Abstract
Objectives
To assess the extent to which stage at diagnosis and adherence to treatment guidelines may explain the persistent differences in colorectal cancer survival between the USA and Europe.
Design
A high-resolution study using detailed clinical data on Dukes’ stage, diagnostic procedures, treatment and follow-up, collected directly from medical records by trained abstractors under a single protocol, with standardised quality control and central statistical analysis.
Setting and participants
21 population-based registries in seven US states and nine European countries provided data for random samples comprising 12 523 adults (15–99 years) diagnosed with colorectal cancer during 1996–1998.
Outcome measures
Logistic regression models were used to compare adherence to ‘standard care’ in the USA and Europe. Net survival and excess risk of death were estimated with flexible parametric models.
Results
The proportion of Dukes’ A and B tumours was similar in the USA and Europe, while that of Dukes’ C was more frequent in the USA (38% vs 21%) and of Dukes’ D more frequent in Europe (22% vs 10%). Resection with curative intent was more frequent in the USA (85% vs 75%). Elderly patients (75–99 years) were 70–90% less likely to receive radiotherapy and chemotherapy. Age-standardised 5-year net survival was similar in the USA (58%) and Northern and Western Europe (54–56%) and lowest in Eastern Europe (42%). The mean excess hazard up to 5 years after diagnosis was highest in Eastern Europe, especially among elderly patients and those with Dukes’ D tumours.
Conclusions
The wide differences in colorectal cancer survival between Europe and the USA in the late 1990s are probably attributable to earlier stage and more extensive use of surgery and adjuvant treatment in the USA.
Elderly patients with colorectal cancer received surgery, chemotherapy or radiotherapy less often than younger patients, despite evidence that they could also have benefited.
Keywords: Epidemiology, Public Health, Statistics & Research Methods
Article summary.
Article focus
Why has population-based survival for colorectal cancer been so much higher in the USA than in Europe?
Can differences in stage, diagnostic procedures and/or treatment explain these wide disparities?
Are evidence-based guidelines for staging and treatment being followed?
Key messages
The stage at diagnosis varied more widely between the participating European countries than between the participating US states.
Evidence-based guidelines do not seem to have been closely followed. The proportion of patients who received surgery with adjuvant chemotherapy and/or radiotherapy was much lower in Europe than in the USA. Elderly patients received surgery, chemotherapy or radiotherapy less often than younger patients, despite evidence that they could have benefited.
The wide US–Europe differences in 5-year net survival from colorectal cancer in the late 1990s were probably attributable to the earlier stage and more extensive use of surgery and adjuvant treatment in the USA. Lower survival in Europe was mainly attributable to the much lower survival in Eastern countries. This study underlines the need for population-based survival estimates derived from systematic clinical records of stage and treatment for all patients.
Strengths and limitations of this study
To our knowledge, this is the first population-based high-resolution study with a direct US–Europe comparison of colorectal cancer survival, using clinical data on investigation and treatment collected directly from medical records by trained abstractors with a single protocol, which was then subjected to standard quality control procedures and analysed centrally with the same statistical methods. Some of these clinical records of investigation, stage and treatment are not complete, systematic or timely because they are not collected through routine cancer surveillance reporting for all patients with cancer.
Most of the diagnostic and therapeutic approaches used in the late 1990s remain in widespread use; mesorectal excision for rectal cancer is more recent. It remains relevant to understand the extent to which investigation and treatment are responsible for the persistent international differences in colorectal cancer survival.
The modelling approach to estimate net survival is a methodological strength.
Northern Europe was represented only by Finland.
Introduction
Five-year relative survival from cancers of the colon and rectum has been reported as 12–14% higher in the USA than in Europe.1 Survival for patients diagnosed during 1985–1989 was higher in each of the 9 US states and metropolitan areas covered at that time by the Surveillance, Epidemiology and End Results (SEER) Program than in any of the 22 European countries participating in the EUROCARE-2 study.2
The differences in 3-year colorectal cancer survival for patients diagnosed during 1990–1991 between 10 territories in five European countries and the nine SEER areas were mainly attributable to the stage at diagnosis.3
The first worldwide analysis of cancer survival (CONCORD1) provided a systematic comparison of survival for adults (15–99 years) diagnosed with cancer of the breast, colon, rectum or prostate in 31 countries during 1990–1994 and followed up to 1999. International differences in age-standardised survival were very wide, even after adjustment for differences in mortality from other causes of death. Colorectal cancer survival was higher in the USA and Canada than in many other countries. Differences between the USA and most European regions were smaller than for patients diagnosed during 1985–1989.2 The largest differences were between the USA and Eastern Europe.
The CONCORD protocol incorporated studies designed to explain the international variations in survival. These ‘high-resolution’ studies involve the systematic collection of detailed clinical and pathological data that are not routinely abstracted by population-based cancer registries from the original medical records of large random samples of patients. The high-resolution study reported here provides a transatlantic comparison of stage, treatment and survival for patients with colorectal cancer.
The aims were (1) to compare the distributions of stage for colorectal cancers in Europe and the USA; (2) to determine whether the transatlantic differences in survival persist and, if so, to assess the extent to which they are attributable to differences in stage at diagnosis and (3) to compare adherence to ‘standard care’4 for colorectal cancer in relation to age, stage and cancer site between the USA and Europe.
Material and methods
Data on stage, diagnostic procedures, treatment and follow-up were collected for a representative sample of about 13 000 patients aged 15–99 years diagnosed with colorectal cancer (International Classification of Diseases, ninth revision (ICD-9)5 codes 1530–1539, 1540–1549) in the USA and Europe during 1996–1998. A single protocol was used, derived from the EUROCARE high-resolution protocols.6
The European data were provided by 14 population-based cancer registries in nine countries, four with national coverage (denoted below with an asterisk (*)). For some analyses, the data were grouped into the four European regions defined by the United Nations (UN, http://unstats.un.org/unsd/methods/m49/m49regin.htm)—Northern Europe: Finland*; Western Europe: France (Côte d’Or) and the Netherlands (North East Netherlands); Southern Europe: Italy (Genova, Ragusa and Varese), Slovenia* and Spain (Granada, Navarra and Tarragona); Eastern Europe: Estonia*, Poland (Cracow and Kielce) and Slovakia*. Estonia is classified by the UN as being in Northern Europe, but cancer survival has resembled that in Eastern European countries7 and Estonia was included here with Eastern Europe. US data were provided by seven statewide registries (California, Colorado, Illinois, Louisiana, New York, Rhode Island and South Carolina) from the National Program of Cancer Registries (NPCR), based at the Centers for Disease Control and Prevention.
