Abstract
Background
Epidemiological studies demonstrate an inverse relation between dietary flavonoid intake and cardiovascular risk. Recent studies with flavonoid-containing beverages suggest that the benefits of these nutrients may relate, in part, to improved endothelial function.
Objective
We hypothesized that dietary supplementation with epigallocatechin gallate (EGCG), a major catechin in tea, would improve endothelial function in humans.
Design
We examined the effects of EGCG on endothelial function in a double blind, placebo-controlled, crossover design study. We measured brachial artery flow-mediated dilation by vascular ultrasound at six time points: prior to treatment with EGCG or placebo, two hours after an initial dose of EGCG (300 mg) or placebo, and after two weeks of treatment with EGCG (150 mg twice daily) or placebo. The order of treatments (EGCG or placebo) was randomized and there was a one-week washout period between treatments.
Results
A total of 42 subjects were enrolled, and brachial artery flow-mediated dilation improved from 7.1±4.1 to 8.6±4.7% two hours after the first dose of 300mg of EGCG (P=0.01), but was similar to baseline (7.8±4.2%, P=0.12) after two weeks of treatment with the final measurements made approximately 14 hours after the last dose. Placebo treatment had no significant effect, and there were no changes in reactive hyperemia or the response to sublingual nitroglycerin. The changes in vascular function paralleled plasma EGCG concentrations, which increased from 2.6±10.9 to 92.8±78.7 ng/ml after acute EGCG (P<0.001), but were unchanged from baseline after two weeks of treatment (3.4±13.1 ng/ml).
Conclusion
EGCG acutely improves endothelial function in humans with coronary artery disease, and may account for a portion of the beneficial effects of flavonoid-rich food on endothelial function.
Keywords: endothelial function, tea, epigallocatechin gallate, coronary artery disease, catechins, flavonoids
Introduction
Fruit and vegetable consumption is associated with reduced risk for cardiovascular disease, and this benefit has been attributed to a number of micronutrients, including flavonoids.[1,2] The majority of epidemiological studies demonstrate a benefit of higher intake of tea and other flavonoid containing beverages.[3–6] While several cohort studies have failed to demonstrate such an association,[7,8] those results may reflect a higher overall intake of tea and other flavonoids in particular cohorts and/or other confounding factors, including socioeconomic status and how tea is prepared and served.[6]
Studies have also examined specific classes of flavonoids, and have demonstrated inverse relations between cardiovascular risk and intake of catechins.[9] The catechins are monomeric flavanols that include epicatechin, epigallocatechin, epigallocatechin gallate, and epicatechin gallate. They are found in a variety of foods including green and black tea, onions, grapes, apples, and chocolate. Many of these foods also contain also contain polymeric flavanols such as thearubingins and theaflavins, which are found in fermented teas, and procyanidins, which are found in red grapes, red wine, apples, and chocolate. The mechanisms accounting for the benefits of flavanol and, specifically, catechin intake remain incompletely defined, however, recent studies suggest that these nutrients may act, in part, by improving endothelial function.[6,10–14]
The endothelium plays a central role in the regulation of vascular homeostasis, and maintenance of the normal vasodilator, anti-inflammatory, anti-thrombotic, and anti-proliferative properties of the endothelium may reduce cardiovascular risk.[15,16] We recently observed that acute and chronic black tea consumption reverses impaired flow-mediated dilation in patients with coronary artery disease,[10] a response that reflects, in part, endothelial production of nitric oxide and other vasodilators.[17] Experimental studies have shown that flavanols, including epigallocatechin gallate (EGCG), enhance production and/or bioavailability of endothelium-derived nitric oxide in cultured endothelial cells and isolated arterial tissue.[18–21] In an effort to better understand the specific components of tea that improve endothelial function, the present study examined the effects of EGCG supplementation on brachial artery endothelial function.
Methods
Study Subjects
We enrolled consecutive, clinically stable, patients with coronary artery disease receiving care at Boston Medical Center. Coronary artery disease was defined angiographically by the presence of at least one coronary stenosis (>50%) or by a documented history of myocardial infarction. We excluded subjects taking supplemental vitamin C (> 60 mg/day) or vitamin E (>30 IU/day) within 30 days of entry. Individuals receiving lipid-lowering or angiotensin converting enzyme inhibitor therapy were required to be on stable doses for at least one month prior to study entry. The Boston University Medical Center Institutional Research Board approved the study, and all volunteers provided written, informed consent.
Study Design
We examined the effect of EGCG on vascular function in a five-week, double blind, placebo-controlled, crossover study. Subjects received EGCG 150 mg twice daily (TEAVIGO®, DSM Nutritional Products, Inc., Parsippany, NJ) and placebo (gelatin capsules visually identical to EGCG capsules, supplied by DSM Nutritional Products, Inc., Parsippany, NJ) for two weeks for each treatment with a one-week washout period between treatments. The order of treatments was determined using computer-generated random numbers. Subjects visited our clinical research unit at the beginning and end of each of the two-week treatment periods. Prior to each visit, they discontinued all vasoactive medications for at least 24 hours and current smokers were asked to refrain from smoking for at least 12 hours. We also asked subjects to completely refrain from tea and red wine consumption during the 5-week study period.
