Abstract
Introduction
Fragile X syndrome (FXS) is the leading cause of inherited intellectual and developmental disability. Policy development relating to carrier screening programmes for FXS requires input from large studies examining not only test uptake but also psychosocial aspects. This study will compare carrier screening in pregnant and non-pregnant populations, examining informed decision-making, psychosocial issues and health economics.
Methods and Analysis
Pregnant and non-pregnant women are being recruited from general practices and obstetric services. Women receive study information either in person or through clinic mail outs. Women are provided pretest counselling by a genetic counsellor and make a decision about testing in their own time. Data are being collected from two questionnaires: one completed at the time of making the decision about testing and the second 1 month later. Additional data are gathered through qualitative interviews conducted at several time points with a subset of participating women, including all women with a positive test result, and with staff from recruiting clinics. A minimum sample size of 500 women/group has been calculated to give us 88% power to detect a 10% difference in test uptake and 87% power to detect a 10% difference in informed choice between the pregnant and non-pregnant groups. Questionnaire data will be analysed using descriptive statistics and multivariate logistic regression models. Interview data will be thematically analysed. Willingness-to-pay and cost effectiveness analyses will also be performed. Recruitment started in July 2009 and data collection will be completed by December 2013.
Ethics and Dissemination
Ethics approval has been granted by the Universities of Melbourne and Western Australia and by recruiting clinics, where required. Results will be reported in peer-reviewed publications, conference presentations and through a website http://www.fragilexscreening.net.au. The results of this study will make a significant contribution to discussions about the wider introduction of population carrier screening for FXS.
Keywords: Genetics, Public Health
Article summary.
Article focus
This article is a protocol of a study that involves offering fragile X syndrome carrier screening to pregnant and non-pregnant women in the general population. We are undertaking a programme evaluation approach using mixed methods to collect data about informed decision-making and predictors of test uptake, with a focus on psychosocial measures. We are also undertaking an economic appraisal.
Key messages
Carrier screening for fragile X syndrome is the subject of debate because of concerns around education and counselling for this complex condition and the potential for psychosocial harms.
This study will inform policy and practice in the area of population carrier screening by examining psychosocial aspects of screening, including informed decision-making; models of screening, through antenatal care or other access points and health economics of carrier screening for fragile X syndrome.
Strengths and limitations of this study
This study seeks to recruit 1000 women in total. This large sample size will give us sufficient power to address the aims of the study.
Collecting quantitative and qualitative data will provide a more in-depth picture of screening for fragile X syndrome.
A limitation of the study is that the data on models of screening may not be applicable to other countries that have different healthcare systems.
Introduction
Population-based screening programmes are available for a number of genetic conditions in the newborn, prenatal and preconception settings. Several guidelines based on specific criteria exist to help assess which genetic conditions are suitable for population screening.1 2 Fragile X syndrome (FXS) is an X linked condition which meets many of the criteria for population screening, as discussed in Hill et al3 However, in many countries, it is still not routine practice to offer carrier screening for FXS. This is because of concerns about the challenges of screening for this complex condition, including the need for genetic counselling and education and the potential psychosocial and other impacts of a positive result, discussed further in Finucane et al.4
FXS is the most commonly inherited cause of intellectual and developmental disability. Virtually all FXS is caused by an expanded CGG trinucleotide repeat in the 5′ untranslated region of the FMR1 gene, which leads to hypermethylation and silencing of the gene.5–9 Currently, the normal range of repeats is defined as 6–44, with 45–54 repeats being considered an intermediate ‘grey zone’ allele (GZ), 55–200 a premutation (PM) and >200 repeats a full mutation (FM).10 11 The repeats in the GZ, PM and FM ranges can expand when passed from mother to child, although not usually from father to child.8 12 13
The FM is associated with intellectual disability, anxiety and features of the autism spectrum and attention/deficit hyperactivity disorders.14 The clinical presentation varies between individuals15 with men usually being more severely affected than women. FXS is not curable but specific treatments exist, which may help a number of the physical16–19 and behavioural symptoms.20 Although there is currently no robust evidence to support specific pharmacological treatments for people with FXS,21 a number of new therapies are being trialled,22–25 which may lead to improved treatments in the future.
In addition to the reproductive risk of having a child with FXS, female FXS PM carriers also have personal health risks: an increased risk of FX-associated primary ovarian insufficiency (FXPOI) with a 20% risk of premature menopause26–29; a higher incidence of mental health issues such as anxiety and depression4; a risk of developing FX-associated tremor/ataxia syndrome (FXTAS), a late onset neurodegenerative condition, which is more common in male PM carriers than in female PM carriers.29–31
The reported prevalence of FMR1 alleles varies. Three large studies examining FMR1 in anonymous newborn samples32–34 found frequencies of the FMR1 FM in males of 1 in 263333 to 1 in 6209.34 The reported rates of PM in females in four large studies12 34–36 ranged from 1 in 15412 in Israel to 1 in 54934 in Canada, with rates of 1 in 17835 and 1 in 20936 reported in the USA. Two large studies reported GZ rates of 1 in 6636 to 1 in 85.34
A number of studies have investigated carrier screening for FXS for women in the general population.12 37–46 Most of these studies focused on uptake of testing, FMR1 allele sizes and expansion rates, reproductive choices and pregnancy outcomes. However, genetic population screening guidelines1 emphasise the importance of examining the psychosocial aspects of screening, including informed decision-making. Only our pilot study43 47 and one other retrospective study39 have measured the psychosocial impacts of screening for FXS and no studies until now have examined informed decision-making.
This study aims to help us better understand the psychosocial aspects of carrier screening for FXS and will
Compare informed decision-making by pregnant and non-pregnant women offered carrier screening for FXS;
Compare uptake and predictors of uptake in pregnant and non-pregnant women offered carrier screening for FXS;
Undertake an economic appraisal of FXS population carrier screening.