For this study, cancer registries in the EUROCARE-3 high-resolution study8 updated follow-up to at least 5 years after diagnosis for all patients. North East Netherlands was not included in EUROCARE-3, but the registry routinely collects high-resolution data and could provide such data on virtually all patients with colorectal cancer.
Most registries provided a random sample of at least 500 patients diagnosed during 1996–1998 (1997 in the USA). The Finnish cases were a population-based sample of patients diagnosed in the Tampere hospital region, which is considered representative of Finland.
Of the 12 941 anonymised records for patients with a malignant neoplasm of the colon or rectum, 418 were excluded: in situ (396, 3.1%: collected in the USA, but not in Europe); unknown sex (22, 0.2%); benign or uncertain behaviour (1), or age less than 15 or 100 years or over (19, 1.5%). In all, 12 523 patients with a primary, invasive and malignant colorectal neoplasm were included in the comparisons of stage and treatment. For survival analyses, a further 118 patients were excluded: cancer registered only from a death certificate (72, 0.6%), unknown vital status (3, 0.02%) and date of last known vital status either unknown or earlier than the date of diagnosis (43, 0.3%), leaving 12 405 patients (99.1% of the 12 523 eligible).
Information on stage, diagnostic examinations and treatment was abstracted from the clinical record, pathology reports, hospital discharge records and other sources, as necessary.
Disease stage was defined according to the tumour, nodes, metastasis (TNM) manual9 and/or Dukes’ stage. Many registries collected TNM and Dukes’ stage, but only Dukes’ stage was available for Kielce (Poland) and Finland, so we used Dukes’ classification in order to include these populations in the stage-specific analyses. Dukes’ stage information was more complete than that in the TNM stage, but TNM was used to reconstruct Dukes’ stage where necessary. For descriptive purposes, we defined patients with ‘advanced stage’ as those with metastatic disease or those who had been operated on, but for whom no pathology report was available. This broad category was not used in stage-specific survival analyses, which are based on Dukes’ stage, where available.
Age was categorised as 15–64, 65–74 and 75–99 years.
We defined resection for curative intent as resection of all macroscopically evident malignant tissue with no macroscopic evidence of surgical margin involvement, excluding polypectomy and transanal excision. Radiotherapy and chemotherapy were dichotomised as administered versus not administered or unknown.
Statistical analysis
We analysed the distribution of stage and the number of lymph nodes examined pathologically.9 We report the proportion of patients resected with curative intent and the distributions of stage-specific treatment for colon or rectal cancer. Data sets were excluded if data on stage and/or treatment were missing for 25% or more of patients: Ragusa was excluded from stage-specific analyses, including those on treatment related to the stage at diagnosis.
Net survival up to 5 years after diagnosis was estimated by geographical area (UN region of Europe, country, registry or US state), age and stage, using flexible parametric excess hazard models.10 Net survival is the survival of patients with cancer in the hypothetical situation where the cancer may be assumed to be the only possible cause of death; it may be interpreted as cancer survival after controlling for competing causes of death. Net survival was estimated with a modelling approach10–12 in which the total hazard of death is considered as the sum of the cancer-related mortality hazard (excess hazard) and the hazard of death from other causes (background hazard). The background hazard is derived from life tables of all-cause mortality by sex, single year of age and calendar year in the general population of the geographical area from which the patients with cancer are drawn. We constructed period life tables for 1994–2004 with the approaches proposed by Baili et al.13
Age was included as a continuous variable in all models, in order to avoid the bias in the estimation of net survival that would otherwise arise from differential loss of the oldest patients to competing hazards of death (informative censoring). The non-linear and time-dependent (interaction with time since diagnosis) effects of age were initially modelled with cubic splines. The proportionality of the effect of tumour stage on the excess hazard was also assessed. Simpler models, with linear and/or proportional effects, were successively tested and selected using the Akaike information criterion for goodness of fit.14 We also estimated the instantaneous excess risk (hazard) of death due to colorectal cancer, after subtracting the hazard from all other causes of death.10–12 15 16 We present the mean excess hazard per 1000 person-years at risk at selected times since diagnosis (1 and 6 months, and 1, 3 and 5 years), by age group as well as by stage at diagnosis, after adjustment for age.
The overall (all ages) net survival estimates were age standardised with the International Cancer Survival Standard (ICSS) weight.17
We used a logistic regression model to estimate the odds of patients with colorectal cancer in each area being resected with curative intent, the odds of patients with colon cancer at Dukes’ stage B or C receiving chemotherapy, and the odds of patients with rectal cancer with Dukes’ stage A–C being treated with radiotherapy, after adjustment for age and/or tumour site and/or sex.
Survival analyses were performed with stpm215 in Stata V.12 (StataCorp LP, College Station, Texas, USA).
Results
We included 12 523 patients with an invasive, primary colorectal cancer: 9186 patients in 14 registries in nine European countries and 3337 patients in seven US states (table 1). Microscopic verification was available for 96–98% of the patients in each of the US states and 93% in Europe, ranging from 85% in Ragusa (Italy) to 99% in Kielce (Poland). The proportion of patients with colorectal cancer who were men was similar in Europe (53%) and the USA (50%), but colon cancer was more frequent in the USA (73%) than in Europe (60%). Data were available on stage at diagnosis for 90–93% of patients on both sides of the Atlantic, ranging from 76% (Finland) to 95% or more in 3 of the 14 European registries and from 90% (Colorado and South Carolina) to 97% (Louisiana) in the USA.
Table 1.
