Abstract
The current generation of blood substitutes tested in clinical trials are red blood cell (RBC) substitutes; that is, they are designed primarily to transport oxygen. The products now being used in advanced-phase clinical trials are derived from hemoglobin (Hb) and are thus often referred to as Hb-based oxygen carriers (HBOCs). The potential benefits of HBOCs are well known (Box 1). The objectives of this overview are to provide the scientific background and rationale for the study design of the USA Multi-center Prehospital HBOC Resuscitation Trial and to present the results and discuss clinical implications.
Box 1. Potential clinical benefits of hemoglobin-based oxygen carriers in trauma care.
Availability
Abundant supply
Universally compatible
Prolonged shelf-life
Storage at room temperature
Safety
No disease transmissions
No antigenic reactions
No immunologic effects
Efficacy
Enhanced oxygen delivery
Improved rheologic properties
Keywords: Hemoglobin-based oxygen carrier, Blood substitute Prehospital resuscitation, Hemorrhagic shock, Trauma
POTENTIAL ROLE OF HEMOGLOBIN-BASED OXYGEN CARRIERS IN TRAUMA CARE
The US Food and Drug Administration (FDA) approval of a new product proceeds through phase I, II, and III studies designed to establish safety and efficacy (Table 1). FDA regulation defines efficacy as follows: “Effectiveness means a reasonable expectation that…the pharmacologic or other effect of the biologic product…will serve a clinically significant function in the diagnosis, cure, mitigation, treatment, or prevention of disease in man.” 1 The Center for Biologics Evaluation and Research (CBER) is the review body for the FDA in the arena of biologies and has published a comprehensive listing of “points to consider in the safety evaluation of HBOCs.”2 These points encompass characterization of the product, animal safety testing, and human studies and address the theoretic concerns of Hb solutions raised previously,3–6 including pulmonary and systemic hypertension, organ dysfunction, oxidative tissue injury, synergy with bacterial pathogens, and immunomodulation. In 1994, CBER convened a workshop with the National Heart, Lung, and Blood Institute and the Department of the Army to develop “points to consider in the efficacy evaluation of HBOCs.”7 Documenting a direct clinical end point for HBOCs is challenging because this end point has never been established for RBCs. Specific recommendations for clinical studies are in three areas: perioperative applications, acute hemorrhagic shock, and regional perfusion. Field trials for postinjury hemorrhagic shock, where RBCs are not available, are difficult because of safety and ethical issues. Decreased perioperative allogeneic RBC transfusion is regarded as a clinical benefit, but the potential risks of HBOCs also need to be defined and evaluated. Regional perfusion studies include reperfusion following ischemia (eg, as an adjunct during coronary angioplasty; the FDA approved Fluosol DA in 1989 as an oxygen-carrying drug for this setting).
Table 1.
Potential role of hemoglobin-based oxygen carriers in trauma care
| Application | Location |
|---|---|
| Perioperative applications | |
| Reduce allogeneic RBC transfusions | ED, angiography, OR, ICU |
| Attenuate transfusion immunodulation | OR, ICU |
| Acute hemorrhagic shock | |
| When stored RBCs are unavailable | Field, ED, OR, ICU, remote hospital, civilian disaster, military conflict |
| More efficient resuscitation | Field, ED, OR, ICU |
| Low-volume resuscitation | Remote hospital, civilian disaster, military conflict |
| Regional perfusion | |
| Enhance oxygen delivery | |
| Ischemic reperfusion tissue/organ | OR, ICU |
| Inflamed tissue | OR, ICU |
| Ex vivo organ perfusion | Hospital, OR |
Abbreviations: ED, Emergency department; OR, Operating room.
CLINICAL EVALUATION OF MODIFIED TETRAMERIC HEMOGLOBIN IN TRAUMA CARE: THE FIRST MULTICENTER TRIAL
Of the modified Hb tetrameric solutions that looked promising in the late 1980s, one formulation was authorized by the FDA for a phase III study in trauma. HemAssist (Baxter, Boulder, Colorado) consisted of Hb tetramers cross-linked between α subunits with bis 3,5 diabromosalicyl fumarate to prevent dissociation into dimers and to reduce oxygen affinity. It is unfortunate that this clinical trial failed.8 Regarded by some as a major setback for HBOCs, it is important to emphasize that this United States multicenter trial of diaspirin cross-linked Hb (DCLHb) for the treatment of severe traumatic hemorrhagic shock was based on the explicit proposal that “DCLHb was tested not as a substitute for blood but rather as an adjunct to the currently used therapies for enhancing oxygen delivery: fluids, blood, and operative intervention.” Although the unexpected outcome raised the issue of comparable study groups, the difference in the primary study end point was concerning: the 28-day mortality for the DCLHb group was 46% (24/52) compared with 17% (8/46) for the control (normal saline) group. Much expert thought and preparation went into the study design of this human trial, but the scientific justification of using a vasoconstricting agent for the initial resuscitation of acute hemorrhagic shock was, in retrospect, questionable.
The investigators rationalized this study design because in preclinical trials, “DCLHb has been shown to be effective in enhancing perfusion in small volumes, suggesting a pharmacologic effect that is independent of hemoglobin.” The pharmacologic effect, however, was not always reported as beneficial. In 1993, Hess and coauthors,9 at the Letterman Army Institute of Research, reported that in a swine model of hemorrhagic shock, DCLHb infusion doubled systemic and pulmonary vascular resistance and that these responses were associated with a fall in cardiac output. In fact, these changes were equivalent to resuscitation with unmodified tetrameric Hb. Hess and colleagues9 concluded, “the decrease in cardiac output associated with the vasoconstriction in the Hb-treated animals was equal to the increase in oxygen-carrying capacity—crystalloid or colloid solutions provided equally rapid correction of the elevated whole blood lactate.” In a follow-up study,10 the infusion of low-dose (4 mL/kg = 14 g Hb) DCLHb into swine subjected to hemorrhagic shock prompted the investigators to further warn “pulmonary hypertension and low peripheral perfusion may offset benefits for trauma patients.” Although the DCLHb trial investigators cited several animal models that appeared to support their study hypothesis, none of these models replicated their study design—a lesson for the future conduct of clinical trials with HBOCs.
The mechanisms responsible for the vasoconstriction resulting from DCLHb administration were investigated before the trauma clinical trial. The increased vascular resistance was believed to be mediated predominantly by the scavenging of nitric oxide (NO), with an additional component of enhanced endothelin release.11–13 Subsequently, alternative mechanisms were proposed, including release-enhanced adrenergic receptor sensitivity and reduced arterial wall shear stress secondary to decreased viscosity.14,15 Development of DCLHb has been terminated, but the relevance of these basic mechanisms to future trauma care with HBOCs is clear.
CLINICAL SAFETY OF POLYMERIZED HEMOGLOBIN IN TRAUMA CARE
At this moment, the HBOCs currently tested in phase III trials are polymerized Hb solutions (Table 2). Polymerization addresses several of the problems inherent in tetrameric Hb: short intravascular retention and reduced colloid osmotic activity. Polymerization also appears to attenuate vasoconstriction associated with the infusion of Hb solutions. A proposed explanation is that tetrameric Hb (65 kd) extravasates through the endothelium to bind abluminal NO, leading to unopposed vasoconstriction, whereas polymerized Hb (>130 kd) remains in the vasculature to bind only luminal NO. Of interest, Hb of the common earthworm, Lumbricus terrestris, is a polymer with a molecular weight of 400 kd that circulates extracellularly.16 Mice and rats undergoing exchange transfusion with this naturally occurring polymeric Hb showed no changes in behavior, and nuclear magnetic resonance spectroscopy of the heart confirmed normal oxygen-carrying capacity.17
Table 2.
Characteristics of current hemoglobin-based oxygen carriers in phase III trials
| Characteristic | Hemopure | PolyHeme | RBCs |
|---|---|---|---|
| Hemoglobin (g%) | 13 | 10 | 13 |
| Unit equivalent (g) | 30 | 50 | 50 |
| Molecular weight (>64 kd) | ≥95% | ≥99% | ≥100% |
| P50 (mm Hg) | 38 | 29 | 26 |
| Hill coefficient | 1.4 | 1.7 | 2.7 |
| Oncotic pressure (mm Hg) | 25 | 23 | 25 |
| Viscosity (cp) | 1.3 | 2.1 | (Whole blood 5–10) |
| Methemoglobin (%) | <10 | <8 | <1 |
| Half-life | 19 h | 24 h | 31 d |
| Shelf-life at 4°C | ≥3 y | ≥1.5 y | 42 d |
| Shelf-life at 21°C | ≥2 y | ≥6 wk | ≥6 h |
Abbreviations: cp, Centipoise; P50, Tension when hemoglobin-binding sites are 50% saturated.
Polymerized HBOCs have undergone extensive preclinical and clinical testing for safety. Hemopure (Biopure Corp, Cambridge, Massachusetts), a polymer of bovine Hb, has been used successfully to reduce allogeneic RBC transfusion in elective cardiac,18 aortic,19 and hepatic20 surgery. One study with abdominal aortic reconstruction raised concern about increased systemic vascular resistance,21 an effect identified in normal volunteers.22 Recent animal studies designed to replicate prehospital hypotensive resuscitation for hemorrhagic shock have been encouraging.23–25 Hemopure has been approved for replacement of acute blood loss in South Africa, but there are no published results to date. PolyHeme (Northfield Laboratories, Evanston, Illinois) has been evaluated predominantly in acutely injured patients. PolyHeme is derived from outdated human stored blood. After lysis of RBCs, the native tetrameric Hb is polymerized with glutaraldehyde. Pyridoxal phosphate is used to obtain a more physiologic P50. The meticulous, multistep biochemical purification of PolyHeme is believed to eliminate the risk of infection transmission. Under FDA guidance, the authors initiated clinical trials in trauma to confirm safety with escalating doses of PolyHeme. In the first clinical trail,26 39 patients received one (n =14), two (n =2), three (n =15), or six (n =8) units of PolyHeme as their initial resuscitation after acute blood loss. Infusion rates ranged from one unit in 175 minutes to six units (300 g) in 20 minutes. Although the RBC Hb fell to 2.9 ± 0.2 g%, total Hb was maintained at 7.5 ± 0.2 g% with PolyHeme. With respect to safety, the patient’s temperature, mean arterial pressure, heart rate, and creatinine clearance did not change during the 72-hour study period. Liver function tests and amylase varied substantially because of patient injuries. Cognizant of the vasoconstriction associated with the DCLHb clinical trial, the authors designed a study to specifically evaluate the vascular response to PolyHeme infusion in acutely injured patients.27 Patients requiring urgent transfusion were administered PolyHeme (up to six units) or stored RBCs during their initial resuscitation. Systemic arterial pressure, pulmonary arterial pressure, cardiac index, and pulmonary capillary wedge pressure were measured every 4 hours post infusion. There were no significant differences between the groups for these indices or the calculated systemic or pulmonary vascular resistance. Additional issues reported with the clinical use of polymerized Hb solutions included interference of laboratory tests that are based on colormetric changes from dissolved plasma Hb, inaccuracy of oxygen saturation monitoring because of methemoglobin, mild elevations of serum amylase (but without evidence of pancreatitis), and skin rashes. None of these issues has been considered a clinically significant adverse event to date.
CLINICAL EFFICACY OF POLYMERIZED HEMOGLOBIN IN TRAUMA CARE
Perioperative Applications: Reduce Allogeneic Red Blood Cell Transfusions in Trauma Care
Prompted by the FDA guidelines to demonstrate efficacy, all HBOC companies pursued what appeared to be the simplest clinically: to reduce the need for allogeneic RBC transfusions. In collaboration with David B. Hoyt, MD, and the University of California at San Diego, the authors conducted a randomized trial in patients requiring urgent transfusion.28 The 44 trauma patients (injury severity score [ISS] = 21 ± 1.3) were allocated to receive stored RBCs or up to six units of PolyHeme as their initial blood replacement. The RBC Hb was equivalent preinfusion (10.4 ± 0.4 g% versus 9.4 ± 0.3 g%); at end infusion, the RBC Hb of the PolyHeme patients fell to 5.8 ± 0.5 g% versus 10.6 ± 0.3 g% in the control subjects. The PolyHeme group received 4.4 ± 0.3 units, resulting in a plasma Hb of 3.9 ± 0.2 g%. The total number of allogeneic RBC transfusions for the control group compared with the PolyHeme group was 10.4 ± 0.9 units versus 6.8 ± 0.9 units (P<.05) through day 1, and 11.3 ± 0.9 units versus 7.8 ± 0.9 units (P = .06) through day 3. After the initial phase, infusion of 4.6 units of stored RBCs in the control group was equivalent to the 5.2 units in the Poly-Heme group. Both volumes presumably represented the infused RBCs or PolyHeme lost during acute hemorrhage before operative control. Subsequent replacement volumes were comparable, ultimately sparing the PolyHeme group approximately four units of allogeneic RBC transfusion.
