Abstract
In our recent work, we proposed an image reconstruction procedure aimed to unify gated imaging and dynamic imaging in nuclear cardiac imaging. With this procedure the goal is to obtain an image sequence from a single acquisition which shows simultaneously both cardiac motion and tracer distribution change over the course of imaging. In this work, we further develop and demonstrate this procedure for fully 5D (3D space plus time plus gate) reconstruction in gated, dynamic cardiac SPECT imaging, where the challenge is even greater without the use of multiple fast camera rotations. For 5D reconstruction, we develop and compare two iterative algorithms: one is based on the modified block sequential regularized EM (BSREM-II) algorithm, and the other is based on the one-step late (OSL) algorithm. In our experiments, we simulated gated cardiac imaging with the NURBS-based cardiac-torso (NCAT) phantom and Tc99m-Teboroxime as the imaging agent, where acquisition with the equivalent of only three full camera rotations was used during the course of a 12-minute postinjection period. We conducted a thorough evaluation of the reconstruction results using a number of quantitative measures. Our results demonstrate that the 5D reconstruction procedure can yield gated dynamic images which show quantitative information for both perfusion defect detection and cardiac motion.
Index Terms: 5D reconstruction, dynamic SPECT, gated SPECT, spatio-temporal reconstruction
I. Introduction
Recent advances in nuclear cardiology imaging have made myocardial perfusion imaging an important means for assessing regional coronary blood flow. Gated SPECT offers an important additional benefit by permitting the evaluation of left ventricular function through quantitative determination of global and regional wall motion [1]. Traditionally, the distribution of the tracer is assumed constant over the entire study, and a stationary image is reconstructed for each gate frame.
A logical and clinically important extension of gated imaging is the problem of reconstructing nuclear cardiac imaging studies using agents for which both the tracer distribution and cardiac function change during the course of imaging. However, this problem faces significant challenges because, besides the reduced data counts for the individual gate intervals due to gating as in traditional SPECT, the acquired projection data are available at only one angular position of the rotating camera for a particular time instance, and therefore, are far from being sufficient for directly reconstructing the dynamic images at a given time point during the course of imaging. Moreover, in a setting without use of fast camera rotation, which is more suitable for clinical applications [2], the fast-changing tracer dynamics can lead to significant inconsistencies in the projection data among the different angular positions.
Despite these difficulties, in the early work in [2] and [3], a reconstruction procedure was developed where the tracer distribution was treated as time-varying in a gated cardiac SPECT acquisition. In this procedure, a dynamic image sequence was first reconstructed independently for each gate frame by use of the dynamic expectation-maximization (dEM) algorithm first proposed in [4], in which temporal constraints were imposed on the dynamic activities at each voxel location; afterward, Wiener filtering was applied across the different gate frames for noise reduction. Motivated by this early work, we proposed for the first time a joint reconstruction approach in [5]–[7] for dynamic gated imaging, where the dynamic images from the different gates are treated collectively as a single signal, and determined from the acquired dynamic data by using maximum a posteriori (MAP) estimation. Such an approach aims to explicitly exploit the statistical correlation among the different gate intervals during reconstruction. Our preliminary results in [7] show that it could yield an image sequence that shows both wall motion and time-varying tracer distribution in the myocardium.
Encouraged by this initial success, in this work we further develop and evaluate our image reconstruction procedure for fully 5D (3D space plus time and gate) cardiac gated dynamic imaging. The purposes are two-fold. First, for initial development of the concept only 2D slices were used in [7] owing to the great complexity of the reconstruction problem; we now extend it to fully 3D volumes. Second, for reconstruction we will develop a fully 5D version of the modified block sequential regularized EM (BSREM-II) algorithm [8], which is much faster than the one-step late (OSL) algorithm we previously used. In the development of the original dEM algorithm [4], it was noted that an ordered-subset (OS) type technique might not be effective for dEM, because the projection data are not consistent at different angular positions due to the fast-changing tracer dynamics. However, in [9] we first demonstrated that an OS type algorithm could still be applied for gated dynamic SPECT images. Based on prior success in this work we develop a 5D BSREM algorithm to speed up the reconstruction. We will compare it against the modified OSL algorithm in the context of fully 5D reconstruction.
In our experiments, we simulated gated cardiac imaging with the NURBS-based cardiac-torso (NCAT) phantom and Tc99m-Teboroxime as the imaging agent, where acquisition with the equivalent of only three full camera rotations was used during the course of a 12 minute post-injection period. In this setting, the projection data were under-determined by a factor of over 20:1 for reconstructing the dynamic images. We conducted a thorough evaluation of the reconstruction results using a number of quantitative measures, including 1) error analysis on the accuracy of the reconstructed myocardium, 2) time activity analysis for quantifying the distinctive time-varying tracer distributions between an introduced perfusion defect and the normal myocardium, and 3) ejection fraction (EF) of the left ventricle (LV) for assessment of LV functions. Our quantitative results demonstrate that, in spite of the great challenges mentioned above, the proposed reconstruction procedure can still lead to an adequate reconstruction of gated dynamic images, which show both perfusion defect detection and cardiac motion. We believe that such a development is an important step toward our ultimate goal of making 5D imaging a useful clinical tool.
We mention briefly that in the literature there has been significant interest in development of spatio-temporal reconstruction methods in gated SPECT in recent years, aimed to dealing with the presence of increased imaging noise caused by gating (e.g., [10]–[13]). Parallel to gated SPECT, there also has been growing research interest in dynamic SPECT imaging (e.g., [14], [15]), aiming to obtain a time-varying tracer distribution in an organ. However, these methods typically deal with only one aspect of tracer change, due to either gating or tracer dynamics, but not both. Further detailed discussions on the challenges and potential benefits of unifying these two imaging modalities were given in [7].
The rest of the paper is organized as follows: the imaging model for gated dynamic SPECT and the proposed reconstruction algorithms are given in Section II. The evaluation methods and criteria are described in Section III. Experiment results are given in Section IV to demonstrate the proposed methods. Finally, conclusions are given in Section V.
II. Gated Dynamic SPECT Reconstruction
A. Imaging Model
As in gated SPECT, the acquired list-mode projection data are binned K into gate intervals by using the ECG signal. Following the notion in [14], we use the angular incremental steps of the rotating SPECT camera to denote the progress of sample time t. The imaging data are described by the following model:
| (1) |
where gt,k, ft,k represent the projection data and the image, respectively, at time interval t for gate frame k, E[·] is the expectation operator, and Ht is the system matrix which is time-varying because of the rotation of the SPECT system.