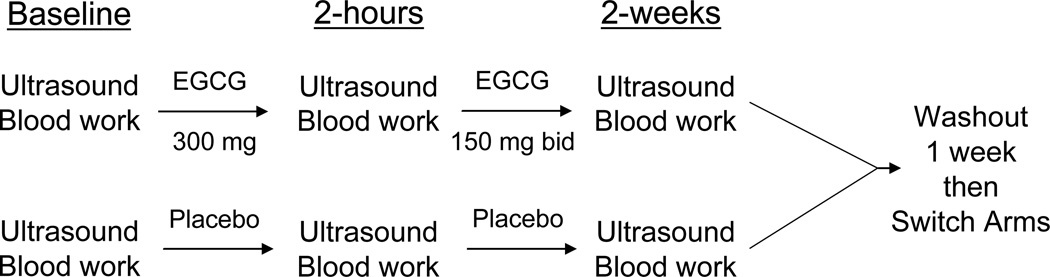
The study design is outlined in Figure 1. We tested vascular function at six time points: 1) before EGCG; 2) two hours after an initial 300mg dose of EGCG; 3) after two weeks of EGCG 150 mg twice daily; 4) before placebo; 5) two hours after placebo; and 6) after two weeks of placebo. The two-hour time point doses of EGCG and placebo were administered with a light snack consisting of a bagel and standardized portions of margarine and jelly. We chose this time point for measurement of the acute effect of EGCG given the known pharmacokinetics in humans (1.5–1.6 hours to peak plasma concentration)[22] and our prior study showing a benefit of tea consumption at two hours.[10] During the two-week treatment portions of the study, subjects were instructed to take EGCG or placebo capsules with their morning and evening meals to limit the risk of gastrointestinal discomfort, based on the manufacturer’s recommendations. We confirmed compliance by pill count. Subjects took their last dose of study medication the evening before the follow-up time points (3 and 6) or approximately 14 hours prior to measurement of vascular function. Thus, the studies performed at the end of two weeks of treatment were completed at the time of “trough” EGCG plasma concentration. At each of the six time points, we collected a blood sample and then measured blood pressure and heart rate using an automatic hemodynamic monitor after the subject rested supine for at least 10 minutes (Johnson and Johnson, Inc., New Brunswick, NJ). The blood pressure and heart rate measurements were made three times five minutes apart and averaged.
Figure 1.
Outline of Study Design. Subjects were randomly assigned to either the EGCG or Placebo arm first and then crossed over to the other treatment. Bid = twice daily.
Vascular Function Assessment
Endothelium-dependent flow-mediated dilation, endothelium-independent nitroglycerin-mediated vasodilation (0.4mg sublingual), and hyperemic flow in the brachial artery were determined by two-dimensional and Doppler ultrasound with the occlusion cuff on the upper arm as previously described.[23,24] The nitroglycerin portion of the study was omitted if systolic blood pressure was less than 100 mmHg, if the subject had a history of migraine headaches, or if he or she reported a previous history of prior adverse reaction to nitroglycerin. Ultrasound images (Powervision 6000, Toshiba Medical, Inc., Tustin, CA) were digitized online using customized hardware (Cardiovascular Engineering, Inc., Holliston, MA). Investigators blinded to treatment assignment performed all analyses using commercially available software (Brachial Analyzer, Medical Imaging Applications, Iowa City, IA). In a recent reproducibility study of our analysis methodology, we found inter- and intra-observer correlation coefficients to be 0.99 and 0.99, respectively, for brachial diameter and 0.93 and 0.89, respectively, for percent flow mediated dilation in a population of twenty individuals. The average differences between two determinations two hours apart for the same individual were 0.03±0.03mm for brachial diameter and 0.93±0.66% for percent flow mediated dilation. The mean difference and standard deviation for two determinations one month apart in twenty-five individuals for flow-mediated dilation were 1.96±1.16%.