Perioperative Applications: Reduce Allogenic Red Blood Cell Transfusions During Initial Resuscitation and Thereby Decrease Acute Respiratory Distress Syndrome and Multiple Organ Failure
With the authors’ long-term interest in the pathogenesis of postinjury multiple organ failure (MOF),29,30 their working hypothesis extended beyond reduced stored blood use during hospitalization. The authors proposed that PolyHeme, in lieu of stored RBCs during initial resuscitation, would attenuate the adverse immunoinflammatory effects of allogeneic RBC transfusion and ultimately reduce the incidence of acute respiratory distress syndrome (ARDS) and MOF. Stored blood is reportedly safer than ever due to comprehensive screening for disease transmission, but the potential adverse effects of packed RBC storage on the immune response to injury and illness are becoming more apparent.31,32 The authors have been interested in the proinflammatory effects of stored RBCs and specifically in their capacity to provoke polymorphonuclear neutrophil (PMN) cytotoxicity. The PMN is a key cellular mediator in the pathogenesis of postinjury MOF. Consequently, PMN functional responses are evaluated as a clinical surrogate for the two-event model of MOF: inflammatory priming and subsequent activation. The two-event construct of postinjury MOF is based on the fundamental concept that injury primes the innate immune system such that a second insult, during this vulnerable window, provokes unbridled systemic inflammation, resulting in organ dysfunction.33 Priming is defined as an enhanced response to a stimulus that is due to prior exposure of the cell to a different agonist.34 In the authors’ ongoing epidemiologic studies,35 they have shown that more than six units of RBC transfusion within the first 12 hours post injury is an independent risk factor for MOF.36 Furthermore, the age of the transfused blood within the first 6 hours post injury correlates with the incidence of MOF.37 Previous studies in the authors’ center have shown that after severe injury, patients at high risk for MOF have circulating PMNs that are primed for cytotoxicity within the first 6 hours post injury, as marked by the increased surface expression CD11b/CD18, p 38 mitogen-activated protein kinase activation, release of cytotoxic products in response to formyl-methionyl-leucyl-phenylalanine, and delayed apoptosis.38
The precise mechanisms linking packed RBC transfusion and PMN priming remain to be established, but many believe that passenger leukocytes accompanying RBCs in storage are important in the generation of proinflammatory agents.39 Plasma from stored RBCs primes PMNs in vitro, and this effect has been shown to increase progressively from 14 to 42 days of storage.40 Some investigators have incriminated cytokines (tumor necrosis factor α, interleukin [IL]-1, IL-6, IL-8, and IL-18) generated during storage,41 whereas the authors have focused on proinflammatory lipids leukotriene β4 and lysophosphatidylcholine) presumably generated from the RBC membrane.42 Metabolites of the arachidonic acid cascade have been strongly implicated in the pathogenesis of transfusion-related acute lung injury.43 Although prestorage leukoreduction of RBCs decreases the generation of cytokines, this process does not eliminate PMN priming.44 Thus, collectively, these studies suggest that a blood substitute devoid of proinflammatory agents will avoid the immunomodulatory consequences of allogeneic RBCs.
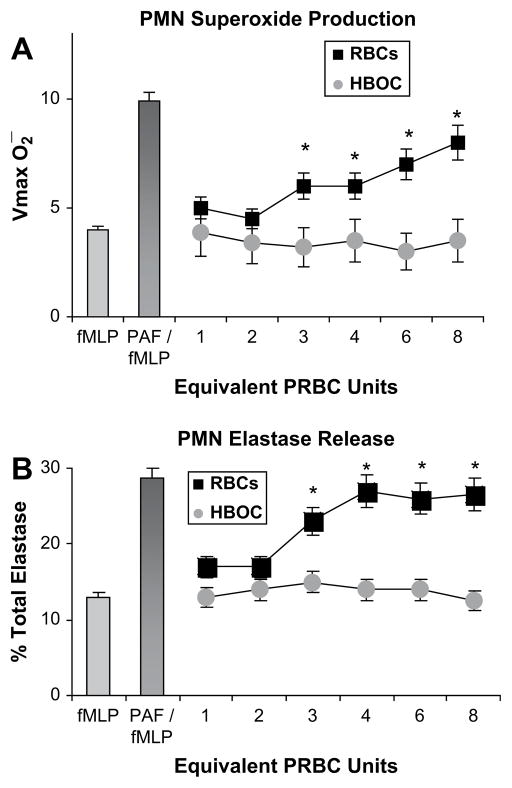
In preparation for clinical trials, the authors conducted in vitro and in vivo studies to test their hypothesis that PolyHeme—free of inflammatory cytokines and lipids—would eliminate the PMN priming previously documented with stored RBCs and translate to reduced ARDS and MOF.45 Human PMNs were isolated from healthy volunteers, and the plasma fraction was separated from packed RBCs at 42 days of storage in the authors’ blood bank (day 42 is the last day that stored RBCs can be transfused clinically, but often, these are the first RBCs infused into trauma patients).46 The isolated PMNs were incubated with RBC plasma or PolyHeme at concentrations calculated to be equivalent up to eights units of transfusion. The plasma fraction representing three or more units of stored RBCs primed the human PMNs for enhanced superoxide production and elastase release (Fig. 1).
Fig. 1.
Isolated human neutrophils (PMNs) were incubated with the plasma fraction from stored RBCs or PolyHeme at concentrations equivalent to one through eight units of acute transfusion. (A) PMN superoxide production. (B) PMN elastase release. Formyl-methionyl-leucyl-phenylalanine (fMLP) is employed as a PMN activator, and platelet-activating factor (PAF; primer) followed by fMLP approximates maximal PMN response. *P<.05.
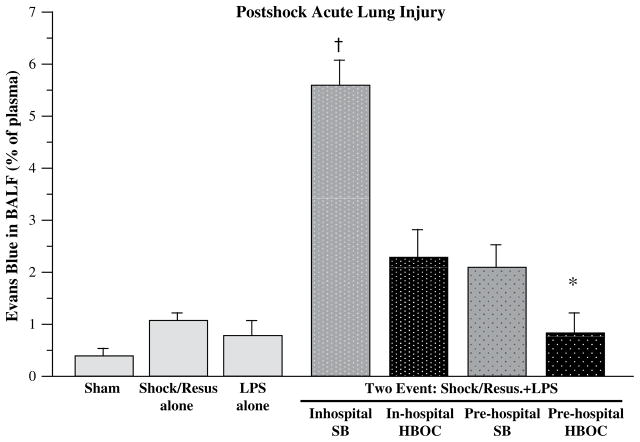
The authors further tested their hypothesis in an established two-event model of MOF: trauma/hemorrhagic shock as a priming event followed by toll-like receptor 4 engagement as an activating event.47 The primary study objective was to contrast HBOCs versus crystalloid in the prehospital phase, but the study groups were expanded to encompass the possible availability of stored blood in the field and the authors’ previous in-hospital phase II clinical work with HBOC resuscitation. Acute lung injury was selected as the primary study end point because ARDS is the first manifestation of postinjury MOF. Rats underwent laparotomy and hemorrhagic shock (30 mm Hg × 45 minutes) and were resuscitated over 2 hours in a clinically relevant design: two times the volume of shed blood (SB) using normal saline in the first 30 minutes; one half the volume of SB in the next 30 minutes; another two times SB volume with normal saline over the remaining 60 minutes. Four study groups represented alternative fluid strategies during the first hour of resuscitation: in-hospital SB (standard resuscitation); in-hospital HBOC; prehospital SB; and prehospital HBOC. Global physiologic response was assessed by way of tissue oxygenation (near infrared spectroscopy) and arterial base deficit; pulmonary response was assessed by way of lung neutrophil (PMN) accumulation and vascular permeability. Pre-hospital HBOC resuscitation provided the most efficient recovery of tissue oxygenation (Fig. 2) and correction of base deficit, had the greatest reduction in pulmonary PMN accumulation, and abrogated acute lung injury (Fig. 3). The findings in this controlled in vivo study further supported the authors’ hypothesis that initial HBOC resuscitation attenuates the postshock inflammatory response and secondary organ dysfunction.
Fig. 2.
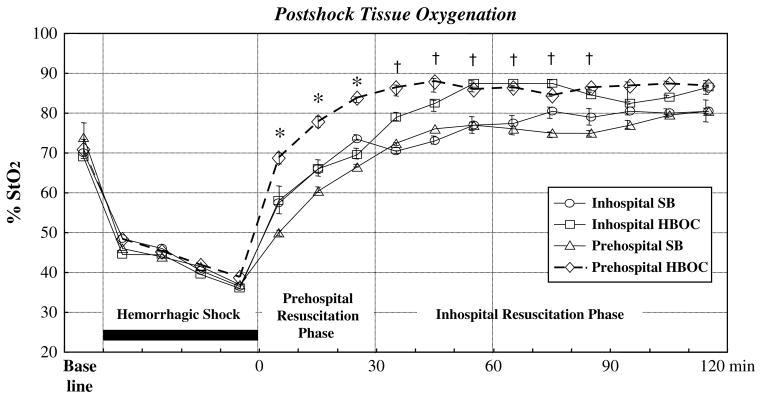
Tissue oxygenation (StO2 = tissue oxygen saturation) was monitored continuously with a near infrared spectroscopy device placed on the animal’s hind limb. Prehospital HBOC and Inhospital HBOC versus Prehospital SB and Inhospital SB, *P<.05 versus other groups; †P<.05.
Fig. 3.
Acute lung injury, determined by Evans blue alveolar extravasation, was evaluated at the end of the study (8 hours post insult). Simulated prehospital HBOC resuscitated abrogated early acute lung injury. *P<.05 versus Inhospital SB; †P<.05 versus all other Two-Event groups. BALF, bronchoalveolar lavage fluid; LPS, lipopolysaccharide; Resus, resuscitated.
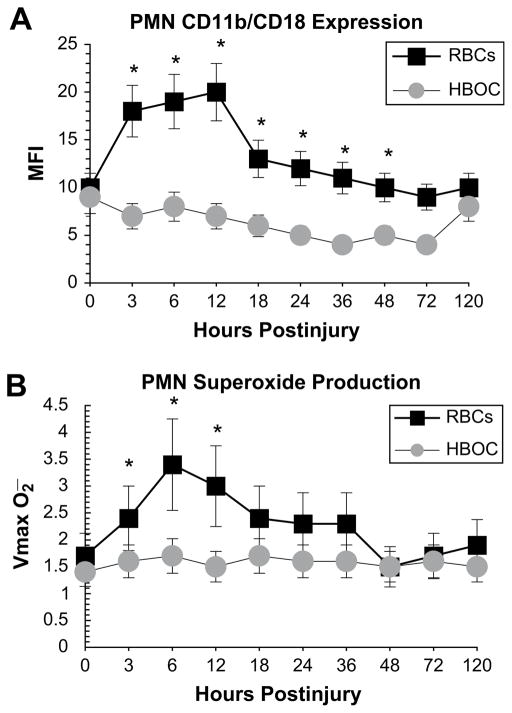
In the authors’ subsequent clinical trial, injured patients requiring urgent transfusion were administered PolyHeme (up to 20 units = 1000 g) or stored RBCs for their initial 12 hours of resuscitation.48 PMN priming was determined by the surface expression of CD11b/CD18 in whole blood and by superoxide production in isolated PMNs. The study groups (stored RBCs [n = 10] versus PolyHeme [n = 9]) were comparable with respect to injury severity (ISS: 27.9 ± 4.5 versus 21.9 ± 2.7), physiologic compromise (emergency department pH: 7.22 ± 0.04 versus 7.19 ± 0.08), and Hb transfusion in the first 24 hours (units: 14.1 ± 2.0 versus 14.5 ± 1). Circulating PMNs from patients resuscitated with stored RBCs manifested evidence of priming through increased CD11b/CD18 expression and enhanced superoxide production (Fig. 4). Three patients (30%) in the stored RBC group died of MOF, whereas all patients in the PolyHeme group survived.
Fig. 4.
Circulating neutrophils (PMNs) from injured patients who underwent initial resuscitation with stored RBCs or PolyHeme. (A) PMN CD11b/CD18 receptor expression in whole blood. (B) PMN superoxide production in isolated cells. *P<.05.
