Abstract
Exposures associated with common agricultural activities may increase risk of stroke. The authors evaluated associations between self-reported agricultural activities including pesticide use and handling of crops and stroke mortality among 51,603 male pesticide applicators enrolled in the Agricultural Health Study (AHS). Vital status was obtained through 2008. Stroke mortality was defined by underlying or contributing cause of death (ICD-9 430–438, ICD-10 I60-I69). Information regarding lifetime pesticide use, working with crops or animals, engagement in other agricultural activities, and potential confounders was self-reported at enrollment. Cox proportional hazards models, with age as the time scale, were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) adjusted for state of residence, smoking status, and alcohol consumption. Median follow-up time was 13 years, during which 308 stroke deaths occurred. No measure of overall or specific pesticide use was positively associated with mortality due to stroke. Stroke mortality was inversely associated with handling hay, grain, or silage at least once each year as reported at enrollment (HR: 0.75; 95% CI: 0.58, 0.98). There was no evidence of an association between pesticide use and stroke mortality. The inverse association between handling of hays and grains and stroke mortality may be due to (1) those engaging in such activities being healthier than those who did not or (2) exposure to some biological agent present in hays and grains. Further investigation of incident stroke, rather than stroke mortality, as well as stroke subtypes are needed to determine the full role of agricultural exposures and stroke.
INTRODUCTION
Stroke is the third leading cause of death in the United States and the second leading cause globally(Grysiewicz et al. 2008). Stroke is responsible for a large proportion of long-term disability and health-care costs. Although there are well-established lifestyle and clinical risk factors for stroke, much of the risk remains unexplained (Ohira et al. 2006; Grysiewicz et al. 2008; Reynolds et al. 2003). Experimental and observational studies indicate that environmental exposures to air pollution, inhaled dust, and exogenous chemicals may contribute to the risk of cardiovascular disease, including stroke (Ha et al. 2007; Yorifuji et al. 2011; Lee et al. 2012; Mastin 2005; Yang et al. 2004). In addition, epidemiologic data provide some evidence to support associations between environmental exposures and well-established stroke risk factors including diabetes, metabolic syndrome, weight gain, and other cardiovascular conditions (Bar-Meir et al. 2007; Montgomery et al. 2008; Roth et al. 1993; Saadeh et al. 1997). Possible biological pathways through which these environmental exposures may operate include oxidative stress and endothelial and vascular damage leading to pro-inflammatory events or through disturbances in coagulation (Bombick et al. 1984; Ha et al. 2007; Hennig et al. 2002; Lovati et al. 1984; Toborek et al. 1995; Ghio et al. 2012; Park et al. 2010).
Farmers and other agricultural workers come into contact with a variety of environmental exposures including pesticides and inhaled dusts from crops and animals as part of their work(Coble et al. 2002; Thomas et al. 2010; Hines et al. 2011; Poole et al. 2010; Burch et al. 2010). Few studies have examined the association between these farming-related exposures and stroke or related mortality. As farmers are known to have lower rates of morbidity and mortality due to cerebrovascular disease and other associated conditions as compared to the general public(Blair et al. 2005; Mills et al. 2009; Waggoner et al. 2011), the Agricultural Health Study (AHS) provides a unique opportunity to examine associations between a range of agricultural exposures and stroke mortality in a large cohort of pesticide applicators, consisting mainly of farmers. The present study aimed to determine if exposure to pesticides or other farming activities is associated with stroke mortality among participants of the AHS.
METHODS
Study Population
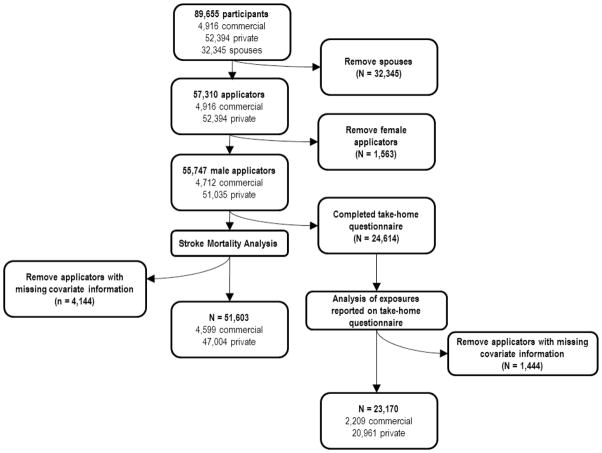
The AHS is a large, prospective cohort study of licensed pesticide applicators and their spouses residing in North Carolina and Iowa (N = 89,655). The study design has been described in detail elsewhere (Alavanja et al. 1996). Briefly, private pesticide applicators (mainly farmers, N=53,394) and their spouses (N = 32,345) and commercial pesticide applicators (N = 4,916) enrolled in the cohort between 1993 and 1997. Applicators enrolled in the study by completing a self-administered questionnaire which collected information about pesticide use, farming activities, demographics, and health, when they applied for, or renewed their pesticide license. Approximately 44% of applicators returned, by mail, a take-home questionnaire, which included additional information regarding work-related activities (copies of both questionnaires are available at the AHS web site http://www.aghealth.org/questionnaires.html).
The present study was restricted to male private and commercial pesticide applicators (Figure 1). Further, 4,144 applicators (7%) (82 stroke deaths) with missing information on either smoking or alcohol consumption were excluded leaving 51,603 applicators for the present analysis.
Fig. 1.
Illustration of participants included in the present analysis of the association between agricultural activities and stroke mortality in the Agricultural Health Study Cohort, 1993–2008.
Stroke Mortality
The cohort is linked regularly to state and national death registries to determine vital status of all participants. Through this linkage, underlying and contributing causes of death were obtained from enrollment through December 31, 2008, the most recent year available. Mortality from stroke was identified as either the underlying or a contributing cause of death on the death certificate, according to the International Classification of Diseases, Ninth Revision (ICD-9), codes (430–438), or Tenth Revision (ICD-10), codes I60-I69 (World Health Organization 2009). ICD-9 codes were converted to equivalent ICD-10 codes. Although information was available for type of stroke (ischemic, hemorrhagic, and unspecified subtypes) for some decedents, results from analyses examining all stroke mortality only are presented due to a high proportion of unspecified cases (65%) a problem that has been previously noted throughout the United States (Cheng et al. 2012).