Biochemical Analysis
We measured serum markers of cardiovascular disease risk at each visit (prior to the standard meal) and we measured plasma EGCG concentrations at the same six time points used for evaluation of vascular function. We also evaluated the safety of EGCG treatment by measuring renal, hepatic, muscle, and thyroid function at baseline and each of the two-week treatment periods. Serum glucose, blood urea nitrogen (BUN), creatinine, sodium, potassium, creatine kinase, calcium, total cholesterol, high density lipoprotein, triglycerides, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase and total bilirubin were determined in freshly collected serum samples by automated analyzer (Bayer Advia 1640) and automated complete blood count was performed in the Boston Medical Center Clinical Laboratories. Thyroid Stimulating Hormone (TSH) and Free Thyroxine (FT4) were determined by chemiluminescence measured by automated analyzer (Bayer-Centaur). LDL cholesterol was calculated using the Friedewald formula.[25] Samples for EGCG concentration were prepared by mixing one ml of heparinized plasma with 1 ml of stabilizing buffer (1% ascorbic acid and 0.01% EDTA). Plasma EGCG concentration was measured by high-performance liquid chromatography with electrochemical detection.[26] Serum C-reactive protein was measured using a high-sensitivity nephelometric method on a commercial basis by the Brigham and Women’s Hospital Department of Laboratory Medicine as previously described with a limit of detection of 0.17 mg/L.[27] Serum intracellular adhesion molecule-1 (ICAM-1) concentrations were measured with a commercially available enzyme linked immunoassay kit (R&D Systems, Minneapolis, MN).[28] Samples for C-reactive protein, ICAM-1, and EGCG were frozen at –80 degrees Celsius until analyzed.
Statistical Analysis
The primary endpoint of the study was the effect of treatment (EGCG and placebo) on brachial artery flow-mediated dilation, expressed as percent change in diameter and calculated as the difference between peak brachial artery diameter at one minute following cuff release and baseline brachial diameter, divided by the baseline brachial artery diameter as previously described.[23,24] Secondary endpoints included the effect of treatment on flow-mediated dilation expressed as absolute change in diameter (millimeters), nitroglycerin-mediated dilation, baseline brachial artery diameter, hyperemic flow, blood pressure, heart rate, and the biochemical analyses. These analyses were completed using two-way mixed repeated measures analysis of variance (ANOVA) with terms for treatment and treatment order. When the overall model was statistically significant (P<0.05), we completed post hoc pairwise comparisons using the Student-Newman Keuls test. The study was prospectively designed to have 90% power (α=0.05) to detect a 1.5 percentage point improvement in flow-mediated dilation with a sample size of 40 subjects. We examined the comparability of the EGCG-First and Placebo-First groups using the unpaired t or χ2 tests for continuous and categorical variables, respectively. Since, the risk factor profile differed between groups, we also completed an analysis of each group separately. We examined the relation between change in flow-mediated dilation and change in EGCG concentration using the Pearson correlation coefficient. Variables are presented as mean ± SD, except as otherwise indicated.
Results
Baseline Characteristics
A total of 54 subjects with coronary artery disease were enrolled into the study. Five subjects did not finish the protocol because of adverse events; one subject suffered sudden cardiac death eleven days into placebo treatment, one subject developed unstable angina during the washout period after completing placebo treatment, one subject had a recurrence of previously treated superficial transitional cell carcinoma of the bladder discovered on routine follow-up cystoscopy, one subject had recurrence of lower extremity cellulitis, and one subject developed a mild rash one day after starting EGCG. With the exception of possibly the rash, these adverse events were judged by the investigators to be unrelated to the study, and all events were reported to Boston University Institutional Research Board and to the study sponsor. Four subjects were dropped from the study for protocol violations. Three other subjects were eliminated from analysis due to poor ultrasound image quality, which was determined in a blinded fashion prior to image analysis.
Table 1 describes the baseline characteristics of this 42-subject cohort and the characteristics of each treatment order subgroup. The two groups were similar in terms of age, gender, and race. Despite the randomized group assignment, however, the EGCG-First subjects had significantly higher systolic and diastolic blood pressures, and total cholesterol concentrations. There also was a non-significant trend for higher fasting glucose concentrations in the EGCG-First group.
Table 1.
Demographics
| Characteristic | All Subjects (n=42) |
Placebo-First (n=21) |
EGCG-First (n=21) |
|---|---|---|---|
| Age (yrs) | 59±9 | 58±10 | 59±8 |
| Men, n (%) | 29 (69%) | 14 (64%) | 15 (75%) |
| Race, n (% Black) | 9 (21%) | 4 (18%) | 5 (25%) |
| Diabetes mellitus, n (%) | 12 (29%) | 5 (23%) | 7 (35%) |
| Family history of coronary disease | 21 (50%) | 10 (45%) | 11 (55%) |
| History of Hypertension | 32 (76%) | 15 (68%) | 17 (85%) |
| History of Smoking (ever) | 32 (76%) | 18 (82%) | 14 (70%) |
| Statin therapy n (%) | 35 (83%) | 19 (91%) | 16 (76%) |
| ACE inhibitor therapy n (%) | 19 (45%) | 8 (38%) | 13 (62%) |
| Systolic blood pressure (mmHg) | 134±28 | 124±31 | 144±18* |
| Diastolic blood pressure (mmHg) | 76±9 | 72±8 | 80±9† |
| Total Cholesterol (mg/dl) | 179±33 | 169±26 | 190±26** |
| LDL Cholesterol (mg/dl) | 101±23 | 94±19 | 108±25 |
| HDL Cholesterol (mg/dl) | 46±10 | 45±11 | 47±9 |
| Triglycerides (mg/dl) | 163±66 | 151±52 | 176±76 |
| Fasting Glucose (g/dl) | 124±43 | 117±41 | 130±45 |
p=0.02 vs. Placebo-First,
p=0.05 vs. Placebo-First,
p=0.04 vs. Placebo-First. Data are mean ± SD or number and percentage, as indicated.
