Abstract
Animal models are making an increasing contribution to our understanding of the psychology and brain mechanisms underlying behavioral inhibition and impulsivity. The aim here was to develop, for the first time, a mouse analog of the stop-signal reaction time task with high translational validity in order to be able to exploit this species in genetic and molecular investigations of impulsive behaviors. Cohorts of mice were trained to nose-poke to presentations of visual stimuli. Control of responding was manipulated by altering the onset of an auditory ‘stop-signal' during the go response. The anticipated systematic changes in action cancellation were observed as stopping was made more difficult by placing the stop-signal closer to the execution of the action. Excitotoxic lesions of medial prefrontal cortex resulted in impaired stopping, while the clinically effective drugs methylphenidate and atomoxetine enhanced stopping abilities. The specific 5-HT2C receptor antagonist SB242084 also led to enhanced response control in this task. We conclude that stop-signal reaction time task performance can be successfully modeled in mice and is sensitive to prefrontal cortex dysfunction and drug treatments in a qualitatively similar manner to humans and previous rat models. Additionally, using this model we show novel and highly discrete effects of 5-HT2C receptor antagonism that suggest manipulation of 5-HT2C receptor function may be of use in correcting maladaptive impulsive behaviors and provide further evidence for dissociable contributions of serotonergic transmission to response control.
Keywords: stop-signal reaction time task, ADHD, mouse models, translation, 5-HT2C receptor antagonism
INTRODUCTION
Inhibition is a fundamental property of behavior and makes an important contribution to adaptive responding in the face of changing environmental circumstances. Without the efficient operation of inhibitory mechanisms behavior can become maladaptive, as seen in a number of disorders where subjects exhibit ‘impulsive' responding (broadly defined as action without forethought), such as ADHD, mania, chronic substance abuse, and schizophrenia (Grant and Potenza, 2012; Robbins et al, 2012; Swann, 2010). Behavioral inhibition is multi-faceted (see reviews by Evenden, 1999; Robbins et al, 2012). Hence, at the psychological level it is possible to contrast the inhibition required to inhibit a well-rehearsed correct motor response in order to execute another that had been previously incorrect (‘reversal learning'), to that required to choose a larger reward in the future rather than a smaller one immediately (‘delayed gratification'), to that needed to forestall a response (‘action restraint'), to that needed to cease a motor response already in motion (‘action cancellation' or ‘stopping'). There is increasing evidence that these psychological distinctions may have partially discrete underlying brain substrates. Work in humans has begun to map out a functional circuitry that consistently implicates sub-territories of the frontal cortex (in particular prefrontal cortex) and associated cortico-striatal loops in dissociable inhibitory functions (Bonelli and Cummings, 2007; Robbins et al, 2012). The main emphasis in terms of neurochemical mechanisms has been on the diffuse ascending monoaminergic systems, where modifications to dopaminergic, noradrenergic and serotonergic transmission can have powerful modulatory effects on behavioral inhibition (Boulougouris and Tsaltas, 2008; Dalley and Roiser, 2012). Consistent with these data, the therapeutic effects of current drugs used clinically to treat ADHD, the amphetamine-based psychostimulants methylphenidate and dextroamphetamine and the selective noradrenaline reuptake inhibitor atomoxetine, are thought to occur mainly via influencing the monoaminergic axis (Arnsten, 2011; Del Campo et al, 2011).
Animal models are making an increasing contribution to our understanding of behavioral inhibition and impulsivity. Inhibitory processes can be assayed effectively in rat models and a variety of behavioral tasks have been used successfully to examine dissociable aspects of behavior (Humby and Wilkinson, 2011; Winstanley, 2011). In general, the data from rat studies has pointed toward similar fronto-striatal circuitries and transmitter systems to those thought to be important in mediating inhibitory functions in humans. Furthermore, the data add weight to the existence of distinct components of behavioral inhibition and impulsivity, insofar as within-subject performance across different tests of inhibition often shows little correlation, and the effects of lesion and drug manipulations can differ across different tasks (Broos et al, 2012). Assays of behavioral inhibition in rats also show a high degree of translational relevance. This has been particularly marked in work developing a rat analog of the stop-signal reaction time task (SSRTT). The SSRTT measures the ability to stop or cancel a motor action once started in response to a ‘stop-signal' and detects inhibitory deficits in pathological conditions such as ADHD (Alderson et al, 2007; Robbins, 2007). Rats can learn this task and show similar speeds of reaction and sensitivity to task manipulations as people; they also show a similar pattern of effects to drug challenges, most notably recapitulating the inhibition-enhancing properties of the clinically effective drugs methylphenidate and atomoxetine (Bari et al, 2009, 2011; Broos et al, 2012; Eagle et al, 2007).