Informed decision-making is complex and involves many factors.48 One measure used in population carrier screening for Down syndrome to estimate informed decision-making is the multidimensional model of informed choice (MMIC),49 which describes an informed choice as a decision made with sufficient knowledge that is value consistent. Our study will not only measure informed choice using MMIC but also collect additional information on factors involved in informed decision-making in the two study questionnaires and through qualitative interviews.
Our study will also provide information on when to offer population carrier screening for FXS by comparing screening in non-pregnant and pregnant women. Population carrier screening guidelines recommend preconception carrier screening,1 but such screening is often embedded in antenatal care, as this provides a convenient (from the perspective of the service provider) point of access, although it may be a more anxious time for women. Research on informed decision-making in prenatal screening, primarily for Down syndrome, has shown that decisions about testing are often not informed.50–53 Our study will be the first to investigate whether rates of informed choice and uptake differ between pregnant and non-pregnant women.
We are testing two hypotheses
A lower proportion of pregnant women will make an informed decision about carrier screening compared with non-pregnant women;
Carrier screening for FXS will result in a higher uptake of testing by pregnant women compared with non-pregnant women.
The findings of this study will contribute valuable data to inform debate on policy and approaches to population carrier screening for FXS.
Methods and analysis
Key elements of study design
Study design
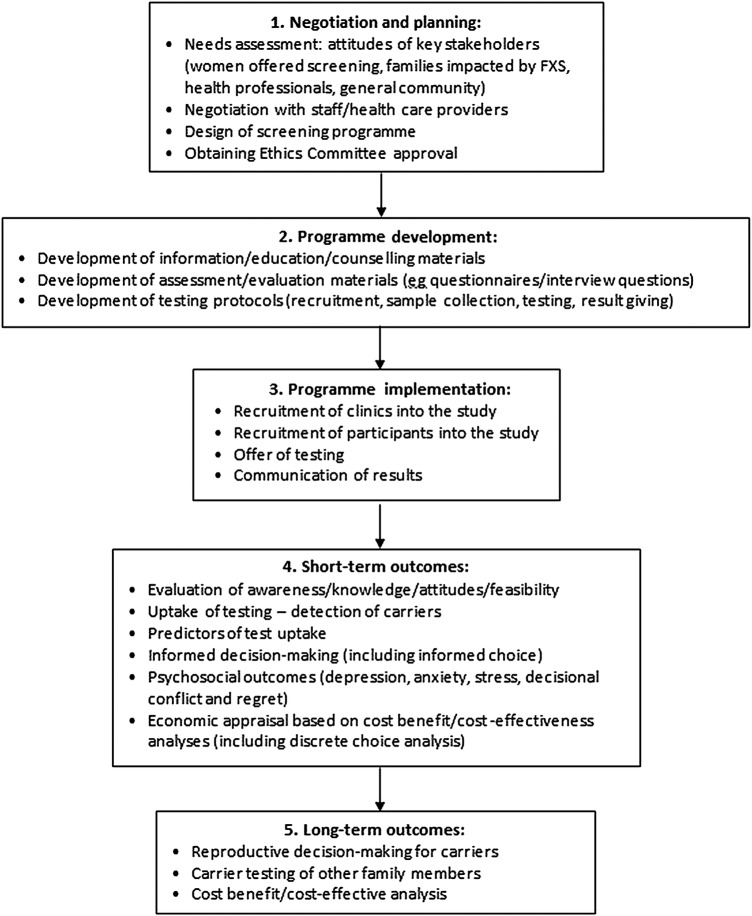
The development and implementation of an effective carrier screening programme is a multistep process requiring a clear theoretical framework. We have developed a programme logic model (see figure 1) to investigate FXS carrier screening incorporating five stages: (1) negotiation and planning; (2) programme development; (3) programme implementation; (4) short-term outcomes and (5) long-term outcomes. The results of our qualitative needs assessment and pilot study, representing stages 1 and 2, have been published previously.43 47 54 55
Figure 1.
Programme logic model to investigate fragile X syndrome carrier screening.
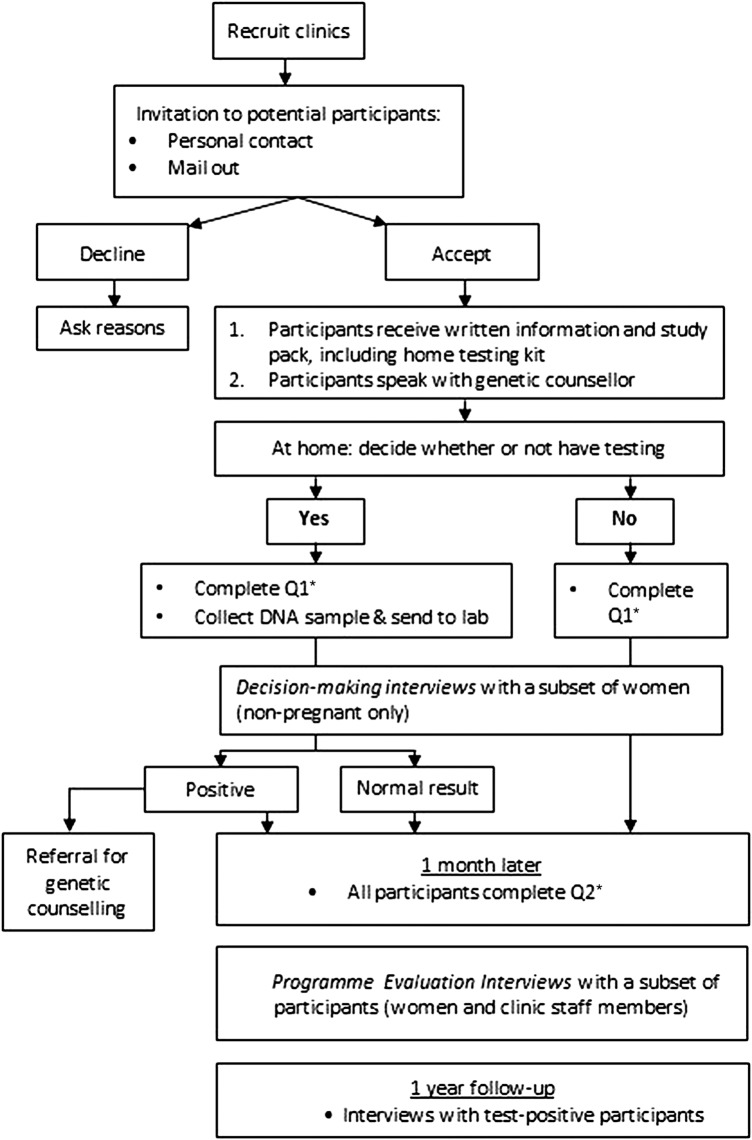
The current study covers stages 3 and 4 and uses a mixed-methods approach to data collection to investigate the short-term outcomes of implementing an FXS carrier screening programme. Figure 2 provides an overview of the study design. Specifically, we will investigate test uptake, informed decision-making, predictors of test uptake, psychosocial outcomes (depression, anxiety, stress, decisional conflict and decisional regret) and health economic factors (willingness-to-pay, WTP).