In the above model the entity ft,k represents the dynamic tracer distribution over time t in the 3D volume when the cardiac phase is at interval k. Our goal is to reconstruct the images ft,k for all t = 1, …, T and k = 1, …, K. Collectively, the set of unknowns {ft,k, t = 1, …, T, k = 1, …, K} represents a 5D quantity.
Note that in the absence of fast camera rotation the projection data gt,k in (1) are available for only a few projection angles (three in our experiments) during a particular time interval t. Thus, the reconstruction problem here is significantly more challenging than in traditional gated SPECT where the tracer distribution is treated as constant during the course of imaging.
As we describe below, in order to deal with this difficulty, we will adopt Farncombe’s dEM approach [14] to regularize the time activities at individual voxels, and develop a joint reconstruction approach in which regularization terms are introduced to exploit the similarity both spatially and temporally among the different gated frames.
In this study the elements hij of the system matrix Ht in (1) are modeled after both the distance-dependent point spread function (PSF) and the attenuation effect of a SPECT system. Specifically, let denote the elements of the system matrix with distance dependent spatial resolution, but without attenuation. Let μ(x, y, z) denote the attenuation map of the object. Then the attenuation effect is modeled in the system matrix as
| (2) |
where the line integral is carried out along the segment between image voxel j and detector bin i. In our experiments, the attenuation map from NCAT phantom was used for the simulation data.
B. Joint Maximum a Posteriori Reconstruction
In this section we introduce the notation of our maximum a posteriori (MAP) estimation framework for subsequent development of the reconstruction algorithms. For convenience, define , i.e., a vector consisting of the dynamic images of gate k, k = 1, …, K; similarly, define to represent all the projection data for gate k. Moreover, define H = diag [H1, H2, …, HT], i.e., a block diagonal matrix formed from the system matrix Ht collectively at t = 1, …, T. Then the dynamic imaging model in (1) can be rewritten as
| (3) |
To combat the highly under-determined nature of the problem, we adopt the following constraint to regulate the dynamic activity at each image voxel: it can only be either constant, increasing only, decreasing only, or first increasing then decreasing, as first proposed in the dEM algorithm [14]. Specifically, consider a voxel j. Let tpj denote the time point at which the image intensity at j reaches its maximum. Then the following temporal constraint must hold:
| (4) |
for j = 1, …, N with N denoting the total voxel number in one gate.
Let Ω be the set of admissible dynamic images defined by the dEM constraint in (4). To further simplify the notation, let , i.e., a vector formed by the dynamic images of all gates; similarly, let . Then the dynamic images are collectively estimated according to the following constrained MAP criterion:
| (5) |
where log p(G|F) is the likelihood function of G parameterized by F, and p(F)is a prior distribution on F.
In this study the likelihood function log p(G|F) is assumed to obey an independent Poisson distribution, i.e.,
| (6) |
where (Ht ft,k), (i), gt,k (i) are the ith entries of Ht ft,k and gt,k, respectively, and B is the total number of bins of the camera.
We use a separable Gibbs prior for p(F), which is defined as follows:
| (7) |
where Us (F), Um (F) are two energy functions defined over space and gate intervals, respectively, and βs and βm are the corresponding scalar weighting factors. The spatial term Us (F) is used to exploit the similarity among neighboring voxels, whereas the gate term Um (F) is used to exploit the similarity among different gate intervals. Such a prior was previously applied for 4D reconstruction of SPECT images [16]; here we extend it for 5D dynamic images. Without interrupting the flow of development, the specific definitions of these two terms are relegated to the Appendix.
It is noted that the prior term in (7) is defined to enforce both spatial and inter-gate smoothing in the reconstructed images. In a similar fashion, one could also introduce an additional term in (7) to explicitly enforce temporal smoothing in the reconstructed dynamic images. However, the temporal constraint introduced earlier in (4) already plays such a role in that it can discourage sudden random fluctuations in the reconstructed time activity curves. Thus, no additional temporal smoothing term is used in (7) in this study.
To facilitate the solution of the constrained optimization problem in (5), we express the dynamic images in terms of their temporal increments (for increasing activity) or decrements (for decreasing activity), as first proposed in [14]. For example, consider the case that the activity at voxel j is increasing, i.e.,0 ≤ f1,k (j) ≤ f2,k (j) … ≤ fT,k (j). This constraint can be expressed in terms of differential increments of ft,k (j) as
Clearly, given these increments, the image activity ft,k (j) can be obtained. For the general case in (4) increment terms will be used for t ≤ tpj and decrement terms will be used for t > tpj.
Let A denote the differential operator for computing the temporal increment (or decrement) at each voxel in F, and let F̃ denote the transformed incremental (or decremental) form of F, i.e.,
| (8) |
Then the dEM constraint in (4) simply becomes F̃ ≥ 0.
For convenience, let Ak denote the corresponding sub-operator of A restricted to gate k, i.e.,f̃k ≡ Ak fk, which is the incremental form of fk. Note that the operator Ak is invertible, that is, from the incremental form f̃k we can readily reconstruct fk as , which consists of simple additive (or subtractive) operations. Note that the operator yields f1, k,…, fT,k simultaneously. Now, for each t = 1, …, T, let denote the sub-operator of such that . In other words, yields only the portion of fk at time t.
Upon some manipulation, the constrained MAP estimate in (5) can be solved in terms of F̃ from maximizing the following objective function:
| (9) |
where (i) denotes the ith projection bin of .
Next, we describe two iterative algorithms for this optimization problem. Once the incremental vector F̃ is obtained, the image frames F can be determined as F = A−1 F̃.
C. Modified Block Sequential Regularized EM Algorithm
For maximizing the objective function in (9), the modified block sequential regularized expectation-maximization (BSREM-II) algorithm [8], [17] is adapted in this study. The BSREM-II algorithm is known to be globally-convergent and typically faster than non-ordered-subset algorithms. To apply it here, we begin with dividing the entire set of projection data G into a number of subsets as in a typical ordered-subset algorithm [18]. With the angular rotating steps of the SPECT camera denoted by time t, it becomes convenient to divide the projection data according to t. Assume a total of L subsets used. Then the objective function in (9) is accordingly decomposed into , in which the sub-objective function Jl (F̃) is defined as Jl (F̃)
| (10) |
where Sl denote the collection of angular positions for the lth subset, l = 1, …, L.
