Abstract
Objectives
Learning as measured by eyeblink classical conditioning is preserved in patients with idiopathic Parkinson's disease, but severely affected in patients with progressive supranuclear palsy. We here sought to clarify whether procedural learning is impaired in multiple system atrophy (MSA), and whether it may be helpful for the differentiation of parkinsonian syndromes.
Design
We investigated learning using (1) eyeblink classical conditioning with a delay (interstimulus interval 0 ms) and a trace (600 ms) paradigm and (2) a serial reaction time task.
Setting
Participants were recruited from academic research centres.
Participants
11 patients with MSA and 11 healthy controls.
Results
Implicit learning in eyeblink classical conditioning (acquisition of conditioned responses) as well as the serial reaction time task measures of implicit learning (reaction time change) are impaired in patients with MSA as compared with controls, whereas explicit learning as measured by the sequence recall of the serial reaction time task is relatively preserved.
Analysis
We hypothesise that the learning deficits of patients with MSA are due to lesions of cerebellar and connected brainstem areas.
Conclusions
A retrospective synopsis of these novel data on patients with MSA and groups of patients with idiopathic Parkinson's disease and progressive supranuclear palsy studied earlier suggest that eyeblink classical conditioning may contribute to the early differentiation of atypical Parkinson syndromes from idiopathic Parkinson's disease. This hypothesis should be tested in a prospective trial.
Keywords: Parkinsons disease, Learning
Article summary.
Strength and limitation of this study
The study differentiates feasible and non-feasible assessments of procedural learning in MSA.
The comparison to other patient groups is clearly retrospective and needs to be validated by a prospective trial.
Introduction
Multiple system atrophy (MSA) is a progressive neurodegenerative disorder characterised by an absent or a poor levodopa responsive parkinsonism, cerebellar dysfunction and autonomic failure.1 A predominantly parkinsonian (MSA-P) and a cerebellar subtype (MSA-C) are recognised. Despite the development of the consensus criteria,2 the differential diagnosis between MSA and other hypokinetic rigid syndromes, such as idiopathic Parkinson's disease (IPD) or progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome, PSP), remains a clinical challenge.3 4
Recent years have seen an increasing focus on non-motor symptoms such as cognitive deficits in parkinsonian disorders. Different patterns of cognitive impairment including deficits in executive function and learning abilities have been described.5–9
A well-established task to study associative, procedural learning10 is eyeblink classical conditioning (EBCC), which some regard as a model of implicit learning.11 Previous studies have shown that learning as measured by EBCC is normal in patients with IPD, but, severely affected in patients with PSP.12–14 In contrast to tracking or pointing tasks,15 16 EBCC has the advantage not to depend on manual motor skills. Learning assessed by the serial reaction time task (SRTT) showed the implicit motor skill close to normal in patients with IPD, whereas patients with PSP were markedly impaired; in contrast, the SRTT sequence recall component as measure of explicit learning was largely preserved in both groups.12 14 We sought to investigate whether implicit learning deficits are specific for PSP or present in MSA as well, thereby comparing the clinical feasibility of SRTT and of EBCC in this patient group.
Methods
Subjects
Eleven patients with MSA were recruited from the outpatient clinics in Munich and Göttingen between 1999 and 2008 (table 1). The clinical diagnosis of ‘probable MSA’ was established following consensus criteria.2 Seven patients with MSA were taking L-Dopa, two dopamine agonists (ropinirole, pramipexole), one amantadine, one budipine, one biperiden, another metixen and two were taking antidepressants. L-Dopa equivalent doses were calculated according to Tomlinson et al17 except for budipine, biperiden and metixen, where no conversion factor was given.
Table 1.
Characteristics of the patients with MSA
| Patient numbers | MSA type | Age (year) | Sex | Duration (year) | L-Dopa response | LED (mg) | UPDRS maximum=108 | Cerebellar maximum=4 | Autonomic maximum=5(f), 6(m) | Pyramidal maximum=2 | Hamilton maximum=69 | MMS maximum=30 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P | 66 | F | 9 | Poor | 0+ | 50 | 0 | 1 | 0 | 11 | 27 |
| 2 | P | 69 | M | 4.5 | Poor | 125 | 20 | 0 | 2 | 0 | 20 | 30 |
| 3 | P | 73 | M | 8 | Absent | 255 | 16 | 0 | 3 | 0 | 15 | 28 |
| 4 | P | 59 | F | 1.5 | Poor | 125# | 30 | 0 | 2 | 1 | 16 | 26 |
| 5 | P | 71 | M | 4 | Absent | 150 | 35 | 0 | 4 | 0 | 6 | 29 |
| 6 | P | 75 | M | 5 | Modest | 524 | 38 | 0 | 3 | 0 | 6 | 29 |
| 7 | P | 75 | F | 3 | Poor | 375 | 40 | 0 | 3 | 0 | 10 | 28 |
| 8 | P | 58 | M | 3 | Poor | 105# | 18 | 0 | 1 | 0 | 2 | 30 |
| 9 | C | 64 | M | 2 | Poor | 900 | 69 | 2 | 2 | 0 | 22 | 27 |
| 10 | C | 56 | M | 2.5 | * | 0 | 5 | 3 | 1 | 0 | 16 | 28 |
| 11 | C | 60 | F | 8 | * | 0 | 30 | 3 | 3 | 1 | 14 | 26 |
| Mean | 66.0 | 4.6 | 31.9 | 0.7 | 2.3 | 0.2 | 12.5 | 28.0 | ||||
| S.D. | 7.1 | 2.6 | 17.7 | 1.3 | 1.0 | 0.4 | 6.2 | 1.4 |
Type: parkinsonian (MSA-P) or cerebellar (MSA-C) predominance; LED, L-Dopa equivalent dose, + indicates additional budipine medication, # additional anticholinergic medication; UPDRS, Unified Parkinson's disease rating scale, motor examination only (high number of points indicates high disability); MMS, Mini-Mental State (30 points are normal, ≤26 is usually considered as cognitive impairment). Cerebellar impairment was evaluated for ataxia (arm, leg), saccades and intention tremor (finger–nose test), autonomic impairment for postural faintness, syncopes, urinary incontinence, urinary retention, faecal incontinence and impotence (males only). Pyramidal tract impairment was scored for hyper-reflexia and Babinski sign. Each item was scored if present with 1, otherwise 0. In the Hamilton scale a low score indicates few depressive symptoms. *Not investigated.