Figure 2.
Overview of study protocol. *See table 1 for details of measures included in questionnaires 1 and 2.
The key elements of the study are that all women will receive a purpose-made brochure and genetic counselling before making a decision about testing; the test is optional, convenient and non-invasive and offered at no charge to the participants. Genetic counselling and the field-tested brochure are included in the protocol, as participants in our pilot study and needs assessment indicated that having sufficient information and the chance to discuss it is important in making an informed decision.43 54 Offering a test that can be performed at home after sufficient time for decision-making is important, as we found in our pilot study that having to return to the clinic for an invasive test was identified as a barrier to testing, although it did allow some time for deliberation.43 Recruiting pregnant and non-pregnant women will allow us to examine if there are any differences in test uptake, informed choice or psychosocial measures between these groups. Our economic appraisal will provide important information to guide policy on offering carrier screening for FXS.
Settings
The study is being conducted in general practices, public and private obstetric clinics and through private obstetric ultrasound services in Melbourne, Victoria and Perth, Western Australia.
General practice
In Australia, women may attend any general practice of their choice and may choose to attend more than one practice. General practitioners (GPs) are the gatekeepers to access secondary and tertiary care services. About 88% of the Australian population visit a GP at least once a year.56 Most GP clinics also operate a reminder system for the National Cervical Screening Programme, which offers women between the ages of 18 and 69 a cervical (Pap) smear test every 2 years. Thus, most GP clinics have a mail-out system in place to send a reminder letter to their female patients every 2 years. This provides one approach to inviting non-pregnant women into the study and could act as a future service model for population carrier screening.
Obstetrics
A range of maternity care models exist in Australia, but they can be broadly divided into private maternity care, public hospital maternity care and shared local health practitioner/public hospital maternity care. The first step in accessing maternity care is to attend a GP in early pregnancy to obtain a referral to a private obstetrician or public hospital. The timing of the first appointment with the maternity care provider varies, but in the public hospital system women are often not seen until the second trimester of pregnancy. In 2009, the majority (96.9%) of Australian women gave birth in hospitals and of these, 69.9% (150 157 women) were in the public system and 30.1% (64 771 women) were in the private system.57
Obstetric ultrasound—first trimester combined screening
Provision of antenatal screening varies across Australia. In Victoria50 and Western Australia,58 first trimester combined screening is available through private pathology laboratories and private ultrasound clinics with some rebate available from the government-funded Medicare system, while second trimester screening is state funded. GPs or private obstetricians refer women to the private ultrasound clinic for a first trimester nuchal fold thickness scan. In Victoria, about 70% of pregnant women have first trimester combined screening (L Bonacquisto, 2013, personal communication) and therefore would be expected to attend a private ultrasound practice. In addition to offering testing at initial presentation in primary care, linking FXS carrier screening to first trimester screening is another potential service model.
Participants
Enrolling women in the study
Women are eligible to enter the study if they are 18 or over and either not pregnant or up to 12 weeks+6 days pregnant at the time of recruitment. For non-pregnant women, the upper age limit is 70, the age at which participation in the National Cervical Screening Programme ends. Women who are unable to speak, read and write English are not eligible to enter the study.
Recruitment occurs in a number of different ways according to the preferences of individual clinics. Non-pregnant women are recruited from general practice clinics. Women are provided with information about the study either personally (by a researcher, GP, practice nurse or receptionist) or they receive the information through the mail. Study information is not provided by researchers to women who attend general practice clinics and who are obviously ill. Pregnant women are recruited from general practice, private ultrasound and private or public obstetric and ultrasound clinics. In general practice, women are provided with information about the study by the GP when they attend their pregnancy confirmation appointment. In private ultrasound clinics, study information is provided by clinic reception staff when women attend their 12-week scan. In private and public obstetric clinics, women are sent the study information in the mail prior to their first appointment or are given the information personally by an obstetrician or midwife. Women who receive information about the study are asked to complete an expression of interest which is faxed to the research team, either indicating why they do not wish to take part or providing their contact details so they can be recruited by a researcher. All recruitment is completed by the research team and all women speak with a research genetic counsellor.
Enrolling clinics in the study
General practice clinics located across the metropolitan areas of Melbourne and Perth are being targeted to try and achieve a geographical spread and a broad representation of different socioeconomic areas. General practices with established shared-care programmes are being identified using registered shared-care provider lists. Professional networks and an in-house database of GPs and obstetricians who have previously ordered prenatal carrier testing for FXS or cystic fibrosis in Victoria are also being used to identify practices that might be interested in participating. We anticipate requiring five general practices, five private obstetric clinics and one obstetric ultrasound clinic to recruit the 1000 women needed for the study.