Calendar period of diagnosis, morphological verification and data on sex, cancer site and stage. Patients with invasive primary colorectal cancer, Europe and the USA
| Dukes’ stage at diagnosis* |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microscopically verified | Males |
Colon |
A |
B |
C |
D |
Not available |
||||||||||||
| EUROPE | Registry | N | Period of diagnosis | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % |
| Estonia | Estonia | 560 | 1997 | 491 | 88 | 250 | 45 | 337 | 60 | 144 | 26 | 151 | 27 | 76 | 14 | 167 | 30 | 22 | 4 |
| Finland | Finland | 523 | 1996–1998 | 478 | 91 | 247 | 47 | 294 | 56 | 61 | 12 | 174 | 33 | 103 | 20 | 60 | 11 | 125 | 24 |
| France | Côte d'Or | 561 | 1996–1997 | 544 | 97 | 302 | 54 | 382 | 68 | 112 | 20 | 209 | 37 | 98 | 17 | 114 | 20 | 28 | 5 |
| Italy | Genova | 589 | 1996 | 529 | 90 | 326 | 55 | 379 | 64 | 71 | 12 | 192 | 33 | 148 | 25 | 131 | 22 | 47 | 8 |
| Ragusa† | 424 | 1996–1998 | 361 | 85 | 233 | 55 | 269 | 63 | |||||||||||
| Varese | 500 | 1997 | 485 | 97 | 266 | 53 | 332 | 66 | 109 | 22 | 148 | 30 | 105 | 21 | 114 | 23 | 24 | 5 | |
| Netherlands | North East Netherlands | 1936 | 1997 | 1821 | 94 | 1002 | 52 | 1240 | 64 | 280 | 14 | 579 | 30 | 463 | 24 | 332 | 17 | 282 | 15 |
| Poland | Cracow | 512 | 1997–1998 | 463 | 90 | 252 | 49 | 285 | 56 | 128 | 25 | 101 | 20 | 82 | 16 | 158 | 31 | 43 | 8 |
| Kielce | 271 | 1996 | 267 | 99 | 147 | 54 | 133 | 49 | 62 | 23 | 67 | 25 | 41 | 15 | 89 | 33 | 12 | 4 | |
| Slovakia | Slovakia | 581 | 1996 | 535 | 92 | 351 | 60 | 315 | 54 | 161 | 28 | 147 | 25 | 75 | 13 | 160 | 28 | 38 | 7 |
| Slovenia | Slovenia | 937 | 1997 | 871 | 93 | 490 | 52 | 474 | 51 | 131 | 14 | 265 | 28 | 243 | 26 | 209 | 22 | 89 | 9 |
| Spain | Granada | 567 | 1996–1997 | 523 | 92 | 312 | 55 | 360 | 63 | 63 | 11 | 191 | 34 | 109 | 19 | 148 | 26 | 56 | 10 |
| Navarra | 588 | 1996–1997 | 558 | 95 | 354 | 60 | 335 | 57 | 100 | 17 | 188 | 32 | 121 | 21 | 120 | 20 | 59 | 10 | |
| Tarragona | 637 | 1996–1997 | 603 | 95 | 339 | 53 | 421 | 66 | 71 | 11 | 174 | 27 | 176 | 28 | 146 | 23 | 70 | 11 | |
| European registries‡ | 9186 | 8529 | 93 | 4871 | 53 | 5556 | 60 | 1493 | 17 | 2586 | 30 | 1840 | 21 | 1948 | 21 | 895 | 10 | ||
| Northern Europe | 523 | 478 | 91 | 247 | 47 | 294 | 56 | 61 | 12 | 174 | 33 | 103 | 20 | 60 | 11 | 125 | 24 | ||
| Western Europe | 2497 | 2365 | 95 | 1304 | 52 | 1622 | 65 | 392 | 16 | 788 | 32 | 561 | 22 | 446 | 18 | 310 | 12 | ||
| Southern Europe† | 4242 | 3930 | 93 | 2320 | 55 | 2570 | 61 | 545 | 14 | 1158 | 30 | 902 | 24 | 868 | 20 | 345 | 8 | ||
| Eastern Europe | 1924 | 1756 | 91 | 1000 | 52 | 1070 | 56 | 495 | 26 | 466 | 24 | 274 | 14 | 574 | 30 | 115 | 6 | ||
| USA | |||||||||||||||||||
| California | 495 | 1997 | 485 | 98 | 242 | 49 | 356 | 72 | 89 | 18 | 137 | 28 | 168 | 34 | 60 | 12 | 41 | 8 | |
| Colorado | 548 | 1997 | 536 | 98 | 296 | 54 | 407 | 74 | 85 | 16 | 162 | 30 | 191 | 35 | 56 | 10 | 54 | 10 | |
| Illinois | 505 | 1997 | 497 | 98 | 239 | 47 | 384 | 76 | 71 | 14 | 144 | 29 | 224 | 44 | 36 | 7 | 30 | 6 | |
| Louisiana | 511 | 1997 | 502 | 98 | 263 | 51 | 374 | 73 | 115 | 23 | 146 | 29 | 146 | 29 | 90 | 18 | 14 | 3 | |
| New York | 492 | 1997 | 473 | 96 | 248 | 50 | 350 | 71 | 91 | 18 | 114 | 23 | 226 | 46 | 21 | 4 | 40 | 8 | |
| Rhode Island | 418 | 1997 | 413 | 99 | 195 | 47 | 302 | 72 | 64 | 15 | 149 | 36 | 160 | 38 | 29 | 7 | 16 | 4 | |
| South Carolina | 368 | 1997 | 358 | 97 | 187 | 51 | 265 | 72 | 68 | 18 | 89 | 24 | 150 | 41 | 26 | 7 | 35 | 10 | |
| US registries | 3337 | 3264 | 98 | 1670 | 50 | 2438 | 73 | 583 | 17 | 941 | 28 | 1265 | 38 | 318 | 10 | 230 | 7 | ||
| Total | 12523 | ||||||||||||||||||
*Dukes' stage A–D correspond to TNM stage categories I-IV.
†Data for Ragusa are not included in the percentages of Dukes' stage for Southern Europe.
‡Northern Europe: Finland; Western Europe: France (Côte d'Or), the Netherlands (North East Netherlands); Southern Europe: Italy (Genova, Ragusa, Varese), Slovenia, Spain (Granada, Navarra, Tarragona); Eastern Europe: Estonia, Poland (Cracow, Kielce), Slovakia.
Early-stage (Dukes’ A or B) colorectal cancers were equally common in the USA (45%) and Europe (47%), but the stage distributions varied widely between the US states and between the European regions. Tumours in Dukes’ stage A were of similar frequency in Europe (17%, range 11–28%) and in the USA (17%, 14–23%), and the proportion of Dukes’ B tumours were also very comparable (Europe 30%, 25–37%; USA 28%, 24–36%). In contrast, Dukes’ C tumours were twice as common in the USA (38%, 29–46%) as in Europe (21%, 24–30%), while Dukes’ D tumours were twice as common in Europe (21%, 11–33%) as in the USA (10%, 7–18%). The proportion of tumours with unspecified stage was slightly higher in Europe (10%, 4–24%) than in the USA (7%, 3–10%). Exclusion of Finland, with 24% of tumours of unknown stage, did not substantially alter the overall stage distributions in Europe (data not shown).
Patients diagnosed at an advanced stage (ie, metastatic cases plus unresected cases for which no data on stage were available) were more common in the four European regions (29%, 24–34%) than in the USA (20%, 16–23%; table 2). In Europe, advanced stage was more common in Southern Europe (30%) and Eastern Europe (34%). The highest proportion of patients at an advanced stage in the USA (23%, California) was similar to the lowest regional proportion in Europe (24%, Western Europe).
Table 2.