Lipids, Inflammatory Markers, and Safety Measures
Table 2 displays the effects of the two treatments on lipid profiles, C-reactive protein concentrations, and ICAM-1 concentrations. As shown, these serum markers remained stable over time and were not affected by EGCG treatment. Furthermore, EGCG treatment had no effect on liver function, renal function, thyroid function, creatine kinase, or serum electrolytes, providing evidence for the safety of EGCG treatment at this dose and duration (data not shown).
Table 2.
Serum and Plasma Markers
| Marker | Baseline Placebo |
2 Weeks Placebo |
Baseline EGCG |
2 Weeks EGCG |
P-Value |
|---|---|---|---|---|---|
| Total Cholesterol (mg/dl) | 179±33 | 176±35 | 178±30 | 176±30 | 0.74 |
| HDL (mg/dl) | 46±10 | 45±12 | 45±10 | 46±10 | 0.57 |
| LDL (mg/dl) | 101±23 | 98±32 | 100±30 | 100±25 | 0.84 |
| Triglycerides (mg/dl) | 163±66 | 163±81 | 162±65 | 150±66 | 0.31 |
| CRP (mg/L) | 3.1±2.5 | 3.0±3.1 | 3.2±2.8 | 2.8±2.6 | 0.75 |
| ICAM-1 (ng/ml) | 290 ± 105 | 284 ± 108 | 259 ± 72 | 278 ± 67 | 0.16 |
Treatments compared by two-way mixed measures ANOVA; n=41 for lipid profile; n=36 for C-reactive protein; n=42 for ICAM-1. Data are mean ± SD.
Vascular Function
Table 3 presents the effects of EGCG and placebo treatment on vascular function. For brachial artery flow-mediated dilation expressed as percent change from baseline (the primary endpoint), there was a significant effect of treatment overall by repeated measures ANOVA. As shown in Table 3, there a significant increase in flow-mediated dilation two hours after a dose of EGCG compared to before EGCG (P=0.01). However, flow-mediated dilation after two weeks of EGCG was not different from before EGCG (P=0.12). Two-hour and two-week placebo treatment had no effect on flow-mediated dilation (P=0.58 and 0.74, respectively). Flow-mediated dilation before EGCG and before Placebo was not different (P=0.11). When flow-mediated dilation was expressed as absolute change (millimeters), which is less dependent on baseline brachial artery diameter,17 the findings were similar with an improvement at the two hour time point (P=0.008), but not after two weeks of EGCG treatment (P=0.85).
Table 3.
Blood Pressure and Vascular Function
| Baseline Placebo |
2 Hours Placebo |
2 Weeks Placebo |
Baseline EGCG |
2 Hours EGCG |
2 Weeks EGCG |
|
|---|---|---|---|---|---|---|
| SBP (mmHg) | 133±28 | 134±18 | 134±20 | 134±17 | 136±20 | 135±17 |
| DBP (mmHg) | 74±8 | 75±9 | 74±8 | 75±9 | 75±10 | 74±8 |
| Brachial diameter (mm) | 4.51±0.77 | 4.52±0.76 | 4.56±0.68 | 4.51±0.68 | 4.53±0.72 | 4.50±0.72 |
| Hyperemic Flow (cm/sec) | 69±21 | 68±22 | 79±26 | 84±52 | 70±27 | 68±25 |
| Flow-mediated dilation (%) | 8.0±4.4 | 8.6±4.2 | 8.2±4.1 | 7.1±4.1 | 8.6±4.7* | 7.8±4.2 |
| Flow-mediated dilation (mm) | 0.34±0.15 | 0.38±0.16 | 0.36±0.15 | 0.31±0.17 | 0.38±0.19† | 0.33±0.14 |
| Nitroglycerin-mediated dilation(%) | 12.1±6.8 | 13.0±7.2 | 11.4±5.7 | 9.9±4.6 | 10.5±4.7 | 10.8±7.0 |
SBP=systolic blood pressure; DBP=diastolic blood pressure;
P=0.01 vs. before EGCG and
P=0.008 vs. before EGCG by post hoc comparison. n=35 for hyperemic flow; n=22 for nitroglycerin-mediated dilation; n=42 for all other measurements. Data are mean ± SD.
As shown in Table 3, EGCG and Placebo had no effect on systolic blood pressure (P=0.80), diastolic blood pressure (P=0.57), baseline brachial artery diameter (P=0.95), or hyperemic flow (n=35, P=0.11). There also was no effect of treatment on the vasodilator response to nitroglycerin (n=22, P=0.15).