Members of the project team provide academic detailing to clinics involved in recruitment. Academic detailing covers background information on FXS, the aims of the project and what the study involves for participants. It is emphasised that the aim of the study is not to test as many women as possible, but rather to understand what factors influence a woman's decision to accept or decline carrier testing for FXS. Clinics are provided with project resources, including study brochures and expression of interest forms.
Australian GPs are primarily funded by a fee for the service system and receive no government funding (personal or infrastructure) for involvement in research. Private obstetricians and ultrasound clinics also receive no government funding for involvement in research. All clinics are offered a small amount of remuneration to cover their costs of involvement in the study, depending on the number of women recruited from their clinic.
Data collection
This research protocol will use mixed-methods data collection that includes genetic testing uptake and outcomes, questionnaires and interviews.
Questionnaires
The questionnaires use validated and psychometrically robust self-reported scales. Table 1 shows which scales are used in questionnaire 1 (Q1), completed after making a decision about carrier testing for FXS, and questionnaire 2 (Q2), completed 1 month after returning Q1.
Table 1.
Questionnaire measures and scales
| Measure/scale | Description | Q1 | Q2 |
|---|---|---|---|
| Knowledge | 10 item scale containing questions on fragile X syndrome (true/false/unsure). A score of 7 or higher is classified as ‘good’ knowledge.55 | √ | √ |
| Attitudes | 5 item scale (0–4) used to assess a woman's attitude to screening (beneficial/harmful; important/unimportant, bad thing/good thing, pleasant/unpleasant, worrying/not worrying). Dichotomous scale: women are classified as having a positive (11–20) or a negative (0–10) attitude towards screening.49 | √ | |
| Multidimensional model of informed choice | Defines an informed choice as a decision made with ‘good’ knowledge, which is consistent with a person's values. Incorporates three dimensions: knowledge, attitudes and uptake. Dichotomous scale: ‘informed choice’ or ‘not informed choice’.49 | √ | |
| Deliberation | 6 item scale measuring the extent to which a decision is deliberated on a 5 point Likert scale (0 = strongly agree—4 = strongly disagree). Dichotomous scale: responses below the midpoint (11 or under) classified as not deliberated and those at or above the midpoint as deliberated.53 | √ | |
| Decisional conflict scale | 16 item scale measuring uncertainty about a course of action on a 5 point Likert scale (0 = strongly agree—4 = strongly disagree). Mean scores are reported with higher scores indicating higher decisional conflict. Scores range from 0 to 100 with scores over 37.5 associated with decision delay or uncertainty about implementation.60 | √ | |
| Depression Anxiety Stress Scale (DASS-21) | 21 item scale divided into 3 subscales measuring depression, anxiety and stress. Responses are classified into 5 categories: 1 (normal) to 5 (extremely severe).61 62 | √ | √ |
| State Trait Anxiety Index (STAI-6) | 6 item scale measuring state anxiety. The maximum score is 80 with scores 31–49 considered as average and scores over 50 indicating elevated state anxiety.59 | √ | √ |
| Health belief | 16 items measuring the importance of a range of factors which may influence decision-making: perceived benefits; perceived susceptibility; perceived severity and perceived barriers in a woman's decision to accept or decline testing for FXS.47 63 | √ | |
| Decisional regret | 5 item scale measuring distress or remorse after a healthcare decision using a 5 point Likert scale (0–4). Scores range from 0–100 with higher scores indicating a higher level of regret.64 | √ | |
| Willingness-to-pay (WTP) | 2 questions (piloted) that address WTP and gross family income. Income question has six income ranges with a tick box. WTP question has 11 item income values with a tick box and sub-questions that address: (1) utility of test (information only or information plus decision-making); and (2) who receives a test result (recipient only or recipient plus shared with health share professionals). | √ | |
| Sociodemographics | Marital status, age, parity, reproductive life-stage, education, occupation, postcode. | √ |
Interviews
To provide in-depth data on participants’ experiences, semi-structured qualitative interviews are conducted with participants at a number of time-points (see table 2). Interviews are conducted by two members of the research team with genetic counselling and qualitative research skills.
Table 2.
Overview of interview schedule
| Time-point | Interview type | Interview description | Selection |
|---|---|---|---|
| After return of Q1, before Q2 and result sent (if tested) | Decision-making interviews | Knowledge, attitudes, factors influencing decision-making, the decision-making process, and perspectives on decisions | Non-pregnant women only; mix of tested and untested women |
| One month after return of Q2 | Programme evaluation interviews (women) | Motivations for participating, factors influencing decision-making, experience of participating in the study including genetic counselling, reflections on decision and views on screening | Mix of tested and untested women from each clinic, including all women with positive test results. Sociodemographic data examined to ensure that selected women are representative of the overall sample |
| After completion of recruitment at any given clinic | Programme evaluation interviews (clinic staff) | Attitudes to population carrier screening for fragile X syndrome (FXS), knowledge of FXS, reflections on offering FXS carrier screening at their clinic, and feedback on the study | Mix of staff from each clinic involved in recruitment |
| 1 year after return of Q2 | 1 year follow-up | Motivations for screening, interpretation of result, perceived value of result, impact of result and reflections on decision | All women with a test-positive result (ie, GZ, PM or FM) |
Data entry quality control
To ensure accuracy of the questionnaire data, every 20th questionnaire entered is checked prior to analysis. The rate of accuracy will be calculated as the number of errors per number of data items entered. To ensure rigour in the qualitative data analysis, transcripts will be independently coded.
Testing
One of the aims of our study was to evaluate the performance of a new innovative assay specifically designed for population screening for FXS.65 Therefore, for the first part of the study, we collected DNA from a saliva sample (Oragene—DNA collection kit) and carried out the gold standard two step diagnostic test8 66 in parallel with the innovative screening test. The routine FXS diagnostic test may involve Southern blotting and therefore can take up to 4 weeks.43 This is performed by the Victorian Clinical Genetic Service laboratory. Refinements to the innovative screening assay67 mean that we are now able to collect DNA from cheek brush samples and have results available in 1 week. This screening assay, marketed by Asuragen, is being performed by Healthscope Pathology.