MSA, multiple system atrophy.
To rule out an immediate impact of medication on the patients’ memory performance, the antiparkinsonian medication was discontinued on the morning of the day of the study. Patients with MSA were compared with 11 healthy control participants, matched for age (t test), and chosen for the absence of neurodegenerative or any other neurological disease, and for the absence of intake of central nervous system-active medication (mean age 59.5±10 years, 6 men, 5 women). A subgroup was already involved in our earlier published study (numbers 2, 3, 5, 6, 8, 9, 11, 12, 14 according to table 2 in Ref. 12). All participants gave written informed consent; the research protocol was approved by the local ethics committee. Neither the patients nor the control participants had any sign of cranial nerve impairment or auditory deficits in routine neurological examination.
Table 2.
| Number | Group | Age (year) | Sex | Duration (year) | UPDRS maximum=108 | BDI maximum=63 | MMS maximum=30 | MDRS maximum=144 |
|---|---|---|---|---|---|---|---|---|
| 1 | C | 57 | M | – | – | 2 | 144 | |
| 2 | C | 60 | F | – | – | 9 | 142 | |
| 3 | C | 50 | M | – | – | 0 | 141 | |
| 4 | C | 64 | F | – | – | 0 | 142 | |
| 5 | C | 58 | M | – | – | 1 | 138 | |
| 6 | C | 73 | M | – | – | 6 | 134 | |
| 7 | C | 49 | F | – | – | 0 | 143 | |
| 8 | C | 45 | M | – | – | 1 | 144 | |
| 9 | C | 53 | M | – | – | 1 | 142 | |
| 10 | C | 73 | F | – | – | 11 | 30 | |
| 11 | C | 72 | F | – | – | 0 | 30 | |
| Mean | 59.5 | 2.6 | ||||||
| S.D. | 10.0 | 3.6 | ||||||
| 1 | IPD | 69 | F | 2 | 45 | 11 | 138 | |
| 2 | IPD | 64 | F | 6 | 39 | 15 | 143 | |
| 3 | IPD | 62 | M | 5 | 21 | 9 | 132 | |
| 4 | IPD | 45 | M | 6 | 28 | 5 | 141 | |
| 5 | IPD | 47 | M | 7 | 31 | 6 | 139 | |
| 6 | IPD | 49 | M | 7 | 25 | 6 | 140 | |
| 7 | IPD | 64 | M | 9 | 47 | 11 | 135 | |
| 8 | IPD | 63 | M | 8 | 16 | 11 | 141 | |
| 9 | IPD | 61 | M | 5 | 33 | 8 | 143 | |
| 10 | IPD | 50 | M | 3 | 44 | 11 | 143 | |
| Mean | 57.4 | 5.8 | 32.9 | 9.3 | 139.5 | |||
| S.D. | 8.7 | 2.1 | 10.7 | 3.1 | 3.7 | |||
| 1 | PSP | 54 | M | 2 | 22 | 6 | 30 | 127 |
| 2 | PSP | 69 | M | 9 | 34 | 0 | 28 | 110 |
| 3 | PSP | 65 | F | 2 | 44 | 13 | 28 | 107 |
| 4 | PSP | 57 | F | 3 | 30 | 35 | 30 | 135 |
| 5 | PSP | 66 | M | 2 | 30 | 13 | 28 | 135 |
| 6 | PSP | 59 | M | 6 | 50 | 4 | 22 | 100 |
| 7 | PSP | 68 | F | 4 | 43 | 50 | 18 | 116 |
| 8 | PSP | 63 | M | 5 | 54 | * | 23 | 112 |
| Mean | 62.6 | 4.1 | 38.4 | 17.3 | 25.9 | 117.8 | ||
| S.D. | 5.4 | 2.5 | 11.1 | 18.4 | 4.4 | 13.1 |
Dementia had been ruled out using the Mattis Dementia Rating Scale (MDRS),47 48 where higher scores out of a maximum of 144 indicate better performance, with a cut-off ≤123 considered as cognitive impairment.49 Depression had been assessed using the Beck Depression Inventory (BDI), where higher scores out of a maximum of 63 points indicate a more severe depressed state, and a score of 15 is regarded as cut off for a self-report of mild depression.12 50 *Not investigated. The MDRS was not available at the German study sites.
IPD, idiopathic Parkinson's disease; MMS, Mini-Mental State; PSP, progressive supranuclear palsy; UPDRS, Unified Parkinson's disease rating scale.
Clinical testing procedures
The Hamilton18 rating scale for depression and the Mini-Mental State Examination19 were used to quantify the affective and general cognitive status, respectively, with pragmatic and established tests. Motor features in patients were evaluated with the Unified Parkinson's Disease Rating Scale (UPDRS, part III).20 Further clinical assessments are listed in table 1.
To investigate eyeblink function we used the blink reflex (BR) and its R2 recovery cycle detailed elsewhere.12 21 22 In brief, a single electrical stimulation of the supraorbital nerve (duration: 0.2 ms) elicits an early unilateral (R1) and a late bilateral (R2) blink response. Paired-pulse supraorbital stimulation probes brainstem interneuronal excitability and yields an inhibition of R2 of the second stimulus. We used interstimulus intervals (ISIs) of 100, 300 and 600 ms on both sides. For EBCC we stimulated the side in which amplitudes and latencies were within or close to the normal range or arbitrarily the right supraorbital nerve if no side-differences were found.