Advanced stage, resection with curative intent, 30-day postoperative mortality and proportion of patients with information on stage: colorectal cancer, Europe and the USA, 1996–1998
| All cases |
Resected with curative intent* |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Advanced stage† |
Deaths within 30 days |
Staged |
||||||||||
| Colon |
Rectum |
|||||||||||
| EUROPE | Registry | N | N | % | N | % | N | % | N | % | N | % |
| European registries‡ | 8762 | 2535 | 29 | 6584 | 75 | 248 | 4 | 3895 | 95 | 2374 | 95 | |
| Northern Europe | 523 | 134 | 26 | 385 | 74 | 16 | 4 | 192 | 84 | 142 | 90 | |
| Western Europe§ | 2497 | 609 | 24 | 2092 | 84 | 24 | 6 | 1299 | 93 | 646 | 92 | |
| Southern Europe¶ | 3818 | 1131 | 30 | 2912 | 76 | 152 | 5 | 1748 | 97 | 1081 | 97 | |
| Eastern Europe | 1924 | 661 | 34 | 1195 | 62 | 56 | 5 | 656 | 98 | 505 | 97 | |
| US registries | 3337 | 676 | 20 | 2832 | 85 | 124 | 4 | 2039 | 97 | 677 | 93 | |
| California | 495 | 112 | 23 | 415 | 84 | 15 | 4 | 294 | 96 | 102 | 93 | |
| Colorado | 548 | 113 | 21 | 468 | 85 | 18 | 4 | 335 | 95 | 109 | 93 | |
| Illinois | 505 | 112 | 22 | 422 | 84 | 21 | 5 | 320 | 97 | 85 | 93 | |
| Louisiana | 511 | 105 | 21 | 431 | 84 | 26 | 6 | 315 | 100 | 111 | 97 | |
| New York | 492 | 80 | 16 | 411 | 84 | 22 | 5 | 287 | 95 | 102 | 94 | |
| Rhode Island | 418 | 78 | 19 | 369 | 88 | 9 | 2 | 268 | 99 | 93 | 94 | |
| South Carolina | 368 | 76 | 21 | 316 | 86 | 13 | 4 | 220 | 96 | 75 | 87 | |
| Total | 12 099 | |||||||||||
†All metastatic cases, plus unresected cases for which no stage data were available.
*Curative intent: surgery not specified as palliative or tumour entirely resected.
‡Northern Europe: Finland; Western Europe: France (Côte d'Or) and the Netherlands (North East Netherlands); Southern Europe: Italy (Genova, Ragusa and Varese), Slovenia and Spain (Granada, Navarra and Tarragona); Eastern Europe: Estonia, Poland (Cracow and Kielce) and Slovakia.
§Data for North East Netherlands (1936) are not included in the proportion of deaths within 30 days of surgery for Western Europe because the date of surgery was not available.
¶Data for Ragusa (424) are not included in the percentages of Dukes’ stage for Southern Europe.
Resection for curative intent was more frequent in the USA (85%) than in Europe (75%). The proportion resected with curative intent was remarkably similar in all seven US states (84–88%). Only Western Europe (84%) showed a proportion as high as that in the USA.
Thirty-day postoperative mortality was 5% or less in the USA and Europe. Among patients resected with curative intent, the proportion with a known stage was around 95% in the USA and Europe, with the lowest proportions in Northern Europe (84–90%; table 2). In many European registries, data on the number of lymph nodes examined after surgery were not available for most patients (see web-appendix table S1).
Adjuvant chemotherapy and radiotherapy were administered more frequently in the USA than in Europe (table 3). Among Dukes’ B patients with colon cancer, 28% received chemotherapy in the USA (21–46%) vs 20% in Europe (4–31%). Among Dukes’ C patients with colon cancer, 56% received chemotherapy in the USA (47–64%) vs 47% in Europe (38–53%). Among Dukes’ A–C patients with rectal cancer, 47% received radiotherapy in the USA (41–52%) vs 37% in Europe (26–45%).
Table 3.
Chemotherapy in Dukes’ B and C colon cancer and radiotherapy in Dukes’ A–C rectal cancer
| Colon Dukes’ B* |
Colon Dukes’ C* |
Rectum Dukes’ A–C* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Among whom, chemotherapy |
N | Among whom, chemotherapy |
N | Among whom, radiotherapy |
|||||
| EUROPE | Registry | N | % | N | % | N | % | |||
| European registries† | 1748 | 343 | 20 | 1130 | 528 | 47 | 1850 | 678 | 37 | |
| Northern Europe | 110 | 11 | 10 | 50 | 21 | 42 | 118 | 34 | 29 | |
| Western Europe | 591 | 23 | 4 | 346 | 133 | 38 | 411 | 183 | 45 | |
| Southern Europe‡ | 736 | 209 | 28 | 529 | 265 | 50 | 797 | 331 | 42 | |
| Eastern Europe | 259 | 80 | 31 | 154 | 81 | 53 | 480 | 124 | 26 | |
| US registries | 727 | 200 | 28 | 913 | 508 | 56 | 484 | 228 | 47 | |
| California | 108 | 29 | 27 | 114 | 54 | 47 | 65 | 31 | 48 | |
| Colorado | 129 | 29 | 22 | 145 | 93 | 64 | 70 | 29 | 41 | |
| Illinois | 112 | 28 | 25 | 171 | 88 | 51 | 65 | 33 | 51 | |
| Louisiana | 105 | 22 | 21 | 106 | 59 | 56 | 76 | 33 | 43 | |
| New York | 86 | 24 | 28 | 157 | 81 | 52 | 84 | 44 | 52 | |
| Rhode Island | 119 | 37 | 31 | 107 | 69 | 64 | 66 | 30 | 45 | |
| South Carolina | 68 | 31 | 46 | 113 | 64 | 57 | 58 | 28 | 48 | |
*Dukes’ stage A–D correspond to TNM stage categories I–IV.
†Northern Europe: Finland; Western Europe: France (Côte d'Or) and the Netherlands (North East Netherlands); Southern Europe: Italy (Genova, Ragusa and Varese), Slovenia and Spain (Granada, Navarra and Tarragona); Eastern Europe: Estonia, Poland (Cracow and Kielce) and Slovakia.
‡Data for Ragusa (424) are not included in the percentages of Dukes’ stage for Southern Europe.
Relative to Southern Europe (2912 patients, reference category), the odds of receiving resection for curative intent (vs any other surgical procedure), after adjustment for age and tumour site, were much lower in Eastern Europe (OR 0.46, 95% CI 0.41 to 0.52), somewhat lower in Northern Europe (OR 0.88, 0.71 to 1.09) and much higher in Western Europe (OR 1.62, 1.43 to 1.85) and in the USA (OR 1.72, 1.52 to 1.94; table 4).
Table 4.
