of C/EBPβ-2 in a human mammary epithelial cell line lead to anchorage independence and invasive properties (Bundy and Sealy, 2003). C/EBPβ is highly expressed in colorectal tumors (Rask et al., 2000) and is associated with ovarian tumor progression (Sundfeldt et al., 1999). Furthermore, C/EBPβ can interact with cyclin D1 and appears to be important for the unique pattern of altered gene expression in human cancers that overexpress cyclin D1 (Lamb et al., 2003).
C/EBPβ is abundantly expressed in keratinocytes (Oh and Smart, 1998) and we previously reported that C/EBPβ−/− mice are completely refractory to skin tumorigenesis induced by carcinogens, such as 7,12-dimethylbenz[a]anthracene (DMBA), that produce oncogenic Ras mutations in epidermal keratinocytes (Zhu et al., 2002). C/EBPβ−/− mice also display elevated levels of DMBA-induced apoptosis suggesting that C/EBPβ has a role in keratinocyte survival in response to oncogenic Ras signaling and/or DNA damage (Zhu et al., 2002; Johnson, 2005). These data demonstrate an important role for C/EBPβ in keratinocytes survival and skin tumorigenesis. However, phenotypes of germline knockouts can be complicated by pleiotropic effects of the protein under study in various tissues and organs (Lewandoski, 2001). For example, germline deletion of Rb results in severe defects in neurogenesis involving increased apoptosis. Subsequent studies have shown that the apoptosis in the CNS is due to a nonautonomous neuronal defect (de Bruin et al., 2003; MacPherson et al., 2003). As C/EBPβ is implicated in the regulation of many different molecular and physiological processes, it is possible that systemic factors are involved in the increased keratinocyte apoptosis as well as the subsequent lack of skin tumorigenesis in carcinogen treated C/EBPβ−/− mice. C/EBPβ null mice (C/EBPβ−/−) have major defects in their immune system (Screpanti et al., 1995; Tanaka et al., 1995) and C/EBPβ is known to be involved in the regulation of numerous cytokines (Poli, 1998) some of which are important in Ras-tumorigenesis (Sparmann and Bar-Sagi, 2005). It is a distinct possibility that the increase in epidermal keratinocyte apoptosis in DMBA-treated C/EBPβ−/− mice and subsequent lack of tumor development could be of a systemic nature, perhaps involving alterations in the expression of nonkeratinocyte-derived cytokines. In addition, C/EBPβ activity is implicated in a number of conditions that are known to affect skin tumorigensis through systemic mechanisms, such as metabolic status (Boutwell, 1983), cytokine and growth factor expression (Klatt and Serrano, 2003), and steroid hormone levels (Porter et al., 2002). Thus, it is critical to determine the contribution of systemic versus keratinocyte autonomous functions of C/EBPβ in skin tumorigenesis.