Alongside the progress made with rat models we, and others, have been active in developing mouse tasks able to assay inhibitory functions. These efforts have been motivated in large part by the current superior tractability of this species in modeling genetic effects on impulsive responding seen in people. Such genetic effects are considerable, spanning monogenic, fully penetrant conditions, such as the familial tauopathy FTDP-17 (frontotemporal dementia and Parkinsonism linked to chromosome 17) to contributions to overall risk in more complex disorders such as ADHD (Bruno et al, 2007; Helms et al, 2008; Lambourne et al, 2007). Our main objective in this work was to develop, for the first time, a mouse analog of the SSRTT and to use the task to assess the effects of frontal brain lesions and drugs on performance focusing on the clinically effective drugs methylphenidate and atomoxetine and antagonism of 5-HT2C receptors. We report that SSRTT performance can be successfully modeled in mice and that performance was influenced by medial prefrontal cortex (mPFC) dysfunction and systemic administrations of methylphenidate and atomoxetine in a qualitatively similar manner to humans and previous rat models. We also provide evidence for novel and highly selective effects of 5-HT2C receptor antagonism in enhancing response control, findings of potential relevance to the increasing interest in 5-HT2C receptors as drug targets in several disorders where inhibitory deficits are present (Meltzer et al, 2012).
MATERIALS AND METHODS
Subjects, Husbandry and Surgical Procedures
In the present work we report on C57BL/6 mice due to their common use as a background strain for genetically modified lines. Male mice (N=36) bred from stocks in the Behavioural Neuroscience Laboratory in the Cardiff School of Psychology and 4 months old at the beginning of the studies were used in the experiments. Further descriptions of general husbandry, handling, and restriction schedules used to motivate performance in the behavioral task are detailed in Supplementary Materials and Methods. Mice (N=22) were randomly assigned to sham-operated (N=11) or lesion groups (N=11) before undergoing surgery under isoflurane anesthesia. Excitotoxic lesions of the medial wall of the prefrontal cortex were made using sterotaxic placements of N-methyl-D-aspartate (NMDA) according to coordinates taken from Franklin and Paxinos (2008) described in detail in the Supplementary Materials and Methods. Animals were left for at least 1 month to recover from surgery before behavioral training. On completion of the study, sham and lesioned mice were perfused with 4% paraformaldehyde, their brains removed, sectioned, and stained with cresyl violet for assessment of lesion location and spread. All procedures were performed in accordance with UK Home Office rules and regulations under PPL(s) 80/1937 and 30/2267 and adhered to local governance and ethical rules.
SSRTT: Training to Baseline
Before training, the animals were habituated to the food reward (10% condensed milk) used in the task as described previously (Humby et al, 1999, 2005). The SSRTT was programmed using ARACHNID software controlling custom-configured nine-hole box apparatus (see Supplementary Materials and Methods for task configuration). Training to baseline involved shaping the mice to respond sequentially at two stimulus locations, using nose-pokes, to give rise to a ‘go' response, and then learn to withhold responding to the second stimulus location when an auditory stop-signal was presented, to give rise to a ‘stop' response. At baseline, in any given session 100 trials (or 20 min) were available with 80% go trials and 20% interpolated stop trials. Training to baseline was achieved through four main stages, ‘single nose-poking', ‘double nose-poking', ‘learning to stop', and ‘training to baseline', with mice moving to the next training stage once they had achieved stable performance criteria. Full details of training are given in the Supplementary Materials and Methods.