All women who choose to have carrier testing are given information about their result based on current best practice. Women with a result in the normal range receive a letter that includes an offer to speak to a genetic counsellor at their local clinical service should they require further information. Women with a test-positive result (GZ, PM or FM) are telephoned and offered face-to-face genetic counselling at their local clinical genetics service. Genetic counselling for women with test-positive results follows usual clinical practice.4 68 Any pregnant woman found to have a PM or FM is given her result and, as part of genetic counselling, is offered prenatal diagnostic testing of the fetus, due to the risk of having a child with FXS. An important outcome of receiving an FXS carrier result is that relatives can access genetic testing, which may lead to identification of other carriers and/or the diagnosis of FXS-related disorders in other family members. Genetic testing is discussed as part of the genetic counselling process and family members are offered genetic counselling and testing where appropriate.
Outcomes
The primary outcomes for the study are test uptake and informed choice. Study participants (denominator) are defined as the number of women recruited in the study who do not actively withdraw at any point. Test uptake is defined as the number of women who accept testing (numerator) divided by the number of study participants and will be reported as a percentage. Informed choice will be reported as the percentage of women in each group (pregnant and non-pregnant, tested and untested) making an informed choice as measured using MMIC.62 MMIC will be measured in Q1 at the time closest to decision-making. Knowledge, a component of MMIC, will be measured in Q1 and Q2 and mean knowledge scores will be reported for each time point.
The study will also examine predictors of test uptake. These multivariate analyses will make use of sociodemographic, family history, health belief and psychosocial items included in Q1.
Psychosocial factors will be examined as secondary measures in this study, including anxiety, depression and stress. These will be administered in both questionnaires to allow them to be measured at the time of decision-making and 1 month later. Decisional conflict will be measured in Q1 and decisional regret in Q2.
State anxiety will be reported as the difference in the mean STAI-6 item short form score of women in each group (pregnant and non-pregnant, tested and untested, normal result vs test positive). Depression, anxiety and stress will be reported as the mean score of women in each group. Decisional conflict and decisional regret will be reported as mean scores.
In the WTP literature, there is keen interest in how WTP dollar values for information may vary in accordance with intended use, who receives the information and capacity-to-pay. Our questions have been designed to address these key issues. Accordingly, WTP data will be reported in a number of ways: (1) intended use (‘information only’ and/or ‘decision-making—personal or medical’); (2) by recipients of information (‘women only’ or ‘women plus healthcare professionals’); (3) for women in the trial as a whole and for each group (pregnant and non-pregnant, tested and untested, normal result vs test positive); as mean dollar values together with associated ranges around each mean to facilitate sensitivity testing.
Sample size
In our pilot study, in which women were required to return on a separate occasion to give a blood sample, test uptake in non-pregnant women was 20%, although 50% indicated that they intended to be tested.43 Based on the relevance to reproductive life-stage, we expect test uptake in the pregnant group to be greater than in the non-pregnant group. Our minimum sample size of 500 women/group will give us 88% power to detect a difference of 10% in test uptake between groups (50% vs 40% or 50% vs 60%). We have less information about the likely percentage of women making an informed choice. If the percentage is 50, with a minimum sample size of 500 per group, an unadjusted analysis would have 87% power to detect a difference of 10% (ie, 50% vs 40% or 50% vs 60%) between groups. If the base rate is greater than or less than 50%, we would have >87% power to detect a difference of 10%. The study will therefore be sufficiently powered to exclude anything other than small percentage differences between groups.
Proposed analysis
Descriptive statistics will be used to describe the sociodemographic, knowledge, attitudes and psychological characteristics of the sample. To compare the uptake of testing by pregnant and non-pregnant women, a multivariate logistic regression model with uptake as the dependent variable, and sociodemographic variables such as age, education and parity, together with pregnant/non-pregnant status and mode of invitation or recruitment as the independent variables, will be estimated. This will ensure that a difference in uptake is not due to differences in socioeconomic composition of the pregnant and non-pregnant samples. Robust SEs will be estimated to take into account the possible effect of clustering due to recruitment methods. ORs will be transformed back to percentage differences.69 A similar analysis will be performed to compare informed choice. To investigate predictors of uptake of testing, a multivariate logistic regression model will be estimated with independent variables including: informed choice, attitudes, number of children, prior awareness of FXS, psychosocial variables, family history of intellectual disability, age and education. Interactions between predictors and pregnancy/non-pregnancy will be examined and, if necessary, separate models will be estimated for pregnant and non-pregnant women.
Interviews are transcribed verbatim and NVivo V.10 (QSR International, Australia) is being used to manage the data and facilitate coding. Coding is being carried out by at least two independent researchers to provide rigour of analysis. The decision-making interviews are being examined using content and thematic analysis. These interviews occur between the return of Q1 and the issue of results (for tested women) and Q2. As such, they involve only non-pregnant women, as we were concerned that an interview at this time before receiving a result or needing to delay sending out the result prior to the interview could be distressing for pregnant women at a time when they might be vulnerable. Data from the post-Q2 interviews are being analysed using directed content analysis.70 The coding framework has been developed using data from the needs assessment phase of the study.43 47 54 As little prior research has explored the experiences of women identified as carrying GZ, PM or FM alleles through population-based carrier screening or the experiences of staff in clinics offering population carrier screening, the interviews will be analysed thematically. This will involve an iterative process where data are coded, compared, contrasted and refined to generate emergent themes71 using an approach we have described previously.54
The economic analysis is matched to the stages of FXS carrier screening described in our programme logic model (figure 1). At this stage, the analysis is concerned with examining stage 3 (programme implementation) and stage 4 (short-term outcomes). Placing a dollar value on the health and non-health outcomes of FXS screening is complex. The immediate result of FXS screening is information. That information might be about a risk to a fetus the woman is carrying, implications for the woman's future health or implications for the woman's future reproductive health and reproductive choices. It is for this reason that we have started with WTP methods to explore the value that individuals place on the information provided. The WTP data will be analysed in accordance with the intervention design and policy issues set out above. The WTP data will also be analysed to see if there is an association between the dollar values and preparedness to undergo testing. Similarly, to the extent feasible, the relationship between sociodemographic variables and WTP will be analysed to see if these variables impact on WTP.