Exposure Assessment
As part of the enrollment questionnaire, participants reported overall lifetime days of pesticide use, including total years and days/year of pesticide use. Participants reported ever use of 50 commonly used pesticides and lifetime frequency and duration of use for 22 of those pesticides. Information regarding use of chemical-resistant gloves while handling pesticides was also reported. Use of chemical-resistant gloves has been demonstrated in the AHS cohort to be the item of personal protective equipment associated with the greatest reduction in pesticide exposure among those examined (Hines et al. 2011; Thomas et al. 2010; Waggoner et al. 2011) and consequently this was considered as a marker of reduced exposure and protective behavior.
Participants reported characteristics of the farm where they live or work including the size of the farm and the number and type of livestock and poultry raised. Information regarding the frequency of performance of agricultural activities was also collected. Participants reported handling hays, grains, or silage, or performing work in swine or poultry confinement areas at least once each year at enrollment.
As part of the take-home questionnaire, participants reported detailed use information on the remaining 28 pesticides for which this information was not provided on the enrollment questionnaire, as well as a history of doctor-diagnosed pesticide poisoning, or having experienced a high-pesticide exposure event, defined as an “incident or experience while using any type of pesticide which caused you unusually high personal exposure.” Individuals responding to the take-home questionnaire provided more detailed information regarding frequency of farm activities including use of diesel and gasoline tractors, trucks, and combines.
Statistical Analyses
The distribution of demographic and health characteristics was examined within the study population. A Poisson regression model was used to generate incidence rate ratios (IRR) adjusted for age in categories and 95% confidence intervals (CI) illustrating the association between each demographic and behavioral characteristic and stroke mortality. Separate directed acyclic graphs (DAGs) were created to represent the association between each category of exposure (pesticide use, handling of crops, presence of animals, farm characteristics) and stroke mortality.
All multivariable analyses were conducted using Cox proportional hazards models with age as the time scale in order to produce hazard ratios (HR) and 95% CIs. For each category of exposure, covariates identified as potential confounders based on DAGs were further examined to determine the association between the covariate and exposure and the covariate and outcome.
Body mass index (<25, 25–29.9, ≥30 kg/m2), ever receiving a doctor diagnosis of diabetes (yes/no), use of chemical-resistant gloves (yes/no), and state of residence (Iowa, North Carolina) were considered as potential effect measure modifiers and race/ethnicity (non-Hispanic white vs. other) and education (high school or less vs. beyond high school) as potential confounders of each association. A backward elimination model building approach was used to determine the final model. No evidence of modification based on stratum-specific estimates and likelihood ratio tests (α=0.10) was observed and consequently the previously mentioned potential modifiers were assessed for confounding. A change-in-estimate (<0.10) approach was used to identify confounders. Final models were adjusted for state of residence (Iowa, North Carolina), smoking status (never, past, current), and alcohol consumption (none, 1–4, 5–30, >30 drinks/month). In order to maintain statistical stability, analyses were only performed for exposures with at least 5 cases in each level of exposure. Consequently, no analyses were performed examining use of individual pesticides with fewer than 5 exposed or unexposed cases.
Our analysis included all stroke-related deaths occurring within the study population during the study period. Some of those included could have experienced a non-fatal stroke prior to enrollment. Therefore, the impact of this choice was assessed by limiting the analysis to only those participants with stroke identified as the underlying cause of death (N=187). In addition, as older participants may be in poorer health at baseline and poor health status may have a stronger effect on stroke death than agricultural exposures, analyses restricted to the study population younger than 65 years old at enrollment were conducted. Results from these additional analyses were similar to those with no restrictions and therefore are not presented here.
Prior to excluding pesticide applicators with missing information on smoking or alcohol consumption, the distribution of missing data was examined (see Table, Supplemental Digital Content 1). Missing data for smoking and alcohol consumption were determined to be not missing completely at random. To determine the impact of this exclusion on results, a set of assumptions regarding the distribution of missing information were tested. All analyses were conducted under the following assumptions: 1) all those missing information were considered current smokers/heavy drinkers; 2) all those missing information were considered never smokers/never drinkers; 3) all cases missing information were current smokers/heavy drinkers and all non-cases were never smokers/never drinkers; and, 4) all cases missing information were never smokers/never drinkers and all non-cases were current smokers/heavy drinkers. These analyses produced similar results to the complete-case analysis and consequently it was determined that exclusion of these individuals did not significantly bias results and therefore, only results from the complete-case analysis are presented here.
All statistical analyses were completed in SAS, version 9.2 (SAS Institute, Inc., Cary, North Carolina) using the AHS data releases P1REL0906.00 and REL0905.00. The AHS was reviewed and approved by institutional review boards at the National Institutes of Health and its contractors. This analysis was also reviewed and approved by the institutional review board at the University of North Carolina at Chapel Hill.
RESULTS
A total of 308 stroke-related deaths occurred among male pesticide applicators between enrollment and the end of 2008 (median 13 years of follow-up). Twenty six percent of deaths due to stroke were recorded as hemorrhagic and 9% ischemic, while the rest were of an “unspecified” or other type. Most known risk factors for stroke were associated with stroke mortality (Table 1). Participants who were older, current smokers, lived in North Carolina, or reported a history of heart disease, diabetes or hypertension had an increased rate of death due to stroke. A slightly J-shaped association between alcohol consumption and stroke mortality was observed.
Table 1.