Odds of colorectal cancer patients with cancer being resected with curative intent, odds of patients with Dukes’ B or C colon cancer being treated with chemotherapy and odds of Dukes’ stage A–C rectal cancer being treated with radiotherapy: by region, age, cancer site or sex
| Resection for curative intent |
Colon Dukes’ B* |
Colon Dukes’ C* |
Rectum Dukes' A–C* |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | OR | 95%CI |
N | OR | 95%CI |
N | OR | 95%CI |
N | OR | 95%CI |
|||||
| Region† | ||||||||||||||||
| Northern Europe | 385 | 0.88 | 0.71 | 1.09 | 110 | 0.29 | 0.15 | 0.56 | 50 | 0.88 | 0.46 | 1.69 | 118 | 0.58 | 0.38 | 0.89 |
| Western Europe | 2092 | 1.62 | 1.43 | 1.85 | 591 | 0.10 | 0.06 | 0.16 | 346 | 0.64 | 0.48 | 0.87 | 411 | 1.22 | 0.95 | 1.56 |
| Southern Europe‡ | 2912 | 1.00 | 736 | 1.00 | 529 | 1.00 | 797 | 1.00 | ||||||||
| Eastern Europe | 1195 | 0.46 | 0.41 | 0.52 | 259 | 0.89 | 0.64 | 1.23 | 154 | 0.89 | 0.61 | 1.32 | 480 | 0.46 | 0.36 | 0.59 |
| USA | 2832 | 1.72 | 1.52 | 1.94 | 727 | 1.25 | 0.97 | 1.60 | 913 | 1.56 | 1.23 | 1.98 | 484 | 1.39 | 1.10 | 1.76 |
| Age (years) | ||||||||||||||||
| 15–64 | 3194 | 1.00 | 674 | 1.00 | 684 | 1.00 | 890 | 1.00 | ||||||||
| 65–74 | 3195 | 0.89 | 0.79 | 0.99 | 797 | 0.61 | 0.48 | 0.77 | 653 | 0.47 | 0.37 | 0.59 | 784 | 0.69 | 0.57 | 0.84 |
| 75–99 | 3027 | 0.48 | 0.43 | 0.53 | 952 | 0.07 | 0.05 | 0.10 | 655 | 0.10 | 0.08 | 0.13 | 616 | 0.30 | 0.24 | 0.38 |
| Site | ||||||||||||||||
| Colon | 6191 | 1.00 | ||||||||||||||
| Rectum | 3225 | 0.73 | 0.66 | 0.79 | ||||||||||||
| Sex | ||||||||||||||||
| Male | 1324 | 1.00 | ||||||||||||||
| Female | 966 | 0.92 | 0.77 | 1.10 | ||||||||||||
*Dukes’ stage A–D correspond to TNM stage categories I–IV.
†Northern Europe: Finland; Western Europe: France (Côte d'Or) and the Netherlands (North East Netherlands); Southern Europe: Italy (Genova, Ragusa and Varese), Slovenia and Spain (Granada, Navarra and Tarragona); Eastern Europe: Estonia, Poland (Cracow and Kielce) and Slovakia.
‡Data for Ragusa (424) are not included in the percentages of Dukes’ stage for Southern Europe
Patients aged 75 years or more were only half as likely to be resected with curative intent as those aged 15–64 years (OR 0.48, 95%CI 0.43 to 0.53), after adjustment for region and tumour site.
Patients with colon cancer (reference category) were resected with curative intent more often than patients with rectal cancer (OR 0.73, 0.66 to 0.79).
Patients with Dukes’ B colon cancer received chemotherapy much less often in Western Europe (OR 0.10, 0.06 to 0.16) and Northern Europe (OR 0.29, 0.15 to 0.56) than in Southern Europe. For patients with Dukes’ C colon cancer, chemotherapy was used less in Western Europe (OR 0.64, 0.48 to 0.87) and more often in the USA (OR 1.56, 1.23 to 1.98) than in Southern Europe.
Radiotherapy was administered to patients with rectal cancer in Dukes’ stage A–C more often in the USA (OR 1.39, 1.10 to 1.76) and less often in Northern Europe (OR 0.58, 0.38 to 0.89) or Eastern Europe (OR 0.46, 0.36 to 0.59), compared to Southern Europe.
Older patients were only 10% as likely to be treated with radiotherapy and chemotherapy.
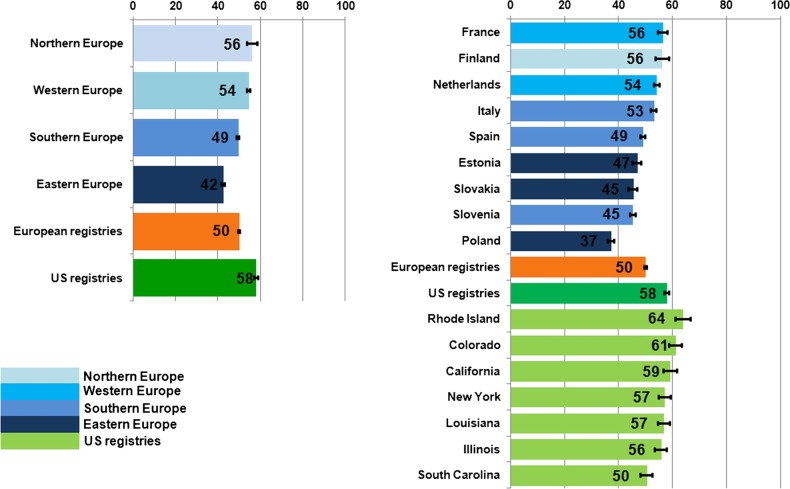
Overall, age-standardised net survival at 5 years was 50% in Europe and 58% in the USA (figure 1). Survival was lower in all European areas than in the USA, and only in Northern Europe was the figure (56%) close to that in the USA. Survival was lower in Western Europe (54%) and in Southern Europe (49%) and lowest in Eastern Europe (42%). Survival varied widely not only between European countries (from 56% in France and Finland to 37% in Poland), but also between US states (from 64% in Rhode Island to 56% in Illinois and 50% in South Carolina).
Figure 1.
Five-year age standardised net survival (%), patients diagnosed with primary invasive colorectal cancer in Europe and the USA in the late 1990s: country and region. Note—Northern Europe: Finland; Western Europe: France (Côte d'Or) and the Netherlands (North East Netherlands); Southern Europe: Italy (Genova, Ragusa and Varese), Slovenia and Spain (Granada, Navarra and Tarragona); Eastern Europe: Estonia, Poland (Cracow and Kielce) and Slovakia.