Longer-term economic modelling using a surrogate is planned for stage 5. We aim to go on to record the actions that the women undertake as a result of their test results and the incidence of births of babies with FXS to women in the study, discussion of test results with family and identification of carriers/affected individuals with cascade testing. This will facilitate full economic appraisal using a range of methods, including discrete choice experiments (DCE). DCE has applicability to this field because non-health outcomes and process attributes are also important and DCE is a logical extension to WTP for inclusion in stage 5.
Ethics and dissemination
Ethics
A plain language statement is provided to all women and to clinics and health professionals involved in recruiting women for the study and a signed consent form is obtained from all participants at the time of recruitment.
Steering group and advisory committee
This study has a designated research team and an advisory group. The advisory group includes representation from the Victorian Department of Health, the Fragile X Association of Australia and clinicians involved in the study. This group meets annually. The research team includes expertise in population health, genetics, primary care, epidemiology, FXS, health economics, pathology and psychology, with the full team meeting quarterly.
Dissemination
This study will be the first of its kind worldwide to address informed decision-making in carrier screening for FXS and to compare screening in pregnant and non-pregnant women. It will inform the development of appropriate clinical service models for offering FXS screening and also provide important exploratory health economic data. We expect to publish one main trial outcome paper and a number of additional papers exploring aspects of the data in more detail. We will also present our findings at a number of international conferences. A report outlining the main findings of the study will also be made available on the study website http://www.fragilexscreening.net.au on completion. The findings of this study will inform policy development about when and how to offer population carrier screening for FXS.
Supplementary Material
Footnotes
Contributors: MM led the writing of the manuscript and coordinated the study. AA contributed to the study design, ethics application, data collection and drafting of the manuscript. VA contributed to the design of the study and advised on the management of women with high DASS and STAI scores. RC and SY were the health economists who were involved in the NHMRC project grant, prepared the text on the WTP analysis and associated economic appraisal and read/approved the manuscript. JC contributed to the initial development of the project concept, provided input to the development of tools used in the project, assisted with the site selection and provided relevant input to the manuscript. MD contributed to the design of the study. SD was involved in the study design while also being responsible for the sample size calculations and the statistical analysis. JE contributed to the study design, conduct of the study in WA and drafting of the manuscript. JH was involved in the study design and contributed to the drafting of the manuscript. MH provided critical input into the design and set-up of the study, as well as the study materials. LS contributed to the initial development of the project concept and was also involved in the design of the study. HS contributed to the design of the study and provided oversight of the diagnostic FXS testing. FT designed a test for FXS, which makes it possible to offer population carrier screening. SM was responsible for the overall design of the study, study materials and contributed to the drafting of the manuscript.
Funding: This work was supported by a National Health and Medical Research Council project grant (607320) and the Victorian Government's Operational Infrastructure Support Programme. Funding was also received from the Shepherd Foundation, Helen Macpherson Smith Trust, the Apex Foundation, the Fragile X Alliance Inc and theme funding from the Murdoch Childrens Research Institute.
Competing interests: None.
Ethics approval: Ethics approval to conduct this study has been granted by the Human Research Ethics Committees of the Universities of Melbourne (HREC 0830733) and Western Australia (RA/4/1/4028). Additionally, approval has been granted by the ethics committees of the following recruitment sites: Family Planning Victoria (09/2); Women's and Newborn Health Service and Charles Gardiner Hospital—King Edward Memorial Hospital (1925/EW); Swan Kalamunda Health Service (2012–160). This project is being carried out according to the National Statement on Ethical Conduct in Human Research (2007) and the Australian Code for the Responsible Conduct of Research (2007) produced by the National Health and Medical Research Council of Australia.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data sharing statement: The submitted manuscript is a protocol paper. As such, data from the study are still being collected and are yet to be analysed or published. Until data collection and analysis are complete and until publication of the study findings, no data will be made available, except in submitted conference abstracts.