General characteristics of 51,603 male pesticide applicators by stroke mortality outcome, Agricultural Health Study, 1993–2008.a
| Casesb | Noncasesc | Person Time | Incidence Rate Ratiod | 95% Confidence Interval | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| (N = 308) | % | (N = 51,295) | % | |||||
| Site | ||||||||
| NC | 194 | 63 | 16591 | 32 | 218426 | 1 | ||
| Iowa | 114 | 37 | 34704 | 68 | 457408 | 0.43 | 0.34 | 0.55 |
| Smoking status | ||||||||
| Never | 116 | 38 | 27230 | 53 | 362046 | 1 | ||
| Past | 135 | 44 | 15530 | 30 | 201973 | 1.21 | 0.94 | 1.55 |
| Current | 57 | 19 | 8535 | 17 | 111815 | 2.37 | 1.72 | 3.27 |
| Age (years) | ||||||||
| <50 | 27 | 9 | 31983 | 62 | 431181 | 1 | ||
| 50–59 | 50 | 16 | 10356 | 20 | 135751 | 5.88 | 3.69 | 9.39 |
| 60–69 | 120 | 39 | 6818 | 13 | 85287 | 22.48 | 14.81 | 34.12 |
| 70+ | 111 | 36 | 2138 | 4 | 23645 | 75.00 | 49.23 | 114.17 |
| Race/Ethnicity | ||||||||
| White, non-Hispanic | 295 | 96 | 49607 | 97 | 653862 | 1 | ||
| Other | 12 | 4 | 1606 | 3 | 20934 | 1.07 | 0.60 | 1.91 |
| Education | ||||||||
| High school of less | 207 | 69 | 28493 | 57 | 372228 | 1 | ||
| Beyond high school | 91 | 31 | 21663 | 43 | 288718 | 1.07 | 0.83 | 1.37 |
| BMI (kg/m2) | ||||||||
| <25 | 80 | 36 | 9573 | 26 | 127407 | 1 | ||
| 25–29.9 | 102 | 46 | 19105 | 51 | 253118 | 0.64 | 0.50 | 0.82 |
| >=30 | 38 | 17 | 8745 | 23 | 115694 | 0.63 | 0.44 | 0.89 |
| Drinks per month at enrollment | ||||||||
| None | 184 | 60 | 16450 | 32 | 214040 | 1 | ||
| 1–4 | 63 | 20 | 13917 | 27 | 184014 | 0.72 | 0.54 | 0.96 |
| 5–30 | 36 | 12 | 13727 | 27 | 182045 | 0.57 | 0.39 | 0.80 |
| >30 | 25 | 8 | 7201 | 14 | 95735 | 0.80 | 0.52 | 1.22 |
| Heart disease | ||||||||
| No | 236 | 81 | 47750 | 95 | 632603 | 1 | ||
| Yes | 55 | 19 | 2590 | 5 | 30975 | 1.41 | 1.04 | 1.90 |
| Diabetes | ||||||||
| No | 242 | 84 | 48897 | 97 | 645814 | 1 | ||
| Yes | 46 | 16 | 1419 | 3 | 17438 | 3.11 | 2.26 | 4.27 |
| Hypertensione | ||||||||
| No | 91 | 59 | 19029 | 84 | 254169 | 1 | ||
| Yes | 62 | 41 | 3665 | 16 | 46840 | 1.74 | 1.25 | 2.42 |
Data not available for all participants due to missing values in some categories.
Cases are defined as deaths with an underlying or contributing cause attributed to stroke on state death certificates by using
International Classification of Diseases, Tenth Revision, codes I60-I69.
Non-cases are participants who did not experience a stroke-related death during study follow-up.
Adjusted for age in categories.
The hypertension question asked on the take-home questionnaire was answered by 23,170 participants (161 stroke deaths).
No consistent positive associations between overall pesticide use and stroke mortality were identified (Table 2). While there was no evidence of an association of overall use of pesticides with stroke mortality, individuals who used chemical-resistant gloves had a slightly reduced rate of stroke death (HR: 0.83; 95% CI: 0.65, 1.05) and those reporting having had a diagnosed pesticide-poisoning event had a slightly increased rate of stroke death (HR: 1.73; 95% CI: 0.77, 3.92) but neither association was statistically significant. When we examined ever use of 50 individual pesticides, there was no evidence of an association between individual chemicals and increased stroke mortality, either for ever use (see Table, Supplemental Digital Content 2) or lifetime days of use (data not shown). Reported use of the herbicide, metribuzin, was associated with a reduced rate of stroke mortality. Grouping of pesticides by functional group or chemical class showed no evidence of an association with stroke mortality.
Table 2.
Associations between pesticide use activities and stroke mortality among 51,603 male private and commercial pesticide applicators, Agricultural Health Study, 1993–2008.a
| Pesticide Use | Casesb | Non-casesc | Person Time | Hazard Ratiod | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|---|---|
| (N = 308) | % | (N = 51,295) | % | |||||
| Lifetime exposure, days | ||||||||
| 0–50 | 49 | 18 | 8788 | 19 | 116646 | 1 | ||
| 51–100 | 46 | 17 | 3648 | 8 | 47366 | 1.56 | 1.04 | 2.33 |
| 101–250 | 82 | 30 | 16957 | 36 | 223916 | 1.13 | 0.79 | 1.62 |
| >250 | 98 | 36 | 18078 | 38 | 237819 | 1.09 | 0.78 | 1.55 |
| Years mixed/applied pesticides | ||||||||
| ≤ 5 years | 27 | 10 | 8120 | 17 | 109332 | 1 | ||
| 6–10 years | 40 | 14 | 7598 | 15 | 101490 | 1.41 | 0.87 | 2.30 |
| 11–20 years | 52 | 19 | 15935 | 32 | 212009 | 0.93 | 0.58 | 1.48 |
| 21–30 years | 69 | 25 | 11427 | 23 | 148579 | 1.14 | 0.72 | 1.78 |
| >30 years | 93 | 33 | 6055 | 12 | 76339 | 1.31 | 0.84 | 2.02 |
| Days/year mixed | ||||||||
| <5 days | 80 | 28 | 8777 | 18 | 115011 | 1 | ||
| 5–9 days | 70 | 25 | 10881 | 22 | 142286 | 0.97 | 0.71 | 1.35 |
| 10–19 days | 74 | 26 | 14041 | 29 | 184932 | 0.98 | 0.71 | 1.34 |
| ≥ 20 days | 58 | 21 | 15078 | 31 | 200930 | 0.90 | 0.64 | 1.26 |
| Use of chemical- resistant gloves | ||||||||
| No | 156 | 51 | 14839 | 29 | 193863 | 1 | ||
| Yes | 152 | 49 | 36456 | 71 | 481971 | 0.83 | 0.65 | 1.05 |
|
| ||||||||
| Casesb | Non-casesc | |||||||
| (N = 191) | (N = 23,009) | |||||||
|
| ||||||||
| High-pesticide-exposure event e,g | ||||||||
| No | 140 | 91 | 18941 | 85 | 251256 | 1 | ||
| Yes | 14 | 9 | 3448 | 15 | 45923 | 0.91 | 0.52 | 1.58 |
| Diagnosed pesticide-poisoning event f,g | ||||||||
| No | 151 | 96 | 22205 | 98 | 294572 | 1 | ||
| Yes | 6 | 4 | 455 | 2 | 6038 | 1.73 | 0.77 | 3.92 |
Data not available for all participants due to missing values in some categories.