Five-year age-standardised net survival was higher in the USA than in Europe for Dukes’ stage A (84%) and B (75%) tumours, but higher in Northern Europe than in the USA for Dukes’ C (52%) and D (12%) tumours (figure 2). The geographical range in survival was much wider for locally advanced disease, from 36% in Eastern Europe to 77% in Northern Europe and 49% in the USA. As with overall survival, stage-specific 5-year survival was similar in Northern, Western and Southern Europe and the USA. In Eastern Europe, survival for node-positive, locally advanced and metastatic tumours was lower than in other European regions and the USA.
Figure 2.
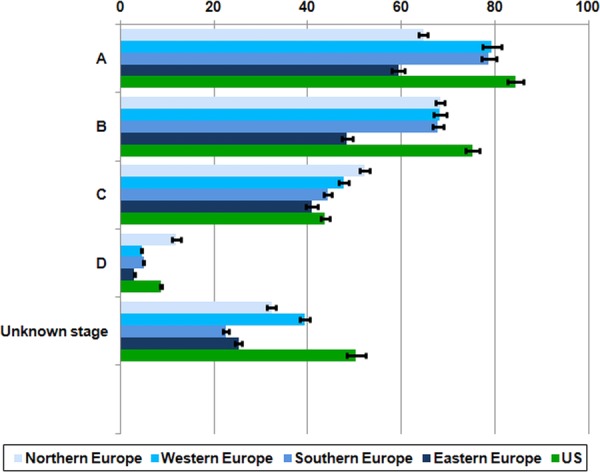
Five-year age-standardised net survival (%), patients diagnosed with primary invasive colorectal cancer in Europe and the USA in the late 1990s: region* and stage at diagnosis. *Northern Europe: Finland; Western Europe: France (Côte d'Or) and the Netherlands (North East Netherlands); Southern Europe: Italy (Genova, Ragusa and Varese), Slovenia and Spain (Granada, Navarra and Tarragona); Eastern Europe: Estonia, Poland (Cracow and Kielce) and Slovakia.
Survival was 5–12% higher in women than in men in all areas, especially in Northern and Western Europe (11–12%; see web-appendix figure S1).
The mean excess hazard of death at 1 and 6 months, and at 1, 3 and 5 years after diagnosis was higher in Eastern Europe than in all other regions, for all ages combined as well as in each of the three age categories (see web-appendix figure S2). The difference was most marked for elderly patients (75–99 years). No striking differences were found between Northern, Western and Southern Europe and the USA. The high excess hazard of death in Eastern Europe was mainly confined to patients with Dukes’ D tumours (see web-appendix figure S3).
Discussion
Transatlantic differences in population-based colorectal cancer survival have raised questions about early diagnosis and the adequacy of investigation and treatment that cannot be addressed with data from clinical trials, which include only selected patient groups.
Patterns-of-care studies and survival studies have been conducted separately in Europe3 6 8 and the USA.18 19 To our knowledge, this is the first population-based high-resolution study that allows direct comparison of colorectal cancer survival between Europe and the USA with clinical data on investigation and treatment collected directly from medical records by trained abstractors with a single protocol, which is then subjected to standard quality control procedures and analysed centrally with the same statistical methods.
The participating cancer registries are population-based registries that register all persons diagnosed in the territory they cover. This study included large, randomly selected subsets of all persons diagnosed with colorectal cancer during 1996–1998 in each territory. These samples are not intended to be ‘representative’ of all patients with colorectal cancer in Europe or the USA, but they are representative of all patients with colorectal cancer diagnosed during 1996–1998 in the territory of each registry and the findings are generalisable to the populations from which they are drawn.
Most of the diagnostic and therapeutic approaches used in the late 1990s remain in widespread use. Understanding their role in international differences in survival remains relevant. Mesorectal excision for rectal cancer is the main exception: it has improved survival from rectal cancer20 21 but its widespread use is more recent. Mesorectal excision was not used in Estonia before 1997, which may partly explain the low survival from rectal cancer.22
The transatlantic 12% difference in the 3-year survival in colorectal cancer survival for patients diagnosed during 1990–19913 was mostly attributed to the differences in stage at diagnosis. In our study of patients diagnosed in the late 1990s, the overall 5-year net survival was still higher in the 7 US states (58%) than in the 14 European regions (42–56%). The widest differences with the USA were seen in Southern (49%) and Eastern Europe (42%).
The two studies differed in design, however: data from the SEER public-use data set in the USA23 were simply adapted to the EUROCARE-2 high-resolution protocol as far as possible. In contrast, data for this study were collected directly from clinical records on both sides of the Atlantic, with a standard protocol. US coverage changed from the five metropolitan areas and four states covered by the SEER Program to seven of the state-wide NPCR registries. In the earlier study, differences in background mortality in the USA were controlled with a single national life table for 1990, weighted for the proportion of African-American patients, white patients and other races. Here, we were able to use state-specific life tables for each of the calendar years 1996–2004.
The tighter control for background mortality and the modelling approach used to estimate net survival are the methodological strengths of this study, but these changes do not explain why the transatlantic differences we observed in 5-year survival are smaller than the differences in 3-year survival for patients diagnosed in the early 1990s.3
Survival varied widely not only among European countries, but also between the seven US states. Survival in Slovenia was lower than in other Southern European countries and more similar to that in Eastern Europe. In the USA, survival was lowest in South Carolina, where African-American patients represent approximately 30% of the population (http://www.ipspr.sc.edu/publication/Older%20SC.pdf).
Apart from patients with Dukes’ B cancers, where survival was similar in Northern, Western and Southern Europe, stage-specific net survival was rather variable. Survival was highest in the USA for Dukes’ stage A and B and in Northern Europe (Finland) for Dukes’ stage C and D. This could be due to some misclassification of stage in Finland, where the stage data were not available for 24% of cases.
The mean excess hazard of death up to 5 years after diagnosis was similar in Europe and the USA for patients with tumours in Dukes’ stage A or B. The hazard was somewhat higher in Eastern Europe for Dukes’ stage C and much higher for Dukes’ D disease, especially in the first 3 years after diagnosis. The very high hazard of death for patients with late-stage disease in Eastern Europe suggests that fewer effective treatment options were available for these patients, although higher levels of comorbidity may also have restricted the choice.
It was not possible to evaluate the impact of the number of examined lymph nodes on the stage-adjusted excess hazard of death, because information on nodal status was so often unavailable (see web-appendix). It is therefore impossible to assess whether stage migration affects the comparison of stage-specific survival between European regions and the USA in the late 1990s, as reported for patients diagnosed in 1990.3
We did not have information on whether or not patients in this study had undergone faecal occult blood testing or sigmoidoscopy before diagnosis. Opportunistic testing with these procedures was common in the USA in the late 1990s. Almost 40% of respondents to the Behavioural Risk Factor Surveillance System (http://www.cdc.gov/mmwr/preview/mmwrhtml/00056494.htm) survey in 1997 reported having had a faecal occult blood test at some time in the past and 42% reported a previous sigmoidoscopy or proctoscopy. Removal of premalignant polyps or in situ neoplasms may thus have been more frequent than in Europe. This would be expected to reduce incidence, shift the spectrum of malignancy to the right and reduce survival in the USA. In fact, incidence in the USA is higher, the stage distribution is less advanced, and survival is higher than in Europe.