SSRTT: Assessment of Task Manipulations, Medial Prefrontal Lesions, and Administration of Drugs
At stable baseline performance, a number of manipulations were assessed. A main task manipulation was to assess the effects of making stopping more or less difficult by having interpolated stop trials where the auditory stop-signal was presented further away or closer to the execution of the response. At baseline, in stop trials the stop-signal was always presented coincident with the beginning of the response (making stopping relatively easy) but in separate probe sessions the position of the stop-signal was presented at different positions relative to the individual correct go reaction times of each mouse, ie at 0, 10, 50 and 90% into the individualized go reaction time, where 90% is close to the execution of the response and stopping therefore more difficult, as described in Carter et al. (2003). Individualized reaction times were required to normalize the relative position of the stop-signal across individuals, this was important as animals can have differing go reaction times. Individual correct go reaction times were monitored within-session and updated, thereby ensuring consistent placement of the stop-signal for each subject across all experimental conditions. Correct go reaction times were determined directly; however, the stop-signal reaction time (SSRT) had to be derived from the distribution of correct go reaction times and the proportion of correctly stopped trials (see Supplementary Materials and Methods for full details). SSRTs were derived from data obtained from sessions with a 50% stop-signal position, when subjects showed ∼50% correct stopping, in order to ensure balanced contributions from underlying psychological and brain processes of going and stopping according to the predominant ‘race' model of behavioral inhibition as assayed in the SSRTT (see Logan, 1994, Eagle and Robbins, 2003b and below).
SSRTT performance was assessed in mice bearing mPFC lesions and, in separate cohorts, following the systemic administration of the drug compounds methylphenidate (threo-methylα-phenyl-2-piperidineacetate), atomoxetine ((R)-N-methyl-γ-(2-methylphenoxy)-benzenepropanamine), and SB242084 (6-chloro-5-methyl-1-[2-(2-methylpyridyl-3-oxy)-pyrid-5-yl carbomyl] indoline). As above, drug effects were monitored in sessions where the stop-signal position was at 50% and subjects showed ∼50% correct stopping. Animals were given a single session with each dose of drug, based on a Latin square design, with at least 4 days of stable performance and baseline criteria between each drug treatment. Methylphenidate HCl (0, 0.3, 1, 1.5, and 3 mg/kg, Sigma, UK) was administered (i.p.) 30 min before testing, atomoxetine HCl (0, 0.6, 1, 3, and 5 mg/kg, Sigma) was also administered (i.p.) 30 min before testing. SB242084 dihydrochloride hydrate (0, 0.5, 1, and 2 mg/kg, Sigma) was administered (s.c.) immediately before the mice were placed into the test chambers. All concentrations were calculated as free base and all drugs made up in physiological saline fresh on the day of use.
Measures and Statistical Analyses
Key measures from the SSRTT were % correct stop trials, SSRT, % correct go trials, and correct go reaction time. Ancillary measures of general task performance included overall number of trials initiated, the latency to initiate a trial and the time taken to enter the food magazine following a successful go or stop trial. For a full listing of measures and definitions see Supplementary Materials and Methods. SSRTs were calculated according to the methods of Logan (1994) and Eagle and Robbins (2003b), thus in sessions where the SSRT was derived, the correct go reaction times were rank-ordered from smallest to largest and the nth value found, where n is the rank-order position based on the proportion of failing to stop correctly in stop trials, corrected for the occurrence of omitted go trials (see Eagle and Robbins, 2003a; Solanto et al, 2001; Tannock et al, 1989). To determine the SSRT, the time the stop-signal was presented was subtracted from the nth correct go reaction time value, where the time the stop-signal was presented was determined as the mean correct go reaction time × % stop-signal position (see Supplementary Materials and Methods for full details). All data were analyzed using SPSS (V.16, SPSS, USA) by ANOVA with within-subjects factor of STOP-SIGNAL POSITION (0% (baseline), 10, 50, and 90%) or DOSE (vehicle and dose of each drug). For the lesion study, an additional factor of GROUP (sham and lesion) was included in the analyses. To compare the latencies to collect the reward for correct go and correct stop trials, a further within-subjects factor of TRIAL TYPE (go or stop) was used. Scores calculated as percentages were arcsine transformed before analysis (see Hogg and Craig, 1995); if any other parameters violated normality Greenhouse–Geisser corrections were used. Criterion level of significance was set at 0.05 level, and all data are shown as mean±SEM.