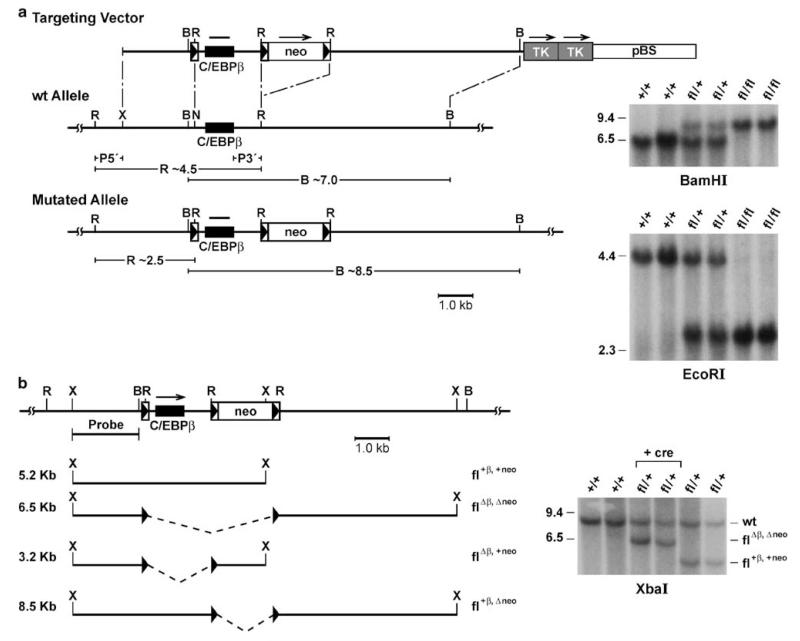
To this aim, we generated a conditional null allele of C/EBPβ by flanking the coding region with LoxP recombination sites (Figure 1a). In contrast to the null mutation (Sterneck et al., 1997) the conditional null allele was transmitted at Mendelian frequency, and C/EBPβfl/fl female mice were fertile. Furthermore, Northern analysis showed that the gene manipulations did not affect C/EBPβ mRNA levels in various organs (data not shown). To confirm recombination competence of the targeted locus in vivo, the mice were mated to β-actin-Cre transgenic mice (Ma et al., 2003) and the genomic DNA from offspring was analysed for the predicted recombination events (Figure 1b). Complete deletion of both the C/EBPβ coding region and the neo gene were observed in the presence of β-actin-Cre. Removal of only the C/EBPβ or the neo gene alone by virtue of the center LoxP site was not observed in these experiments (also confirmed by PCR analysis, not shown).
Figure 1.
Targeted conditional mutation of the C/EBPβ gene in mice. (a) Diagram of the replacement type targeting vector, wild-type allele and mutated allele; and Southern analysis of genomic DNA from mice with the indicated genotypes. Homologous recombination in embryonic stem cells at the 5′ side of the gene was screened by a probe external to the targeting vector (P5′) that detects the conversion of a 4.5 kb EcoRI fragment into 2.5 kb and, at the 3′ side, a probe (P3′) that detects the alteration of a 7.0 kb BamHI fragment into an 8.5 kb fragment. Correctly targeted ES cells were obtained at a frequency of 1/39 and used to generate heterozygous mutant mice as described (Tessarollo, 2001). Boxed arrowheads indicate LoxP recognition sites; TK, pGK-thymidine kinase cassettes, neo, neomycin resistance gene. The subjects used in this study were of mixed 129S1 and C57BL/6 strain background. (b) Diagram of potential recombination events in the presence of cre recombinase, and Southern blot analysis with the indicated probe of XbaI digested genomic DNA from mice with the indicated genotypes (‘+ cre’ indicates β-actin-cre transgenic). B, BamHI; X, XbaI; N, NotI; R, EcoRI.
To achieve keratinocyte-specific ablation, the C/EBPβfl/fl mice were crossed with K5-Cre transgenic mice, in which Cre recombinase expression is directed to the epidermis and other stratified epithelia by the keratin 5 promoter (Ramirez et al., 2004). As shown in Figure 2a, C/EBPβ protein was not detected in the epidermis of K5-Cre;C/EBPβfl/fl mice (faster mobility band in epidermis is non specific) but was present at normal levels in the other organs examined. Furthermore, K5-Cre; C/EBPβfl/fl females were fertile and lactation competent, indicating that ovarian and mammary gland defects present in C/EBPβ−/− mice (Sterneck et al., 1997; Robinson et al., 1998) did not occur in the K5-Cre; C/EBPβfl/fl mice. In addition, K5-Cre; C/EBPβfl/fl mice exhibited normal immunostaining for C/EBPβ in pulmonary alveolar macrophages of lung sections histological sections (data not shown) and no cases of splenomegaly (0/19), which is due to elevated IL-6 levels in C/EBPβ null mice (Screpanti et al., 1996) were observed in K5-Cre;C/EBPβfl/fl mice. To further examine the efficiency of Cre-induced recombination within the epidermis, we conducted immunohistochemical staining for C/EBPβ. In wild-type mouse skin, C/EBPβ staining was observed in the nuclei of cells of the epidermis, hair follicles, and sebaceous glands (Figure 2b). In contrast, C/EBPβ was not detected in the epidermis, hair follicles, and sebaceous glands of K5-Cre;C/EBPβfl/fl mouse skin (Figure 2c). Epithelial cells of these structures are considered to be derived from a pluripotent K5 expressing stem cell. Thus the staining pattern in K5-Cre;C/EBPβfl/fl mice suggests that the deletion of C/EBPβ occurred in epidermal stem cells, the putative target cells for skin carcinogenesis.