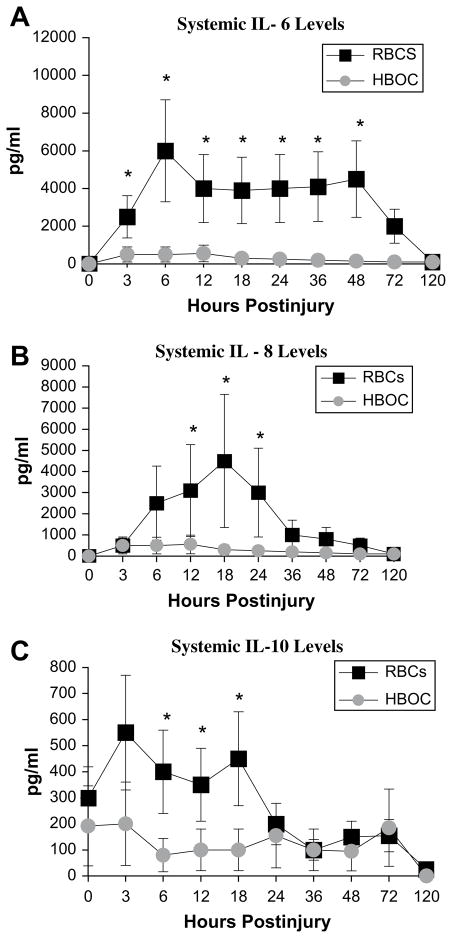
To further investigate the impact of early resuscitation with PolyHeme in lieu of stored RBCs, the authors extended the clinical trial to evaluate the systemic levels of proinflammatory cytokines (IL-6, IL-8), counterregulatory cytokines (IL-10, IL-11), and markers of endothelial activation (sICAM, sE-selectin).49 The study groups (stored RBCs [n = 7] versus PolyHeme [n = 18]) were comparable with respect to injury severity. Patients resuscitated with stored RBCs had higher levels of the proinflammatory cytokines IL-6 and IL-8 and higher levels of the counterregulatory cytokine IL-10 (Fig. 5), with a trend toward higher sICAM and sE-selectin levels. The authors did not enroll a sufficient number of injured patients to definitively address the ultimate study objective—reduction of postinjury MOF; however, the incidence of MOF in the acutely injured patients given PolyHeme during their initial resuscitation for whom the authors had complete data (n = 20) was 15%, contrasted with a predicted incidence of 37% (P<.05) based on their MOF prediction model.45 In sum, these clinical trials in trauma patients suggest that PolyHeme, used in the early resuscitation of patients who have hemorrhagic shock, attenuates the immunodysfunction associated with stored RBC transfusion and thereby reduces the incidence of postinjury MOF.
Fig. 5.
Systemic IL-6 (A), IL-8 (B), and IL-10 (C) from injured patients who underwent initial resuscitation with stored RBCs or PolyHeme. *P<.05.
Acute Hemorrhagic Shock: When Stored Red Blood Cells are Unavailable in Trauma Care
The most compelling indication for HBOC is the scenario in which stored RBCs are unavailable. This potential benefit for military use has largely driven the development of HBOCs, but there are also a number of key applications in civilian trauma care. Most conspicuous is the role in prehospital care, particularly for extended transport times, but there are also remote hospitals throughout the country in which stored blood is simply not available or is rapidly depleted when multiple casualties are encountered. Well-designed animal models have strongly suggested that prehospital low-volume resuscitation with HBOCs can save lives.23–25
Despite the evidence, the scientific design and ethical conduct of clinical trials to establish efficacy of HBOCs when RBCs are unavailable remain a challenge.50,51 To best approximate this scenario, the authors compared the 30-day mortality in 171 trauma patients given up to 20 units (1000 g) of PolyHeme with a historic control group of 300 surgical patients who refused stored RBCs on religious grounds.52 The trauma patients received rapid infusion of 1 to 2 units (n = 45), 3 to 4 units (n = 45), 5 to 9 units (n= 47), or 10 to 20 units (n = 34) of PolyHeme; 40 patients had a nadir RBC Hb of 3 g% or less (mean 1.5 ± 0.7 g%). Total Hb was adequately maintained (mean 6.8 ± 1.2 g%) by way of plasma Hb added by PolyHeme. The 30-day mortality was 25.0% (10/40) in the PolyHeme group compared with 64.5% (20/31) in the control group (Fig. 6).
Fig. 6.
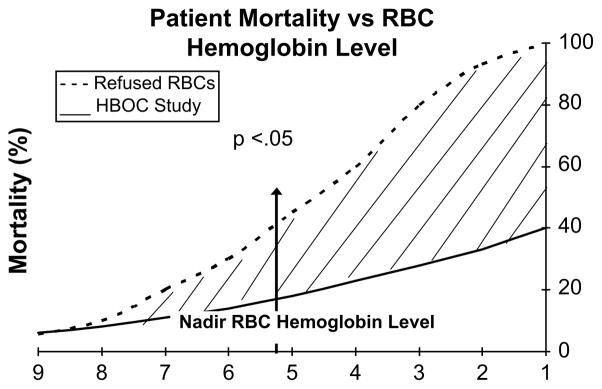
Comparison of 30-day mortality in surgical patients who refused stored RBC transfusion versus injured patients who were initially resuscitated with PolyHeme. Computer-generated curves are based on nadir Hb levels. Mortality was significantly less (P<.05) in the PolyHeme group when RBC Hb was 5.3 g% or less (critical anemia).
A personal experience with PolyHeme during the authors’ in-hospital FDA-approved phase II studies convinced them that the time had arrived for licensing of HBOCs for trauma care.45 An 18-year-old man arrived by ground ambulance at the emergency department in extremis after a gunshot wound to the abdomen from a high-velocity elk-hunting rifle (30.06, hollow soft-point 220 gr, muzzle energy 2840 ft/lb). Because of immediate availability, 10 units of PolyHeme (maximal dose permitted at that time) were administered during the first 14 minutes of in-hospital resuscitation, representing greater than 91% of total circulating Hb at end infusion (RBC Hb = 0.7 g%). The missile entered the left midabdomen and exited posteriorly. At laparotomy, the authors encountered an avulsed shattered left kidney with secondary aortic and vena caval perforations, a partially transected superior mesenteric vein, and destructive injuries to the patient’s distal duodenum, proximal jejunum, midileum, and descending and sigmoid colon. In addition, the patient had massive soft tissue loss in the retroperitoneum, including the psoas and paraspinous muscles, and suffered a concussive spinal cord lesion with resultant paraplegia. The patient received an additional 40 units of packed RBCs during initial laparotomy but, ultimately, this man survived to discharge without organ failure. The authors believe that the immediate infusion of this HBOC was pivotal in maintaining sufficient oxygen delivery during the critical period of massive blood loss to help save this man’s life.
PHASE III USA MULTICENTER PREHOSPITAL HBOC RESUSCITATION TRIAL
The optimal resuscitation fluid for acute blood loss remains unclear, and the practical options for prehospital care have been limited to expansion of the circulating blood volume. The issue is magnified in the combat scenario in which access to blood transfusion is further delayed.53 Resurgent interest in defining optimal field resuscitation has challenged the long-standing practice of unbridled crystalloid loading,54 citing the potential risk of exacerbating hemorrhage by way of dislodging hemostatic clots55 and diluting plasma coagulation factors. Conversely, the magnitude of oxygen debt following hemorrhagic shock correlates directly with adverse outcome.56–58 The availability of HBOCs offers a new strategy for this clinical “catch 22.” Consequently, with this background and preliminary data, the authors conducted a multicenter prehospital trial in the United States.59
The objective of this trial was to assess survival of patients in hemorrhagic shock by comparing treatments initiated at the scene: PolyHeme versus standard of care (crystalloid in the field followed by stored RBCs at hospital arrival). The protocol was based on two potential survival benefits: (1) early replacement of oxygen-carrying capacity in a setting where blood is unavailable, and (2) the use of PolyHeme in lieu of allogeneic RBCs during the first 12 hours post injury to reduce the immunoinflammatory response and subsequent organ dysfunction.
Study Design
In this controlled, open-label trial, patients were randomized in the prehospital setting to the PolyHeme group or the control group (Control). The inclusion criteria were presumed acute blood loss from trauma, class III hemorrhagic shock (systolic blood pressure [SBP] ≤90 mm Hg), and age 18 years or older. Exclusion criteria included imminent death, cardiopulmonary resuscitation, severe head injury (Glasgow Coma Scale [GCS] ≤5), pregnancy, or religious objection to blood products. Patient enrollment occurred under FDA regulation 21CFR§50.24 (Box 2), providing for exception from informed consent.60 Study sites were level I trauma centers. The study design is outlined in Fig. 7. PolyHeme patients received up to six units (50 g Hb/unit) of Poly-Heme beginning at the scene of injury and during the first 12 hours post injury. If needed, stored RBCs were given thereafter. Control patients received crystalloid in the field and stored RBCs as needed in the hospital. Transfusion triggers were based on recent National Institutes of Health Glue Grant protocols for the resuscitation of hemorrhagic shock.61
Box 2. Exception from informed consent requirements for emergency research in the United States.
Human subjects are in a life-threatening situation; available treatments are unsatisfactory.
Obtaining informed consent is not feasible.
Participation in the research holds out the prospect of direct benefit to the subjects.
The clinical investigation could not practicably be performed without the waiver.
The investigational plan defines the length of the potential therapeutic window; the investigator has committed to attempting to contact a legally authorized representative during that window.
Fig. 7.
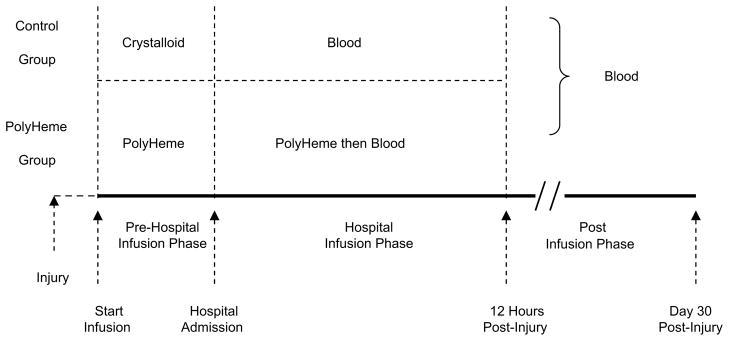
Study design. Patients were randomized (50:50) into each treatment group. PolyHeme patients received PolyHeme in the field, then PolyHeme (up to six units) for up to 12 hours, and then blood in the hospital, as needed. Control patients received crystalloid in the field, then RBC transfusion in the hospital as needed. (Adapted from Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg 2009;208:1–13; with permission.)
Study End Points
The primary efficacy end point was day 30 mortality. Secondary efficacy end points were day 30 mortality for injury-type subgroups (blunt versus penetrating), day 1 mortality, allogeneic blood use through day 1, and the incidence of MOF through day 30. MOF scores were calculated using the Denver MOF score,36 which evaluated four organ systems (lung, kidney, liver, and heart), each graded zero to three with an MOF threshold of four or greater after 48 hours post injury. Primary and secondary safety end points included day 1 mortality, day 30 mortality, adverse events (AEs), and serious AEs (SAEs).
Statistical Analysis
The primary efficacy analysis was a dual superiority/noninferiority assessment of day 30 mortality.62–67 The study design assumed a mortality rate of 17% for the control group based on published series.8,54,68,69 The superiority hypothesis assumed that PolyHeme patients would have a 7% lower mortality rate compared with Control patients. The superiority outcome was based on the potential benefit of (1) providing an oxygen carrier during prehospital critical anemia, and (2) avoiding allogeneic blood transfusion–related MOF in the first 12 hours. The noninferiority hypothesis assumed that PolyHeme patients would have no more than a 7% higher mortality rate compared with Control patients. Different noninferiority margins were considered, but 7% was chosen based on available medical literature, the feasibility of the study, earlier work in acute blood loss patients compared with historical control subjects,52 and a study of injured and bleeding patients who were administered blood en route to the medical center.70 The implication of a noninferiority outcome is that PolyHeme would not be used interchangeably with available RBCs. Rather, in contrast with traditional noninferiority trials, a noninferiority outcome in this trial would allow the benefit to be extrapolated to settings in which RBCs are needed but not available.
Using a one-sided 0.05 alpha level, the study was powered for both hypotheses at 720 patients. An independent data monitoring committee (IDMC) comprising three experts in trauma, critical care, and biostatistics convened following enrollment of 60, 120, 250, and 500 patients to perform blinded safety analyses. A blinded adaptive power analysis was performed after enrollment of 250 patients to ensure that no increase in the trial size was necessary. In the final analysis, the confidence interval on the difference between mortalities was used in a nonparametric analysis of covariance,62–67 adjusting for the following covariates: age, sex, mechanism of injury, ISS, GCS, field SBP, and amount of prerandomization crystalloid. The alpha level was adjusted to correct for the IDMC interim analyses.
Results
Enrollment occurred from January 2004 to July 2006 at 29 level I trauma centers. Demographics and baseline characteristics were comparable between groups (Table 3), with the exception of coagulation status. Within the 12-hour postinjury interval, 53% of patients in the PolyHeme group received one to two units, 15% received three to five units, and 32% received six units. Of the Control patients, 7% received one to two units of RBCs, 10% received three to five units, and 34% received at least six units in the initial 12-hour postinjury interval. At 6 and 12 hours post injury, PolyHeme patients had a mean [Hb] of 10.4 g/dL and 10.3 g/dL, respectively; Control patients had a mean [Hb] of 11.2 g/dL and 10.8 g/dL, respectively.