EBCC-implicit learning
The procedures were virtually identical to and detailed in the earlier studies from our group.12 14 In brief, an unconditioned stimulus (UCS), that is, an electric pulse over the supraorbital nerve, invariably induces an eyeblink reflex. It is paired with a preceding conditioned stimulus (CS), that is, a tone, which by itself may elicit early startle responses (so called α blinks, within 200 ms after the tone). With repeated presentation of the tone and the electric pulse, a conditioned eyeblink response is expected to occur before the onset of the electrical stimulus. In the current study, the tone (CS, 400 ms duration) was generated by a custom built sound generator (Department of Electronic Engineering, University of Goettingen) and presented through earphones (Cherry Inc, Japan) at an intensity perceived as very loud by each participant, usually 80 dB. A brief electrical stimulus to the supraorbital nerve eliciting BR served as UCS. We tested the EBCC with two different ISIs between the end of the tone and the beginning of the electric pulse, either ISI 0 ms (delay paradigm) or 600 ms (trace paradigm), in randomised order. For each paradigm we administered six learning blocks, each with CS and UCS in trials 1–9, UCS only in trial 10 (to control for random blinks) and CS only in trial 11 (to test for a persistent learning effect). To control for extinction of learned responses a seventh block consisted of 11 trials with CS only.23 The intertrial interval was randomised between 10 and 30 s.
Electromyographic (EMG) activity was recorded using silver-silverchloride cup electrodes fixed with adhesive tape over the lower eyelid (active electrode) and over the ipsilateral temple (reference electrode); with a sampling rate of 10 kHz.12 14 EMG signals were fed into a recording device and filtered at 100 Hz and 5 kHz (SynAmps, Neuroscan Inc, Virginia, USA). To detect any ongoing muscular activity we recorded 400 ms before and 1600 ms after CS onset.
Serial reaction time task
The SRTT is established as a test of implicit learning.12 24 Participants were sitting in front of a computer screen, and were told that single asterisks would appear in one of four positions on a computer screen. They were instructed to press a marked key on a computer keyboard that was underneath the position of the asterisk on the screen. The asterisks were presented in three random blocks (blocks 1, 2 and 7) and four identical sequence blocks (blocks 3–6), in each of which a sequence of 10 elements (CBDABCDCBA) was presented 10 times. After each block, participants were asked to repeat the last 10 asterisk positions manually on the computer keyboard, which may have accentuated explicit aspects of the task.25 26 We analysed reaction time, errors and number of correctly repeated parts of the sequence. This test was difficult for many patients: Only six patients completed the test as required, one patient discontinued after block 1 and was excluded from the analysis. Two others discontinued after block 4, and one after block 3. To enable some kind of statistical analysis, the result that these patients reached in their last sequence block was carried forward to the following sequence blocks, and the result of the second random block was assumed for block 7. One patient apparently responded with random typing to the letters presented and was therefore excluded from the analysis.
Comparison of patients with MSA with PSP and IPD studied earlier
While clearly retrospective, we have taken the liberty to compare the patients with MSA results obtained here with the group of patients with PSP that we studied in 200114 and a subgroup of patients with IPD studied in 1999 with identical electrophysiological methods (numbers 1–4 and 6–11 according to table 1,12 selected to match as good as possible the current MSA group with regard to the disease severity (according to UPDRS part III)), even though retrospective matching based in part on different scales used in different laboratories is certainly not perfect. Demographical data are cited in table 2. In figures 1, 2 and 4A, data from these patients with IPD and PSP are given in dashed lines.
Figure 1.
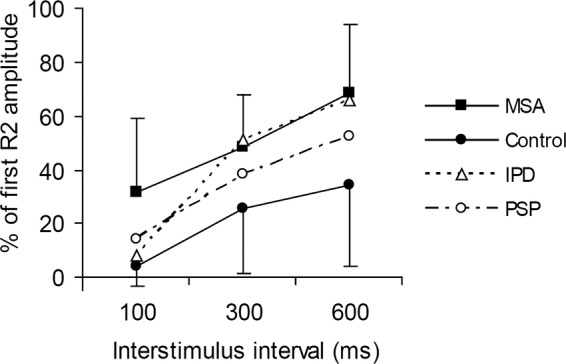
Blink reflex recovery cycle with interstimulus intervals of 100, 300 and 600 ms. In patients with multiple system atrophy (MSA), the inhibition of the ipsilateral R2 response to the second stimulus is weaker than in the control group. Data of patients with MSA and controls are indicated as average value and single SD. Data for patients with idiopathic Parkinson's disease and progressive supranuclear palsy (dashed lines) were taken from our earlier studies using identical methods.12 14
Figure 2.
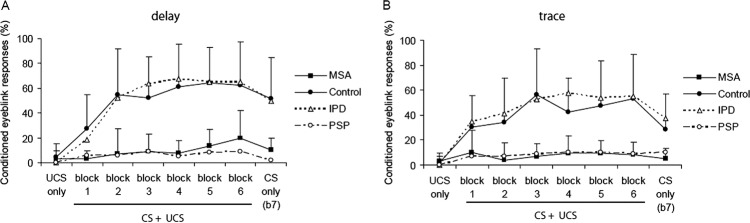
Conditioned eyeblink responses in two different paradigms: left, delay (interstimulus interval 0 ms), right, trace paradigm (interstimulus interval 600 ms). In both paradigms, the number of conditioned responses was significantly lower in patients with multiple system atrophy (MSA) and progressive supranuclear palsy (PSP) than in the control and idiopathic Parkinson's disease (IPD) groups indicating impaired implicit learning. CS, conditioned stimulus (tone), UCS, unconditioned stimulus (electrical stimulation to the supraorbital nerve). Data of patients with MSA and controls are indicated as average value and single SD. Data for patients with IPD and PSP (dashed lines) were taken from our earlier studies using identical methods.12 14
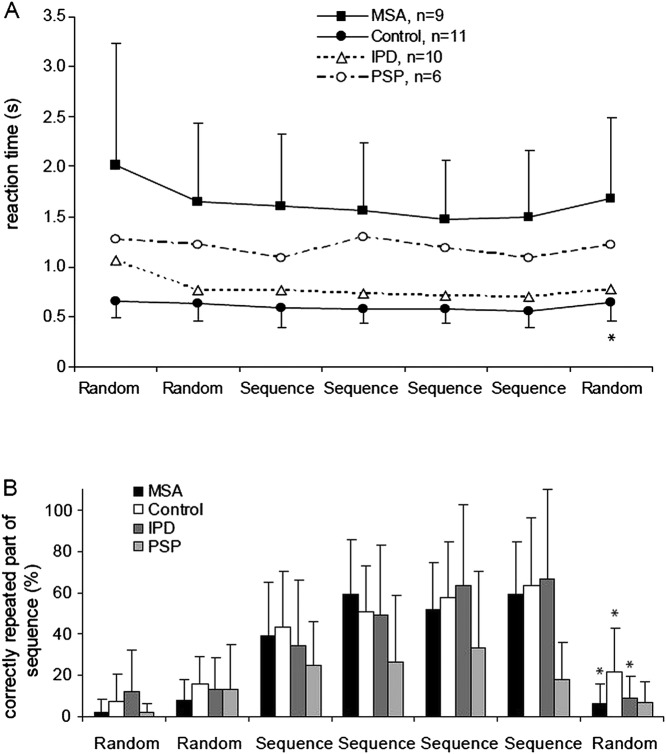
Figure 4.