RESULTS
SSRTT Performance in Mice
An initial group of 14 mice were trained in the SSRTT. Summary data for the four main stages of training to baseline performance are detailed in Supplementary Table S1. On average it took ∼44 sessions for the mice to achieve stable baseline performance, defined as >70% of possible trials initiated, >80% correct go responses, and >80% correct stopping performance, the latter with the auditory stop-signal coincident with the start of the go response. All animals displayed an increasing degree of stimulus control during task acquisition across the four main stages of training, resulting at baseline in rapid (3.5±0.2 s) and high levels (73.4±2.7%) of responding to the initiating stimulus, go latencies comparable to those reported for rat and human subjects (656±29 ms), high levels of successful stopping during stop trials (84.2±2.4%), and efficient patterns of behavior in relation to nose-poke responses/trial initiation (1.3±0.03) and motivation as indexed by the rapid collection of reward following a successful trial (1.4±0.2 s; Supplementary Table S1).
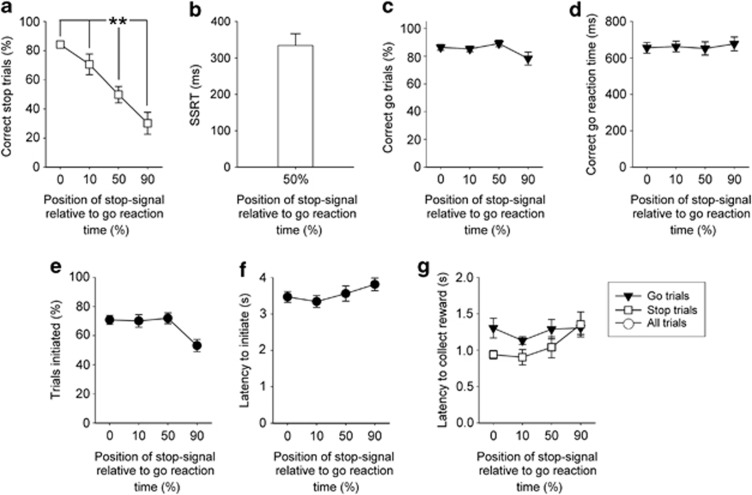
The effects of altering the position of the auditory stop-signal during stop trials are illustrated in Figure 1. As predicted, making stopping more difficult by presenting the stop-signal progressively closer to the execution of the response (10, 50, and 90% into the individualized go reaction time) led to systematic reductions in the ability to stop (Figure 1a, main effect of STOP-SIGNAL POSITION, F3,39=13.36, p<0.001); confirmed by pairwise comparisons showing that successful stopping at the 50% stop-signal position was significantly different from baseline (0%), and the 10 and 90% stop-signal positions (p<0.01). SSRTs, derived in sessions where the stop-signal was placed 50% into the correct go reaction time (see Materials and Methods), were in the order of 350 ms, and similar to those reported in human and rat tasks (Figure 1b). Performance in the interpolated go trials was in general indifferent to the presence of stop trials in the session, with no effects on correct go reaction times (Figure 1d, main effect of STOP-SIGNAL POSITION, F3,39=0.91, n.s.) and a slight reduction in correct go responses limited to the sessions where stopping was most difficult (Figure 1c, main effect of STOP-SIGNAL POSITION, F3,39=2.85, p<0.05).
Figure 1.
Effects of altering the stop-signal position on SSRTT performance. Stopping was made more difficult by presenting an auditory ‘stop-signal' progressively closer to the execution of the response (a). SSRT, the latency to stop, was determined in sessions where the stop-signal was presented half way (50%) into the individualized go reaction time, generating values comparable to studies in other species (b). Stopping and going behaviors were dissociable as the effects of moving the stop-signal onset were specific to stopping behavior with other task measures unaffected by this manipulation including correct go trials (c), correct go reaction time (d), number of trials initiated (e), the latency to initiate a trial (f) and reward collection latencies (g). Data are mean±SEM, n=14. **p<0.01 and *p<0.05 for pairwise differences related to stop-signal position.