Table 3.
Baseline characteristics and demographics
| Characteristic | As Randomized (N = 714)
|
As Treated (N =714)
|
Per Protocol (N = 590)
|
Major Protocol Violations (N = 124)
|
||||
|---|---|---|---|---|---|---|---|---|
| PolyHeme (N = 350) | Control (N = 364) | PolyHeme (N = 349) | Control (N = 365) | PolyHeme (N = 279) | Control (N = 311) | PolyHeme (N = 71) | Control (N = 53) | |
| Age (y)a | 36.3 (0.8) | 37.9 (0.9) | 35.9 (0.8) | 38.3 (0.9) | 36.7 (0.9) | 38.2 (0.9) | 35.0 (1.7) | 35.9 (2.3) |
|
| ||||||||
| Age category (APACHE)b | ||||||||
|
| ||||||||
| ≤44 y | 242 (69) | 251 (69) | 247 (71) | 246 (67) | 195 (70) | 211 (68) | 47 (66) | 40 (76) |
|
| ||||||||
| 45–54 y | 65 (19) | 57 (16) | 62 (18) | 60 (16) | 48 (17) | 51 (16) | 17 (24) | 6 (11) |
|
| ||||||||
| 55–64 y | 27 (8) | 27 (7) | 25 (7) | 29 (8) | 21 (8) | 24 (8) | 6 (9) | 3 (6) |
|
| ||||||||
| 65–74 y | 11 (3) | 16 (4) | 10 (3) | 17 (5) | 10 (4) | 14 (5) | 1 (1) | 2 (4) |
|
| ||||||||
| ≥75 y | 5 (1) | 13 (4) | 5 (1) | 13 (4) | 5 (2) | 11 (4) | 0 (0) | 2 (4) |
|
| ||||||||
| Maleb | 272 (78) | 289 (79) | 268 (77) | 293 (80) | 218 (78) | 252 (81) | 54 (76) | 37 (70) |
|
| ||||||||
| Ethnicityb | ||||||||
|
| ||||||||
| Caucasian | 160 (46) | 170 (47) | 156 (45) | 174 (48) | 127 (46) | 151 (49) | 33 (47) | 19 (36) |
|
| ||||||||
| African American | 124 (35) | 120 (33) | 124 (36) | 120 (33) | 97 (35) | 102 (33) | 27 (38) | 18 (34) |
|
| ||||||||
| Hispanic | 53 (15) | 61 (17) | 57 (16) | 57 (16) | 43 (15) | 48 (15) | 10 (14) | 13 (25) |
|
| ||||||||
| Asian | 10 (3) | 7 (2) | 9 (3) | 8 (2) | 9 (3) | 4 (1) | 1 (1) | 3 (6) |
|
| ||||||||
| Other | 3 (<1) | 6 (2) | 3 (<1) | 6 (2) | 3 (1) | 6 (2) | 0 | 0 |
|
| ||||||||
| Height (cm)a | 174.5 (0.6) | 173.4 (0.6) | 174.3 (0.6) | 173.6 (0.6) | 174.8 (0.7) | 173.9 (0.6) | 173.4 (1.7) | 170.1 (2.3) |
|
| ||||||||
| Weight (kg)a | 82.4 (1.1) | 83.2 (1.2) | 82.6 (1.1) | 83.0 (1.2) | 82.9 (1.3) | 84.0 (1.2) | 80.0 (2.5) | 78.1 (3.6) |
|
| ||||||||
| BMI (kg/m2)a | 27.0 (0.4) | 27.7 (0.4) | 27.1 (0.3) | 27.6 (0.4) | 27.1 (0.4) | 27.9 (0.4) | 26.5 (0.9) | 26.6 (1.2) |
|
| ||||||||
| ISSa | 19.9 (0.8) | 19.4 (0.7) | 20.1 (0.7) | 19.2 (0.7) | 19.1 (0.8) | 19.1 (0.8) | 22.9 (1.9) | 21.2 (1.9) |
|
| ||||||||
| ISS categoryb | ||||||||
|
| ||||||||
| Mild/moderate (<9) | 56 (16) | 60 (16) | 51 (15) | 65 (18) | 47 (17) | 53 (17) | 9 (13) | 7 (13) |
|
| ||||||||
| Serious (9–15) | 107 (31) | 101 (28) | 107 (31) | 101 (28) | 87 (31) | 90 (29) | 20 (28) | 11 (21) |
|
| ||||||||
| Severe (16–24) | 61 (17) | 74 (20) | 60 (17) | 75 (21) | 51 (18) | 67 (22) | 10 (14) | 7 (14) |
|
| ||||||||
| Critical/maximal (25–75) | 126 (36) | 123 (34) | 129 (37) | 120 (33) | 94 (34) | 99 (32) | 32 (45) | 24 (49) |
|
| ||||||||
| Maximal (36–75) | 48 (14) | 41 (11) | 46 (13) | 43 (12) | 36 (13) | 36 (12) | 12 (17) | 5 (9) |
|
| ||||||||
| Unsurvivable (75) | 3 (<1) | 1 (<1) | 3 (<1) | 1 (<1) | 1 (<1) | 1 (<1) | 2 (3) | 0 |
|
| ||||||||
| Mechanism of injuryb | ||||||||
|
| ||||||||
| Blunt | 166 (47) | 174 (48) | 165 (47) | 175 (48) | 138 (49) | 154 (50) | 28 (39) | 20 (40) |
|
| ||||||||
| Penetrating | 184 (53) | 186 (52) | 183 (53) | 187 (52) | 141 (51) | 156 (50) | 43 (61) | 30 (60) |
|
| ||||||||
| Transport modeb | ||||||||
|
| ||||||||
| Air | 121 (35) | 122 (34) | 117 (34) | 126 (35) | 99 (35) | 112 (36) | 22 (31) | 10 (19) |
|
| ||||||||
| Ground | 229 (65) | 242 (66) | 232 (66) | 239 (65) | 180 (65) | 199 (64) | 49 (69) | 43 (81) |
|
| ||||||||
| Median Transport Time (min)a | 26 | 26 | 26 | 26 | 27 | 26 | 24 | 26 |
|
| ||||||||
| SBP randomization (mm Hg)a | 77.9 (0.7) | 77.8 (0.6) | 77.2 (0.6) | 78.4 (0.7) | 77.2 (0.7) | 77.3 (0.6) | 82.5 (3.3) | 82.5 (2.7) |
|
| ||||||||
| SBP <60 mm Hgb | 17 (5) | 19 (5) | 16 (5) | 20 (6) | 12 (4) | 19 (6) | 4 (6) | 1 (2) |
|
| ||||||||
| Zero/no SBP obtainedb | 30 (9) | 19 (5) | 27 (8) | 22 (6) | 0 | 0 | 27 (39) | 22 (41) |
|
| ||||||||
| GCS randomizationa | 13.7 (0.1) | 13.6 (0.1) | 13.7 (0.1) | 13.7 (0.1) | 13.8 (0.1) | 13.7 (0.1) | 13.4 (0.3) | 13.3 (0.4) |
|
| ||||||||
| GCS randomization categoryb | ||||||||
|
| ||||||||
| ≤5 | 4 (1) | 2 (<1) | 4 (1) | 2 (<1) | 0 | 0 | 4 (6) | 2 (4) |
|
| ||||||||
| 6–8 | 17 (5) | 18 (5) | 18 (5) | 17 (5) | 15 (5) | 17 (5) | 2 (3) | 1 (2) |
|
| ||||||||
| 9–12 | 35 (10) | 49 (13) | 34 (10) | 50 (14) | 28 (10) | 40 (13) | 7 (10) | 9 (17) |
|
| ||||||||
| 13–15 | 294 (84) | 295 (81) | 293 (84) | 296 (81) | 236 (85) | 254 (82) | 58 (82) | 41 (77) |
|
| ||||||||
| Hb at randomization (g/dL)a,c | 13.2 (0.2) | 13.0 (0.2) | 13.2 (0.2) | 13.0 (0.2) | 13.3 (0.2) | 13.0 (0.2) | 12.8 (0.6) | 12.7 (0.7) |
|
| ||||||||
| PT at randomization (s)a | 29.4 (2.6)d | 20.9 (1.7) | 29.8 (2.6)d | 20.0 (1.6) | 28.3 (2.8)d | 20.2 (1.7) | 37.4 (8.2) | 28.5 (7.5) |
|
| ||||||||
| aPTT at randomization (s)a | 67.4 (7.3)d | 46.6 (4.4) | 68.6 (7.2)d | 44.3 (4.2) | 63.8 (7.7)d | 43.1 (4.1) | 92.6 (22.0) | 80.1 (24.0) |
|
| ||||||||
| Hb at ED admission (g/dL)a,b | 12.3 (0.1)d | 11.5 (0.2) | 12.3 (0.1)d | 11.5 (0.2) | 12.5 (0.2)d | 11.4 (0.2) | 11.6 (0.4) | 11.8 (0.4) |
|
| ||||||||
| PT at ED admission (s)a | 20.7 (1.2) | 21.0 (1.3) | 20.7 (1.2) | 20.9 (1.3) | 20.1 (1.3) | 21.0 (1.4) | 23.4 (3.1) | 20.3 (2.7) |
|
| ||||||||
| aPTT at ED admission (s)a | 49.4 (3.8) | 49.3 (4.1) | 49.3 (3.7) | 49.4 (4.1) | 46.0 (3.9) | 49.3 (4.5) | 63.2 (10.1) | 49.0 (9.2) |
Research board approved the informed consent document and procedures. Additional protection of the rights and welfare of the subjects include community consultation, public disclosure, and establishment of an independent data monitoring committee. Consent to continue the study is obtained from the patient as soon as possible.
Abbreviations: aRTT, Activated partial thromboplastin time; ED, Emergency department; PT, Prothrombin time.
Values are expressed as mean (SE).
Values are expressed as n (%); percentages are based on the number of patients in each treatment group divided by number of patients who have nonmissing values.
To convert to g/L, multiply by 0.1.
P<0.05 compared with Control.
Data from Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg 2009;208:1–13.
Cohort Analyses
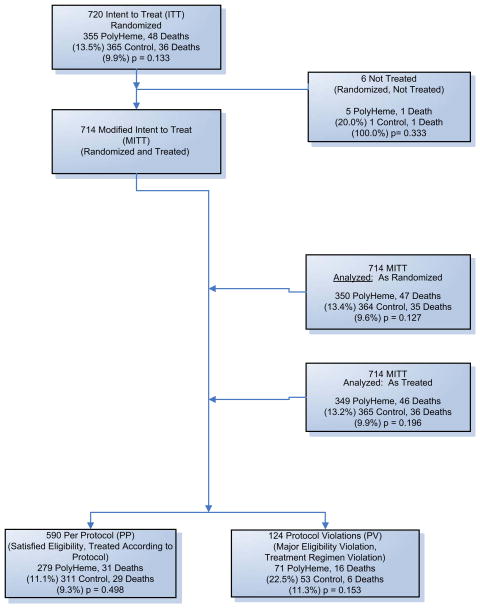
Three prespecified cohorts were analyzed (Fig. 8). Six of the 720 patients received no study treatment. The primary efficacy analysis was based on the remaining 714 patients analyzed according to the treatment to which they were randomized (“As Randomized”). Safety was analyzed in the 714 patients based on the treatment actually received (“As Treated”). The “Per Protocol” cohort included 590 randomized patients who did not violate predefined major eligibility or treatment regimen criteria. There were 124 (17%) patients who had major protocol violations (71 PolyHeme, 53 Control).
Fig. 8.
Study patient enrollment. Patients were assessed for eligibility by paramedics in the field at the scene of injury; 722 patients were enrolled in the study. Two patients had their data withheld by the local institutional review board. The Modified Intent to Treat (MITT) population comprises 720 patients. The MITT population is analyzed two ways: “As Randomized,” in which patients are analyzed in the group to which they were randomized (regardless of which treatment the patients received), and “As Treated,” in which patients are analyzed according to the treatment they actually received. Of the 720 patients, 590 satisfied all the eligibility criteria and were treated according to the protocol; 124 violated eligibility criteria or did not adhere to the treatment regimen described in the protocol. (Adapted from Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg 2009;208:1–13; with permission.)
Primary Efficacy End Point
Day 30 mortality rates and confidence intervals for the three cohorts and the “Major Protocol Violations” group are shown in Table 4 and Fig. 9. There was no significant difference in mortality between the PolyHeme and Control groups. The upper limit of the confidence interval exceeded the 7% noninferiority threshold in the As Randomized (7.65%) and the As Treated cohorts (7.06%). In the Per Protocol cohort, the upper limit of the confidence interval was below the 7% threshold (6.21%).
Table 4.