(A) Reaction time in a serial reaction time task (SRTT). An implicit learning effect is indicated by the reaction time increase between the last sequence block (6) and the following random block (7). (B) Explicit learning in the SRTT was tested after each block by manual retrieval of the sequence (repetition of the last 10 key presses) and revealed no significant difference between groups. Data are indicated as average value and single SD. Data for patients with idiopathic Parkinson's disease (IPD) and progressive supranuclear palsy (PSP) were taken from our earlier studies using identical methods.12 14 Asterisks indicate a significant difference for the comparison of blocks 6 and 7 (p<0.05, post hoc t test).
Data analysis
UPDRS scores in the patient groups, and age in all four groups, were compared using factorial analysis of variances (ANOVAs) with group (three or four levels) as between-participant factor. R2 latencies were measured off-line. For analysis of the BR recovery cycle, we entered the R2 amplitudes of the second pulse normalised to the R2 amplitudes of the first pulse into a repeated measure ANOVA with ‘interstimulus interval’ (three levels: 100; 300, 600 ms) as within-subject factor and ‘group’ (two levels: control and MSA or four levels: control, MSA, IPD and PSP) as between-subject factor.12 21 22 In the EBCC, EMG bursts were regarded as present if their peak-to-peak amplitude exceeded baseline noise by at least 1.5-fold and reached at least 50 µV. They were counted as α blinks, that is, startle responses or conditioned responses (CRs) if they occurred within the appropriate time window (α blinks: within 200 ms after onset of tone (CS); CRs: within 200 ms before electrical stimulus (UCS)). For the tone-alone-trials we extended the time window until 300 ms after the end of the UCS to detect delayed CRs.27 Random blinks were counted as EMG bursts occurring in the CR time window in the absence of a CS, that is, in the UCS only trials. Their occurrence rate was reported numerically. We analysed the percentage of conditioned eyeblink responses repeated measures ANOVAs with ‘block’ (six levels: blocks 1–6) as within-subject factor and ‘group’ (two levels: control and MSA; or four levels: control, MSA, IPD and PSP) and ‘paradigm’ (two levels: delay vs trace) as between-subject factors. In addition, we repeated the ANOVAs for conditioned eyeblink responses with the individual average α blink rate across blocks 1–6 as covariate. We calculated separate repeated measures ANOVAs for the tone-alone trials (trial 11, blocks 1–6), with ‘block’ (six levels: blocks 1–6) as within-subject factor and ‘group’ (two levels: control and MSA; or four levels: control, MSA, IPD and PSP) and ‘paradigm’ (two levels: delay vs trace) as between-subject factors. For α blink rate, we calculated a repeated-measures ANOVA with ‘block’ (seven levels: blocks 1–6 and CS only block) as within-subject factor and ‘group’ (two levels: control and MSA; or four levels: control, MSA, IPD and PSP) and ‘paradigm’ (two levels: delay vs trace) as between-subject factors.
For SRTT, we analysed reaction time, accuracy errors and retrieval using separate repeated measures ANOVAs with ‘block’ (seven levels: blocks 1–7) as within-subject factor and ‘group’ (two levels: control and MSA; or four levels: control, MSA, IPD and PSP) as between-subject factor. Post hoc, we compared the effect change from the last sequence block 6 to random block 7, which is considered a measure of implicit learning, within-group and with uncorrected, two-tailed t tests.
In all analyses, Mauchly's sphericity test was performed and Greenhouse-Geisser correction was applied when necessary. The level of significance was set at p<0.05. Post hoc t tests were calculated for the four-group comparisons and Bonferroni-corrected. A correlation between two parameters was determined by calculating Pearson's correlation coefficient and was reported if it was higher than 0.75 or lower than −0.75. The results are given as mean values±one SD.
Results
Rating scales
Details of age, sex, disease duration, L-Dopa response and rating scales of patients with MSA are displayed in table 1. UPDRS scores for motor impairment placed the patients in an intermediately impaired range. The Hamilton depression score revealed mild depressive symptoms (mean 12.5±6.2 of 69 points) and the Mini-Mental State scored between 26 and 30 points (mean 28±1.4) indicating mild cognitive impairment in more than half of the patients. These results are comparable to the IPD and PSP groups reported earlier.12 14 The UPDRS score did not differ between the three patient groups (factorial ANOVA; no effect of group, no post hoc difference on Bonferroni-corrected t tests); in addition, all four groups did not differ with regard to age.
BR pathways
BR responses in patients with MSA showed normal latencies (ipsilateral R1: 11.6±1 ms, ipsilateral R2: 31.5±4.9 ms contralateral R2: 34.4±4.1 ms). An R2 recovery cycle could be obtained in all patients (figure 1) with no significant side difference between the ipsilateral and contralatral R2 recovery. Patients with MSA showed significantly less R2 inhibition compared with the control group (repeated-measures ANOVA MSA-controls, effect of group, F(1, 20)=15.0 p=0.001).