The specificity of the stop-signal manipulation to stopping behavior was further confirmed by the general lack of effects on other task parameters. There was a significant reduction on overall trials initiated when stopping was most difficult (Figure 1e, main effect of STOP-SIGNAL POSITION, F3,39=7.53, p<0.001) and an accompanying nonsignificant tendency toward slower initiation latencies (Figure 1f, main effect of STOP-SIGNAL POSITION, F3,39=2.06, n.s.). There were no significant differences in the time taken to enter the food magazine following a successful trial (Figure 1g, main effect of STOP-SIGNAL POSITION, F3,39=2.32, n.s.), but magazine latencies were quicker in stop trials than go trials due to the mundane reason of correct stop trials requiring only one nose-poke (main effect of TRIAL TYPE, F1,13=4.37, p<0.05).
Lesions to mPFC Impair the Ability to Stop
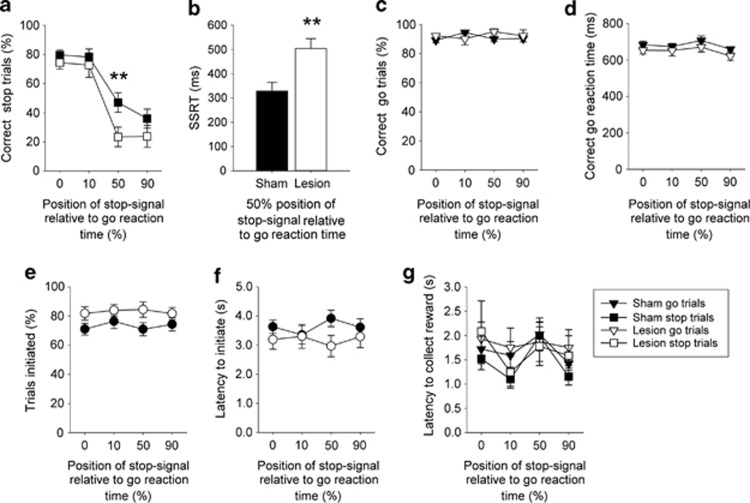
A separate cohort of 22 mice, half of which bore excitotoxic lesions of mPFC and half sham lesions (for details of the location and extent of the lesions see Supplementary Figure S1) were trained in the SSRTT. Data from two of the mPFC-lesioned mice were removed from the analysis due to incorrect lesion placement. There were no between-group differences in learning the SSRTT to baseline criteria (Supplementary Table S2). However, as illustrated in Figure 2a, lesioned animals showed impairments in correct stopping as stopping was made more difficult. Post-hoc analysis of the significant GROUP × STOP-SIGNAL POSITION interaction (F3,54=3.68, p<0.05) indicated that the effects of the lesion were most prominent at the 50% stop-signal position; differences between lesion and sham animals at the 90% stop-signal position were less pronounced probably as a result of floor effects. The lesioned animals were also slower to stop as indicated by SSRTs derived in sessions where the stop-signal was placed 50% into the correct go reaction time (Figure 2b, t18=3.21, p<0.01). The effects of the lesion were specific to stopping, as there were no effects on correct go responses (Figure 2c, main effect of GROUP, F1,18=1.31, n.s.) or correct go reaction times (Figure 2d, main effect of GROUP, F1,18=1.49, n.s.). Furthermore, in general, there were no lesion-associated effects on other indices of task performance, such as the overall number of trials initiated (Figure 2e, main effect of GROUP, F1,18=2.95, n.s.), the latency to initiate a trial (Figure 2f, main effect of GROUP, F1,18=1.15, n.s.), or the time taken to collect the food reward following a successful go or stop trial (Figure 2g, main effect of GROUP, F1,18=0.27, n.s.).
Figure 2.
Effects of bilateral mPFC lesions on SSRTT performance. Deficits in stopping emerged as stopping was made more difficult in animals bearing excitotoxic lesions of medial prefrontal cortex; indexed by both correct stop trials (a) and SSRT (b). In contrast, the brain lesion had minimal effects on going behaviors (c and d) or on ancillary task measures indexing general task performance (e and f) and motivation (g). Data are mean±SEM, mPFC group, n=9 and sham operated group, n=11. **p<0.01 for pairwise differences between sham and lesioned groups. Note that two mPFC lesioned mice were removed from the study due to poor lesion placement (see Supplementary Figure S1).