Adjuvant chemotherapy for colon cancer and adjuvant radiotherapy for rectal cancer were used more widely in the USA than in Europe. Despite the evidence available in the late 1990s on the lack of efficacy of adjuvant chemotherapy for Dukes’ B colon cancer, 30% of patients with colon cancer received it in the USA, and 20% overall in Europe. In Finland and Western Europe, however, adjuvant chemotherapy was rare, in line with the contemporary recommendations, while in Southern and Eastern Europe, adjuvant chemotherapy was used as frequently as in the USA.
In contrast, there were striking differences in the use of adjuvant chemotherapy for Dukes' C stage colon cancer in the late 1990s, particularly within Europe. Given the wide consensus on its effectiveness since 1990, we did not expect to find that such a strong recommendation would be so poorly followed. Comorbidity and greater toxicity are not valid reasons for the underuse of adjuvant chemotherapy in the elderly: toxicity is not greater24 25 and quality of life is not worse.26
Elderly patients were 90% less likely to receive adjuvant chemotherapy than younger patients. Clinical attitudes appear to differ between the USA and Europe, where the proportion of patients receiving adjuvant chemotherapy is much lower. This suggests that a higher proportion of older patients with Dukes’ C colon cancer who are fit enough to undergo surgery should receive adjuvant chemotherapy, particularly in Europe.
Radiotherapy is known to be an effective complement to surgery for rectal cancer, in particular to reduce the risk of local recurrence; preoperative radiotherapy is preferable to postoperative radiotherapy27 and it is recommended in Europe and the USA.28–31 We were unable to distinguish between the impact of preoperative and postoperative radiotherapy, because this information was not systematically available, but fewer patients received radiotherapy in Europe than in the USA and the practice in Europe was strikingly heterogeneous, even within a given country. Age was a strong predictor of the use of radiotherapy. Some older patients are unsuitable for radiotherapy because of comorbidity, but their 70% lower odds of receiving it cannot be explained by comorbidity alone; radiotherapy has not yet been deployed to its full potential for older patients with rectal cancer. It is not clear why the evidence on the benefits of radiotherapy was so poorly followed in many regions.
Surgical resection offers the only approach to a definitive cure for colorectal cancer. The proportion of patients resected with curative intent was very similar in the seven US states (84–88%), but it varied widely between the nine European countries (from 56% to 86%) and was particularly low in Eastern Europe (mean 62%). A more aggressive approach to surgical treatment for elderly patients with colorectal cancer in Europe could improve this situation, although European patients were more often diagnosed at an advanced stage or with unresectable disease. Performance status and comorbidity can influence whether a patient is considered fit for resection, but data on these factors were not available. The quality of life in Canadian patients aged over 80 years who underwent surgery for colorectal cancer was generally comparable to that of younger patients.32
In this large, population-based study in Europe, however, age alone often seems to have been a limiting factor in the treatment of colorectal cancer. Elderly patients were generally treated less often with surgery, chemotherapy or radiotherapy, despite the evidence that they could benefit from these treatments. Treatment decisions should be taken in the context of multidisciplinary meetings, including a comprehensive geriatric assessment: age alone should not exclude a patient from receiving surgery and/or adjuvant treatment.
Differences in colorectal cancer survival between Europe and the USA in the late 1990s were still wide and may be attributable to the earlier stage at diagnosis, higher levels of surgery and more extensive use of adjuvant treatment in the USA.
Evidence-based guidelines do not seem to have been followed as closely as they should be: chemotherapy was used too often for Dukes’ B disease and not often enough for Dukes’ C disease, especially among elderly patients.
The need for population-based survival estimates derived directly from the clinical records on the stage at diagnosis and treatment is recognised by clinicians and epidemiologists. A recent comparison of stage-specific cancer survival with population-based data33 was complicated by inconsistent coding of the stage34; several registries had to be excluded because fewer than half the tumour records contained data on stage. In this high-resolution study, the stage data were remarkably complete (76–94% in Europe and 93% in the USA), because they were collected directly from clinical records. Ideally, the medical records of patients with cancer would systematically include data on investigations and stage at diagnosis; cancer registries would obtain those data for all patients and the stage would be coded consistently. Until then, high-resolution studies would appear to offer the most reliable approach to obtain data on stage and treatment, and to assess survival by stage at diagnosis.
If good evidence is required on whether all patients receive guideline-compliant investigation and treatment, and whether this makes a difference to survival, then cancer registries will need to be able to obtain timely and high-quality data on the investigations, the stage and the treatment for all patients with cancer.
Supplementary Material
Acknowledgments
Some of the data for this study were collected with the support of the Compagnia di San Paolo, Turin, Italy. Alleanza Contro il Cancro, the Italian Cancer Network (http://www.alleanzacontroilcancro.it) supported a CONCORD Working Group meeting for this study in London, 29–30 September 2010. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Footnotes
Contributors: CA, MS, GG and MPC contributed to the study design. CL, JF, TA, EA, MBL, SB, RC, ME, JPF, JG, SG, TH, MPZ, JR, CSD, MJSa, MJSc, TS, GT, RT, MV, HJW and XCW contributed to data collection. CA performed data quality control. PB prepared the life tables; CA, BR and MPC performed the data analyses. CA, BR, CL, JF, HKW, LCR, TA, ME, MV and MPC contributed to the interpretation of the findings. CA, BR and MPC drafted the article and CL, JF, HKW, LCR, MJS, MBL, MS, TA, XCW, CLo and GG contributed to the revisions of the manuscript. All authors have read and approved the final version of the manuscript.
Funding: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Cancer Prevention and Control, Cancer Surveillance Branch, Atlanta, GA; Louisiana Tumor Registry, School of Public Health, Louisiana State University, New Orleans, LA (cooperative agreement #U58/CCU 62(306); CDC/NPCR contractor. Atlanta, GA; New York State Cancer Registry, New York State Department of Health, Albany, NY (cooperative agreement #U58/CCU 220322); Colorado Central Cancer Registry, Colorado Department of Public Health and Environment, Denver, CO (cooperative agreement #U58/CCU 820326); Illinois State Cancer Registry, Illinois Department of Public Health, Springfield, IL (cooperative agreement #U58/CCU 520378); South Carolina Central Cancer Registry, Columbia; SC (cooperative agreement #U58/CCU 420312); California Cancer Registry, Sacramento, CA (cooperative, agreement #U58/CCU 920352); Rhode Island Cancer Registry, Rhode Island Department of Health, Providence, RI (cooperative agreement #U58/CCU 520378); University of Kentucky, Lexington KY (UKRF 3049024672-12-568). Support was also obtained from the Health Department of the Navarra Government, Spain (research grant 79/2000). The participation of Estonia was partly supported by the Estonian Ministry of Education and Research (SF0940026s07).