As noted above, subjects in the EGCG-First group had significantly higher blood pressures and total cholesterol concentrations than the Placebo-First group. At the beginning of the study, flow-mediated dilation was 6.2 ± 3.6% in the EGCG-First group and 8.0 ± 4.9% in Placebo-First group, but this difference was not statistically significant (P = 0.19). To explore the possibility that the differences in baseline risk factors might have confounded results, we completed an analysis of each of the two groups separately, recognizing that the small sample size for the individual groups (n=21 each) would limit statistical power. As for the group as a whole, there was significant improvement in flow-mediated dilation two hours following EGCG treatment in the Placebo-First group (P = 0.02), while flow-mediated dilation after two weeks of treatment was similar to before treatment (P=0.80). No significant differences were found with respect %FMD in the EGCG-First group, although directionally of %FMD between baseline and two hours after EGCG administration was similar to the group as a whole.
Vascular Function and Plasma EGCG Concentrations
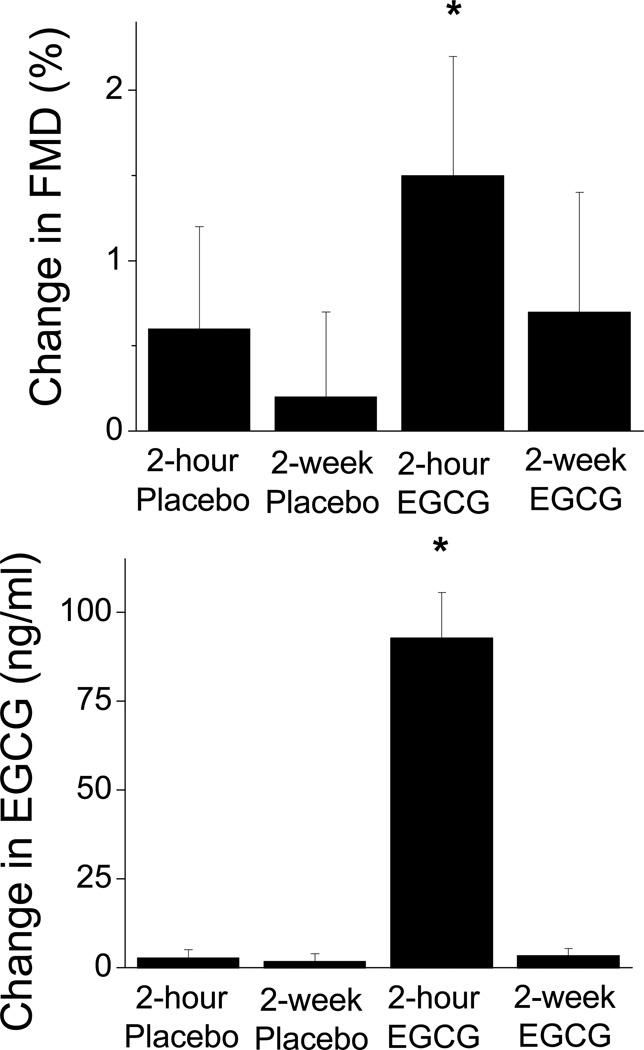
Overall, treatment affected EGCG concentration (P<0.001 by ANOVA). Plasma concentrations of EGCG were undetectable before placebo and were 2.8±13.7, 1.9±12.2 ng/ml after short-term and long-term Placebo treatment, respectively (P=NS). EGCG concentrations were 2.6±10.9 before EGCG and 92.8±78.7 and 3.4±13.1 ng/ml at the two-hour and two-week time points, respectively. As shown in Figure 2, only short-term EGCG produced a significant change in EGCG concentration compared to before treatment (P<0.001), and this change paralleled the change in vascular function. There was no correlation between change in flow-mediated dilation and change in EGCG concentration at the two-hour time point (P=0.52).
Figure 2.
Upper Panel: Changes in brachial artery flow-mediated dilation (FMD) compared to pre-treatment are displayed for Placebo and EGCG. FMD improved two hours after a single 300 mg dose of EGCG (*P=0.01), while FMD was not changed after two weeks of EGCG 150 mg twice daily (P=0.12). Placebo had no effect at the two-hour and two-week time points. Lower Panel: Changes in plasma EGCG concentration compared to pre-treatment concentration are displayed for Placebo and EGCG (n=38). Plasma ECGC concentrations were increased two hours after a single 300 mg dose of EGCG (P<0.001), while trough plasma EGCG concentrations (approximately 14 hours after the last dose) were unchanged after two weeks of EGCG 150 mg twice daily. Data are mean ± SEM.