Figure 2.
Loss of C/EBPβ expression in the epidermis of K5-Cre;C/EBPβfl/fl mice. (a) Western blot analysis of C/EBPβ in multiple organs/tissues from wild type, C/EBPβ−/− (Sterneck et al., 1997), C/EBPβfl/fl, and K5-Cre;C/EBPβfl/fl mice. Male K5-Cre;C/EBPβfl/+ and female C/EBPβfl/+ mice were mated to generate the subjects which were genotyped using the following PCR primers; Floxed allele, 5′-GAGCCACCGCGTCCTCCAGC-3′ and 5′-GGTCGGTGCGCGTCATTGCC-3′; Cre allele, 5′-CGATG CAA CGAGTGATGAGGTTC-3′ and 5′-CAACTACGGCCACTTGCACG-3′. Epidermis was removed from the dermis as described (Oh and Smart, 1998) and all organs/tissues were homogenized in RIPA buffer containing 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, pH 7.4, 1 mM PMSF, 1 mM SOV, 1 × GIBCO complete protease inhibitor cocktail. Samples (20 μg) were separated by SDS-PAGE and western analysis was conducted as described (Zhu et al., 1999). (b) Immunohistochemical staining for C/EBPβ in skin of wild type and (c) K5-Cre;C/EBPβfl/fl mice. Mouse skins samples were frozen in OCT compound and frozen 5 μm sections were immunohistohistochemically stained using C/EBPβ antibody (1:25000) (Santa Cruz C-19) as described (Oh and Smart, 1998). HF – -hair follicle, E – epidermis, and SG – sebaceous gland.
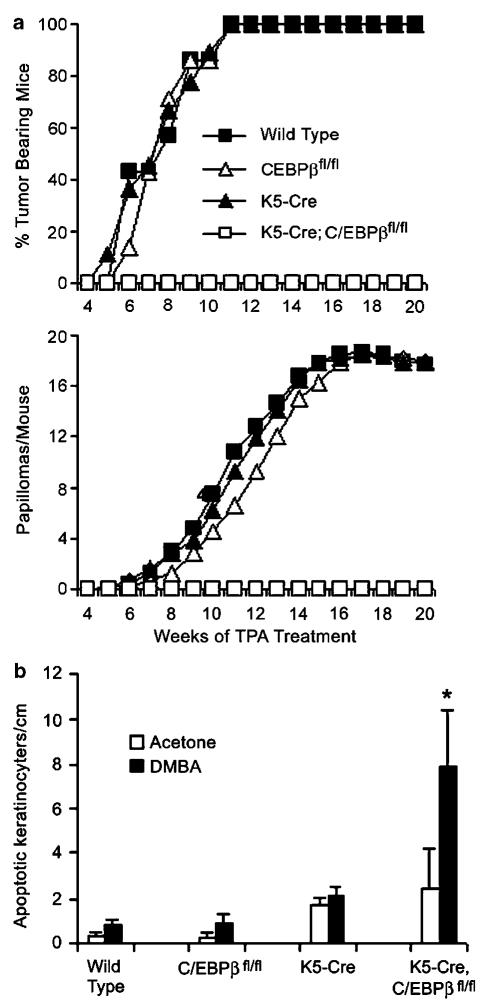
To determine the response to two-stage skin carcinogenesis, K5-Cre;C/EBPβfl/fl mice and controls consisting of K5-Cre, C/EBPβfl/fl and wild-type mice were treated with DMBA followed by thrice weekly treatment with the tumor promoter, 12-O-tetradecanylphobol-13-acetate (TPA). As shown in Figure 3a, all three control groups displayed a 100% tumor incidence and a tumor multiplicity of B18 squamous papillomas/mouse. In contrast, K5-Cre;C/EBPβfl/fl mice did not develop any tumors. Similar results were obtained in an independent repeat experiment (data not shown). These results demonstrate that keratinocyte-specific ablation of C/EBPβ is sufficient to confer complete resistance to DMBA-induced skin tumorigenesis.