Day 30 mortality by subgroups
| Characteristic | As Randomized (N =714)
|
As Treated (N = 14)
|
Per Protocol (N =590)
|
Major Protocol Violations (N = 124)
|
||||
|---|---|---|---|---|---|---|---|---|
| PolyHeme (N = 350) | Control (N = 364) | PolyHeme (N = 349) | Control (N = 365) | PolyHeme (N = 279) | Control (N = 311) | PolyHeme (N = 71) | Control (N = 53) | |
| Mortality by mechanism of injurya | ||||||||
|
| ||||||||
| Blunt | 30/166 (18.1)d | 18/174 (10.3) | 30/165 (18.2)d | 18/175 (10.3) | 22/138 (15.9) | 17/154 (11.0) | 8/28 (28.6) | 1/20 (5.0) |
|
| ||||||||
| Penetrating | 17/184 (9.2) | 17/186 (9.1) | 16/183 (8.7) | 18/187 (9.6) | 9/141 (6.4) | 12/156 (7.7) | 8/43 (18.6) | 5/30 (16.7) |
|
| ||||||||
| Mortality by ISS categorya,b | ||||||||
|
| ||||||||
| Mild/moderate (1–8) | 1/56 (1.8) | 1/60 (1.7) | 0/51 (0) | 2/65 (3.1) | 0/47 (0) | 0/53 (0) | 1/9 (11.1) | 1/7 (14.3) |
|
| ||||||||
| Serious (9–15) | 4/107 (3.7) | 2/101 (2.0) | 4/107 (3.7) | 2/101 (2.0) | 3/87 (3.4) | 2/90 (2.2) | 1/20 (5.0) | 0/11 (0) |
|
| ||||||||
| Severe (16–24) | 8/61 (13.1) | 5/74 (6.8) | 8/60 (13.3) | 5/75 (6.7) | 6/51 (11.8) | 4/67 (6.0) | 2/10 (20.0) | 1/7 (14.3) |
|
| ||||||||
| Critical (25–75) | 34/126 (27.0) | 25/123 (20.3) | 33/129 (25.6) | 26/120 (21.7) | 22/94 (23.4) | 22/99 (22.2) | 12/32 (37.5) | 3/24 (12.5) |
|
| ||||||||
| Mortality regression model interaction termsc | ||||||||
|
| ||||||||
| By randomization SBP | 0.687 | 0.751 | 0.919 | ND | ||||
|
| ||||||||
| By APACHE age category | 0.333 | 0.236 | 0.462 | ND | ||||
|
| ||||||||
| By mechanism of injury | 0.187 | 0.146 | 0.350 | ND | ||||
|
| ||||||||
| By ISS | 0.981 | 0.645 | 0.863 | ND | ||||
|
| ||||||||
| By GCS | 0.536 | 0.703 | 0.172 | ND | ||||
|
| ||||||||
| By prerandomization crystalloid | 0.194 | 0.106 | 0.101 | ND | ||||
|
| ||||||||
| By sex | 0.312 | 0.914 | 0.779 | ND | ||||
Abbreviation: ND, Not determined.
Values are expressed as number of deaths (%).
Six patients did not have an ISS calculated: four were patients who had non-trauma-related blood loss; two were trauma patients who died early post injury and had inadequate documentation of their injuries.
P values.
P<.05 compared with Control.
Data from Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg 2009;208:1–13.
Fig. 9.
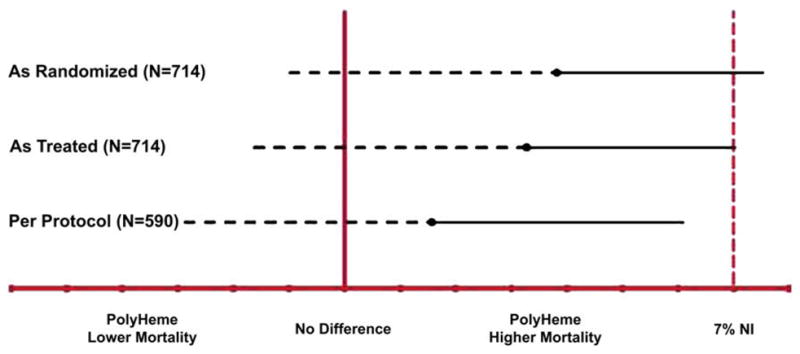
Noninferiority and inferiority mortality analyses. The solid circle represents the point estimate for the observed mortality difference between the PolyHeme and Control groups for each cohort. The dashed black line represents the one-sided inferiority analysis using a final critical alpha level of 0.0144, having split the 0.05 starting alpha level using the Pocock spending function approach between four planned interim analyses and one final analysis. The solid black line represents the one-sided noninferiority (NI) analysis using a final critical alpha level of 0.044, having split the 0.05 starting alpha level using the O’Brien-Fleming spending function approach, as described by Lan and DeMets,63 between one planned interim analysis for superiority after 500 patients had been enrolled and one final analysis. “7%NI” and the vertical red dashed line refer to the noninferiority boundary at 7% higher mortality in the PolyHeme group compared with Control. The As Randomized cohort includes the 714 patients (350 PolyHeme, 364 Control); the As Treated cohort includes 714 patients (349 PolyHeme, 365 Control); and the Per Protocol cohort includes 590 patients (279 PolyHeme, 311 Control). (Adapted from Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg 2009;208:1–13; with permission.)
Following the day 30 assessment, four patient deaths in the As Randomized cohort were reported. One patient died on day 32 who was randomized to Control and received PolyHeme. A second patient died on day 34 who was randomized to and received Control. A third patient died on day 41 who was randomized to and received Control. A fourth patient died on day 194 who was randomized to and received Poly-Heme. Two additional patients died (one PolyHeme and one Control) who were randomized but did not receive any treatment with study fluids (saline, PolyHeme, or RBCs).
Secondary Efficacy End Points
Day 1 mortality was not significantly different between PolyHeme and Control in any cohort (As Randomized: 35/350 [10.0%] versus 27/364 [7.4%]; As Treated: 34/349 [9.7%] versus 28/365 [7.7%]; Per Protocol: 20/279 [7.2%] versus 22/311 [7.1%]). This end point is an important one because day 1 represents a clinically relevant prolonged interval of delayed access to RBCs. The administration of PolyHeme, up to six units in the first 12 hours, simulated such a delay in access to stored RBCs.
Blunt deaths were statistically higher in the PolyHeme group, but there was no treatment-by-covariate interaction for mechanism of injury in any cohort (see Table 4). Mortality differed considerably between PolyHeme and Control patients who had blunt injury and protocol violations (see Table 4). By comparison, the mortality between PolyHeme and Control patients sustaining penetrating wounds was virtually identical, whereas the distribution of major protocol violations was equivalent.
Exposure to allogeneic stored blood was a secondary efficacy end point. In the initial 12 hours post injury, 238 (68%) PolyHeme patients did not require RBCs compared with 183 (50%) Control patients (P<.001). By day 1, 201 (57%) PolyHeme patients and 174 (48%) Control patients did not require RBCs (P<.001). In addition, the time to first exposure to RBCs in those patients who received RBCs was markedly different. The median time to first unit of RBCs in the PolyHeme group was 7.6 hours (453.0 minutes) compared with 1.5 hours (88.5 minutes) in Control. In the Per Protocol cohort, the median time to first unit of RBCs in the PolyHeme group was 14.1 hours (848.0 minutes) compared with 1.5 hours (89.0 minutes) in Control.
Incidence of MOF was low in this study and not significantly different between groups: 26 of 350 (7.4%) in the PolyHeme group versus 20 of 364 (5.5%) in Control. Of the patients who developed MOF, a similar proportion from each group received at least six units of RBCs (PolyHeme 23/26, Control 18/20). There was a strong association in both groups between receiving six or more units of RBCs in the first 12 hours post trauma and MOF (PolyHeme odds ratio: 6.76; P<.001; Control odds ratio: 4.83, P = .002), as was found by the authors previously.36
Safety Analyses
As expected in seriously injured patients, AEs were reported in virtually all: 93% (324/349) of PolyHeme patients and 88% (322/365) of Control patients (P = .041). Investigator-reported AEs occurring in 20% or more of patients included anemia, pyrexia, hypocalcemia, hypokalemia, hyperglycemia, thrombocytopenia, leukocytosis, and tachycardia. SAEs were reported in 40% (141/349) of PolyHeme patients and in 35% (126/365) of Control patients (P = .122; Table 5).
Table 5.
Investigator-reported adverse events
| Event | As Treated (N = 714) | |
|---|---|---|
| PolyHeme (N = 349) | Control (N = 365) | |
| AEs | 324 (93)b | 322 (88) |
| SAEs | 141 (40) | 126 (35) |
| Most common SAEs (>2%) | ||
| Pneumonia | 27 (8) | 21 (6) |
| Hemorrhagic shock | 20 (6) | 16 (4) |
| Respiratory failure | 21 (6) | 17 (5) |
| Hypercoagulable state | 18 (5) | 12 (3) |
| Coagulopathy | 13 (4)b | 4 (1) |
| Sepsis | 12 (3) | 11 (3) |
| MI | 10 (3)b | 2 (1) |
| MI AEsa | 11 (3)b | 3 (1) |
| MI | 7 | 2 |
| NSTEMI | 3 | 0 |
| Non–Q wave MI | 0 | 1 |
| Acute traumatic MI | 1 | 0 |
| Requiring intervention | 1 | 1 |
| Death within 30 d | 3 | 1 |
| Cardiovascular events | ||
| Heart failure/CHF/PE/fluid overload/hypervolemia | 20 (6) | 20 (5) |
| Cardiac arrest/EMD/VFib/V-arrhythmia/VT | 15 (4) | 9 (2) |
| CVA/cerebral ischemia/cerebral infarction | 3 (1) | 1 (1) |
| MOF in 30 d (adjudicated) | 26 (7) | 20 (6) |
| Renal (creatinine >1.8 mg/dL) | 13/26 (50) | 9/20 (45) |
| Hepatic (total bilirubin >2.0 mg/dL) | 20/26 (77) | 15/20 (75) |
| Cardiac (inotropes) | 9/26 (35) | 4/20 (20) |
| Pulmonary (PaO2/FIO2 <240) | 24/26 (92) | 19/20(95) |
Values are expressed as n (%): the number of patients who had the event divided by the total number of patients in each treatment group.
Abbreviations: CHF, Congestive heart failure; CVA, Cerebrovascular accident; EMD, Electro-mechanical dissociation; FIO2, Fraction of inspired oxygen; MI, Myocardial infarction; NSTEMI, Non–ST-segment MI; PE, Pulmonary embolism; V, Ventricular; VFib, Ventricular fibrillation; VT, Ventricular tachycardia.
Two MI events were not considered “serious” by the investigator.
P<.05 compared with Control.
Data from Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg 2009;208:1–13.
Hypertension (Table 6) was reported more frequently as an AE in the PolyHeme group compared with Control (18% versus 12%, P = .028); however, the overall incidence of substantially abnormal episodes of systolic and diastolic hypertension on arrival to the hospital or through 6 hours post injury was low (5% PolyHeme versus 3% Control and 7% PolyHeme versus 7% Control, respectively). The lack of significant evidence of vasoconstriction is consistent with the authors’ previous work.27 The incidence of renal failure was also low and comparable between groups (3% in PolyHeme versus 2% in Control). There was also no difference in the incidence of nausea (17% in PolyHeme versus 15% in Control) or vomiting (13% in PolyHeme versus 12% in Control). Hyperamylasemia was reported in one PolyHeme patient versus no Control patients, and acute pancreatitis was reported in one PolyHeme patient and in two Control patients.
Table 6.
Systemic blood pressure
| Characteristic | As Treated (N = 714)
|
Per Protocol (N = 590)
|
||
|---|---|---|---|---|
| PolyHeme (N = 350) | Control (N = 364) | PolyHeme (N = 279) | Control (N = 311) | |
| Markedly abnormal high SBP (>180 mm Hg) | ||||
|
| ||||
| At admissiona | 6 (2) | 5 (1) | 5 (2) | 4 (1) |
|
| ||||
| At 6 h post injurya | 11 (3) | 5 (1) | 10 (4) | 4 (1) |
|
| ||||
| At admission or 6 h post inurya | 16 (5) | 10 (3) | 14 (5) | 8 (3) |
|
| ||||
| Markedly abnormal high DBP (>105 mm Hg) | ||||
|
| ||||
| At admissiona | 23 (7) | 18 (5) | 17 (6) | 14 (5) |
|
| ||||
| At 6 h post injurya | 3 (1) | 8 (2) | 3 (1) | 5 (2) |
|
| ||||
| At admission or 6 h post inurya | 26 (7) | 25 (7) | 20 (7) | 18 (6) |
|
| ||||
| SBP at admission (mm Hg)b | 115 (2) | 113 (2) | 115 (2) | 113 (2) |
|
| ||||
| SBP at 6 h post injury (mm Hg)b | 129 (2)c | 122 (2) | 129 (2)c | 122 (2) |
|
| ||||
| DBP at admission (mm Hg)b | 71 (1)c | 68 (1) | 71 (1) | 68 (1) |
|
| ||||
| DBP at 6 h post injury (mm Hg)b | 73 (1)c | 66 (1) | 73 (1)c | 66 (1) |
Abbreviations: DPB, Diastolic blood pressure.