During each iteration, the elements of F̃ are updated as follows:
| (11) |
where n is the iteration index, l is the sub-iteration index, ℘ is the projection operator onto with ε being a small positive number and U being an upper bound in [8], αn is a relaxation parameter, and Λ is a diagonal scaling operator defined below.
In (11), the diagonal elements of are given by
| (12) |
j = 1, …, N and pj is chosen to be
| (13) |
where (i, j+(t−1)N) denotes the entry of the matrix at position (i, j + (t − 1) N).
Finally, the elements of the gradient vector ∇ Jl (F̃n,l − 1) in (11) are given by
| (14) |
Note that the nonnegative constraint F̃ ≥ 0 is automatically satisfied by the projection step in (11). In our experiments, L = 8 was used; the subsets were formed such that the projections in two consecutive subsets were separated as far as possible. The total number of iterations used was 10. The step size αn was set to 1/(n/15 + 1) with n being the iteration index.
D. Modified One-Step Late Reconstruction Algorithm
For comparison, we also implemented a fully 5D version of the one-step late (OSL) EM algorithm [19] for the maximization problem in (9). In this algorithm, at each iteration the image voxels are updated as follows:
| (15) |
where
| (16) |
where n denotes the iteration number. At each step, the step size αn is first set to 1, then successively reduced by half if necessary to ensure that the objective function is nondecreasing.
III. Evaluation Methods
A. Data Sets
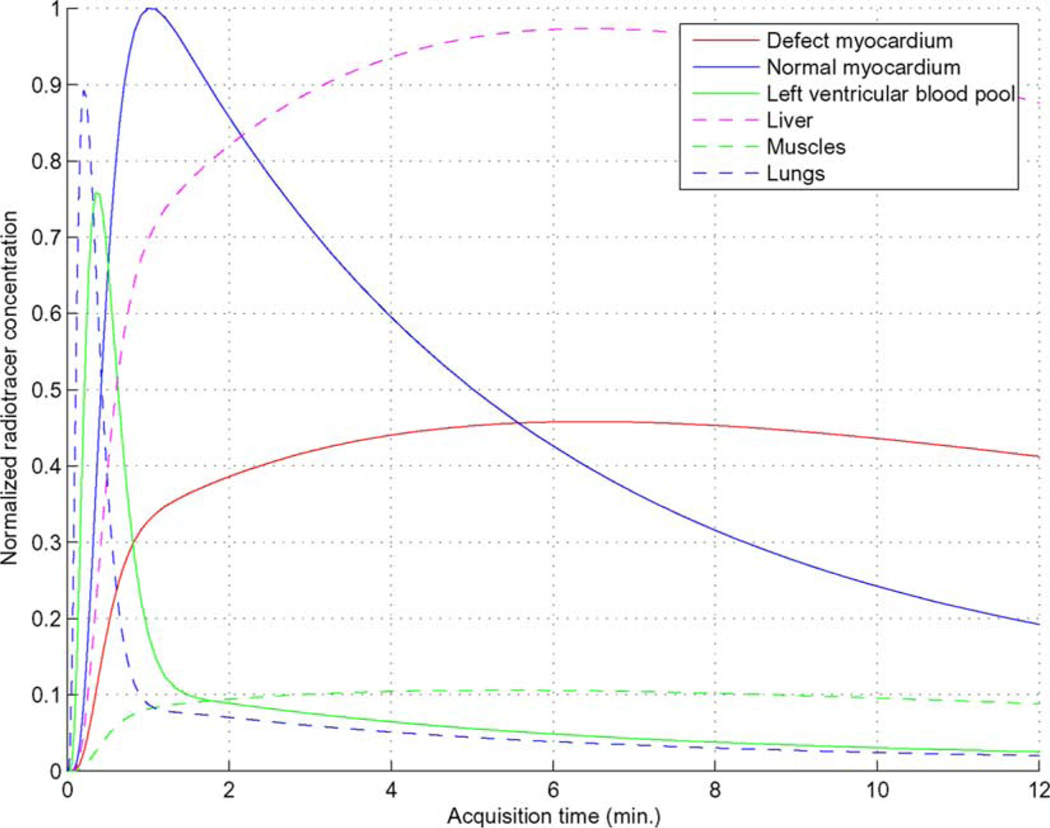
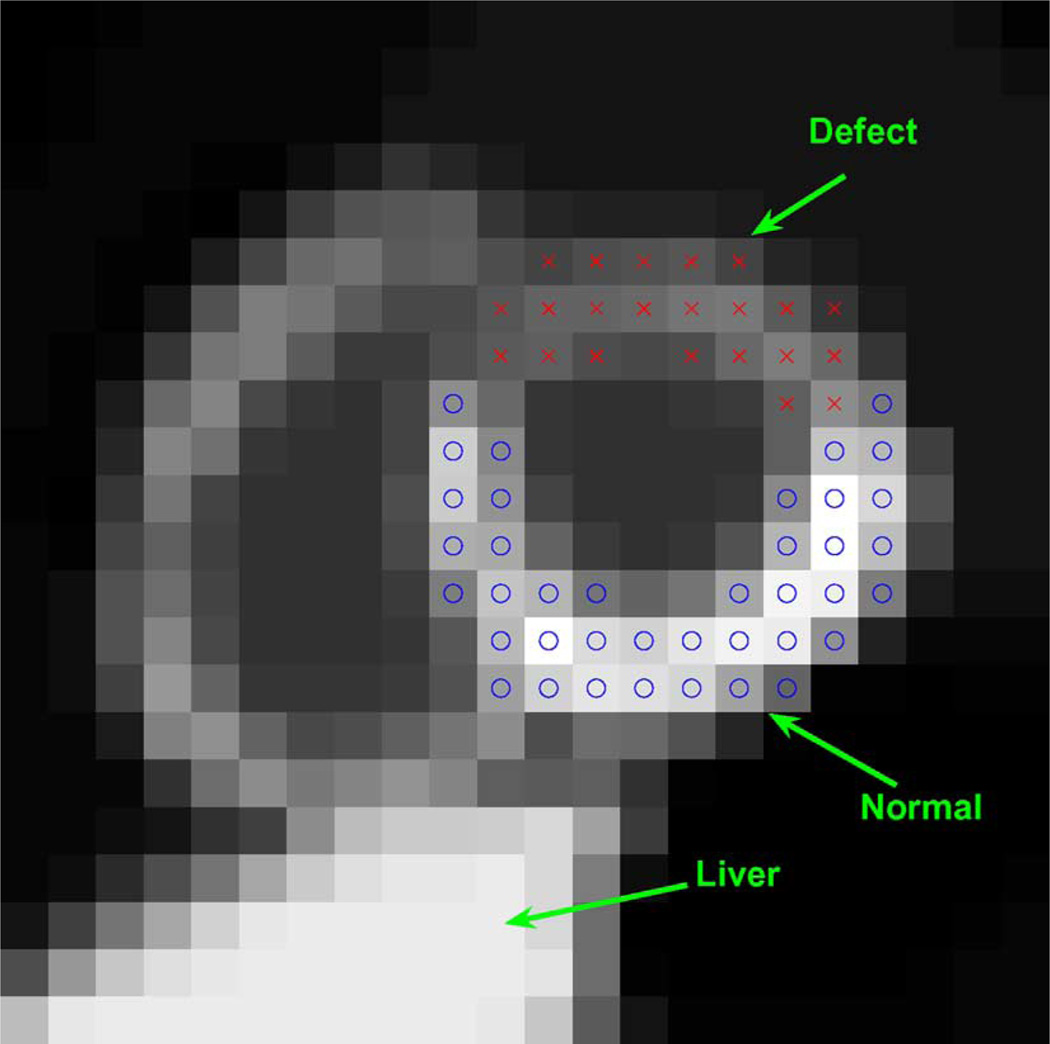
To demonstrate the proposed 5D reconstruction method, we used the NURBS-based cardiac-torso (NCAT) v2.0 phantom [20] with a tracer-kinetic model simulating imaging with Tc99m labeled Teboroxime, as shown in Fig. 1, where different time activities were introduced among the different organs as in [2]. A perfusion defect was introduced in the anterior wall of the left ventricle (LV), as illustrated in Fig. 2 in a short axis slice of the LV for gates #1. Note that the defect has slower uptake and washout rates than the normal myocardium, and there is a high concentration in the liver.