Figure 3.
Resistance to DMBA-induced tumorigenesis and increased apoptosis in epidermal keratinocytes for K5-Cre;C/EBPβfl/fl mice. (a) DMBA/TPA skin tumorigenesis study. Wild type, K5-Cre, C/EBPβfl/fl, and K5-Cre;C/EBPβfl/fl mice (6-8 weeks old, six to eight mice per group) were initiated with a single dose of 200 nmol DMBA/200 μl acetone, followed 1 week late with thrice weekly treatment with 5 nmol 12-O-tetradecanoylphorbol-13-acetate (TPA)/200 μl acetone. All experiments were conducted in compliance with institutional guidelines. (b) Apoptosis in DMBA treated skin. Mice (three mice/group) were treated with 200 nmol DMBA/200 μl acetone or with acetone alone. Dorsal skins were collected 24 h after treatment and fixed in 10% neutral buffered formalin. The number of apoptotic keratinocytes/cm length of epidermis was determined as described (Zhu et al., 2002). Interfollicular keratinocytes were scored from three H&E stained sections (~1.5 cm length)/mouse using the following criteria; presence of dark pyknotic nuclei, cytoplasmic eosinophilia and the absence of cellular contacts. Data are expressed as mean±s.d. of three mice. * significantly different from all other DMBA-treated groups (P<0.01) as determined by Student’s t test.
To determine if reduced keratinocyte survival, as previously observed in C/EBPβ−/− mice, is associated with the resistance phenotype, apoptotic cells were scored in DMBA-treated skins. As shown in Figure 3b, both K5-Cre and K5-Cre;C/EBPβfl/fl mice displayed elevated basal levels of apoptosis in acetone vehicle treated epidermis (similar to untreated epidermis, data not shown) indicating that Cre expression alone causes keratinocyte apoptosis. However, unlike DMBA-treated K5-Cre;C/EBPβfl/fl mice, K5-Cre keratinocytes did not display a large net increase in apoptosis in response to DMBA treatment. DMBA-treated K5-Cre;C/EBPβfl/fl mice displayed a 9- to 13-fold net increase in the number of apoptotic basal keratinocytes compared to DMBA-treated C/EBPβfl/fl, K5-Cre or wild-type mice. Similar results were obtained with deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining (data not shown). These results demonstrate that a keratinocyte-intrinsic role of C/EBPβ is critical for cell survival subsequent to DMBA treatment.
C/EBP proteins must undergo dimerization for DNA binding and promoter activation. As a result of the high degree of conservation in their bZIP domains, C/EBP family members form both homodimers and heterodimers (Rask et al., 2000). C/EBPδ, another member of the C/EBP family, is upregulated in mouse skin papillomas and squamous cell carcinomas (Kim and Fischer, 1998). On the other hand, C/EBPδ might act as a tumor suppressor by virtue of its role in genome maintenance (Huang et al., 2004). To determine whether the resistance to skin tumorigenesis is a property shared with other C/EBP knockout mice and to determine whether C/EBPδ has a role in skin tumorigeneis perhaps as a homodimer or as a hererodimer with C/EBPβ, we conducted skin tumorigenesis studies in C/EBPδ−/− mice. As shown in Figure 4, the response of C/EBPδ−/− mice was indistinguishable from that of wild-type mice in both tumor incidence and multiplicity. Similar results were obtained with N-methyl-N′-nitro-N-nitrosoguanidine (MNNG)/TPA treatment (data not shown). These data demonstrate that C/EBPδ does not have a role in skin tumor development. This result along with earlier studies demonstrating that C/EBPα protein levels are greatly diminished in squamous papillomas, squamous carcinomas, and squamous cell carcinoma cell lines while C/EBPβ protein levels are retained (Shim et al., 2005) support a unique role of C/EBPβ among the C/EBP family in skin tumorigenesis.