Values are expressed as n (%); percentages are based on the number of patients in each treatment group divided by number of patients who had nonmissing values.
Values are expressed as mean (SE).
P<.05 compared with Control.
Data from Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg 2009;208:1–13.
The risk of adverse myocardial-related events associated with HOBCs is particularly germane in light of a recent meta-analysis suggesting an increased risk of myocardial infarction (MI) with HBOCs in clinical trials.71 There was no difference between groups in the incidence of combined cardiovascular events related to cardiac failure, malignant dysrhythmias, or cerebral ischemic/thrombotic complications (see Table 5). The number of investigator-reported MIs was 11 in the PolyHeme group versus three in Control; none was considered by the investigator to be “possibly” or “probably” related to PolyHeme. Three MIs in the PolyHeme group did not fit the classic clinical profile: two were not substantiated by ECGs, enzymes, or autopsy reports and a third followed ligation of a coronary artery during repair of a ventricular stab wound. Cardiology recommended cardiac catheterization for one patient in each group; otherwise, no interventions were necessary. Three PolyHeme patients and one Control patient who had MIs died by day 30; the cause of death was MI in two patients (a 55-year-old man on day 1 and a 56-year-old man on day 19), cardiac arrhythmia in one patient (an 88-year-old woman on day 8), and MOF in one patient (a 63-year-old man on day 30). This overall low incidence of MI (2%) contrasts with the high rate of abnormal ECGs (75% in PolyHeme, 78% in Control), markedly abnormal creatine kinase–myocardial band (CK-MB) isoenzymes (65% in PolyHeme, 68% in Control), and markedly abnormal troponin I (26% in PolyHeme, 19% in Control).
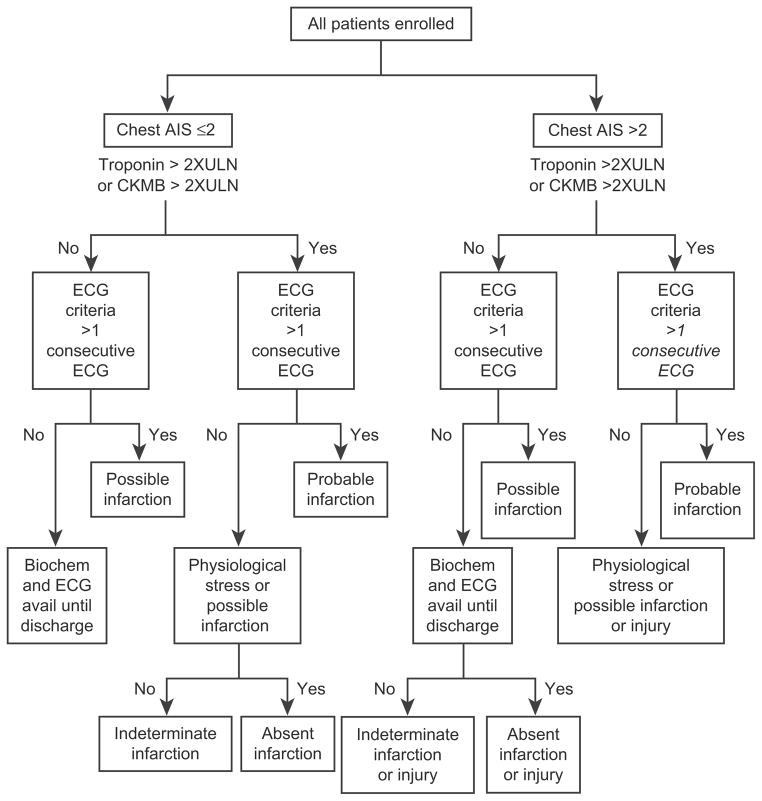
Because of the disparity between high rates of ECG abnormalities and troponin and CK-MB elevation but low MI incidence, a post hoc independent Cardiac Event Subcommittee of experts in cardiology and resuscitation medicine blinded to treatment was convened to adjudicate these results. The committee developed a standardized decision algorithm to classify study patients as to the likelihood of MI (Fig. 10). Patients were stratified by chest trauma (defined as chest abbreviated injury score [AIS] ≤2 or >2). Patients who had an AIS of two or less were classified as having “absent infarction,” “indeterminate infarction,” “possible infarction,” “probable infarction,” or “physiologic stress or possible infarction.” Patients who had an AIS greater than two were classified as having “absent infarction or injury,” “indeterminate infarction or injury,” “possible infarction or injury,” “probable infarction or injury,” or “physiologic stress or possible infarction or injury.” The addition of the term “injury” to the possible and probable classifications of patients who had an AIS greater than two was done in recognition of the fact that chest trauma in and of itself can cause elevations of cardiac enzymes and biomarkers in addition to abnormal ECGs.72,73 Standardized methods of assessing ischemia/infarction on ECG recordings using ST elevation, ST depression, and T wave changes; ECG changes associated with prior MI; and left bundle branch block were employed. When a single abnormal ECG or biomarker was available before the patient died, the patient was classified as “possible infarction.” When a patient had a cardiac arrest before an ECG or laboratory results were obtained, the patient was classified as indeterminate infarction, with a footnote that the patient died before testing. Each case was reviewed initially by two subcommittee members. In the event that classifications by these two members differed, all members reviewed the case. The final classification was determined by consensus.
Fig. 10.
MI algorithm. An independent data monitoring committee cardiac subcommittee reviewed all patient records in the Modified Intent to Treat–As Randomized cohort in a treatment-blinded fashion. Patients were assigned an MI category according to the algorithm based on ECG criteria and chest abbreviated injury score (AIS), troponin, and CK-MB data. 2XULN, two times upper limit of normal. (Adapted from Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg 2009;208:1–13; with permission.)
Regional wall motion abnormality or ischemia on stress testing will be incorporated if available.
The committee found a higher incidence of probable infarction in patients who had or did not have chest trauma than was reported by the investigators (Table 7). More patients were designated probable infarction in the PolyHeme group. The committee also reported the incidence of possible infarction in patients who did or did not have chest injury. When all of the categories of probable and possible infarction were grouped together, more than half of the patients in each group had some evidence of MI. The patients who had these combined designations were numerically higher in Control (193 [55.3%] patients versus 190 [52.1%] patients). There were more patients who had absent infarction in the PolyHeme group (84 versus 64) and more patients who had indeterminate infarction in Control (72 versus 111). In summary, a relationship of PolyHeme to elevated cardiac biomarkers and abnormal ECG findings was not supported by the data. A relationship of PolyHeme to MI could not be clearly ascertained from the totality of the cardiac data in this study, despite the higher number of investigator-reported MIs.
Table 7.
Independent Data Safety Monitoring Cardiac Sub committee Assignation of Myocardial infarction
| Cardiac Committee Determination | As Treated (n = 714)
|
|||
|---|---|---|---|---|
| PolyHeme (n = 349)
|
Control (n = 365)
|
|||
| n | % | n | % | |
| Chest AIS ≤ 2 | ||||
|
| ||||
| Probable infarction | 19 | 5.4 | 11 | 3.0 |
|
| ||||
| Physiologic stress or possible infarction | 58 | 16.6 | 67 | 18.4 |
|
| ||||
| Possible infarction | 5 | 1.4 | 6 | 1.6 |
|
| ||||
| Total possible or probable infarction | 82 | 23.4 | 84 | 23.0 |
|
| ||||
| Absent infarction | 61 | 17.5 | 52 | 14.2 |
|
| ||||
| Indetermine infarction | 49 | 14.0 | 79 | 21.6 |
|
| ||||
| Chest AIS > 2 | ||||
|
| ||||
| Probable infarction or injury | 23 | 6.6 | 19 | 5.2 |
|
| ||||
| Physiologic stress or possible infarction or injury | 81 | 23.2 | 80 | 21.9 |
|
| ||||
| Possible infarction or injury | 1 | 0.3 | 6 | 1.6 |
|
| ||||
| Total possible or probable infarction or injury | 105 | 30.1 | 105 | 28.8 |
|
| ||||
| Absent infarction or injury | 22 | 6.3 | 12 | 3.3 |
|
| ||||
| Indetermine infarction or injury | 30 | 8.6 | 33 | 9.0 |
Values expressed as n (%); percentages are based on the number of patients in each treatment group divided by number of patients who had nonmissing values.
Data from Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg 2009;208:1–13.
CLINICAL IMPLICATIONS OF THE PHASE III PREHOSPITAL HBOC RESUSCITATION TRIAL
Mortality at day 1 or day 30 was not statistically different between injured patients in shock who received up to six units of PolyHeme in lieu of blood for up to 12 hours following injury and Control patients who received the standard of care, including early blood transfusion. Mortality is best understood in the context of the novel study design and conduct of this trial, which presented multiple challenges. The protocol specified enrollment of patients who were bleeding and in shock; therefore, it was necessary to conduct the study under federal regulation 21§CFR50.24, allowing an exception from informed consent.60 In addition, the choice of the control group and the basis for statistical assessment of efficacy were complex. The ideal control group to assess efficacy of an oxygen carrier when blood is not available would comprise severely injured, massively bleeding patients who have critically low [Hb] levels and substantially delayed access to blood and the resulting high mortality. Prior clinical experience with PolyHeme in hospitalized patients26–28,48,49,52,74–78 has shown that PolyHeme can provide life-sustaining oxygen-carrying capacity at otherwise life-threatening Hb levels.52 Because of the possibility that the control group would include patients who had mixed injury severity, variable volumes of blood loss, and early access to blood, dual primary end points of superiority and noninferiority were employed. Thus, Control patients not sustaining critical anemia would not represent the scientifically appropriate control in which statistical superiority would be anticipated; such is the basis for extrapolating the observed results to the intended population.
Superiority in day 30 survival was not observed, likely due to several factors: short transport times, enrollment of patients who were not severely injured (only 34% of the Control group required more than six units of RBCs in the first 12 hours), and the occurrence of protocol violations. In the Per Protocol population, the results fell within the 7% noninferiority boundary, with a mortality difference of two patients between groups. The Per Protocol population represents the clearest opportunity to assess potential treatment effects because all other variables are well matched. The difference between the Per Protocol and As Randomized outcomes is influenced by patients who had major prespecified protocol violations (17%) related to eligibility or treatment regimen in this open-label study. Tables 5 and 6 illustrate that more Poly-Heme patients had indicators of poor prognosis on enrollment, indicating potential futile resuscitation efforts. This is most evident in the subset of Major Protocol Violations patients who had blunt injury; a close review of this subset highlights an imbalance in critical variables between the PolyHeme and Control groups. The Major Protocol Violations patients receiving PolyHeme were characterized by higher ISS (31 versus 26), by lower randomization SBP (80 mm Hg versus 97 mm Hg), by lower randomization GCS (12 versus 14), and by more severe acidosis on arrival to the hospital (base deficit −8.8 mEq/L versus −6.5 mEq/L)—all of which correlate with a higher mortality. PolyHeme administration cannot influence ISS, randomization SBP, or GCS because these are obtained before infusion. Furthermore, more Poly-Heme patients than Control patients had markedly abnormal coagulation status (prothrombin time >17seconds) on enrollment (29% versus 20%). This clinical observation is important and is another potential indication of difference in injury severity. The imbalances in these critical variables alone could potentially account for the observed mortality difference between PolyHeme and Control in the blunt-injury Major Protocol Violations patients.
Transfusion avoidance is particularly relevant to the intended use of PolyHeme for treatment until definitive care and blood become available. Transfusion of six units of allogeneic blood in the first 12 hours has been invoked as the dominant risk factor in the development of postinjury MOF.32,36 PolyHeme has been shown to attenuate the immunoinflammatory effects of stored RBCs,47–49 forming the basis for infusing up to six units of PolyHeme in 12 hours. PolyHeme patients had a statistically significant reduction in the incidence of blood use during the 12- and 24-hour intervals. Although there was no difference in MOF between the groups, 23 of 26 PolyHeme patients and 18 of 20 Control patients who developed MOF also received six units of banked blood. Conceivably, higher allowed doses of PolyHeme within the 12-hour therapeutic window, in lieu of blood, might have reduced the incidence of MOF by averting the adverse immunoinflammatory effects of stored RBCs.