Cases are defined as deaths with an underlying or contributing cause attributed to stroke on state death certificates by using International Classification of Diseases, Ninth Revision (430–438), or Tenth Revision (I60-I69) codes.
Non-cases are participants who did not experience a stroke-related death during study follow-up.
Results were obtained from Cox proportional hazards regression adjusted for state, smoking status, and alcohol consumption.
Participants were asked the question, “Have you ever had an incident or experience while using any type of PESTICIDE which caused you unusually high personal exposure?”
Participants were asked if a doctor had ever diagnosed them with pesticide poisoning.
Questions asked on the take-home questionnaire were answered by 23,170 participants (161 stroke deaths).
Estimates of associations between other farming factors and stroke mortality varied around the null with some significant associations observed (Table 3). Individuals who handled grain, hay, or silage had a reduced rate of stroke death (HR: 0.75; 95% CI: 0.58, 0.98). This reduction in stroke mortality was observed when all three activities were combined and when assessed independently. Individuals reporting work on farms that were 5 or more acres had a slightly reduced rate of stroke. As number of livestock increased, the association with stroke mortality approached unity. When type of livestock present on the farm was considered, a slightly reduced hazard of stroke was present for those reporting cattle (dairy or beef) as a major income producing crop. No positive or negative associations were found between stroke mortality and the use of diesel or gasoline trucks or tractors, combines, or work in animal confinement facilities (data not shown).
Table 3.
Associations between agricultural activities, characteristics and stroke mortality among 51,603 male pesticide applicators, Agricultural Health Study, 1993–2008.a
|
|
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Agricultural activity | Casesb | Non-casesc | Person Time | Hazard Ratiod | 95% Confidence Interval | |||
| (N = 308) | % | (N = 51,295) | % | |||||
| Handle stored grain | 124 | 40 | 35350 | 69 | 466566 | 0.72 | 0.56 | 0.94 |
| Handle stored hay | 109 | 35 | 28382 | 55 | 374820 | 0.77 | 0.61 | 0.98 |
| Handle silage | 26 | 8 | 10597 | 21 | 139209 | 0.70 | 0.47 | 1.06 |
| Handle grain or hay or silage | 131 | 43 | 36044 | 70 | 475744 | 0.75 | 0.58 | 0.98 |
| Acres farmed the year before enrollment | ||||||||
| <5 acres | 64 | 23 | 3967 | 9 | 52267 | 1 | ||
| 5–49 | 45 | 16 | 4247 | 9 | 55428 | 0.59 | 0.40 | 0.86 |
| 50–199 | 72 | 26 | 8226 | 18 | 106935 | 0.83 | 0.58 | 1.17 |
| 200–499 | 59 | 21 | 12913 | 28 | 169839 | 0.68 | 0.46 | 1.01 |
| ≥ 500 | 37 | 13 | 17108 | 37 | 226657 | 0.54 | 0.34 | 0.84 |
| Poultry on farme | 20 | 8 | 4298 | 8 | 57124 | 0.91 | 0.58 | 1.44 |
| No. of livestock on farm | ||||||||
| None/Did not work on farm | 138 | 53 | 15468 | 34 | 202428 | 1 | ||
| <50 | 43 | 16 | 6165 | 13 | 80772 | 0.74 | 0.52 | 1.04 |
| 50–99 | 20 | 8 | 3784 | 8 | 49504 | 0.69 | 0.43 | 1.11 |
| 100–499 | 30 | 11 | 8859 | 19 | 116742 | 0.82 | 0.54 | 1.23 |
| ≥500 | 31 | 12 | 11587 | 25 | 153914 | 1.03 | 0.67 | 1.57 |
| Type of livestock on farmf | ||||||||
| Cattle | 83 | 27 | 20566 | 40 | 270789 | 0.73 | 0.56 | 0.94 |
| Swine | 52 | 17 | 16285 | 32 | 216588 | 1.07 | 0.78 | 1.46 |
| Other | 12 | 4 | 2500 | 5 | 33130 | 1.17 | 0.65 | 2.08 |
Data not available for all participants due to missing values in some categories.
Cases are defined as deaths with an underlying or contributing cause attributed to stroke on state death certificates by using International Classification of Diseases, Ninth Revision (430–438), or Tenth Revision (I60-I69) codes.
Non-cases are participants who did not experience a stroke-related death during study follow-up.
Results were obtained from Cox proportional hazards regression adjusted for state, smoking status and alcohol consumption.
The number of poultry present on farm was examined but collapsed into a dichotomous variable (poultry present/absent) based on a small number of cases in each category.
Does not include poultry.
DISCUSSION
Stroke is a leading cause of morbidity and mortality in the United States and abroad (Grysiewicz et al. 2008). Few studies have characterized associations between agricultural exposures and the risk of stroke mortality. Data from the AHS was used to examine the hypothesis that pesticide applicators (mainly farmers) who perform work-related activities that may bring them into contact with exogenous chemicals or particulate matter may have an increased rate of stroke mortality compared with those who do not engage in these activities. There is some evidence that pesticides and particulate matter, including organic dusts and diesel exhaust, may be associated with stroke and consequently stroke mortality, through inflammatory processes producing tissue damage leading to various disease states, coagulation disturbances, and through effects on the cardiac system (Anderson et al. 2011; Bhatnagar 2006; Hennig et al. 2002; Hong et al. 2002; Lovati et al. 1984; Roth et al. 1993; Saadeh et al. 1997; Seaton et al. 1999; Toborek et al. 1995; Ha et al. 2007; Montgomery et al. 2008; Toren et al. 2007; Park et al. 2010). However, in the present analysis no evidence was observed to support the hypothesis that the occupational activities examined here are associated with an elevated rate of stroke mortality among pesticide applicators participating in this large U.S. cohort study.