Fig. 1.
Time activity curves introduced for different organs.
Fig. 2.
A short axis slice of NCAT phantom for gate #1 with a simulated perfusion defect (indicated by x’s) in the anterior wall of the left ventricle.
In our simulation, a triple-head camera system was used, and 64 rotation stops covering a total of 360° by each head with 64 × 64 projection bins at each stop were used during 12-minute data acquisition. A total of eight gates were used. The field of view was 40.6 cm; the voxel size is 0.634 cm in each dimension, resulting in a 64 × 64 × 64 image matrix. The system had a distance dependent blur of approximately 13 mm full width at half-maximum (FWHM) at the center. The attenuation factor was included. Poisson noise was introduced corresponding to a count level of 8 million total counts (for all projections and over all time).
In our experiments, the proposed reconstruction procedure was tested using 30 different noise realizations. For each noise run, a sequence of dynamic volumetric images was obtained over 64 time points during the 12-minute period for each of the eight gates.
B. Reconstruction Methods
To demonstrate the respective effects of the spatial prior and the motion-compensated gate prior, the following different methods were used to reconstruct the image sequences: 1) MAP reconstruction with βm = 0 in (7), which corresponds to reconstruction of individual gates separately using spatial smoothing alone (denoted as “MAP-S”); 2) MAP reconstruction with βs = 0, which corresponds to joint reconstruction of the different gates using gate smoothing prior only (denoted as “MAP-T”); and 3) MAP reconstruction with βs ≠ 0 and βm ≠ 0, joint reconstruction of the different gates using both spatial and gate smoothing prior (denoted as “MAP-ST”). The starting point for these iterative methods was determined in a similar fashion as in dEM [7], [14]: first, a static (average) image was obtained for each gate by EM reconstruction; then, the activity at each pixel was initialized to increase linearly from one half of its static image value at the beginning to its full static value at its peak position, and then decrease linearly afterward to one half of its static value at the end of acquisition time. As reference for comparison, the noiseless images (reconstructed with EM from complete, noiseless projection data) are used (denoted as “Ideal”).
In our experiments we used a motion-compensated gate smoothing prior as defined in Appendix A. For this prior, the image motion was determined from the noisy projection data. First, the MAP-S method was applied to reconstruct the dynamic sequence separately for each gate interval. The resulting images were then summed along the dynamic time axis to get eight gate frames, which were subsequently processed by a lowpass filter (order-3 Butterworth filter with 0.2 cycles/voxel cutoff frequency). Afterward, the 3D optical flow method was applied to estimate the motion between the different gate frames [16].
Furthermore, for the dynamic operator A in (8), we applied the same approach as in dEM [7], [14] to determine the peak time points tpj used in the dynamic constraints. That is, for each gating interval two initial reconstructions were first performed to obtain the dynamic images: one using increasing-only constraints and the other using decreasing-only constraints over the entire imaging period. The resulting two TACs at each pixel from these two reconstructions will have an overlapped plateau, of which the middle point was used as the peak location tpj.
C. Evaluation Measures
We first evaluated the accuracy of the reconstruction results using several quantitative measures, including: 1) the mean squared error (MSE) of the reconstructed myocardium, and 2) bias-standard deviation plots for two selected ROIs (one normal and the other with a perfusion defect, as shown in Fig. 3) on the heart wall.
Fig. 3.
Different regions of interest (ROIs) used in evaluation including MSE, TAC, bias-variance analysis and CNR. Normal: “o”; defect: “x”.
In addition, to demonstrate that the proposed procedure can yield information simultaneously for both time-varying tracer distribution and wall motion, we quantified the visibility of the perfusion defect relative to the normal heart wall by computing its contrast to noise ratio (CNR); in addition, we also compared the time activity curves (TAC) for the normal and perfusion ROIs from the reconstructed dynamic images, which show clear distinction between the two. Furthermore, we also quantified the reconstruction using ejection fraction (EF) of the LV, a measure pertinent to clinical assessment of LV functions.
Below we describe these measures in details.
1) MSE
To quantify the overall accuracy of the reconstructed dynamic images of the myocardium, we computed the MSE of the reconstructed gated dynamic images as
| (17) |
where f̂t,k is the reconstructed, ft,k is the reference (Ideal), ROI denotes a 20 × 19 × 17 volume containing the entire myocardium (of which three slices are shown in Fig. 3), and M is the total number of voxels in the ROI. The MSE in (17) is a direct measure of the accuracy of the reconstructed image values in the presence of imaging noise in the myocardium.