Safety of HBOCs remains a topic of considerable debate.71 The early history with HBOCs included efforts to remove the stromal contaminants believed to be responsible for the observed safety concerns. Savitsky and colleagues,79 however, described vasoconstriction and organ dysfunction in healthy volunteers with a stroma-free tetrameric Hb preparation. Subsequent efforts have focused on removal of small molecular weight tetrameric Hb as a way of improving safety.2,7 In addition, there has been considerable attention paid to the adverse experiences with HBOCs and the potential mechanisms of NO scavenging,9–11,80–81 the generation of reactive oxygen species,3 and reflex autoregulation.6 PolyHeme is polymerized to improve intravascular persistence and then purified to remove virtually all unpolymerized tetramer (<1%) and decrease the interactions with NO that could lead to vasoconstriction.82–90
Safety data in Table 7 show a higher incidence of AEs and SAEs in the PolyHeme group. Of particular concern was that investigators reported more MIs in the Poly-Heme group in this open-label study, although the overall low incidence of MI contrasts with the high incidence of enzymes, biomarkers, and abnormal ECGs. Because of the disparity between high numbers of reported ECG abnormalities and troponin and CK-MB elevation but low reported MI rates, an independent Cardiac Event Subcommittee was convened to adjudicate these results. The committee found a higher incidence of probable infarction in patients who had or did not have chest trauma than was reported by the investigators. More patients were designated probable infarction in the PolyHeme group. The committee also reported the incidence of possible infarction in patients who had and did not have chest injury. When all of the categories of probable and possible infarction were grouped together, more than half of the patients in each group had some evidence of MI. Troponin elevations have been reported in 29% to 44% of critically ill and trauma populations.72,73 Because blood samples and ECGs were obtained serially in this study following admission, the high rates of CK-MB, troponin, and abnormal ECGs may reflect the transitory sequelae of chest trauma in many of these patients rather than myocardial ischemia due to coronary artery disease. Furthermore, analysis of a comprehensive grouping of cardiovascular AEs shows no difference between the groups in the incidence of events related to ischemia, pump failure, or dysrhythmias. Incidences of significant hypertensive episodes and renal failure were low and comparable between groups.
Safety must be assessed in the context of the potential benefit of PolyHeme; that is, improved survival in patients who have life-threatening Hb levels when blood is not available or not an option. This benefit was demonstrated in prior clinical experience with PolyHeme in hospitalized patients26–28,48,49,52,74 and in multiple emergency, compassionate treatments.75,77,78 The results from this study suggest some increase in frequency of AEs compared with the use of blood. Consequently, PolyHeme would not be used interchangeably with RBCs but would be used when the likelihood of dying without oxygen-carrying replacement is so great that the potential life-sustaining benefit would exceed any potential risks. A recent meta-analysis pooled data from multiple different products used in multiple different clinical settings and concluded that there was no role for any of the HBOCs currently in clinical development.71 Such a statistical tool of meta-analysis comparing various products under the umbrella of an entire class can be a useful tool to raise questions that merit answers with respect to the class; however, meta-analysis is not designed to provide answers about specific products or to examine fully the risk-to-benefit ratio of any particular product, particularly for the intended clinical use.91 Rather, that is the role of clinical trials such as the current study with PolyHeme that are focused and conducted to assess the safety and efficacy in relation to a proposed indication—in this case, an indication that addresses a critical, unmet need when there is no available alternative. In this setting, it is possible for an HBOC such as PolyHeme to provide a clinically meaningful benefit, even though the outcome may be less favorable than would be seen with stored blood.
There is an undisputed need for a universally compatible, oxygen-carrying product with long-term storage capability and reduced risk of disease transmission for use when RBCs are not available or not an option. Military battlefield casualties,92 disaster scenarios,93 blood incompatibility and shortages, and religious objection94 represent additional situations in which PolyHeme can address this critical unmet medical need. The combination of PolyHeme’s life-sustaining capability, the logistic benefits, and the acceptable benefit-to-risk calculus for the intended indication represents an opportunity to provide an alternative to transfusion for patients at high risk of death when stored RBCs are not available.
Acknowledgments
Supported in part by Northfield Laboratories, Inc. and National Institutes of Health Grants P50GM49222, T32GM08315, and U54GM62119.
Footnotes
References
- 1.Department of Health and Human Services/US Food and Drug Administration. Biologics Licensing 21 CFR601.25(d) (2) Federal Register. 2004 Apr 1; [Google Scholar]
- 2.Points to consider in the safety evaluation of hemoglobin-based oxygen carriers. Center for Biologics Evaluation and Research. Transfusion. 1991;31:369–71. doi: 10.1046/j.1537-2995.1991.31491213306.x. [DOI] [PubMed] [Google Scholar]
- 3.Alayash AI. Oxygen therapeutics—can we tame hemoglobin? Nat Rev Drug Discov. 2004;3:152–9. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 4.Creteur J, Sibbald W, Vincent JL. Hemoglobin solutions—not just red blood cell substitutes. Crit Care Med. 2000;28:3025–34. doi: 10.1097/00003246-200008000-00058. [DOI] [PubMed] [Google Scholar]
- 5.McFaul SJ, Bowman PD, Villa VM. Hemoglobin stimulates the release of proinflammatory cytokines from leukocytes in whole blood. J Lab Clin Med. 2000;135:263–9. doi: 10.1067/mlc.2000.105180. [DOI] [PubMed] [Google Scholar]
- 6.Winslow RM. Current status of blood substitute research—towards a new paradigm. J Intern Med. 2003;253:508–17. doi: 10.1046/j.1365-2796.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 7.Points to consider on efficacy evaluation of hemoglobin and perfluorocarbon based oxygen carriers. Center for Biologics Evaluation and Research. Transfusion. 1994;34:712–3. doi: 10.1046/j.1537-2995.1994.34894353469.x. [DOI] [PubMed] [Google Scholar]
- 8.Sloan EP, Koenigsberg M, Gens D, et al. Diaspirin cross-linked hemoglobin (DCLHb) in the treatment of severe traumatic hemorrhagic shock—a randomized controlled efficacy trial. JAMA. 1999;282:1857–64. doi: 10.1001/jama.282.19.1857. [DOI] [PubMed] [Google Scholar]
- 9.Hess JR, MacDonald VW, Brinkley WW. Systemic and pulmonary hypertension after resuscitation with cell-free hemoglobin. J Appl Phys. 1993;74:1769–78. doi: 10.1152/jappl.1993.74.4.1769. [DOI] [PubMed] [Google Scholar]
- 10.Poli de Figueiredo LF, Mathru M, Solanki D, et al. Pulmonary hypertension and systemic vasoconstriction may offset the benefits of acellular hemoglobin blood substitutes. J Trauma. 1997;42:847–56. doi: 10.1097/00005373-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Gulati A, Sen AP, Sharma AC, et al. Role of ET and NO in resuscitative effect of diaspirin cross-linked hemoglobin after hemorrhage in rat. Am J Phys. 1997;273:H827–36. doi: 10.1152/ajpheart.1997.273.2.H827. [DOI] [PubMed] [Google Scholar]
- 12.Rohlfs RJ, Bruner E, Chiu A, et al. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J Biol Chem. 1998;273:12128–34. doi: 10.1074/jbc.273.20.12128. [DOI] [PubMed] [Google Scholar]
- 13.Schultz SC, Grady B, Cole F, et al. A role for endothelin and nitric oxide in the pressor response to diaspirin cross-linked hemoglobin. J Lab Clin Med. 1993;122:301–8. [PubMed] [Google Scholar]
- 14.Boura C, Caron A, Longrois D, et al. Volume expansion with modified hemoglobin solution, colloids, or crystalloid after hemorrhagic shock in rabbits: effects in skeletal muscle oxygen pressure versus arterial blood velocity and resistance. Shock. 2003;19:176–82. doi: 10.1097/00024382-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Wettstein R, Cabrales P, Erni D, et al. Resuscitation from hemorrhagic shock with MalPEG-albumin: comparison with MalPEG-hemoglobin. Shock. 2004;22:351–7. doi: 10.1097/01.shk.0000135253.14076.d9. [DOI] [PubMed] [Google Scholar]
- 16.Fushitan K, Imai K, Riggs AF. Oxygen properties of hemoglobin from the earthworm Lumbricus terrestris. J Biol Chem. 1986;261:8414–23. [PubMed] [Google Scholar]
- 17.Hirsch RE, Jelicks LA, Wittenberg BA, et al. A first evaluation of the natural high molecular weight polymeric Lumbricus terrestris hemoglobin as an oxygen carrier. Artif Cells Blood Substit Immobil Biotechnol. 1997;25:429–44. doi: 10.3109/10731199709118932. [DOI] [PubMed] [Google Scholar]
- 18.Levy JH, Goodnough LT, Greilich PE, et al. Polymerized bovine hemoglobin solution as a replacement for allogeneic red blood cell transfusion after cardiac surgery: results of a randomized, double-blind trial. J Thorac Cardiovasc Surg. 2002;124:35–42. doi: 10.1067/mtc.2002.121505. [DOI] [PubMed] [Google Scholar]
- 19.LaMuraglia GM, O’Hara PJ, Baker WH, et al. The reduction of the allogenic transfusion requirement in aortic surgery with hemoglobin-based solution. J Vasc Surg. 2000;31:299–308. doi: 10.1016/s0741-5214(00)90161-7. [DOI] [PubMed] [Google Scholar]
- 20.Standl T, Burmeister MA, Horn EP, et al. Bovine haemoglobin-based oxygen carrier for patients undergoing haemodilution before liver resection. Br J Anaesth. 1998;80:189–94. doi: 10.1093/bja/80.2.189. [DOI] [PubMed] [Google Scholar]
- 21.Kasper SM, Walter M, Grune F, et al. Effects of a hemoglobin-based oxygen carrier (HBOC-201) on hemodynamics and oxygen transport in patients undergoing preoperative hemodilution for elective abdominal aortic surgery. Anesth Analg. 1996;83:921–7. [PubMed] [Google Scholar]
- 22.Hughes GS, Jr, Antal EJ, Locker PK, et al. Physiology and pharmacokinetics of a novel hemoglobin-based oxygen carrier in humans. Crit Care Med. 1996;24:756–64. doi: 10.1097/00003246-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Handrigan MT, Bentley TB, Oliver JD, et al. Choice of fluid influences outcome in prolonged hypotensive resuscitation after hemorrhage in awake rats. Shock. 2005;23:337–43. doi: 10.1097/01.shk.0000156667.04628.1f. [DOI] [PubMed] [Google Scholar]
- 24.Manning JE, Katz LM, Brownstein MR, et al. Bovine hemoglobin-based oxygen carrier (HBOC-201) for resuscitation of uncontrolled, exsanguinating liver injury in swine. Shock. 2000;13:152–9. doi: 10.1097/00024382-200013020-00010. [DOI] [PubMed] [Google Scholar]
- 25.McNeil JD, Smith DL, Jenkins DH, et al. Hypotensive resuscitation using a poly-merized bovine-based oxygen carrying solution leads to reversal of anaerobic metabolism. J Trauma. 2001;50:1063–75. doi: 10.1097/00005373-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Gould SA, Moore EE, Moore FA, et al. Clinical utility of human polymerized hemoglobin as a blood substitute after acute trauma and urgent surgery. J Trauma. 1997;43:325–32. doi: 10.1097/00005373-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JL, Moore EE, Offner PJ, et al. Resuscitation of the injured patient with polymerized stroma-free hemoglobin does not produce systemic or pulmonary hypertension. Am J Surg. 1998;176:612–7. doi: 10.1016/s0002-9610(98)00275-x. [DOI] [PubMed] [Google Scholar]
- 28.Gould SA, Moore EE, Hoyt DB, et al. The first randomized trial of human polymer-ized hemoglobin as a blood substitute in acute trauma and emergent surgery. J Am Coll Surg. 1998;187:113–22. doi: 10.1016/s1072-7515(98)00095-7. [DOI] [PubMed] [Google Scholar]
- 29.Botha AJ, Moore FA, Moore EE, et al. Postinjury neutrophil priming and activation—an early vulnerable window. Surgery. 1995;118:358–65. doi: 10.1016/s0039-6060(05)80345-9. [DOI] [PubMed] [Google Scholar]
- 30.Ciesla DJ, Moore EE, Johnson JL, et al. A 12 year prospective study of postinjury multiple organ failure. Arch Surg. 2005;140:432–40. doi: 10.1001/archsurg.140.5.432. [DOI] [PubMed] [Google Scholar]
- 31.Aiboshi J, Moore EE, Ciesla DJ, et al. Blood transfusion and the two-insult model of postinjury multiple organ failure. Shock. 2001;15:302–6. doi: 10.1097/00024382-200115040-00009. [DOI] [PubMed] [Google Scholar]