Competing interests: None.
Ethics approval: The study was approved by the US Centers for Disease Control (CDC, Atlanta, Georgia, USA), Institutional Review Board #3551.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Additional results are available on the web appendix. No additional data are available.
References
- 1.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 2008;9:730–56 [DOI] [PubMed] [Google Scholar]
- 2.Gatta G, Capocaccia R, Coleman MP, et al. Toward a comparison of survival in American and European cancer patients. Cancer 2000;89:893–900 [PubMed] [Google Scholar]
- 3.Ciccolallo L, Capocaccia R, Coleman MP, et al. Survival differences between European and US patients with colorectal cancer: role of stage at diagnosis and surgery. Gut 2005;54:268–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIH Consensus Conference Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990;264:1444–50 [PubMed] [Google Scholar]
- 5.World Health Organisation International Classification of Diseases, 1975, 9th revision. Geneva: WHO, 1977 [Google Scholar]
- 6.Gatta G, Capocaccia R, Sant M, et al. Understanding variations in colorectal cancer survival in Europe: a EUROCARE high-resolution study. Gut 2000;47:533–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sant M, Allemani C, Santaquilani M, et al. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999: results and commentary. Eur J Cancer 2009;45(Suppl 6):931–91 [DOI] [PubMed] [Google Scholar]
- 8.Gatta G, Zigon G, Aareleid T, et al. Patterns of care for European colorectal cancer patients diagnosed in 1996–98: a EUROCARE high-resolution study. Acta Oncol 2010;49:776–83 [DOI] [PubMed] [Google Scholar]
- 9.Spiessl B, Beahrs OH, Hermanek P, et al. TNM Atlas: illustrated guide to the TNM/pTNM classification of malignant tumours. Berlin: Springer Verlag, 1992 [Google Scholar]
- 10.Nelson CP, Lambert PC, Squire IB, et al. Flexible parametric models for relative survival, with application in coronary heart disease. Stat Med 2007;26:5486–98 [DOI] [PubMed] [Google Scholar]
- 11.Estève J, Benhamou E, Raymond L. Statistical methods in cancer research, volume IV. Descriptive epidemiology (IARC Scientific Publications No. 128) Lyon: International Agency for Research on Cancer, 1994 [PubMed] [Google Scholar]
- 12.Pohar Perme M, Stare J, Estève J. On estimation in relative survival. Biometrics 2012;68:113–20 [DOI] [PubMed] [Google Scholar]
- 13.Baili P, Micheli A, De Angelis R, et al. Life-tables for world-wide comparison of relative survival for cancer (CONCORD study). Tumori 2008;94:658–68 [DOI] [PubMed] [Google Scholar]
- 14.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Control 1974;19:716–23 [Google Scholar]
- 15.Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J 2009;9:265–90 [Google Scholar]
- 16.Danieli C, Remontet L, Bossard N, et al. Estimating net survival: the importance of allowing for informative censoring. Stat Med 2012;31:775–86 [DOI] [PubMed] [Google Scholar]
- 17.Corazziari I, Quinn MJ, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–16 [DOI] [PubMed] [Google Scholar]
- 18.Alley LG, Chen VW, Wike JM, et al. CDC and NPCR's breast, colon, and prostate cancer data quality and patterns of care study: overview and methodology. J Registry Manag 2007;34:148–57 [Google Scholar]
- 19.Cress RD, Sabatino SA, Wu XC, et al. Adjuvant chemotherapy for patients with stage III colon cancer: results from a CDC-NPCR Patterns of Care study. Clin Med Oncol 2009;3:107–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapiteijn E, Putter H, Van de Velde CJ. Impact of the introduction and training of mesorectal excision on recurrence and survival of rectal cancer in the Netherlands. Br J Surg 2002;89:1142–9 [DOI] [PubMed] [Google Scholar]
- 21.Heald RJ. Total mesorectal excision is optimal surgery for rectal cancer: a Scandinavian consensus. Br J Surg 1995;82:1297–9 [DOI] [PubMed] [Google Scholar]
- 22.Innos K, Soplepmann J, Suuroja T, et al. Survival for colon and rectal cancer in Estonia: role of staging and treatment. Acta Oncol 2012;51:521–7 [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute Incidence—SEER 9 public-use data, 2002: cases diagnosed 1973–2000. National Institutes of Health. 2003. Bethesda, MD: National Institutes of Health, 2003. Ref Type: Electronic Citation [Google Scholar]
- 24.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091–7 [DOI] [PubMed] [Google Scholar]
- 25.Kohne CH, Grothey A, Bokemeyer C, et al. Chemotherapy in elderly patients with colorectal cancer. Ann Oncol 2001;12:435–42 [DOI] [PubMed] [Google Scholar]
- 26.Bouvier AM, Jooste V, Bonnetain F, et al. Adjuvant treatments do not alter the quality of life in elderly patients with colorectal cancer: a population-based study. Cancer 2008;113:879–86 [DOI] [PubMed] [Google Scholar]
- 27.Glimelius B, Gronberg H, Jarhult J, et al. A systematic overview of radiation therapy effects in rectal cancer. Acta Oncol 2003;42:476–92 [DOI] [PubMed] [Google Scholar]
- 28.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–23 [DOI] [PubMed] [Google Scholar]
- 29.Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006;24:4620–5 [DOI] [PubMed] [Google Scholar]
- 30.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638–46 [DOI] [PubMed] [Google Scholar]
- 31.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40 [DOI] [PubMed] [Google Scholar]
- 32.Mastracci TM, Hendren S, O'Connor B, et al. The impact of surgery for colorectal cancer on quality of life and functional status in the elderly. Dis Colon Rectum 2006;49:1878–84 [DOI] [PubMed] [Google Scholar]
- 33.Maringe C, Walters S, Rachet B, et al. Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000–7. Acta Oncol 2013;52:919–32 [DOI] [PubMed] [Google Scholar]
- 34.Walters S, Maringe C, Butler J, et al. Comparability of stage data in cancer registries in six countries: lessons from the International Cancer Benchmarking Partnership. Int J Cancer 2013;132:676–85 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.