References
- 1.Godard B, Leo ten K, Evers-Kiebooms G, et al. Population genetic screening programmes: principles, techniques, practices, and policies. Eur J Hum Genet 2003;11:S49–87 [DOI] [PubMed] [Google Scholar]
- 2.Teutsch SM, Bradley LA, Palomaki GE, et al. The evaluation of genomic applications in practice and prevention (EGAPP) initiative: methods of the EGAPP working group. Genet Med 2009;11:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill MK, Archibald AD, Cohen J, bmjopen-2013-003660.R1 A systematic review of population screening for fragile X syndrome. Genet Med 2010;12:396–410 [DOI] [PubMed] [Google Scholar]
- 4.Finucane B, Abrams L, Cronister A, et al. Genetic counseling and testing for FMR1 gene mutations: practice guidelines of the national society of genetic counselors. J Genet Couns 2012;21:752–60 [DOI] [PubMed] [Google Scholar]
- 5.Pieretti M, Zhang FP, Fu YH, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 1991;66:817–22 [DOI] [PubMed] [Google Scholar]
- 6.Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991;65:905–14 [DOI] [PubMed] [Google Scholar]
- 7.Bell MV, Hirst MC, Nakahori Y, et al. Physical mapping across the fragile X: hypermethylation and clinical expression of the fragile X syndrome. Cell 1991;64:861–6 [DOI] [PubMed] [Google Scholar]
- 8.Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 1991;67:1047–58 [DOI] [PubMed] [Google Scholar]
- 9.Yu S, Mulley J, Loesch D, et al. Fragile-X syndrome: unique genetics of the heritable unstable element. Am J Hum Genet 1992;50:968–80 [PMC free article] [PubMed] [Google Scholar]
- 10.HGSA Best practice fragile X testing and analysis guidelines for Australasian laboratories, 2012
- 11.Kronquist KE, Sherman SL, Spector EB. Clinical significance of tri-nucleotide repeats in Fragile X testing: a clarification of American College of Medical Genetics guidelines. Genet Med 2008;10:845–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkenstadt M, Ries-Levavi L, Cuckle H, et al. Preconceptional and prenatal screening for fragile X syndrome: experience with 40,000 tests. Prenat Diagn 2007;27:991–4 [DOI] [PubMed] [Google Scholar]
- 13.Nolin SL, Lewis FA, III, Ye LL, et al. Familial transmission of the FMR1 CGG repeat. Am J Hum Genet 1996;59:1252–61 [PMC free article] [PubMed] [Google Scholar]
- 14.Cornish K, Turk J, Hagerman R. The fragile X continuum: new advances and perspectives. J Intellect Disabil Res 2008;52(Pt 6):469–82 [DOI] [PubMed] [Google Scholar]
- 15.Hagerman R, Hagerman P. Fragile X syndrome: diagnosis, treatment, and research. Baltimore: Johns Hopkins University Press, 2002 [Google Scholar]
- 16.Davids JR, Hagerman RJ, Eilert RE. Orthopaedic aspects of fragile-X syndrome. J Bone Joint Surg Am 1990;72:889–96 [PubMed] [Google Scholar]
- 17.Hagerman RJ, Altshul-Stark D, McBogg P. Recurrent otitis media in the fragile X syndrome. Am J Dis Child 1987;141:184–7 [DOI] [PubMed] [Google Scholar]
- 18.Hatton DD, Buckley E, Lachiewicz A, et al. Ocular status of boys with fragile X syndrome: a prospective study. J AAPOS 1998;2:298–302 [DOI] [PubMed] [Google Scholar]
- 19.Wirojanan J, Jacquemont S, Diaz R, et al. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J Clin Sleep Med 2009;5:145–50 [PMC free article] [PubMed] [Google Scholar]
- 20.Hagerman RJ, Berry-Kravis E, Kaufmann WE, et al. Advances in the treatment of fragile X syndrome. Pediatrics 2009;123:378–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rueda JR, Ballesteros J, Tejada MI. Systematic review of pharmacological treatments in fragile X syndrome. BMC Neurol 2009;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry-Kravis EM, Hessl D, Rathmell B, et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci Transl Med 2012;4:152ra27. [DOI] [PubMed] [Google Scholar]
- 23.Jacquemont S, Curie A, Des Portes V, et al. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med 2011;3:64ra1. [DOI] [PubMed] [Google Scholar]
- 24.Paribello C, Tao L, Folino A, et al. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol 2010;10:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kesler SR, Lightbody AA, Reiss AL. Cholinergic dysfunction in fragile X syndrome and potential intervention: a preliminary 1H MRS study. Am J Med Genet 2009;149A:403–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz CE, Dean J, Howard-Peebles PN, et al. Obstetrical and gynecological complications in fragile X carriers: a multicenter study. Am J Med Genet 1994;51:400–2 [DOI] [PubMed] [Google Scholar]
- 27.Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study—preliminary data. Am J Med Genet 1999;83:322–5 [PMC free article] [PubMed] [Google Scholar]
- 28.Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet 2000;97:189–94 [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, et al. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet 2009;17:1359–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 2001;57:127–30 [DOI] [PubMed] [Google Scholar]
- 31.Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet 2003;72:869–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coffee B, Keith K, Albizua I, et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet 2009;85:503–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez-Carvajal I, Walichiewicz P, Xiaosen X, et al. Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. J Mol Diagn 2009;11:324–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levesque S, Dombrowski C, Morel ML, et al. Screening and instability of FMR1 alleles in a prospective sample of 24,449 mother-newborn pairs from the general population. Clin Genet 2009;76:511–23 [DOI] [PubMed] [Google Scholar]
- 35.Hantash FM, Goos DM, Crossley B, et al. FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency in the United States. Genet Med 2011;13:39–45 [DOI] [PubMed] [Google Scholar]
- 36.Tassone F, Iong KP, Tong TH, et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med 2012;4:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spence WC, Black SH, Fallon L, et al. Molecular fragile X screening in normal populations. Am J Med Genet 1996;64:181–3 [DOI] [PubMed] [Google Scholar]
- 38.Pesso R, Berkenstadt M, Cuckle H, et al. Screening for fragile X syndrome in women of reproductive age. Prenat Diagn 2000;20:611–14 [DOI] [PubMed] [Google Scholar]