Conditioned eyeblink responses
All patients with MSA showed few random blinks as assessed by the UCS only trials (3±6.7% across both paradigms). The CRs were first analysed for blocks 1–6 excluding the tone alone trials (see below). In either paradigm patients with MSA showed significantly fewer CRs than the control group (figure 2; repeated-measures ANOVA MSA-control, effect of group, F(1, 39)=37.1, p<0.0001; effect of block, F(3.4, 39)=7, p<0.0001; interaction of group by block, F(3.4, 266)=3.325, p=0.017, no main effect of paradigm). Adding the rate of α blinks as covariate to the ANOVA did not abolish the effect of group (F(1, 38)=31.5, p<0.0001).
These results were supported by a separate analysis of the tone alone trials (trial 11, blocks 1–6), in which the MSA group yielded an average number of CRs of 14±17% in the delay and 12±17% in the trace paradigm, which was significantly less than the control group with 73±23% and 55±27% of CRs, respectively (ANOVA MSA-control, effect of group, F(1,37)=59.1, p<0.0001). There was again no main effect of paradigm, that is, trace and delay paradigm did not differ from each other.
α Blinks
In either paradigm patients with MSA tended to show less α blinks than controls (repeated measures ANOVA, effect of group F(1,38)=4, p=0.054; figure 3). The mean percentage of α blinks across all blocks in patients with MSA was 17.6±4.6% in the delay and 14.4±4.1% in the trace paradigm, for control participants 31.5±11.1% and 35.2±11.3%, respectively. There were significantly more α blinks in earlier blocks than in late blocks for both paradigms (effect of block, F(4.3, 163.7)=8.5, p<0.0001).
Figure 3.
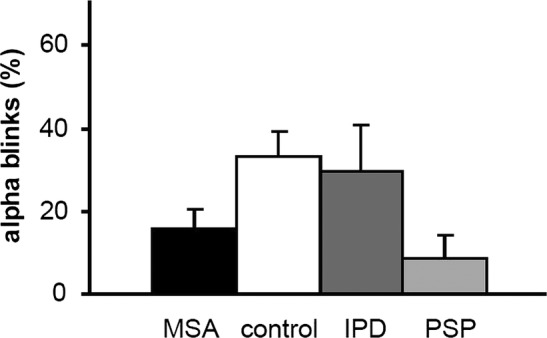
Occurrence of ‘α blinks’. These bursts are a startle reaction to the tone (conditioned stimulus) and are less frequent in patients with multiple system atrophy and progressive supranuclear palsy (PSP) than in the control and idiopathic Parkinson's disease (IPD) groups. Data for patients with IPD and PSP were taken from our earlier studies using identical methods.12 14 Data are indicated as average value and single SD and were pooled for both paradigms.
Serial reaction time task
Reaction time
Patients with MSA showed longer reaction times compared with controls (repeated measures ANOVA MSA-control, effect of group, F(1,18)=20.2, p<0.0001; and a trend for an interaction of group by block, F(1.52, 27.34)=2.77, p=0.10, figure 4A). In both groups reaction times decreased from block 1 to the sequence blocks 3–6 (effect of block, F(1.52, 27.34)=5.5, p=0.016). The reaction time increase from sequence block 6 to random block 7, which is considered a measure of implicit learning, was significant in the control group only (t test p<0.01; MSA p=0.1).
Accuracy errors
The average error rate of patients with MSA across blocks was 19.7±4.2%, which is significantly higher compared with controls with a rate of 2.6±0.8% (repeated measures ANOVA MSA-control, effect of group, F(1,18)=10.1, p=0.005). In both groups error rates decreased from the first random to the sequence blocks (effect of block, F(3.37, 42.66)=3.9, p=0.022) and tended to increase between the last sequence block and the random block 7 without being significant.
Retrieval of sequence
There was no significant difference between patients with MSA and controls in the measures of sequence detection (manual sequence retrieval, ANOVA MSA-control, effect of group, F(1,18)=0.7, p=0.42). Both groups remembered more items of the sequence in postblock reproduction of the last 10 items during the course of the experiment (ANOVA, effect of block, F(3.58, 64.48)=31, p<0.001). A small percentage of repetition was seen even before the sequence was presented, which indicates the baseline guessing rate (figure 4B).
Correlation analyses for patients with MSA
We did not find a significant correlation between the average number of CRs across blocks 3–6 (steady state) and the duration of disease, the Hamilton scale score, the Mini Mental Status Examination (MMST), the motor examination of the UPDRS, the cerebellar or autonomic impairment as assessed by the scores (see table 1) for either paradigm. The percentage of manual retrieval in the SRTT did not correlate with any of these parameters either.
Retrospective comparison of all patient groups (MSA, PSP and IPD) and the control group
In the BR recovery cycle, the R2 inhibition was not different between the patient groups (figure 1). For the EBCC paradigms, the lack of eyeblink conditioning in patients with MSA was similar to the PSP group and differed significantly from the increasing occurrence of CRs in patients with IPD and controls (ANOVA, effect of group, F(3,63)=23.2, p<0.0001; interaction of group by block, F(11.1, 233)=3.6, p<0.0001; figure 2). Post hoc t test with Bonferroni correction confirmed a difference between patients with MSA and PD, and between patients with MSA and control participants. Adding the rate of α blinks as covariate did not abolish the effect of the group (F(1, 32)=16.7, p<0.0001). Also in the tone alone trials, patients with MSA and PSP showed fewer CRs than the IPD and control groups (ANOVA, effect of group, F(3,64)=19, p<0.0001; interaction of group by block, F(15 320)=1.8, p=0.04). MSA and PSP groups both showed fewer α blinks than patients with IPD and controls (ANOVA, effect of group, F(3,61)=3.5, p=0.02; interaction of group by block (F(12.73, 259)=2, p=0.025; see figure 3). However, with post hoc, Bonferroni-corrected t tests these differences were not significant.
For the differentiation of MSA and PSP from IPD we considered the mean percentage of CRs in the steady state part of the EBCC learning curve (blocks 3–6). The mean percentage of CRs allows a non-overlapping separation of the MSA and PSP groups from IPD in the trace paradigm with a cut-off at 26% (figure 5). In the delay paradigm, the separation between groups was less complete. Whereas all patients with PSP exhibited less than 26% of CRs, the fraction of patients with MSA with values equal or below 26% was 0.91. This corresponds to sensitivity values for the separation of PSP and MSA from IPD of 100% and 91%, respectively. If the specificity is defined as the fraction of patients with IPD with CRs values above 26%, the specificity in the delay paradigm is 75%. As reported above, the sensitivity and specificity values for the trace paradigm are 100%. The slightly better performance of patients with IPD13 as compared with controls was not significant.