Discussion
This study demonstrated that a single 300 mg dose of EGCG improves brachial artery flow-mediated dilation in patients with coronary artery disease. Acute EGCG treatment had no effect on reactive hyperemia (the stimulus for endothelial-dependent brachial artery vasodilation, primarily reflecting dilation of forearm microvessels), baseline diameter, or the dilator response to nitroglycerin, suggesting that the benefit cannot be attributed to changes in the stimulus for dilation, vascular geometry, or the general dilator capacity of vascular smooth muscle. Flow-mediated dilation at baseline was lower than our laboratory reference values for healthy subjects of comparable age (9.2 ± 6.2%, n=66 subjects mean age 59 without coronary disease, diabetes mellitus, or hypertension). Collectively these results suggest that acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. In contrast to the benefit of acute treatment, we observed no change in vascular function following two weeks of EGCG treatment when the measurements were made about 14 hours after the last dose. These findings parallel the changes in plasma EGCG concentrations, which were significantly elevated two hours after acute treatment, but were unchanged from baseline at the two-week time point.
The findings of the present study are consistent with several prior studies demonstrating improved endothelial function following acute consumption of a flavonoid-containing beverage.[10,13,29] We previously reported that brachial artery flow-mediated dilation improves two hours after consumption of black tea using the same methodology and patient population that we used in the present study.[10] It is notable that the relative improvement in flow-mediated dilation in our prior tea study (57%) was greater than the present study (21% following 300 mg of EGCG).[10] Although the total catechin content of the tea consumed in our prior study was only 60 mg (17.5 mg of which was EGCG), the total polyphenol content of the tea was higher (733 mg), possibly accounting for the proportionally greater effect. The importance of non-catechin components of tea is further emphasized by our prior observation that changes in flow-mediated dilation did not correlate with changes in plasma catechin concentrations following tea consumption.[30] Agewall and colleagues reported a similar relative improvement in flow-mediated dilation (44%) one hour after consumption of de-alcoholized wine with total polyphenol content of 1110 mg in healthy volunteers.[29] Heiss and colleagues observed a doubling of brachial artery flow-mediated dilation two hours after consumption of flavanol-rich cocoa with a catechin plus procyanidins content of 282 mg (total polyphenols were not reported).[13] Collectively, these findings suggest that EGCG has the ability to acutely improve endothelial function, but that other polyphenols or other components in these beverages have a similar effect. Thus, improvement in vascular function may relate most strongly to total polyphenols content of these beverages.
In addition to an acute effect, consumption of flavonoid-containing beverages, including black tea, also has a cumulative effect on endothelial function following chronic consumption that persists for at least 14 hours after the last beverage consumption.[10–12,31,32] The lack of after two weeks of EGCG treatment in the present study could reflect the relatively low dose in comparison to the total flavonoid content in flavonoid rich beverages. Moreover, the findings are consistent with the relatively short plasma half life of EGCG (approximately 4 hours)[22,33] as reflected by the return of plasma concentrations to baseline the morning after the last dose of EGCG. Thus, the effects of flavonoid-containing beverages on the vascular endothelium are unlikely to be attributable to catechins and are more likely attributable to other tea components or metabolites that persist in plasma and/or accumulate in vascular tissue.
Recent experimental studies provide information about how catechins and other polyphenols might improve vasodilator function. Endothelium-dependent relaxation and cyclic guanosine-3’,5’-monophosphate (cGMP) accumulation were greater in arterial tissue isolated from rats consuming de- alcoholized red wine or a catechin-rich diet compared to a control diet, and these effects were attributable to an increase in the activity, but not the expression of endothelial nitric oxide synthase (eNOS).[19] Epicatechin had comparable effects on eNOS activity in cultured endothelial cells.[34] Interestingly, polymeric procyanidins increased eNOS activity to a greater extent than monomers in aortic endothelial cells,[18] potentially providing insight into the greater effects of whole beverages compared to pure monomeric catechin. Recently, Lorenz and colleagues specifically examined the effects of EGCG on endothelial function.[21] They observed that EGCG produces eNOS-dependent relaxation of isolated rat aorta and increases eNOS activity in cultured endothelial cells. Under these conditions, activation of eNOS is associated with activation of phosphatidylinositol-3-hydroxy kinase and Akt and phosphorlyation of eNOS at serine 1179. These effects occurred in response to EGCG 1–50 µM, concentrations that are reasonably comparable to the plasma concentrations achieved in the present study (0.2 µM), particularly since we were unable to examine concentrations in vascular tissue. Thus, these experimental studies are consistent with our findings in human subjects and suggest that EGCG increases nitric oxide synthesis in endothelial cells. Further studies are needed to elucidate the upstream signals that account for this effect.
Flavonoids have a number of other effects that might reduce cardiovascular disease risk. Of recent interest is the contribution of systemic inflammation to atherosclerosis, and several studies suggest that catechins and other flavonoids have anti-inflammatory effects. We investigated the possibility that EGCG might have such an effect in human subjects by examining changes in the soluble form of the endothelial adhesion molecule ICAM-1, which is shed from the endothelial surface and correlates with cardiovascular risk factors and prevalent cardiovascular disease.[28] We also measured changes in C-reactive protein, a general marker of systemic inflammation and increasingly accepted marker of cardiovascular risk.[33] We observed no effect of EGCG on ICAM-1 or C-reactive protein in the present study, but these findings may be limited by the relatively short period of treatment. The results are consistent with previous data in smokers showing no significant change in C-reactive protein with four weeks of tea consumption.[34] In addition, the lack of effect on these markers likely relates to the relatively low dose of catechin and inability to achieve sustained plasma concentrations with 2 weeks of treatment.