No association was observed between total lifetime days or years of pesticide use or use of any of 50 individual pesticides and stroke mortality. Although some crude rate ratios for use of individual pesticides appeared to indicate an increased rate of stroke mortality for individuals reporting use of specific pesticides, this association was no longer observed once age was considered. Use of one herbicide, metribuzin, was associated with a significantly lower rate of stroke mortality. Although, a reduced rate of stroke mortality was observed for individuals reporting ever using metribuzin, no clear biological mechanism is available to explain this and no other herbicides exhibited a similar association.
The results reported here differ from two previous studies that noted increased stroke-related mortality among individuals occupationally exposed to pesticides.(Cantor et al. 1999; Charles et al. 2010) Both studies relied on job title to define exposure to pesticides and did not assess information regarding use of or exposure to individual pesticides. Among Japanese-American men enrolled in the Honolulu Heart Study, a 70% increased rate of stroke mortality was observed among those determined to have the highest level of exposure to pesticides compared to those with no exposure (Charles et al. 2010). Similarly, a study of aerial pesticide applicators reported an almost two-fold elevation in stroke-related mortality as compared to flight instructors in the United States (Cantor et al. 1999). When overall lifetime pesticide use was examined, no association with stroke mortality was observed. The differences in the findings in the present study and the two previous studies may be due to differences in the demographic make-up of the study populations, calendar year of exposure, and the occupations examined. Further, the routes and definitions of exposures differed between the present and previous studies. The previous study relied on primary job title to determine likelihood of exposure to pesticides, a method that has been demonstrated to lead to misclassification of exposure (MacFarlane et al. 2009), while in the present study information regarding pesticide use was ascertained from participants.
In addition to the previous stroke mortality studies, Lee and colleagues (2012) reported a positive association between elevated plasma concentrations of three organochlorine (OC) pesticides and incident stroke among an elderly population in Sweden (Lee et al. 2012). OCs were measured in plasma up to 5 years prior to stroke diagnosis among individuals aged 70 and older. Those in the ≥95th percentile of a summary measure of OC pesticides had 9 times the odds of stroke compared to those in the lowest quartile (OR= 9.5 95% CI: 2.3, 38.9). Although these results were statistically significant they were based on a small number of cases (6 exposed; 5 unexposed) in a highly selected group (healthy individuals 70 and over). No evidence of an elevated rate of stroke mortality associated with specific OC pesticides or OCs as a group was noted in the present sample of younger individuals in the AHS followed for a longer period. Future studies with longer follow-up time and good characterization of timing of incident stroke will be useful to further explore this hypothesis.
In contrast to Lee et al. (2012), it was not possible in the present study to examine biomarkers of pesticide exposure and consequently relied on self-reported information to define exposure, and therefore some misclassification may be present. However, several steps have been taken to assess the potential for misclassification in the larger cohort. Internal validation studies showed 70–90% repeatability of self-reported pesticide use one-year after completion of the enrollment questionnaire (Blair et al. 2002). In another study, self-reported use of pesticides was found to compare reasonably well with pesticide registration information (Hoppin et al. 2002). A high reliability of report of covariates including smoking, alcohol consumption and reported disease history, and agricultural factors was also demonstrated (Blair et al. 2002). Despite the general high quality characterization of agricultural exposures for this study, it is important to note that some exposure misclassification undoubtedly occurs and that in a prospective cohort study it is likely to bias relative risks toward the null (Blair et al. 2011). Further, the AHS study is unique in that it is one of the largest prospective studies to include detailed information regarding agricultural exposures and covariates.
The present analysis examined stroke mortality defined by causes of death as recorded on the death certificate. This approach has demonstrated high specificity but a range of sensitivities in capturing stroke-related deaths with a clear improvement in recent years, indicating there may be some misclassification of stroke-related death (Corwin et al. 1982; Iso et al. 1990). In order to improve ascertainment of cases of stroke death, both underlying and contributing causes of death listed on the death certificate were considered. Although no associations between many of the agricultural activities examined and stroke-related mortality was observed, it was not possible to explore the timing, severity, or subtype of stroke and therefore exposures associated with non-fatal stroke or a specific stroke subtype are not examined here.
In addition to pesticide use, agricultural activities that would provide opportunity for exposure to particulate matter and, specifically, organic material from animals and crops were examined. No evidence to support a positive association between any of these activities and stroke mortality was found. A reduced rate of stroke mortality among farmers who reported handling hay, grain, and silage at least once each year was observed. In considering this finding it is important to note, individuals could only be classified as having handled hays, grains, and silage at least once each year as of enrollment or not. No further information regarding frequency or intensity of these activities was available, and therefore only this crude measure of exposure was assessed.
The inverse associations observed here may be explained, in that, participants reporting handling hay, grain or silage at least once each year at enrollment were in general different than those who did not reported such activities. For example, those who reported engaging in such activities were more active or healthier, and remained that way over time, and therefore demonstrated decreased risk of mortality related to stroke than those who did not report such activities. To this end, those who reported engaging in these activities were younger and a smaller proportion reported diabetes, heart disease, and hypertension diagnoses at enrollment than those who did not report engaging in these activities.
It is also possible that activities involving handling hay, grain, and silage provide chronic low-dose exposure to biological agents such as endotoxin (Spaan et al. 2006). Although little research in humans exists, some experimental research indicates that chronic exposure to low-levels of endotoxin may protect against, or reduce severity of, a range of injury thought to be associated with inflammatory processes including stroke and many of its risk factors (Biswas et al. 2009; Draisma et al. 2009; Drake et al. 2011; Eising et al. 1996; Fan et al. 2004; Iadecola et al. 2011; Liebers et al. 2006; Marsh et al. 2009; McColl et al. 2009; McColl et al. 2008; Rosenzweig et al. 2007; Doyle et al. 2012). However, the potential mechanism and impact of endotoxin exposure on stroke remains unclear.
A reduced rate of stroke mortality was also observed among farmers reporting cattle (beef or dairy) as a major income producing crop. Handling of hays, grains, and silage is very common among individuals producing cattle. It was found that only 4% of individuals reporting cattle as a major income producing crop did not handle hay, grain, or silage, with only 5 cases of stroke death included in this group. Therefore, it is difficult to separate the independent effects of exposure to cattle and handling of hays, grains, and silage.