2) Bias-Variance Analysis
We also quantified the reconstruction accuracy of the heart wall in reconstructed images using bias-variance analysis. Two ROIs (one normal, one defect; each of size 2 × 2 × 2), as shown in Fig. 3, were used. The results were then summarized by using a bias-standard deviation plot.
Specifically, for a reference image f we use f̅ROI to denote its average intensity in a specified ROI, i.e.,
| (18) |
where M is the total number of pixels within the ROI. Our goal is to quantify the reconstruction accuracy of this quantity from different noisy realizations.
Let f̂(q), q = 1, 2, …, Q be the estimates of f from Q different noise realizations, then the estimate of f̅ROI, denoted by μ̂, is computed as follows:
| (19) |
The percent bias and standard deviation (std) of the estimator in (19) are then respectively estimated as
| (20) |
| (21) |
In our experiments, a total of 30 noise realizations were used for generating the bias-standard deviation plots.
3) CNR
To quantify the visibility of the perfusion defect in the presence of reconstruction noise, we computed the CNR for the perfusion defect relative to the normal heart wall throughout the course of dynamic time. In particular, we computed the average and peak CNR values as follows:
| (22) |
| (23) |
where mn (t) and md (t) are the average values of the normal myocardium and the defect, respectively, at time t, and σn (t) and σd (t) denote their corresponding standard deviation values.
In our experiments the dynamic images of the first gate was used for computing these quantities, of which the defect and normal ROIs are shown in Fig. 3. For each noise realization, mn (t) and md (t) were computed from these two ROIs; the variances and were estimated using an enlarged set of voxels surrounding each ROIs for stabilization. A total of 30 noise realizations were used.
4) TAC Analysis
We also compared the TACs of the reconstructed 5D gated dynamic images from different reconstruction methods with that of the Ideal. The TACs were computed for the two ROIs (normal and perfusion) in Fig. 3 over 30 noise realizations. The ROIs were selected from the first gate of reconstructed images with optimal parameters. The TAC analysis is used to demonstrate that the proposed procedure can discriminate the time-varying tracer distribution in the reconstructed dynamic sequence.
5) LVEF
We also quantified the reconstruction using ejection fraction (EF), a measure pertinent to clinical assessment of LV functions. To obtain LVEF, a reconstructed 5D image sequence was first collapsed into a gated sequence by summing along the dynamic time axis; the clinical software package 4DM-SPECT was then used to compute the EF from this collapsed sequence.
It is noted that with the clinical software the image analysis parameters may not be optimized for the reconstruction methods used, owing to the different resolution properties of the images. To accommodate this, we applied a training step (with a different set of noise realizations) prior to using the software, based on which the 4D images were first pre-processed with a lowpass Gaussian filter.
IV. Results AND Discussions
A. Reconstruction Accuracy
1) MSE Results
In Fig. 4 we summarize the MSE of the reconstructed myocardium ROI obtained with different values of the spatial and gate parameters βs and βm. In this plot, each curve was obtained by varying βs = [0, 0.2, 0.4, 1, 1.5] × 10−6 while βm was held constant. These results were obtained from an average of 30 different noise realizations, with MSE computed for each realization as defined in (17).
Fig. 4.
MSEs of the reconstructed myocardium ROI obtained from 30 noise realizations.
The results in Fig. 4 show that use of gate smoothing prior (i.e., βm ≠ 0) resulted in more accurate reconstruction of the myocardium than spatial-only smoothing MAP-S (i.e., βm = 0). As expected, the largest error was obtained when neither spatial nor gate smoothing was used (i.e., βs = 0 and βm = 0, which corresponds to dEM reconstruction); on the contrary, the best results were achieved when βm = 3 × 10−5 was used, for which the optimal MSE was obtained with βs = 2 × 10−7.
It is also noted that with βm = 3 × 10−5 the best MSE values were obtained when there was little or no spatial smoothing; in fact, MAP-T (i.e., βs = 0) achieved almost the optimal value. These results show that while additional spatial smoothing could further improve the reconstruction accuracy, its merit seems to diminish with increased gate smoothing (e.g., βm = 3 × 10−5). This can be explained as follows. The LV heart wall is limited to only a few pixels thick in some gates; thus, while spatial smoothing can be effective for noise reduction, it can also lead to spatial blurring of the LV wall. With motion-compensated gate smoothing, the benefit of spatial smoothing simply becomes outweighed by its impact on the heart wall.
2) Bias-Variance Plots
In Fig. 5 we show the bias and standard-deviation plots for the normal ROI on the LV wall. These plots were obtained from 30 noise realizations, and the dynamic images at t = 1.78 min, 4.78 min, and 10.03 min were used which represented the early, middle and late stages of the imaging period. Similar results were also obtained for other time points. As in Fig. 4, each curve in Fig. 5 was obtained by varying the spatial parameter βs ([0, 0.2, 0.4, 1, 1.5] × 10−6) while the gate parameter βm was held constant.
Fig. 5.
Bias-standard deviation plots of normal ROIs obtained with 30 noise realizations at three different time points. In each curve, βs was increased from left to right. (a) At time 1.78 min. (b) At time 4.78 min. (c) At time 10.03 min.
From these results it can be seen that use of spatial smoothing reduces the variance at the expense of increased bias; at a given bias level, use of gate prior can significantly reduce the variance. Moreover, the reconstructed images exhibit a larger bias at the early stage of image period, which is consistent with the TAC results to be shown later in Fig. 6.
Fig. 6.
Time activity curves from normal ROI and defect ROI by different methods. (a) MAP-S. (b) MAP-ST.
B. Perfusion Defects versus Normal Myocardium
1) CNR of Perfusion Defect
In Table I we summarize the obtained CNR results of the perfusion defect versus its surrounding normal myocardium. These results were averaged from 30 noise realizations. As can be seen, the best CNR results were obtained with MAP-ST, while MAP-T achieved similar results. Also, although MAP-S achieved comparable values on average, it had significantly larger standard deviation, which reflects the effect of increased noise on the visibility of the perfusion defect.
TABLE I.