The Clinically Effective Drugs Methylphenidate and Atomoxetine Improve Stopping Abilities
The effects of clinically effective drugs methylphenidate and atomoxetine on SSRTT performance in a cohort of intact animals are shown in Figures 3 and 4. To enable cross-species comparisons, we used the same dose ranges used in previous SSRTT studies in rats (Bari et al, 2009; Eagle et al, 2007). These doses ranges have been shown to be effective in other behavioral tasks in mice (Davis and Gould, 2007; Griffin et al, 2013) and lead to systematic changes in monoamine release in mouse mPFC assessed using microdialysis (Koda et al, 2010). Furthermore, the chosen dose range of methylphenidate gives rise in the mouse to approximate plasma levels obtained following therapeutic dosing in patients (Balcioglu et al, 2009). Drug effects were monitored in sessions where the stop-signal was placed 50% into the correct go reaction time. As shown in Figure 1a, this was where the mice showed an average ∼50% successful stopping performance, and hence where there were balanced contributions from underlying psychological and brain processes of going and stopping (Logan, 1994).
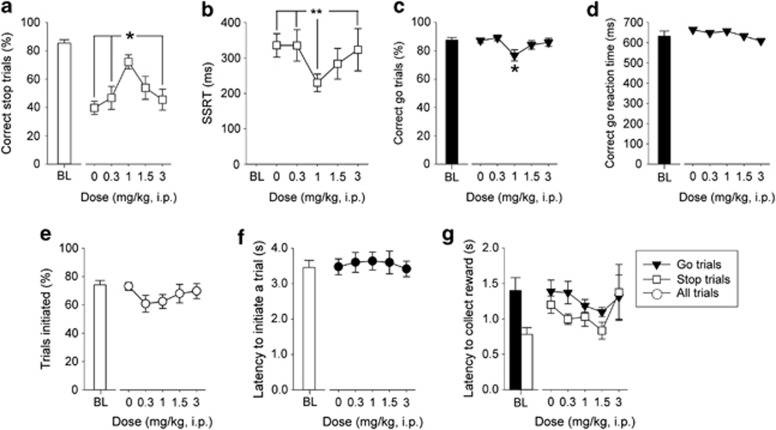
Figure 3.
Effects of methylphenidate on SSRTT performance. Methylphenidate (threo-methyl α-phenyl-2-piperidineacetate, Sigma, UK) was administered (i.p.) 30 min before testing in sessions where the stop-signal position was set at 50% of the go response. Animals were given a single session with each dose of drug, based on a Latin square design, with at least 4 days of stable performance and baseline criteria between each treatment. All concentrations were calculated as free base and made up in physiological saline fresh on the day of use. Increasing doses of methylphenidate up to 1 mg/kg improved stopping behavior (a) and decreased SSRT (b), whereas higher doses were not as effective. There was little effect of methylphenidate on correct go trials, excepting a small decrease at 1 mg/kg (c), or on correct go reaction time (d), the number of trials initiated (e), the latency to initiate a trial (f) or reward collection latencies (g). Baseline data (BL—mean of the five sessions immediately preceding each drug treatment session) when the stop-signal presentation was concurrent with the start of the go response (ie, at 0%) are shown for illustrative purposes and were not included in the statistical analysis. Data are mean±SEM, n=13. **p<0.01 and *p<0.05 for pairwise differences related to dose of drug.
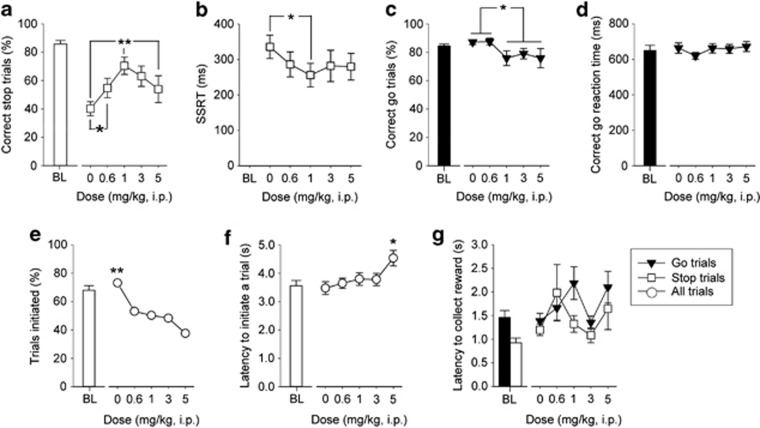
Figure 4.