- 39.Ryynanen M, Heinonen S, Makkonen M, et al. Feasibility and acceptance of screening for fragile X mutations in low-risk pregnancies. Eur J Hum Genet 1999;7:212–16 [DOI] [PubMed] [Google Scholar]
- 40.Toledano-Alhadef H, Basel-Vanagaite L, Magal N, et al. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet 2001;69:351–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cronister A, DiMaio M, Mahoney MJ, et al. Fragile X syndrome carrier screening in the prenatal genetic counseling setting. Genet Med 2005;7:246–50 [DOI] [PubMed] [Google Scholar]
- 42.Anido A, Carlson LM, Taft L, et al. Women's attitudes toward testing for fragile X carrier status: a qualitative analysis. J Genet Couns 2005;14:295–306 [DOI] [PubMed] [Google Scholar]
- 43.Metcalfe S, Jacques A, Archibald A, et al. A model for offering carrier screening for fragile X syndrome to nonpregnant women: results from a pilot study. Genet Med 2008;10:525–35 [DOI] [PubMed] [Google Scholar]
- 44.Geva E, Yaron Y, Shomrat R, et al. The risk of fragile X premutation expansion is lower in carriers detected by general prenatal screening than in carriers from known fragile X families. Genet Test 2000;4:289–92 [DOI] [PubMed] [Google Scholar]
- 45.Fanos JH, Spangner KA, Musci TJ. Attitudes toward prenatal screening and testing for Fragile X. Genet Med 2006;8:129–33 [DOI] [PubMed] [Google Scholar]
- 46.Musci TJ, Caughey AB. Cost-effectiveness analysis of prenatal population-based fragile X carrier screening. Am J Obstet Gynecol 2005;192:1905–12 [DOI] [PubMed] [Google Scholar]
- 47.Archibald AD, Jaques AM, et al. It's something I need to consider: decisions about carrier screening for fragile X syndrome in a population of non-pregnant women. Am J Med Genet A 2009;149A:2731–8 [DOI] [PubMed] [Google Scholar]
- 48.Bekker H, Thornton JG, Airey CM, et al. Informed decision making: an annotated bibliography and systematic review. Health Technol Assess 1999;3:1–156 [PubMed] [Google Scholar]
- 49.Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expect 2001;4:99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaques AM, Halliday JL, Bell RJ. Do women know that prenatal testing detects fetuses with Down syndrome? J Obstet Gynaecol 2004;24:647–51 [DOI] [PubMed] [Google Scholar]
- 51.Jaques AM, Sheffield LJ, Halliday JL. Informed choice in women attending private clinics to undergo first-trimester screening for Down syndrome. Prenat Diagn 2005;25:656–64 [DOI] [PubMed] [Google Scholar]
- 52.Rowe HJ, Fisher JR, Quinlivan JA. Are pregnant Australian women well informed about prenatal genetic screening? A systematic investigation using the Multidimensional Measure of Informed Choice. Aust N Z J Obstet Gynaecol 2006;46:433–9 [DOI] [PubMed] [Google Scholar]
- 53.Van den Berg M, Timmermans DR, Ten Kate LP, et al. Informed decision-making in the context of prenatal screening. Patient Educ Couns 2006;63:110–17 [DOI] [PubMed] [Google Scholar]
- 54.Archibald AD, Hickerton CL, Jaques AM, et al. It's about having the choice: stakeholder perceptions of population-based genetic carrier screening for fragile X syndrome. Am J Med Genet A 2013;161A:48–58 [DOI] [PubMed] [Google Scholar]
- 55.Ames AG, Jaques A, Ukoumunne OC, et al. Development of a fragile X syndrome (FXS) knowledge scale: towards a modified multidimensional measure of informed choice for FXS population carrier screening. Health Expect Published Online First: 2012/10/17.10.1111/hex.12009 [DOI] [PMC free article] [PubMed]
- 56.Britt H, Miller G. ed General practice in Australia, health priorities and policies 1998 to 2008. Canberra: Australian Institute of Health and Welfare and the University of Sydney, 2009 [Google Scholar]
- 57.Li Z, McNally L, Hilder L, et al. Australia's mothers and babies 2009. Perinatal statistics series no. 25. Cat. no. PER 52. Sydney: Australian Institute of Health and Welfare National Perinatal Epidemiology and Statistics Unit, 2011 [Google Scholar]
- 58.Breheny N, O'Leary P, Dickinson J, et al. Statewide evaluation of first trimester screening for Down syndrome and other fetal anomalies in Western Australia. Genomics Occasional Paper 5. Prenatal Diagnosis Committee Department of Health Western Australia, 2005 [Google Scholar]
- 59.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol 1992;31(Pt 3):301–6 [DOI] [PubMed] [Google Scholar]
- 60.O'Connor A. User Manual—Decisional Conflict Scale (16 item statement format). Ottawa: Ottawa Hospital Research Institute. [Google Scholar]
- 61.Crawford JR, Henry JD. The Depression Anxiety Stress Scales (DASS): normative data and latent structure in a large non-clinical sample. Br J Clin Psychol 2003;42(Pt 2):111–31 [DOI] [PubMed] [Google Scholar]
- 62.Lovibond SH, Lovibond PF. Manual for the depression anxiety stress scales 2nd edn Sydney: Psychology Foundation, 1995 [Google Scholar]
- 63.Janz NK, Becker MH. The health bBelief model: a decade later. Health Educ Q 1984;11:1–47 [DOI] [PubMed] [Google Scholar]
- 64.O'Connor A. User manual—decision regret scale. Ottawa: Ottawa Hospital Research Institute [Google Scholar]
- 65.Tassone F, Pan R, Amiri K, et al. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn 2008;10:43–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khaniani MS, Kalitsis P, Burgess T, et al. An improved diagnostic PCR assay for identification of cryptic heterozygosity for CGG triplet repeat alleles in the fragile X gene (FMR1). Mol Cytogenet 2008;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L, Hadd A, Sah S, et al. An information-rich CGG repeat primed PCR that detects the full range of fragile X expanded alleles and minimizes the need for southern blot analysis. J Mol Diagn 2010;12:589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McConkie-Rosell A, Finucane B, Cronister A, et al. Genetic counseling for fragile X syndrome: updated recommendations of the National Society of Genetic Counselors. J Genet Couns 2005;14:249–70 [DOI] [PubMed] [Google Scholar]
- 69.Localio AR, Margolis DJ, Berlin JA. Relative risks and confidence intervals were easily computed indirectly from multivariable logistic regression. J Clin Epidemiol 2007;60:874–82 [DOI] [PubMed] [Google Scholar]
- 70.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–88 [DOI] [PubMed] [Google Scholar]
- 71.Charmaz K. Constructing grounded theory: a practical guide through qualitative analysis. Los Angeles: Sage, 2006 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.