Consistent with other published studies, the age-adjusted stroke mortality rate standardized to the 2000 U.S. population among male participants in the AHS cohort (19.1 stroke deaths/100,000 person-years) was lower than that observed for non-Hispanic, white males older than 15 in the general public (61 stroke deaths/100,000 person-years) and in Iowa (65 stroke deaths/100,000 person-years) and North Carolina (71 stroke deaths/100,000 person-years) during a similar period (1999–2010).(Centers for Disease Control and Prevention 2012). A previous study of this cohort reported a standardized mortality ratio (SMR) for cerebrovascular and arterial diseases of 0.51 (95% CI: 0.46, 0.57) and similar SMR for associated conditions including diabetes and hypertension (Waggoner et al. 2011). Because farmers have been observed to have lower rates of conventional risk factors for stroke (e.g., tobacco use, alcohol use) and increased physical activity levels, than the general public (Blair et al. 1992; Stellman et al. 1988; Sterling et al. 1978), the AHS is a unique population in which to examine associations between agricultural exposures and stroke mortality. Participants in the AHS reside in states where the underlying rates of stroke mortality are quite different allowing for the examination of these associations in different areas of the country (Lanska et al. 1995; Lanska 1993; United States Geological Survey 2002). As expected, participants from North Carolina displayed a higher rate of stroke mortality than participants from Iowa (Lanska et al. 1995; NHLBI). Despite the differences between the two states, the associations between stroke mortality and known risk factors or exposures were similar, potentially due to real similarities or a lack of power to detect differences between the states.
The present analysis is one of the first explorations of associations between agricultural activities and stroke mortality. The findings of no significant association between a number of well-characterized pesticide use parameters and stroke mortality over a median 13-years of follow-up and the potential for a reduced rate of stroke mortality among those reporting handling of hay, grain, and silage warrant further investigation to determine if the results represent a true effect or are a product of some bias. Analyses conducted in large populations with improved exposure assessment and the ability to examine incident stroke and stroke outcomes by subtype will improve the present knowledge regarding the role of environmental and occupational exposures in the burden of stroke, an important cause of morbidity and mortality globally.
Supplementary Material
Acknowledgments
The authors thank Stuart Long for assistance with data analysis. We especially thank the participants of the Agricultural Health Study for their continued support.
Footnotes
Disclosure of Funding: This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01-ES049030) and the National Institutes of Health National Cancer Institute (Z01-CP010119). JLR was supported by the National Institute of Environmental Health Sciences (NIEHS; award no. T32ES007018.
Conflict of Interest: The authors declare that they have no conflict of interest.
Contributor Information
Jessica L. Rinsky, Email: jrinsky@email.unc.edu.
Aaron Blair, Email: blaira@mail.nih.gov.
Ka He, Email: kahe@indiana.edu.
Laura E. Beane Freeman, Email: freemala@mail.nih.gov.
Honglei Chen, Email: chenh2@niehs.nih.gov.
References
- Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, Pennybacker M, Rothman N, Dosemeci M, Bond AE, Blair A. The agricultural health study. Environ Health Perspect. 1996;104(4):362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J Med Toxicol. 2011;8(2):166–175. doi: 10.1007/s13181-011-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Meir E, Schein O, Eisenkraft A, Rubinshtein R, Grubstein A, Militianu A, Glikson M and Medical Corps Israel Defense Forces Cbrn Medical Branch. Guidelines for treating cardiac manifestations of organophosphates poisoning with special emphasis on long qt and torsades de pointes. Crit Rev Toxicol. 2007;37(3):279–285. doi: 10.1080/10408440601177855. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A. Environmental cardiology: Studying mechanistic links between pollution and heart disease. Circ Res. 2006;99(7):692–705. doi: 10.1161/01.RES.0000243586.99701.cf. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Lopez-Collazo E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009;30(10):475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Blair A, Sandler DP, Tarone R, Lubin J, Thomas K, Hoppin JA, Samanic C, Coble J, Kamel F, Knott C, Dosemeci M, Zahm SH, Lynch CF, Rothman N, Alavanja MC. Mortality among participants in the agricultural health study. Ann Epidemiol. 2005;15(4):279–285. doi: 10.1016/j.annepidem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Blair A, Tarone R, Sandler D, Lynch CF, Rowland A, Wintersteen W, Steen WC, Samanic C, Dosemeci M, Alavanja MC. Reliability of reporting on life-style and agricultural factors by a sample of participants in the agricultural health study from iowa. Epidemiology. 2002;13(1):94–99. doi: 10.1097/00001648-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Blair A, Thomas K, Coble J, Sandler DP, Hines CJ, Lynch CF, Knott C, Purdue MP, Zahm SH, Alavanja MC, Dosemeci M, Kamel F, Hoppin JA, Freeman LB, Lubin JH. Impact of pesticide exposure misclassification on estimates of relative risks in the agricultural health study. Occup Environ Med. 2011;68(7):537–541. doi: 10.1136/oem.2010.059469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair A, Zahm SH, Pearce NE, Heineman EF, Fraumeni JF., Jr Clues to cancer etiology from studies of farmers. Scand J Work Environ Health. 1992;18(4):209–215. doi: 10.5271/sjweh.1578. [DOI] [PubMed] [Google Scholar]
- Bombick DW, Matsumura F, Madhukar BV. Tcdd (2,3,7,8-tetrachlorodibenzo-p-dioxin) causes reduction in the low density lipoprotein (ldl) receptor activities in the hepatic plasma membrane of the guinea pig and rat. Biochem Biophys Res Commun. 1984;118(2):548–554. doi: 10.1016/0006-291x(84)91337-8. [DOI] [PubMed] [Google Scholar]