Figure 5.
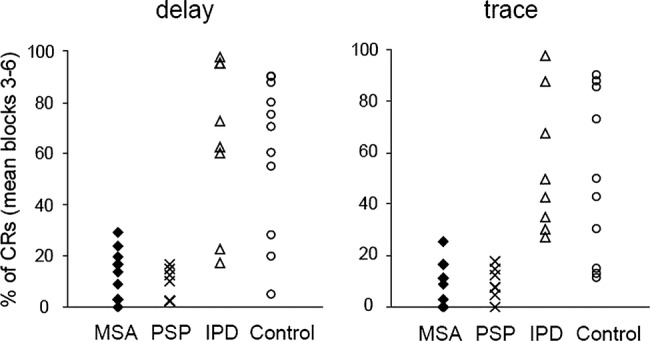
Overview of the mean percentage of conditioned eyeblink responses (CRs) across blocks 3–6 in the eyeblink classical conditioning (EBCC) paradigms in patients with multiple system atrophy (MSA) and control participants from this study and in patients with idiopathic Parkinson's disease (IPD) and progressive supranuclear palsy (PSP) from earlier studies.12 14 With the trace paradigm a complete separation between IPD and the atypical parkinsonian syndromes MSA and PSP is achieved (cut-off 26%), whereas in the delay paradigm there is a small overlap between these groups. Overall, patients with IPD perform slightly better than control participants,13 further enhancing the group distinction between IPD and atypical syndromes.
In the SRTT, patients with MSA showed significantly longer reaction times than the patients with IPD, but no significant difference compared with the PSP group (ANOVA: effect of group F(3,30)=7.4, p=0.001; see figure 4A). With regard to the error rate, patients with MSA performed again very similar to the patients with PSP, who showed 19.5±1.8% accuracy errors, but significantly worse than the patients with IPD (error rate 4.8±1.7%; ANOVA, effect of group, F(3,32)=6.1, p=0.002). The sequence recall measurements revealed no statistically significant differences between groups.
Discussion
The differentiation of IPD and atypical parkinsonian syndromes (MSA and PSP) constitutes a challenge for neurologists, as the motor symptoms often present very similarly, particularly in the early stages. Additional markers such as imaging have been evaluated,28 29 but these provide insufficient sensitivity values or are technically challenging. In addition, macroscopically discernible structural changes as detectable by MRI are likely to occur some time after functional loss has begun. Therefore functional tests might be better suited because they reveal deficits before discernible structural changes occur. In this study we focus on the differential learning abilities tested by eyeblink conditioning (EBCC) and an SRTT. First, the results of the patients with MSA will be discussed, followed by a comparison with PSP and the putative impact for differentiation from IPD.
The patients with MSA showed severely impaired implicit learning in the trace as well as in the delay eyeblink conditioning paradigm, with SDs in the range of other studies,10 30 whereas non-learning associated BR latencies as indicators of oculomotor pathways were normal.
Histopathological alterations in both subtypes of MSA include the substantia nigra, putamen, descending and ascending fibre tracts of the motor system, olivary and pontine nuclei, brainstem-cerebellar circuits as well as cerebellar structures (hemispheres and vermis).31––33 This has been confirmed in vivo by diffusion tensor imaging of white matter microstructure.34 We suggest that damage of cerebellar structures and associated brainstem-cerebellar circuits are responsible for the failure of EBCC learning in patients with MSA. This assumption is supported by the findings of impaired EBCC in patients with cerebellar damage,27 35––37 positron-emission tomography measurements in healthy humans showing changes in glucose metabolism in the cerebellum and pons during EBCC23 38 as well as in experiments studying the influence of selective pharmacological blockade of cerebellar input on EBCC in rabbits.39 Most patients in our study were clinically characterised by MSA-P and not MSA-C. However, the failure in BR acquisition in both groups points to a subclinical cerebellar involvement in MSA-P, which is in accordance with the histopathological studies.32 33 EBCC therefore seems to detect cerebellar involvement at a subclinical stage.
In addition to the cerebellum, several studies indicate that acquisition of CR in the trace paradigm depends on forebrain areas such as the hippocampus or prefrontal cortex, in particular for longer ISIs.40 41 In our study, the failure of CR acquisition in patients with MSA was slightly more pronounced in the trace compared with the delay paradigm. Therefore, lesions of the frontal lobe, which have been suggested by neuropsychological testing6 42 and confirmed histopathologically in a variety of MSA cases,43 44 may have contributed to impaired EBCC acquisition in the trace paradigm.
An alternative explanation that was brought up by an anonymous reviewer is that the tone may be a less salient CS to the patients with MSA than to the control group. The reduced number of α blinks would support this assumption. Following that very elegant line of thought, the EBCC group difference between patients with MSA and control participants would have to do less with implicit learning and more with responsiveness and associative processes related to external stimuli. While this may have some relevance, adding the number of α blinks to the ANOVAs on conditioned eyeblink responses did not abolish the between-group differences.
In the SRTT the patients with MSA showed severe impairment in terms of prolonged reaction time, high error rate and, in some patients, early discontinuation due to fatigue and exhaustion. In contrast to the control group they showed no significant reaction time increase between blocks 6 (random) and 7 (sequence) and this is indicative of implicit learning deficits. On the other hand they showed good performance on the parameters of sequence recall (explicit learning). This preservation of SRTT explicit learning parts may be explained by the relative preservation of posterior association (temporal and parietal) cortex and hippocampus in MSA. It has to be interpreted with some caution, though, given limitations of spatial working memory in MSA.42 However, the validity of the SRTT learning results is limited by the discontinuation of patients and our ‘last observation carried forward approach’ (see Methods section). In addition, the patients’ wide range of motor impairment, which may interfere with the motor part of the task, and the fact that sequence learning and movement preparation seem to share similar attentional and working memory resources45 have to be considered. Therefore the SRTT seems to be inappropriate to assess learning abilities in patients with MSA. This is in contrast to the EBCC, which is independent of the motor performance of patients. Furthermore, EBCC circuits are located anatomically closer to the affected brainstem regions.