Average and Peak Contrast-to-Noise-Ratio (CNR) From Different Methods: 1) MAP-S (βs = 1 × 10−6, βm = 0), 2) Map-T (βs = 0, βm = 3 × 10−5), 3) MAP-ST (βs = 2 × 10−7, βm = 3 × 10−5). The Mean and Standard Deviation (SD) Were Obtained From 30 Noise Realizations
| Methods | (SD) | CNRmax (SD) |
|---|---|---|
| MAP-S | 2.43 (1.89) | 9.56 (6.99) |
| MAP-T | 2.44 (1.00) | 9.22 (3.41) |
| MAP-ST | 2.81 (1.10) | 10.57 (3.94) |
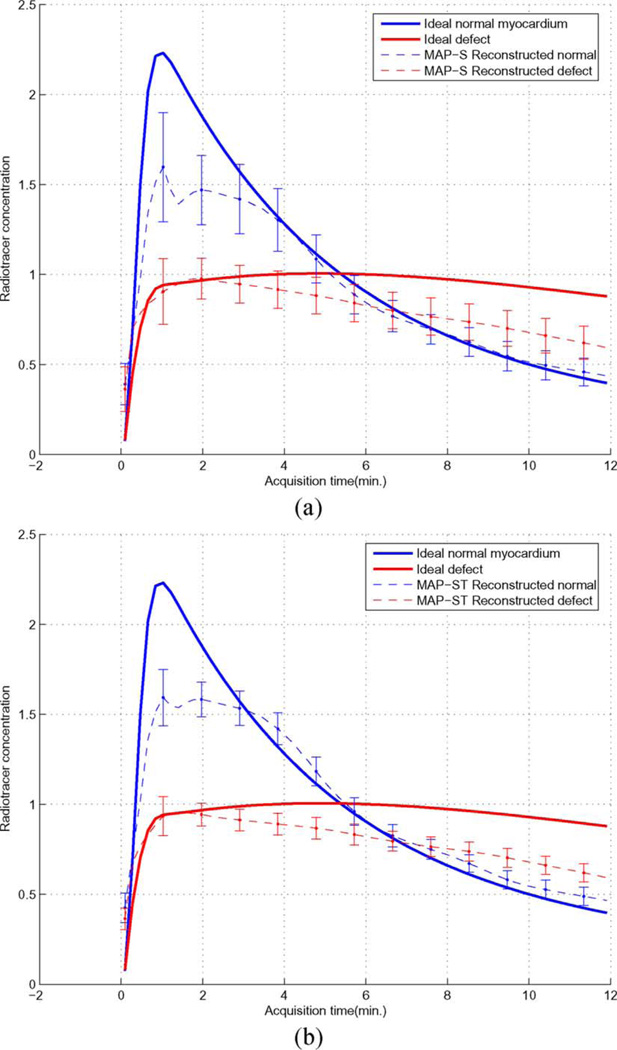
2) Time Activity Curves (TACs)
In Fig. 6 we show the TACs for both the defect and normal ROIs in the myocardium (shown in Fig. 3) obtained from 30 noise realizations (MAP-S: βs = 1 × 10−6 ; MAP-ST: βs = 2 × 10−7 and βm = 3 × 10−5). For comparison, the TACs from Ideal reconstruction are also shown. As can be seen, the reconstructed dynamic images could yield a good separation between the normal and perfusion defect TACs. Compared to MAP-S, MAP-ST achieved notably smaller variance values throughout the imaging period.
It is observed from Fig. 6 that the peak of the reconstructed normal myocardium TAC shows a consistent negative bias. Interestingly, this is also consistent with the relatively larger bias observed earlier in Fig. 5 for the early stage of the imaging period. We believe that this bias is mainly caused by the slow rotation of the camera, which results in temporal under-sampling of the fast uptake of the normal myocardium TAC at the early stage of imaging period.
Moreover, it can also be observed from Fig. 6 that the reconstructed defect TAC is consistently underestimated toward the end of the imaging period. We believe that this is likely caused by the reduced data counts in the late stage of the imaging period; the increased noise in the image data would compromise the estimate of the peak time tpj in the dynamic constraint in (4), leading to premature decline in the defect activity.
One consequence of the biases observed above is that it will reduce the contrast between normal myocardium and defect in the reconstructed images. Nevertheless, MAP-ST yields improved results for both normal and defect TACs. This is also consistent with the CNR results given earlier in Table I.
TABLE II.
LV Ejection Fraction (EF) Values From Different Methods: 1) MAP-S (βs = 1 × 10−6, βm = 0), 2) Map-T (βs = 0, βm = 1 × 10−5), And 3) MAP-ST (βs = 2 × 10−7, βm = 5 × 10−5). These Results Were Obtained From 30 Noise Realizations
| Methods | EF (SD) | |
|---|---|---|
| Ground truth | 59.71 | |
| MAP-S | 64.17 (7.29) | |
| MAP-T | 53.07 (6.09) | |
| MAP-ST | 57.25 (5.12) | |
C. Ejection Fraction of LV
In Table II we show the EF results obtained from the gated dynamic images. These results were averaged from 30 noise realizations (with standard deviation values given in parentheses). As reference, the ground truth of these parameters of the NCAT phantom is also listed. It is observed that all three methods could lead to fairly accurate estimates of the EF, with MAP-ST achieving the smallest variance. These results demonstrate that the reconstructed 5D images could indeed yield valid information for LV function assessment.
D. Reconstructed Images
We show in Fig. 7 a set of dynamic images of the LV volume in short axis view for a number of selected gates and time points obtained with the following reconstruction methods from a typical noise realization: 1) MAP-S (βs = 1 × 10−6 and βm = 0), 2) MAP-T (βs = 0 and βm = 3 × 10−5), 3) MAP-ST (βs = 2 × 10−7 and βm = 3 × 10−5). For comparison, the corresponding Ideal images are also shown. For clarity, only the heart region is shown for four different gates (#1, #3, #5, #7) and nine time sample points from the uptake to the washout period. In Fig. 7, the images along the horizontal direction show the effect of cardiac motion, while along the vertical direction they show the effect of tracer dynamics over time.
Fig. 7.
Reconstructed dynamic images by different reconstruction methods: (1) Ideal: EM reconstruction from complete, noiseless projection data; (2) MAP-S: MAP with spatial smoothing (βs = 1 × 10−6, βm = 0); (3) MAP-T: MAP with temporal smoothing (βs = 0, βm = 3 × 10−5); and (4) MAP-ST: MAP with both spatial and temporal smoothing (βs = 2 × 10−7, βm = 3 × 10−5). For each method, the ordinate represents the time post-injection, and the abscissa represents the gate number.
For better visual comparison, in Fig. 8 we show these images for two time points (t = 1.78 min and t = 4.03 min). These images were normalized such that the myocardium has the same maximum for the two time points (as in clinical display). From these images it can be seen that MAP-S suffers from noticeable distortions in the heart wall; both MAP-T and MAP-ST have led to an improved reconstruction. It is also noted that MAP-T and MAP-ST results are similar. This is confirmed by the MSE results given above.