- Burch JB, Svendsen E, Siegel PD, Wagner SE, von Essen S, Keefe T, Mehaffy J, Martinez AS, Bradford M, Baker L, Cranmer B, Saito R, Tessari J, Linda P, Andersen C, Christensen O, Koehncke N, Reynolds SJ. Endotoxin exposure and inflammation markers among agricultural workers in colorado and nebraska. J Toxicol Environ Health A. 2010;73(1):5–22. doi: 10.1080/15287390903248604. [DOI] [PubMed] [Google Scholar]
- Cantor KP, Silberman W. Mortality among aerial pesticide applicators and flight instructors: Follow-up from 1965–1988. Am J Ind Med. 1999;36(2):239–247. doi: 10.1002/(sici)1097-0274(199908)36:2<239::aid-ajim3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Centers Multiple cause of death 1999–2009 on cdc wonder online database. National Center for Health Statistics; 2012. [cited May 29, 2012]. Available from http://wonder.cdc.gov/mcd-icd10.html. [Google Scholar]
- Charles LE, Burchfiel CM, Fekedulegn D, Gu JK, Petrovitch H, Sanderson WT, Masaki K, Rodriguez BL, Andrew ME, Ross GW. Occupational exposure to pesticides, metals, and solvents: The impact on mortality rates in the honolulu heart program. Work. 2010;37(2):205–215. doi: 10.3233/WOR-2010-1071. [DOI] [PubMed] [Google Scholar]
- Cheng TJ, Chang CY, Lin CY, Ke DS, Lu TH, Kawachi I. State differences in the reporting of ‘unspecified stroke’ on death certificates: Implications for improvement. Stroke. 2012;43(12):3336–3342. doi: 10.1161/STROKEAHA.112.670877. [DOI] [PubMed] [Google Scholar]
- Coble J, Hoppin JA, Engel L, Elci OC, Dosemeci M, Lynch CF, Alavanja M. Prevalence of exposure to solvents, metals, grain dust, and other hazards among farmers in the agricultural health study. J Expo Anal Environ Epidemiol. 2002;12(6):418–426. doi: 10.1038/sj.jea.7500248. [DOI] [PubMed] [Google Scholar]
- Corwin LE, Wolf PA, Kannel WB, McNamara PM. Accuracy of death certification of stroke: The framingham study. Stroke. 1982;13(6):818–821. doi: 10.1161/01.str.13.6.818. [DOI] [PubMed] [Google Scholar]
- Doyle KP, Buckwalter MS. The double-edged sword of inflammation after stroke: What sharpens each edge? Ann Neurol. 2012;71(6):729–731. doi: 10.1002/ana.23579. [DOI] [PubMed] [Google Scholar]
- Draisma A, Pickkers P, Bouw MP, van der Hoeven JG. Development of endotoxin tolerance in humans in vivo. Crit Care Med. 2009;37(4):1261–1267. doi: 10.1097/CCM.0b013e31819c3c67. [DOI] [PubMed] [Google Scholar]
- Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, Hulme S, Georgiou RF, Hinz R, Gerhard A, Vail A, Prenant C, Julyan P, Maroy R, Brown G, Smigova A, Herholz K, Kassiou M, Crossman D, Francis S, Proctor SD, Russell JC, Hopkins SJ, Tyrrell PJ, Rothwell NJ, Allan SM. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun. 2011;25(6):1113–1122. doi: 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eising GP, Mao L, Schmid-Schonbein GW, Engler RL, Ross J. Effects of induced tolerance to bacterial lipopolysaccharide on myocardial infarct size in rats. Cardiovasc Res. 1996;31(1):73–81. [PubMed] [Google Scholar]
- Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10(2):71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev. 2012;15(1):1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: Incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26(4):871–895. vii. doi: 10.1016/j.ncl.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Ha MH, Lee DH, Jacobs DR. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: Results from the national health and nutrition examination survey, 1999–2002. Environ Health Perspect. 2007;115(8):1204–1209. doi: 10.1289/ehp.10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, Robertson LW. Proinflammatory properties of coplanar pcbs: In vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002;181(3):174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Hines CJ, Deddens JA, Coble J, Kamel F, Alavanja MC. Determinants of captan air and dermal exposures among orchard pesticide applicators in the agricultural health study. Ann Occup Hyg. 2011;55(6):620–633. doi: 10.1093/annhyg/mer008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YC, Lee JT, Kim H, Kwon HJ. Air pollution: A new risk factor in ischemic stroke mortality. Stroke. 2002;33(9):2165–2169. doi: 10.1161/01.str.0000026865.52610.5b. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, Yucel F, Dosemeci M, Sandler DP. Accuracy of self-reported pesticide use duration information from licensed pesticide applicators in the agricultural health study. J Expo Anal Environ Epidemiol. 2002;12(5):313–318. doi: 10.1038/sj.jea.7500232. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nat Med. 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso H, Jacobs DR, Jr, Goldman L. Accuracy of death certificate diagnosis of intracranial hemorrhage and nonhemorrhagic stroke. The minnesota heart survey. Am J Epidemiol. 1990;132(5):993–998. doi: 10.1093/oxfordjournals.aje.a115742. [DOI] [PubMed] [Google Scholar]
- Lanska DJ. Geographic distribution of stroke mortality in the united states: 1939–1941 to 1979–1981. Neurology. 1993;43(9):1839–1851. doi: 10.1212/wnl.43.9.1839. [DOI] [PubMed] [Google Scholar]
- Lanska DJ, Kuller LH. The geography of stroke mortality in the united states and the concept of a stroke belt. Stroke. 1995;26(7):1145–1149. doi: 10.1161/01.str.26.7.1145. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lind PM, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind L. Background exposure to persistent organic pollutants predicts stroke in the elderly. Environ Int. 2012;47:115–120. doi: 10.1016/j.envint.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Liebers V, Bruning T, Raulf-Heimsoth M. Occupational endotoxin-exposure and possible health effects on humans. Am J Ind Med. 2006;49(6):474–491. doi: 10.1002/ajim.20310. [DOI] [PubMed] [Google Scholar]
- Lovati MR, Galbussera M, Franceschini G, Weber G, Resi L, Tanganelli P, Sirtori CR. Increased plasma and aortic triglycerides in rabbits after acute administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1984;75(1):91–97. doi: 10.1016/0041-008x(84)90079-6. [DOI] [PubMed] [Google Scholar]