Our study had a number of limitations. Endothelial function was examined in a peripheral artery and extension of the findings to more clinically relevant circulations must be made with caution. However, prior studies suggest a correlation between endothelial function of the brachial artery and that of coronary circulation.[35] We observed an imbalance in risk factor profile in the two treatment groups, which likely is chance finding that relates to the relatively small sample size. However, all subjects received both treatments and the results were directionally similar in both groups, and thus, there is no indication that this imbalance affected the results. Although not statistically significant, flow-mediated dilation was numerically lower before EGCG than before placebo, making it harder to interpret the results. Based on manufacturer experience, EGCG was administered after a standardized meal to avoid gastro-intestinal side effects, and it is possible that the food interfered with EGCG absorption and blunted the improvement in endothelial function, however, plasma levels of EGCG were markedly increased two hours after EGCG administration, confirming bioavailability. Finally, the study was completed in subjects with established cardiovascular disease. The effects of EGCG supplementation in lower risk populations and in patients not taking medications that known to influence endothelial function, including angiotensin-II converting enzyme inhibitors[36] and HMG-CoA reductase inhibitors[37–39] will require further study.
The present study may have clinical relevance. Endothelial dysfunction in the coronary and peripheral circulations is increasingly recognized as a useful barometer of cardiovascular risk,[15] and many interventions that improve endothelial function have been proven to lower cardiovascular disease risk.[16] The results of the present study provide further evidence that the inverse association between flavonoid consumption and cardiovascular disease may relate, in part, to improved endothelial function. Many of the prior studies examining flavonoid consumption and endothelial function involved ingestion of large amounts of beverage that would be difficult for most individuals to sustain over the long term. Administration of appropriate amounts of the active components of these beverages in supplement form clearly has therapeutic potential, although which components or combination of components will yield the greatest effect remains unknown. The present study demonstrates a benefit of acute EGCG treatment on endothelial function. Further studies will be needed to determine the relative merits of flavonoid consumption as supplements or whether consumption of these compounds in whole foods and beverages is preferable, as is currently recommended by the American Heart Association.[40]
Acknowledgments
This study was funded by a grant from DSM Nutritional Products, Inc. James G. Elliot is a full-time employee of DSM Nutritional Products, Inc., the company that supplied epigallocatechin gallate capsules for this study. Individual authors has the following contributions to this study: experimental design (MEW, JGE, DFK, JFK, JAV), data collection (MEW, MH, JAV), data analysis (MEW, JFK, JAV), manuscript writing (MEW, NH, JGE, JAV), and advice and consultation (NH, EA, JFK) The authors gratefully acknowledge the expert technical assistance of Carolyn Maxwell, Joseph Palmisano, and Ryan Beal.
Footnotes
No other author has a conflict of interest to report.
References
- 1.Bazzano LA, He J, Ogden LG, et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr. 2002;76:93–99. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- 2.Krauss RM, Eckel RH, Howard B, et al. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 3.Hertog MGL, Feskens EJM, Hollman PCH, et al. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 4.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 5.Peters U, Poole C, Arab L. Does tea affect cardiovascular disease? a meta-analysis. Am J Epidemiol. 2001;154:495–503. doi: 10.1093/aje/154.6.495. [DOI] [PubMed] [Google Scholar]
- 6.Vita JA. Tea consumption and cardiovascular disease: effects on endothelial function. J Nutr. 2003;133:3293S–3297S. doi: 10.1093/jn/133.10.3293S. [DOI] [PubMed] [Google Scholar]
- 7.Rimm EB, Katan MB, Ascherio A, et al. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann Intern Med. 1996;125:384–389. doi: 10.7326/0003-4819-125-5-199609010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Hertog MG, Sweetnam PM, Fehily AM, et al. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. Am J Clin Nutr. 1997;65:1489–1494. doi: 10.1093/ajcn/65.5.1489. [DOI] [PubMed] [Google Scholar]
- 9.Arts IC, Hollman PC, Feskens EJ, et al. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74:227–232. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- 10.Duffy SJ, Keaney JF, Holbrook M, Jr, et al. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104:151–156. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- 11.Hodgson JM, Puddey IB, Burke V, et al. Regular ingestion of black tea improves brachial artery vasodilator function. Clin Sci (Lond) 2002;102:195–201. [PubMed] [Google Scholar]