Fig. 8.
Normalized reconstructed dynamic images by different reconstruction methods at two time points: (a) at time 1.78 min and (b) at time 4.03 min.
In addition, we also show in Fig. 9 the reconstructed LV from MAP-ST method at three time points (1.78 min, 6.09 min and 11.91 min, representing early, middle, and late stages of the imaging period). Interestingly, the following can be observed: the perfusion defect region (Fig. 3) was initially dimmer than the rest of the LV wall in the early stage (1.78 min), reflecting slower uptake; it then reversed to become brighter in the late stage (11.91 min), reflecting slower washout. These results were consistent with the dynamic TACs in Fig. 1. Such a temporal behavior in tracer redistribution provides an important indicator of the perfusion defect.
Fig. 9.
MAP-ST reconstructed dynamic images at three time points (1.78 min, 6.09 min and 11.91 min, representing early, middle, and late stages of the imaging period).
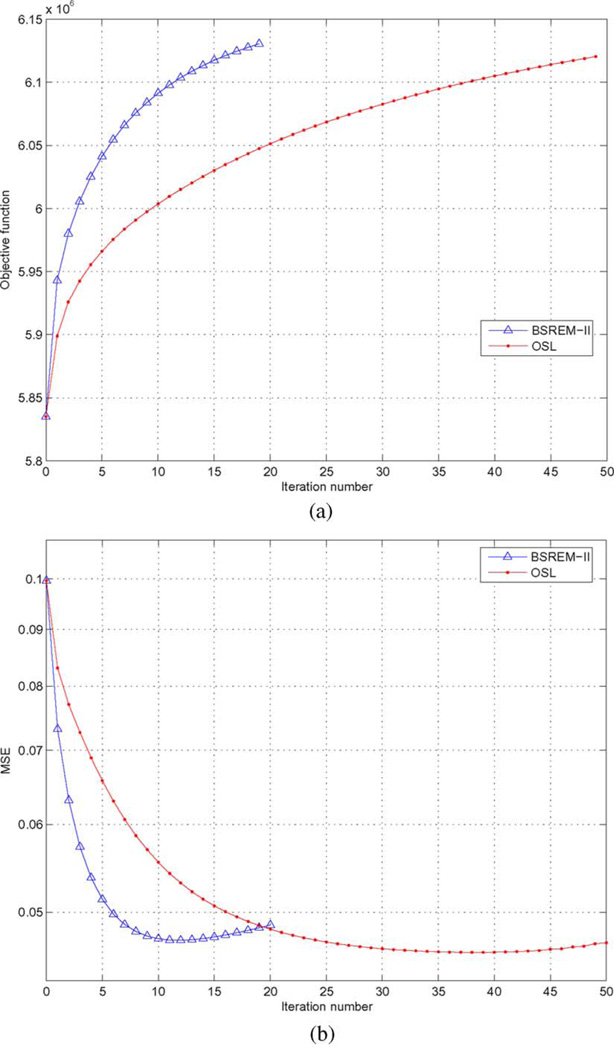
E. Numerical Convergence
Finally, to demonstrate the numerical convergence of the reconstruction algorithms, in Fig. 10(a) we plot the objective function J (F̃) in (9) versus the number of iterations for both BSREM-II and OSL in a typical run (βs = 0, βm = 2 × 10−5); in Fig. 10(b) we show the corresponding MSE of the reconstructed myocardium ROI versus the number of iterations. The results reported above were all obtained by BSREM-II with 10 iterations.
Fig. 10.
(a) Objective function values versus the number of iterations for BSREM-II and OSL with same priors βs = 0 and βm = 2 × 10−5; (b) MSE versus the number of iterations for BSREM-II and OSL with same prior βs = 0 and βm = 2 × 10−5.
V. CONCLUSION
In this study we developed a fully 5D image reconstruction procedure for gated dynamic cardiac SPECT imaging in the absence of multiple fast camera rotations. To combat the difficulty associated with the extremely ill-conditioned nature of the undersampled noisy data, we applied a dynamic constraint on the temporal activities in addition to spatial and motion-compensated gated regularization in the reconstruction procedure. We developed a modified BSREM algorithm for fully 5D reconstruction, which was demonstrated to be numerically more efficient than a previously developed OSL algorithm for gated dynamic SPECT. Our evaluation results demonstrate that with fully 5D reconstruction one can indeed obtain a sequence of gated dynamic images from a single acquisition, which provides information simultaneously for both time-varying tracer distribution in the myocardium and wall motion. Encouraged by these results, in future studies we plan to further develop and evaluate the reconstruction procedure by inclusion of other data degrading factors toward the ultimate goal of making 5D imaging a useful clinical tool.
Our numerical results indicate that the image activity can be underestimated at early stage in the normal myocardium and late stage in perfusion defect. Short of use of fast-rotation data, one possible solution to this problem could be to use list-mode data, which will likely improve the time resolution; in such a case, the likelihood function in (6) will need to be expressed in terms of an inhomogeneous Poisson rate function which relates to the tracer distribution function. This will be a subject of future study. In addition, in this work our main goal has been to study the feasibility of 5D imaging for reconstruction of the dynamic activities of the myocardium from slow-rotation SPECT data. As a result, in our study we have focused on the accuracy of reconstructed TACs of the myocardium (vis-a-vis normal and perfusion defects). As a next step, it would be interesting to further investigate other TACs such as the blood pool input function for use in the context of quantitative compartmental modeling.
Acknowledgments
This work was supported by the National Institutes of Health under Grant HL65425.
Appendix
Definition of Image Priors
The spatial energy function Us (F) in (7) is defined as
| (24) |
where ft,k (j) is the jth voxel in frame ft,k, 𝒩j is the unit-distance neighborhood around voxel j, and N is the number of voxels in ft,k. This quadratic penalty will enforce local spatial intensity smoothness in each image frame.
The energy function Um (F) in (7) is defined as
| (25) |
where Ml→k denotes the motion-compensated prediction operator from gate frame l to frame k, C is a normalization constant for unit DC gain, γ is a parameter to control the degree of smoothing among temporal frames, and ∥ … ∥ is the ℓ2 norm. In (25) the filter coefficients are defined so that temporally neighboring frames will have more contribution to the current frame than frames that are further apart. In our experiment, γ = 1 was used. In our previous work [5] the temporal prior used corresponds to a special case of (25) with γ = 0.