Figure 4.
C/EBPδ knockout mice show no resistance to DMBA-induced skin tumorigenesis. Wild type and C/EBPδ−/− (Sterneck et al., 1997) female mice (6 weeks old, 20 mice per group; 129S1xC57BL/6-F1) were treated as described in Figure 3a.
Taken together, our results firmly establish an intrinsic keratinocyte function for C/EBPβ in cell survival and mouse skin tumorigenesis. C/EBPβ also functions as a prosurvival factor in myc/Ras transformed macrophages in vitro (Wessells et al., 2004) and in Wilms tumor cells (Li et al., 2005). In an attempt to characterize the in vitro role of C/EBPβ in keratinocytes survival, we previously treated primary keratinocytes isolated from C/EBPβ−/− and wild-type mice with various carcinogens or transfected/infected these primary keratinocytes with oncogenic Ras. Preliminary data indicated that in all cases C/EBPβ−/− primary keratinocytes did not display an enhanced apoptotic response compared to wild-type keratinocytes (unpublished results). Since our current results eliminate the possibility of a systemic effect of C/EBPβ as an explanation for the altered apoptotic skin phenotype in vivo and for the lack of an apoptotic phenotype in isolated keratinocytes, the DMBA-induced increase in keratinocytes apoptosis in skin may require the in vivo organizational structure of the epidermis. Thus, efforts to delineate the mechanism(s) of resistance to tumorigenesis and increased apoptosis in the absence of C/EBPβ can now be focused directly on the keratinocyte as well as the organizational structure of the skin. From a cancer therapy perspective, it will be of interest to determine whether blocking C/EBPβ function in a pre-existing tumor will result in apoptosis and subsequent tumor regression. Importantly, the conditional C/EBPβfl/fl mouse model introduced here, can now be used to assess the effect of C/EBPβ deletion at various stages of tumor development, and thus address the potential of C/EBPβ as a therapeutic target in skin as well as other target organs.
Acknowledgements
We would like to thank Dr Peter Johnson for his scientific input; Barbara Shankle and Daniel Logsdon for expert technical assistance; Dr Lino Tessarollo for β-actin-cre mice and help with generating the C/EBPβfl/+ mice and Drs Mac Law and Phillip Sannes for their help with histological identification of pulmonary macrophages. This work was supported in part by National Cancer Institute Grant CA46637 (to RCS).