- 12.Stein JH, Keevil JG, Wiebe DA, et al. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999;100:1050–1055. doi: 10.1161/01.cir.100.10.1050. [DOI] [PubMed] [Google Scholar]
- 13.Heiss C, Dejam A, Kleinbongard P, et al. Vascular effects of cocoa rich in flavan-3-ols. JAMA. 2003;290:1030–1031. doi: 10.1001/jama.290.8.1030. [DOI] [PubMed] [Google Scholar]
- 14.Engler MB, Engler MM, Chen CY, et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 15.Vita JA, Keaney JF., Jr Endothelial function: A barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 16.Widlansky ME, Gokce N, Keaney JF, Jr, et al. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 17.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 18.Karim M, McCormick K, Kappagoda CT. Effects of cocoa extracts on endothelium-dependent relaxation. J Nutr. 2000;130:2105S–2108S. doi: 10.1093/jn/130.8.2105S. [DOI] [PubMed] [Google Scholar]
- 19.Benito S, Lopez D, Saiz MP, et al. A flavonoid-rich diet increases nitric oxide production in rat aorta. Br J Pharmacol. 2002;135:910–916. doi: 10.1038/sj.bjp.0704534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aldini G, Carini M, Piccoli A, et al. Procyanidins from grape seeds protect endothelial cells from peroxynitrite damage and enhance endothelium-dependent relaxation in human artery: new evidences for cardio-protection. Life Sci. 2003;73:2883–2898. doi: 10.1016/s0024-3205(03)00697-0. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz M, Wessler S, Follmann E, et al. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J Biol Chem. 2004;279:6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 22.Lee MJ, Maliakal P, Chen L, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 23.Duffy SJ, Vita JA, Holbrook M, et al. Effect of acute and chronic tea consumption on platelet aggregation in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2001;21:1084–1089. doi: 10.1161/01.atv.21.6.1084. [DOI] [PubMed] [Google Scholar]
- 24.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 26.Lee MJ, Wang ZY, Li H, et al. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol Biomarkers Prev. 1995;4:393–399. [PubMed] [Google Scholar]
- 27.Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 28.Keaney JF, Massaro JM, Larson MG, Jr, et al. Heritability and correlates of intercellular adhesion molecule-1 in the Framingham Offspring Study. J Am Coll Cardiol. 2004;44:168–173. doi: 10.1016/j.jacc.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 29.Agewall S, Wright S, Doughty RN, et al. Does a glass of red wine improve endothelial function? Eur Heart J. 2000;21:74–78. doi: 10.1053/euhj.1999.1759. [DOI] [PubMed] [Google Scholar]
- 30.Widlansky ME, Duffy SJ, Hamburg NM, et al. Effects of black tea consumption on plasma catechins, markers of oxidative stress and inflammation in patients with coronary artery disease. Free Radic Biol Med. 2004;38:499–506. doi: 10.1016/j.freeradbiomed.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Chou EJ, Keevil JG, Aeschlimann S, et al. Effect of ingestion of purple grape juice on endothelial function in patients with coronary heart disease. Am J Cardiol. 2001;88:553–555. doi: 10.1016/s0002-9149(01)01738-6. [DOI] [PubMed] [Google Scholar]
- 32.Fisher ND, Hughes M, Gerhard-Herman M, et al. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens. 2003;21:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Van Amelsvoort JM, Van Hof KH, Mathot JN, et al. Plasma concentrations of individual tea catechins after a single oral dose in humans. Xenobiotica. 2001;31:891–901. doi: 10.1080/00498250110079149. [DOI] [PubMed] [Google Scholar]
- 34.Huang Y, Chan NW, Lau CW, et al. Involvement of endothelium/nitric oxide in vasorelaxation induced by purified green tea (-)epicatechin. Biochim Biophys Acta. 1999;1427:322–328. doi: 10.1016/s0304-4165(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 35.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 36.Prasad A, Husain S, Quyyumi AA. Abnormal flow-mediated epicardial vasomotion in human coronary arteries is improved by angiotensin-converting enzyme inhibition: a potential role of bradykinin. J Am Coll Cardiol. 1999;33:796–804. doi: 10.1016/s0735-1097(98)00611-1. [DOI] [PubMed] [Google Scholar]
- 37.Egashira K, Hirooka Y, Kai H, et al. Reduction in serum cholesterol with pravastatin improves endothelium-dependent coronary vasomotion in patients with hypercholesterolemia. Circulation. 1994;89:2519–2524. doi: 10.1161/01.cir.89.6.2519. [DOI] [PubMed] [Google Scholar]
- 38.Leung WH, Lau CP, Wong CK. Beneficial effect of cholesterol-lowering therapy on coronary endothelium-dependent relaxation in hypercholesterolemic patients. Lancet. 1993;341:1496–1500. doi: 10.1016/0140-6736(93)90634-s. [DOI] [PubMed] [Google Scholar]
- 39.Anderson TJ, Meredith IT, Yeung AC, et al. The effect of cholesterol lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 40.Kris-Etherton PM, Lichtenstein AH, Howard BV, et al. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]