With all the limitations of such a retrospective comparison of data acquired in different patient groups by the same authors, the implicit learning impairment in patients with MSA as clearly revealed by the EBCC and with some limitation by the SRTT parallels the previously reported findings in patients with PSP.14 Interestingly, MSA and PSP are characterised by different histopathological alterations, α-synuclein-positive inclusions versus τ-positive aggregations, which lead to the presumption of different pathophysiological mechanisms. However, the common involvement of cerebellar structures in both diseases31 46 seems to be responsible for the clinical phenomenology independent of the cellular mechanism.
In contrast to patients with MSA and PSP, patients with IPD show normal12 or even enhanced13 acquisition of CRs in EBCC. EBCC may therefore contribute to distinguish IPD from these atypical parkinsonian syndromes. As the development of cerebellar and related neuropathology in MSA or PSP often occurs prior to or even without clinical manifestation,33 46 we propose impaired EBCC particularly in the trace paradigm as an early indicator of neurodegeneration of areas beyond those typically affected in IPD. However, as the retrospective synopsis is clearly limited, the pivotal questions of whether EBCC can serve as predictor for the development of typical or atypical disease and whether EBCC is a useful addition to imaging techniques in establishing an early differential diagnosis are unanswered yet and require further prospective investigation.
Supplementary Material
Acknowledgments
The authors would like to thank Professor Mark Hallett for commenting on an earlier draft of the manuscript.
Footnotes
Contributors: All authors were involved in substantial contributions to conception and design, acquisition of the data or analysis and interpretation of the data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. MSo, CT and WP conceived the study. MSc, FvL and MSo were responsible for obtaining the data. FvL and MSo conducted the data analysis. All authors contributed to decisions on the interpretation of results. FvL and MSo contributed to the drafting of the manuscript. CT and WP were responsible for editing and providing guidance on the paper. All authors were responsible for critically revising the paper; and approved the final version of the manuscript prior to submission.
Funding: This work was supported by the Deutsche Forschungsgemeinschaft (DFG, grant SO 429/2-2 (MSo)), the Bernstein Center for Computational Neuroscience (grant # 01GQ0432 (WP)) and the University of Göttingen (Heidenreich von Siebold-Programm (FvL)).
Competing interests: None.
Ethics approval: Ethics committee of the Medical Faculty of the University of Goettingen.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Kollensperger M, Geser F, Ndayisaba JP, et al. Presentation, diagnosis, and management of multiple system atrophy in Europe: final analysis of the European multiple system atrophy registry. Mov Disord 2010;25:2604–12 [DOI] [PubMed] [Google Scholar]
- 2.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poewe W, Wenning G. The differential diagnosis of Parkinson's disease. Eur J Neurol 2002;9(Suppl 3):23–30 [DOI] [PubMed] [Google Scholar]
- 4.Hughes AJ, Daniel SE, Ben-Shlomo Y, et al. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002;125(Pt 4):861–70 [DOI] [PubMed] [Google Scholar]
- 5.Lange KW, Tucha O, Alders GL, et al. Differentiation of parkinsonian syndromes according to differences in executive functions. J Neural Transm 2003;110:983–95 [DOI] [PubMed] [Google Scholar]
- 6.Burk K, Daum I, Rub U. Cognitive function in multiple system atrophy of the cerebellar type. Mov Disord 2006;21:772–6 [DOI] [PubMed] [Google Scholar]
- 7.Robbins TW, James M, Owen AM, et al. Cognitive deficits in progressive supranuclear palsy, Parkinson's disease, and multiple system atrophy in tests sensitive to frontal lobe dysfunction. J Neurol Neurosurg Psychiatry 1994;57:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol 2010;9:1200–13 [DOI] [PubMed] [Google Scholar]
- 9.Balas M, Balash Y, Giladi N, et al. Cognition in multiple system atrophy: neuropsychological profile and interaction with mood. J Neural Transm 2010;117:369–75 [DOI] [PubMed] [Google Scholar]
- 10.Hoffland BS, Kassavetis P, Bologna M, et al. Cerebellum-dependent associative learning deficits in primary dystonia are normalized by rTMS and practice. Eur J Neurosci 2013;2013:1–6 [DOI] [PubMed] [Google Scholar]
- 11.Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem 2003;10:427–55 [DOI] [PubMed] [Google Scholar]
- 12.Sommer M, Grafman J, Clark K, et al. Learning in Parkinson's disease: eyeblink conditioning, declarative learning, and procedural learning. J Neurol Neurosurg Psychiatry 1999;67:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daum I, Schugens MM, Breitenstein C, et al. Classical eyeblink conditioning in Parkinson's disease. Mov Disord 1996;11:639–46 [DOI] [PubMed] [Google Scholar]
- 14.Sommer M, Grafman J, Litvan I, et al. Impairment of eyeblink classical conditioning in progressive supranuclear palsy. Mov Disord 2001;16:240–51 [DOI] [PubMed] [Google Scholar]
- 15.Soliveri P, Brown RG, Jahanshahi M, et al. Learning manual pursuit tracking skills in patients with Parkinson's disease. Brain 1997;120(Pt 8):1325–37 [DOI] [PubMed] [Google Scholar]
- 16.Hwang EJ, Smith MA, Shadmehr R. Dissociable effects of the implicit and explicit memory systems on learning control of reaching. Exp Brain Res 2006;173:425–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–53 [DOI] [PubMed] [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98 [DOI] [PubMed] [Google Scholar]