References
- Boutwell RK. Cancer Res. 1983;43:2465s–2468s. [PubMed] [Google Scholar]
- Bundy LM, Sealy L. Oncogene. 2003;22:869–883. doi: 10.1038/sj.onc.1206216. [DOI] [PubMed] [Google Scholar]
- de Bruin A, Wu L, Saavedra HI, Wilson P, Yang Y, Rosol TJ, et al. Proc Natl Acad Sci USA. 2003;100:6546–6551. doi: 10.1073/pnas.1031853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AM, Montagna C, Sharan S, Ni Y, Ried T, Sterneck E. Oncogene. 2004;23:1549–1557. doi: 10.1038/sj.onc.1207285. [DOI] [PubMed] [Google Scholar]
- Johnson PF. J Cell Sci. 2005;118:2545–2555. doi: 10.1242/jcs.02459. [DOI] [PubMed] [Google Scholar]
- Kim Y, Fischer SM. J Biol Chem. 1998;273:27686–27694. doi: 10.1074/jbc.273.42.27686. [DOI] [PubMed] [Google Scholar]
- Klatt P, Serrano M. Carcinogenesis. 2003;24:817–826. doi: 10.1093/carcin/bgg057. [DOI] [PubMed] [Google Scholar]
- Lamb J, Ramaswamy S, Ford HL, Contreras B, Martinez RV, Kittrell FS, et al. Cell. 2003;114:323–334. doi: 10.1016/s0092-8674(03)00570-1. [DOI] [PubMed] [Google Scholar]
- Lewandoski M. Nat Rev Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- Li W, Kessler P, Yeger H, Alami J, Reeve AE, Heathcott R, et al. Cancer Res. 2005;65:2592–2601. doi: 10.1158/0008-5472.CAN-04-1532. [DOI] [PubMed] [Google Scholar]
- Ma W, Tessarollo L, Hong SB, Baba M, Southon E, Back TC, et al. Cancer Res. 2003;63:5320–5328. [PubMed] [Google Scholar]
- MacPherson D, Sage J, Crowley D, Trumpp A, Bronson RT, Jacks T. Mol Cell Biol. 2003;23:1044–1053. doi: 10.1128/MCB.23.3.1044-1053.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H-S, Smart RC. J Invest Dermatol. 1998;110:939–945. doi: 10.1046/j.1523-1747.1998.00199.x. [DOI] [PubMed] [Google Scholar]
- Poli V. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- Porter KL, Chanda S, Wang HQ, Gaido KW, Smart RC, Robinette CL. Toxicol Sci. 2002;69:42–48. doi: 10.1093/toxsci/69.1.42. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Page A, Gandarillas A, Zanet J, Pibre S, Vidal M, et al. Genesis. 2004;39:52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- Ramji DP, Foka P. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask K, Thorn M, Poten F, Kraaz W, Sundfeldt K, Hedin L, et al. Int J Cancer. 2000;86:337–343. doi: 10.1002/(sici)1097-0215(20000501)86:3<337::aid-ijc6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Robinson GW, Johnson PF, Hennighausen L, Sterneck E. Genes Dev. 1998;12:1907–1916. doi: 10.1101/gad.12.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screpanti I, Musiani P, Bellavia D, Cappelletti M, Aiello FB, Maroder M, et al. J Exp Med. 1996;184:1561–1566. doi: 10.1084/jem.184.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, et al. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim M, Powers KL, Ewing SJ, Zhu S, Smart RC. Cancer Res. 2005;65:861–867. [PubMed] [Google Scholar]
- Shuman JD, Sebastian T, Kaldis P, Copeland TD, Zhu S, Smart RC, et al. Mol Cell Biol. 2004;24:7380–7391. doi: 10.1128/MCB.24.17.7380-7391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparmann A, Bar-Sagi D. Cancer Cell. 2005;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Sterneck E, Tessarollo L, Johnson PF. Genes Dev. 1997;11:2153–2162. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundfeldt K, Ivarsson K, Carlsson M, Enerback S, Janson PO, Brannstrom M, et al. Br J Cancer. 1999;79:1240–1248. doi: 10.1038/sj.bjc.6690199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, et al. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- Tessarollo L. Methods Mol Biol. 2001;158:47–63. doi: 10.1385/1-59259-220-1:47. [DOI] [PubMed] [Google Scholar]
- Wessells J, Yakar S, Johnson PF. Mol Cell Biol. 2004;24:3238–3250. doi: 10.1128/MCB.24.8.3238-3250.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Oh HS, Shim M, Sterneck E, Johnson PF, Smart RC. Mol Cell Biol. 1999;19:7181–7190. doi: 10.1128/mcb.19.10.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Yoon K, Sterneck E, Johnson PF, Smart RC. Proc Natl Acad Sci USA. 2002;99:207–212. doi: 10.1073/pnas.012437299. [DOI] [PMC free article] [PubMed] [Google Scholar]