Contributor Information
Xiaofeng Niu, Email: niuxiaof@gmail.com, Department of Electrical and Computer Engineering, Illinois Institute of Technology, Chicago, IL 60616 USA.
Yongyi Yang, Email: yy@ece.iit.edu, Department of Electrical and Computer Engineering, Illinois Institute of Technology, Chicago, IL 60616 USA.
Mingwu Jin, Email: mingwu.jin@ucdenver.edu, Department of Radiology and C-TRIC, School of Medicine, University of Colorado Denver, Aurora, CO 80045 USA.
Miles N. Wernick, Email: wernick@iit.edu, Department of Electrical and Computer Engineering, Illinois Institute of Technology, Chicago, IL 60616 USA.
Michael A. King, Email: michael.king@umassmed.edu, Division of Nuclear Medicine, University of Massachusetts Medical Center, Worcester, MA 01655 USA.
References
- 1.Garcia EG. Imaging guidelines for nuclear cardiology procedures Part I. J. Nucl. Cardiol. 1996;vol. 3:G1–G46. [PubMed] [Google Scholar]
- 2.Feng B, Pretorius PH, Farncombe TH, Dahlberg ST, Narayanan MV, Wernick MN, Celler AM, Leppo JA, King MA. Simultaneous assessment of cardiac perfusion and function using 5-dimensional imaging with Tc-99m Teboroxime. J. Nucl. Cardiol. 2006;vol. 13(no. 3):354–361. doi: 10.1016/j.nuclcard.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Feng B, Pretorius PH, Farncombe TH, Dahlberg ST, Narayanan MV, Wernick MN, Celler AM, King MA, Leppo JA. Imaging time-varying Tc-99m Teboroxime localization and cardiac function simultaneously by five-dimensional (5D) gated-dynamic SPECT imaging and reconstruction. J. Nucl. Cardiol. 2003;vol. 10:S11–S12. [Google Scholar]
- 4.Farncombe TH, King MA, Celler AM, Blinder S. A fully 4D expectation maximization algorithm using Gaussian diffusion based detector response for slow camera rotation dynamic SPECT. Proc. 6th Meeting Fully Three-Dimesional Image Reconstruct. Radiol. Nucl. Med. 2001:129–132. [Google Scholar]
- 5.Jin M, Yang Y, Wernick MN, King MA. Reconstruction of dynamic gated cardiac SPECT. IEEE Nucl. Sci. Symp. Conf. Rec. 2005:2342–2345.
- 6.Jin M, Yang Y, Wernick MN, King MA. Motion-compensated dynamic image reconstruction for gated cardiac SPECT. Proc. IEEE Intr. Symp. Biomed. Imag.:Macro to Nano. 2006:267–270. [Google Scholar]
- 7.Jin M, Yang Y, King MA. Reconstruction of dynamic gated cardiac SPECT. Med. Phys. 2006;vol. 33(no. 11):4384–4394. doi: 10.1118/1.2358201. [DOI] [PubMed] [Google Scholar]
- 8.Ahn S, Fessler JA. Globally convergent image reconstruction for emission tomography using relaxed ordered subsets algorithms. IEEE Trans. Med. Imag. 2003;vol. 22(no. 5):613–626. doi: 10.1109/TMI.2003.812251. [DOI] [PubMed] [Google Scholar]
- 9.Jin M, Yang Y, Wernick MN, King MA. Fast dynamic image reconstruction for gated cardiac SPECT. IEEE Nucl. Sci. Symp. Conf. Rec. 2006:2281–2284.
- 10.Gilland DR, Mair BA, Bowsher JE, Jaszczak RJ. Simultaneous reconstruction and motion estimation for gated cardiac ECT. IEEE Trans. Nucl. Sci. 2002;vol. 49(no. 5):2344–2349. [Google Scholar]
- 11.Lalush DS, Tsui BMW. Block-iterative techniques for fast 4D reconstruction using a prior motion models in gated cardiac SPECT. Phys. Med. Biol. 1998;vol. 43:875–886. doi: 10.1088/0031-9155/43/4/015. [DOI] [PubMed] [Google Scholar]
- 12.Brankov JG, Yang Y, Wernick MN. Spatio-temporal processing of gated cardiac SPECT images using deformable mesh modeling. Med. Phys. 2005;vol. 32(no. 9):2839–2849. doi: 10.1118/1.2013027. [DOI] [PubMed] [Google Scholar]
- 13.Gravier E, Yang Y. Motion-compensated reconstruction of tomographic image sequences. IEEE Trans. Nucl. Sci. 2005;vol. 52(no. 1):51–56. [Google Scholar]
- 14.Farncombe TH. Ph.D. dissertation. West Mall Vancouver, BC, Canada: Univ. of British Columbia; 2000. Functional dynamic SPECT imaging using a single slow camera rotation. [Google Scholar]
- 15.Reutter BW, Gullberg GT, Huesman RH. Effects of temporal modelling on the statistical uncertainty of spatiotemporal distributions estimated directly from dynamic SPECT projections. Phys. Med. Biol. 2002;vol. 47:2673–2683. doi: 10.1088/0031-9155/47/15/309. [DOI] [PubMed] [Google Scholar]
- 16.Gravier E, Yang Y, King MA, Jin M. Fully 4D motion-compensated reconstruction of cardiac SPECT images. Phys. Med. Biol. 2006;vol. 51:4603–4619. doi: 10.1088/0031-9155/51/18/010. [DOI] [PubMed] [Google Scholar]
- 17.Pierro ARD, Yamagishi MEB. Fast EM-like methods for maximum ’A Posteriori’ estimates in emission tomography. IEEE Trans. Med. Imag. 2001;vol. 20:280–288. doi: 10.1109/42.921477. [DOI] [PubMed] [Google Scholar]
- 18.Herman GT, Meyer LB. Algebraic reconstruction techniques can be made computationally efficient. IEEE Trans. Med. Imag. 1993;vol. 12:600–609. doi: 10.1109/42.241889. [DOI] [PubMed] [Google Scholar]
- 19.Lange K. Convergence of EM image reconstruction algorithms with Gibbs smoothing. IEEE Trans. Med. Imag. 1990;vol. 9:439–446. doi: 10.1109/42.61759. [DOI] [PubMed] [Google Scholar]
- 20.Segars WP. Ph.D. dissertation. Chapel Hill, NC: Univ. of North Carolina; 2001. Development of a new dynamic NURBS-based cardiac-torso (NCAT) phantom. [Google Scholar]