- MacFarlane E, Glass D, Fritschi L. Is farm-related job title an adequate surrogate for pesticide exposure in occupational cancer epidemiology? Occup Environ Med. 2009;66(8):497–501. doi: 10.1136/oem.2008.041566. [DOI] [PubMed] [Google Scholar]
- Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, Harrington CA, Stenzel-Poore MP. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: A critical role for irf3. J Neurosci. 2009;29(31):9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastin JP. Environmental cardiovascular disease. Cardiovasc Toxicol. 2005;5(2):91–94. doi: 10.1385/ct:5:2:091. [DOI] [PubMed] [Google Scholar]
- McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. 2009;158(3):1049–1061. doi: 10.1016/j.neuroscience.2008.08.019. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28(38):9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KT, Blair A, Freeman LE, Sandler DP, Hoppin JA. Pesticides and myocardial infarction incidence and mortality among male pesticide applicators in the agricultural health study. Am J Epidemiol. 2009;170(7):892–900. doi: 10.1093/aje/kwp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery MP, Kamel F, Saldana TM, Alavanja MC, Sandler DP. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural health study, 1993–2003. Am J Epidemiol. 2008;167(10):1235–1246. doi: 10.1093/aje/kwn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHLBI. Stroke belt initiative. National Heart, Lung, and Blood Institute; [cited. Available from http://www.nhlbi.nih.gov/health/prof/heart/other/sb_spec.pdf. [Google Scholar]
- Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH, Jr, Folsom AR. Risk factors for ischemic stroke subtypes: The atherosclerosis risk in communities study. Stroke. 2006;37(10):2493–2498. doi: 10.1161/01.STR.0000239694.19359.88. [DOI] [PubMed] [Google Scholar]
- Park SK, Son HK, Lee SK, Kang JH, Chang YS, Jacobs DR, Lee DH. Relationship between serum concentrations of organochlorine pesticides and metabolic syndrome among non-diabetic adults. J Prev Med Public Health. 2010;43(1):1–8. doi: 10.3961/jpmph.2010.43.1.1. [DOI] [PubMed] [Google Scholar]
- Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, Mehaffy J, Reynolds SJ. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health A. 2010;73(10):684–700. doi: 10.1080/15287390903578539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: A meta-analysis. J Am Med Assoc. 2003;289(5):579–588. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
- Rosenzweig HL, Minami M, Lessov NS, Coste SC, Stevens SL, Henshall DC, Meller R, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning protects against the cytotoxic effects of tnfalpha after stroke: A novel role for tnfalpha in lps-ischemic tolerance. J Cereb Blood Flow Metab. 2007;27(10):1663–1674. doi: 10.1038/sj.jcbfm.9600464. [DOI] [PubMed] [Google Scholar]
- Roth A, Zellinger I, Arad M, Atsmon J. Organophosphates and the heart. Chest. 1993;103(2):576–582. doi: 10.1378/chest.103.2.576. [DOI] [PubMed] [Google Scholar]
- Saadeh AM, Farsakh NA, Kal-Ali M. Cardiac manifestations of acute carbamate and organophosphate poisoning. Heart. 1997;77(5):461–464. doi: 10.1136/hrt.77.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton A, Soutar A, Crawford V, Elton R, McNerlan S, Cherrie J, Watt M, Agius R, Stout R. Particulate air pollution and the blood. Thorax. 1999;54(11):1027–1032. doi: 10.1136/thx.54.11.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan S, I, Wouters M, Oosting I, Doekes G, Heederik D. Exposure to inhalable dust and endotoxins in agricultural industries. J Environ Monit. 2006;8(1):63–72. doi: 10.1039/b509838f. [DOI] [PubMed] [Google Scholar]
- Stellman SD, Boffetta P, Garfinkel L. Smoking habits of 800,000 american men and women in relation to their occupations. Am J Ind Med. 1988;13(1):43–58. doi: 10.1002/ajim.4700130104. [DOI] [PubMed] [Google Scholar]
- Sterling TD, Weinkam JJ. Smoking patterns by occupation, industry, sex, and race. Arch Environ Health. 1978;33(6):313–317. doi: 10.1080/00039896.1978.10667354. [DOI] [PubMed] [Google Scholar]
- Thomas KW, Dosemeci M, Hoppin JA, Sheldon LS, Croghan CW, Gordon SM, Jones ML, Reynolds SJ, Raymer JH, Akland GG, Lynch CF, Knott CE, Sandler DP, Blair AE, Alavanja MC. Urinary biomarker, dermal, and air measurement results for 2,4-d and chlorpyrifos farm applicators in the agricultural health study. J Expo Sci Environ Epidemiol. 2010;20(2):119–134. doi: 10.1038/jes.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toborek M, Barger SW, Mattson MP, Espandiari P, Robertson LW, Hennig B. Exposure to polychlorinated biphenyls causes endothelial cell dysfunction. J Biochem Toxicol. 1995;10(4):219–226. doi: 10.1002/jbt.2570100406. [DOI] [PubMed] [Google Scholar]
- Toren K, I, Bergdahl A, Nilsson T, Jarvholm B. Occupational exposure to particulate air pollution and mortality due to ischaemic heart disease and cerebrovascular disease. Occup Environ Med. 2007;64(8):515–519. doi: 10.1136/oem.2006.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Geological Survey. Pesticide use maps. USGS. 2012;2002 [cited May 5, 2012 2012]. Available from http://water.usgs.gov/nawqa/pnsp/usage/maps/compound_listing.php?year=02. [Google Scholar]
- Waggoner JK, Kullman GJ, Henneberger PK, Umbach DM, Blair A, Alavanja MC, Kamel F, Lynch CF, Knott C, London SJ, Hines CJ, Thomas KW, Sandler DP, Lubin JH, Beane Freeman LE, Hoppin JA. Mortality in the agricultural health study, 1993–2007. Am J Epidemiol. 2011;173(1):71–83. doi: 10.1093/aje/kwq323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. International statistical classification of diseases and related health problems. World Health Organization; 2009. 10th revision. Version for 2007. [Google Scholar]
- Yang CY, Chen YS, Yang CH, Ho SC. Relationship between ambient air pollution and hospital admissions for cardiovascular diseases in kaohsiung, taiwan. J Toxicol Environ Health A. 2004;67(6):483–493. doi: 10.1080/15287390490276502. [DOI] [PubMed] [Google Scholar]
- Yorifuji T, Kawachi I, Sakamoto T, Doi H. Associations of outdoor air pollution with hemorrhagic stroke mortality. J Occup Environ Med. 2011;53(2):124–126. doi: 10.1097/JOM.0b013e3182099175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.