- 20.Fahn S, Elton R; Committee amotUD Recent developments in Parkinson's disease. Florham Park, NJ: Macmillan Healthcare Information, 1987 [Google Scholar]
- 21.Sommer M, Wobker G, Ferbert A. Voluntary eyelid contraction modifies the blink reflex recovery cycle. Acta Neurol Scand 1998;98:29–35 [DOI] [PubMed] [Google Scholar]
- 22.Kimura J. The blink reflex. In: Kimura J, ed. Electrodiagnosis in diseases of nerve and muscle: principles and practice. Philadelphia: Davis, F. A., 1989:307–31 [Google Scholar]
- 23.Molchan SE, Sunderland T, McIntosh AR, et al. A functional anatomical study of associative learning in humans. Proc Natl Acad Sci U S A 1994;91:8122–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pascual-Leone A, Grafman J, Clark K, et al. Procedural learning in Parkinson's disease and cerebellar degeneration. Ann Neurol 1993;34:594–602 [DOI] [PubMed] [Google Scholar]
- 25.Moisello C, Crupi D, Tunik E, et al. The serial reaction time task revisited: a study on motor sequence learning with an arm-reaching task. Exp Brain Res 2009;194:143–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bo J, Jennett S, Seidler RD. Working memory capacity correlates with implicit serial reaction time task performance. Exp Brain Res 2011;214:73–81 [DOI] [PubMed] [Google Scholar]
- 27.Topka H, Valls-Sole J, Massaquoi SG, et al. Deficit in classical conditioning in patients with cerebellar degeneration. Brain 1993;116(Pt 4):961–9 [DOI] [PubMed] [Google Scholar]
- 28.Mahlknecht P, Hotter A, Hussl A, et al. Significance of MRI in diagnosis and differential diagnosis of Parkinson's disease. Neurodegener Dis 2010;7:300–18 [DOI] [PubMed] [Google Scholar]
- 29.von Lewinski F, Werner C, Jorn T, et al. T2*-weighted MRI in diagnosis of multiple system atrophy. A practical approach for clinicians. J Neurol 2007;254:1184–8 [DOI] [PubMed] [Google Scholar]
- 30.Holloway JL, Trivedi P, Myers CE, et al. Enhanced conditioned eyeblink response acquisition and proactive interference in anxiety vulnerable individuals. Front Behav Neurosci 2012;6:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braak H, Rub U, Del Tredici K. Involvement of precerebellar nuclei in multiple system atrophy. Neuropathol Appl Neurobiol 2003;29:60–76 [DOI] [PubMed] [Google Scholar]
- 32.Wenning GK, Tison F, Elliott L, et al. Olivopontocerebellar pathology in multiple system atrophy. Mov Disord 1996;11:157–62 [DOI] [PubMed] [Google Scholar]
- 33.Ozawa T, Paviour D, Quinn NP, et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain 2004;127(Pt 12):2657–71 [DOI] [PubMed] [Google Scholar]
- 34.Nair SR, Tan LK, Mohd Ramli N, et al. A decision tree for differentiating multiple system atrophy from Parkinson's disease using 3-T MR imaging. Eur Radiol 2013;10:1459–66 [DOI] [PubMed] [Google Scholar]
- 35.Gerwig M, Dimitrova A, Kolb FP, et al. Comparison of eyeblink conditioning in patients with superior and posterior inferior cerebellar lesions. Brain 2003;126(Pt 1):71–94 [DOI] [PubMed] [Google Scholar]
- 36.Gerwig M, Haerter K, Hajjar K, et al. Trace eyeblink conditioning in human subjects with cerebellar lesions. Exp Brain Res 2006;170:7–21 [DOI] [PubMed] [Google Scholar]
- 37.Daum I, Schugens MM, Ackermann H, et al. Classical conditioning after cerebellar lesions in humans. Behav Neurosci 1993;107:748–56 [DOI] [PubMed] [Google Scholar]
- 38.Logan CG, Grafton ST. Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron-emission tomography. Proc Natl Acad Sci USA 1995;92:7500–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attwell PJ, Ivarsson M, Millar L, et al. Cerebellar mechanisms in eyeblink conditioning. Ann N Y Acad Sci 2002;978:79–92 [DOI] [PubMed] [Google Scholar]
- 40.Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science 1998;280:77–81 [DOI] [PubMed] [Google Scholar]
- 41.Takehara-Nishiuchi K, Kawahara S, Kirino Y. NMDA receptor-dependent processes in the medial prefrontal cortex are important for acquisition and the early stage of consolidation during trace, but not delay eyeblink conditioning. Learn Mem 2005;12:606–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbins TW, James M, Lange KW, et al. Cognitive performance in multiple system atrophy. Brain 1992;115(Pt 1):271–91 [DOI] [PubMed] [Google Scholar]
- 43.Konagaya M, Konagaya Y, Sakai M, et al. Progressive cerebral atrophy in multiple system atrophy. J Neurol Sci 2002;195:123–7 [DOI] [PubMed] [Google Scholar]
- 44.Armstrong RA, Lantos PL, Cairns NJ. Multiple system atrophy: laminar distribution of the pathological changes in frontal and temporal neocortex—a study in ten patients. Clin Neuropathol 2005;24:230–5 [PubMed] [Google Scholar]
- 45.Marinelli L, Perfetti B, Moisello C, et al. Increased reaction time predicts visual learning deficits in Parkinson's disease. Mov Disord 2010;25:1498–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jellinger KA, Bancher C. Neuropathology. In: Litvan I, Agid Y, eds. Progressive supranuclear palsy. New York: University Press, 1992:45–88 [Google Scholar]
- 47.Coblentz JM, Mattis S, Zingesser L. Presenile dementia. Clinical aspects and evaluation of cerebrospinal fluid dynamics. Arch Neurol 1973;29:299–308 [DOI] [PubMed] [Google Scholar]
- 48.Schmidt R, Freidl W, Fazekas F, et al. The Mattis Dementia Rating Scale: normative data from 1,001 healthy volunteers. Neurology 1994;44:964–6 [DOI] [PubMed] [Google Scholar]
- 49.Llebaria G, Pagonabarraga J, Kulisevsky J, et al. Cut-off score of the Mattis Dementia Rating Scale for screening dementia in Parkinson's disease. Mov Disord 2008;23:1546–50 [DOI] [PubMed] [Google Scholar]
- 50.Beck AT, Steer RA, Garbin MG. Inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.