- 32.Silliman CC, Moore EE, Johnson JL, et al. Transfusion of the injured patient: proceed with caution. Shock. 2004;21:291–9. doi: 10.1097/00024382-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Moore EE, Moore FA, Harken HA, et al. The two-event construct of postinjury multiple organ failure. Shock. 2005;24:S71–4. doi: 10.1097/01.shk.0000191336.01036.fe. [DOI] [PubMed] [Google Scholar]
- 34.Ingraham LM, Allen JM, Higgins CP, et al. Metabolic membrane and functional responses of human polymorphonuclear leukocytes to platelet activating factor. Blood. 1982;59:1259–66. [PubMed] [Google Scholar]
- 35.Souaia A, Moore FA, Moore EE, et al. Early predictors of post-injury multiple organ failure. Arch Surg. 1994;129:38–45. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- 36.Moore FA, Moore EE, Sauaia A. Blood transfusion—an independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–5. [PubMed] [Google Scholar]
- 37.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for post-injury multiple organ failure. Am J Surg. 1999;178:570–2. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 38.Biffl WL, Moore EE, Zallen G, et al. Neutrophils are primed for cytotoxicity and resist apoptosis in injured patients at risk for multiple organ failure. Surgery. 1999;126:198–202. [PubMed] [Google Scholar]
- 39.Bordin JO, Heddle NM, Blajchman MA. Biologic effects of leukocytes present in transfused cellular blood products. Blood. 1994;84:1703–21. [PubMed] [Google Scholar]
- 40.Nielsen HJ, Reimert CM, Pedersen AM, et al. Time-dependent spontaneous release of white cell and platelet derived bioactive substances from stored human blood. Transfusion. 1996;36:960–5. doi: 10.1046/j.1537-2995.1996.36111297091738.x. [DOI] [PubMed] [Google Scholar]
- 41.Shanwell A, Dristiansson M, Remberger M, et al. Generation of cytokines in red cell concentrates during storage is prevented by pre-storage white cell reduction. Transfusion. 1997;36:678–84. doi: 10.1046/j.1537-2995.1997.37797369441.x. [DOI] [PubMed] [Google Scholar]
- 42.Silliman CC, Paterson AJ, Dickey WO, et al. The association of biologically active lipids with the development of transfusion-related acute lung injury. Transfusion. 1997;37:719–26. doi: 10.1046/j.1537-2995.1997.37797369448.x. [DOI] [PubMed] [Google Scholar]
- 43.Silliman CC, Voelkel NF, Allard JD, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–67. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biffl WL, Moore EE, Offner PJ, et al. Plasma from aged stored red blood cells delays neutrophil apoptosis and primes for cytotoxicity: abrogation by post storage washing but not prestorage leukoreduction. J Trauma. 2001;50:426–31. doi: 10.1097/00005373-200103000-00005. [discussion: 432] [DOI] [PubMed] [Google Scholar]
- 45.Moore EE. Blood substitutes: the future is now. J Am Coll Surg. 2003;196:1–17. doi: 10.1016/s1072-7515(02)01704-0. [DOI] [PubMed] [Google Scholar]
- 46.Partrick DA, Moore EE, Barnett CC, et al. Human polymerized hemoglobin as a blood substitute avoids transfusion-induced PMN priming for superoxide and elastase release. Shock. 1997;7:24. [Google Scholar]
- 47.Masuno T, Moore EE, Cheng AM, et al. Prehospital hemoglobin based oxygen carrier (HBOC) resuscitation attenuates postinjury acute lung injury. Surgery. 2005;B8:335–41. doi: 10.1016/j.surg.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Johnson JL, Moore EE, Offner PJ, et al. Resuscitation with a blood substitute abrogates pathologic postinjury neutrophil cytotoxic function. J Trauma. 2004;50:449–56. doi: 10.1097/00005373-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Johnson JL, Moore EE, Gonzalez RJ, et al. Alteration of the postinjury hyperin-flammatory response by means of resuscitation with a red cell substitute. J Trauma. 2003;54:133–40. doi: 10.1097/00005373-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 50.Huston P, Peterson R. Withholding proven treatment in clinical research. N Engl J Med. 2001;345:912–3. doi: 10.1056/NEJM200109203451210. [DOI] [PubMed] [Google Scholar]
- 51.McRae AD, Weijer C. Lessons from everyday lives: a moral justification for acute care research. Crit Care Med. 2002;30:1146–51. doi: 10.1097/00003246-200205000-00032. [DOI] [PubMed] [Google Scholar]
- 52.Gould SA, Moore EE, Hoyt DB, et al. The life-sustaining capacity of human polymer-ized hemoglobin when red cells may be available. J Am Coll Surg. 2002;195:445–55. doi: 10.1016/s1072-7515(02)01335-2. [DOI] [PubMed] [Google Scholar]
- 53.Gawande A. Casualties of war—military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471–8. doi: 10.1056/NEJMp048317. [DOI] [PubMed] [Google Scholar]
- 54.Bickell WH, Wall MJ, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–9. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 55.Sondeen JL, Coopes VG, Holcomb JB. Blood pressure at which rebleeding occurs after resuscitation in swine and aortic injury. J Trauma. 2004;54:S110–7. doi: 10.1097/01.TA.0000047220.81795.3D. [DOI] [PubMed] [Google Scholar]
- 56.Claridge JA, Schulman AM, Young JS. Improved resuscitation minimizes respiratory dysfunction and blunts interleukin-6 and nuclear factor-kB activation after traumatic shock. Crit Care Med. 2002;30:1815–9. doi: 10.1097/00003246-200208000-00024. [DOI] [PubMed] [Google Scholar]
- 57.Dunham CM, Siegel JH, Weitreter L, et al. Oxygen debt and metabolic acidemia as quantitative predictors of mortality and severity of the ischemic insult in hemorrhagic shock. Crit Care Med. 1991;19:231–41. doi: 10.1097/00003246-199102000-00020. [DOI] [PubMed] [Google Scholar]
- 58.Shoemaker WC, Appel PL, Kram HB. Tissue oxygen debt as a determinant of lethal and nonlethal postoperative organ failure. Crit Care Med. 1988;16:1117–20. doi: 10.1097/00003246-198811000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg. 2009;208:1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 60.Department of Health and Human Services/US Food and Drug Administration. Protection of human rights: informed consent and waiver of informed consent in certain emergency research. Final rules. 21 CFR Parts 50, 56, 312, 314, 601, 612, and 814. Federal Register. 1996;61:51497–531. [Google Scholar]
- 61.Moore FA, McKinley BA, Moore EE, et al. Inflammation and the host response to injury, a large-scale collaborative project: guidelines for shock resuscitation. J Trauma. 2006;61:82–9. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 62.Dunnett CW, Gent M. Alternative to the use of two-sided tests in clinical trials. Stat Med. 1996;15:1729–38. doi: 10.1002/(SICI)1097-0258(19960830)15:16<1729::AID-SIM334>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 63.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–63. [Google Scholar]
- 64.Morikawa T, Yoshida M. A useful testing strategy in phase III trials: combined test of superiority and test of equivalence. J Biopharm Stat. 1995;5:297–306. doi: 10.1080/10543409508835115. [DOI] [PubMed] [Google Scholar]
- 65.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 66.Pocock SJ. Group sequential methods in the design and analysis of clinical trials. Biometrika. 1977;645:191–9. [Google Scholar]
- 67.Wang SK, Tsiatis AA. Approximately optimal one-parameter boundaries for group sequential trials. Biometrics. 1987;43:193–9. [PubMed] [Google Scholar]
- 68.Heckbert SR, Vedder NB, Hoffman W, et al. Outcome after hemorrhagic shock in trauma patients. J Trauma. 1998;45:545–9. doi: 10.1097/00005373-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 69.Mattox KL, Maningas PA, Moore EE, et al. Prehospital hypertonic saline/dextran infusion for post-traumatic hypotension. The USA Multicenter Trial. Ann Surg. 1991;213:482–91. doi: 10.1097/00000658-199105000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barkana Y, Stein M, Maor R, et al. Prehospital blood transfusion in prolonged evacuation. J Trauma. 1999;45:176–80. doi: 10.1097/00005373-199901000-00030. [DOI] [PubMed] [Google Scholar]
- 71.Natanson C, Kern SJ, Lurie P, et al. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299:2304–12. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin M, Mullenix P, Rhee P, et al. Troponin increases in the critically injured patients: mechanical trauma or physiologic stress? J Trauma. 2005;58:1086–91. doi: 10.1097/01.ta.0000190249.19668.37. [DOI] [PubMed] [Google Scholar]
- 73.Velmahos GC, Karaiskskis M, Salim A, et al. Normal electrocardiography and serum troponin I levels preclude the presence of clinically significant blunt cardiac injury. J Trauma. 2004;54:45–51. doi: 10.1097/00005373-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 74.Moore EE, Cheng AM, Moore HB, et al. Hemoglobin-based oxygen carriers in trauma care: scientific rationale for the US Multicenter Prehospital Trial. World J Surg. 2006;30:1247–57. doi: 10.1007/s00268-005-0499-6. [DOI] [PubMed] [Google Scholar]
- 75.Cothern C, Moore EE, Offner PJ, et al. Bood substitute and erythropoietin therapy in severely anemic Jehovah’s Witness. N Engl J Med. 2002;346:1097–8. doi: 10.1056/NEJM200204043461420. [DOI] [PubMed] [Google Scholar]
- 76.Norris EJ, Ness PM, Williams GM. Use of a human polymerized hemoglobin solution as an adjunct to acute normovolemic hemodilution during complex abdominal aortic reconstruction. J Clin Anesth. 2003;15:220–3. doi: 10.1016/s0952-8180(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 77.Raff JP, Dobson CE, Tsai HM. Transfusion of polymerised human haemoglobin in a patient with severe sickle-cell anemia. Lancet. 2002;360:454–65. doi: 10.1016/S0140-6736(02)09640-X. [DOI] [PubMed] [Google Scholar]
- 78.Smith SE, Toor A, Rodriquez T, et al. The administration of polymerized human hemoglobin (pyridoxylated) to a Jehovah’s Witness after submyeloablative stem cell transplantation complicated by delayed graft failure. Compr Ther. 2006;32:172–5. doi: 10.1007/s12019-006-0008-3. [DOI] [PubMed] [Google Scholar]
- 79.Savitsky JP, Docze J, Black J, et al. A clinical trial of stroma-free hemoglobin. Clin Pharmacol Ther. 1978;23:73–80. doi: 10.1002/cpt197823173. [DOI] [PubMed] [Google Scholar]
- 80.Stamler JS, Jia L, Eu JP, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–7. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 81.Yu B, Raher MJ, Volpato GP, et al. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–90. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gould SA, Moss GS. Clinical development of human polymerized hemoglobin as a blood substitute. World J Surg. 1996;20:1200–7. doi: 10.1007/s002689900183. [DOI] [PubMed] [Google Scholar]
- 83.Gould SA, Rosen A, Sehgal L, et al. The effect of altered hemoglobin-oxygen affinity on oxygen transport by hemoglobin solution. J Surg Res. 1980;28:246–51. doi: 10.1016/0022-4804(80)90123-7. [DOI] [PubMed] [Google Scholar]
- 84.Gould SA, Rosen AL, Sehgal LR, et al. Polymerized pyridoxylated hemoglobin: efficacy as an O2 carrier. J Trauma. 1986;26:903–8. doi: 10.1097/00005373-198610000-00007. [DOI] [PubMed] [Google Scholar]
- 85.Moss GS, Gould SA, Sehgal LR, et al. Hemoglobin solution—from tetramer to polymer. Surgery. 1984;95:249–55. [PubMed] [Google Scholar]
- 86.Gould SA, Sehgal LR, Rosen AL, et al. The efficacy of polymerized pyridoxylated hemoglobin solution as an O2 carrier. Ann Surg. 1990;211:394–8. doi: 10.1097/00000658-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosen AL, Gould SA, Sehgal LR, et al. Effect of hemoglobin solution on compensation to anemia in the erythrocyte-free primate. J Appl Phys. 1990;68:938–43. doi: 10.1152/jappl.1990.68.3.938. [DOI] [PubMed] [Google Scholar]
- 88.Sehgal LR, Rosen AL, Gould SA, et al. Preparation and in vitro characteristics of polymerized pyridoxylated hemoglobin. Transfusion. 1983;23:158–62. doi: 10.1046/j.1537-2995.1983.23283172857.x. [DOI] [PubMed] [Google Scholar]
- 89.Sehgal LR, Gould SA, Rosen AL, et al. Polymerized pyridoxylated hemoglobin: a red cell substitute with normal oxygen capacity. Surgery. 1984;95:433–8. [PubMed] [Google Scholar]
- 90.Wilkerson DK, Rosen AL, Sehgal LR, et al. Limits of cardiac compensation in anemic baboons. Surgery. 1988;103:665–70. [PubMed] [Google Scholar]
- 91.LeLorier J, Gregoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536–42. doi: 10.1056/NEJM199708213370806. [DOI] [PubMed] [Google Scholar]
- 92.Holcomb JB, McMullin NR, Pearse L, et al. Causes of death in the U.S. Special Operations Forces in the global war on terrorism. Ann Surg. 2007;245:1–6. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schimdt PJ. Blood and disaster: supply and demand. N Engl J Med. 2002;346:617–20. doi: 10.1056/NEJM200202213460813. [DOI] [PubMed] [Google Scholar]
- 94.Carson JL, Noveck H, Berlin JA, et al. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–8. doi: 10.1046/j.1537-2995.2002.00123.x. [DOI] [PubMed] [Google Scholar]