Effects of atomoxetine on SSRTT performance. Atomoxetine ((R)-N-methyl-γ-(2-methylphenoxy)-benzenepropanamine, Sigma, UK) was administered (i.p.) 30 min before testing in sessions where the stop-signal position was at 50% of the go response. Animals were given a single session with each dose of drug, based on a Latin square design, with at least 4 days of stable performance and baseline criteria between each treatment. All concentrations were calculated as free base and made up in physiological saline fresh on the day of use. Increasing doses of atomoxetine up to 1 mg/kg improved stopping behavior (a) and decreased SSRT (b) whereas higher doses were not as effective. Atomoxetine also led to a small attenuation in correct go trials at higher doses (c), decreased the number of trials initiated (e) and increased the latency to initiate a trial at the highest dose of 5 mg/kg (f). There were no effects of atomoxetine on the correct go reaction time (d) or reward collection latencies (g). Baseline data (BL—mean of the five sessions immediately preceding each drug treatment session) when the stop-signal presentation was concurrent with the start of the go response (ie, at 0%) are shown for illustrative purposes and were not included in the statistical analysis. Data are mean±SEM, n=13. **p<0.01 and *p<0.05 for pairwise differences related to dose of drug
Systemically administered methylphenidate had complex dose-related effects on correct stopping (Figure 3a) with a progressive enhancement in stopping performance, relative to vehicle, at the two lower doses of 0.3 and 1 mg/kg dose, and a progressive reduction of effects back down to vehicle levels at the two higher doses of 1.5 and 3 mg/kg (main effect of DOSE, F4,48=5.52, p<0.001). Pairwise comparisons confirmed that stopping at the 1 mg/kg dose was significantly different (p<0.05) to vehicle, 0.3, and 3 mg/kg and close to significance (p=0.07) in comparison to the 1.5 mg/kg dose of methylphenidate. These behavioral effects were reflected, to an extent, in the SSRT (Figure 3b) with the quickest stopping latencies associated with the mid, 1 mg/kg, dose of methylphenidate (main effect of DOSE, F4,48=3.08, p<0.025). Again, pairwise comparisons confirmed that SSRT at the 1 mg/kg dose was significantly different (p<0.01) to vehicle, 0.3, and 3 mg/kg. The effects of methylphenidate were specific to stopping; there was a small reduction in correct go responding following administration of 1 mg/kg methylphenidate (Figure 3c, main effect of DOSE, F4,48=3.07, p<0.05) but correct go reaction times were not affected at any dose of the drug (Figure 3d, main effect of DOSE, F4,48=1.54, n.s.). General indices of task performance were also unaffected by any dose of methylphenidate used, including overall trials initiated (Figure 3e, main effect of DOSE, F4,48=1.53, n.s.), latency to initiate a trial (Figure 3f, main effect of DOSE, F4,48=0.30, n.s.), and time taken to enter the food magazine following a successful stop or go trial (Figure 3g, main effect of DOSE, F4,48=1.08, n.s.).
Systemic administration of atomoxetine gave rise to a similar qualitative pattern of effects on stopping behavior to those seen following methylphenidate, with dose-related, biphasic effects on correct stopping (Figure 4a, main effect of DOSE, F4,48=4.11, p<0.01); pairwise comparisons confirmed that stopping at the 1 mg/kg dose was significantly different (p<0.01) to vehicle and 5 mg/kg and that the 0.6-mg/kg dose was significantly different to vehicle (p<0.05). In contrast to methylphenidate, however, biphasic effects on SSRT were not as prominent (Figure 4b, main effect of DOSE, F4,48=2.62, p<0.05), with pairwise comparisons showing a significant effect limited to the difference between 1 mg/kg atomoxetine and vehicle (p<0.05). There were small reductions in correct go responses at higher doses of atomoxetine (Figure 4c, main effect of DOSE, F4,48=2.97, p<0.05) but correct go reaction times were unaffected at any dose of drug (Figure 4d, main effect of DOSE, F4,48=1.55, n.s.). Atomoxetine also led to effects on general aspects of task performance, including reductions in the overall number of trials initiated at all doses (Figure 4e, main effect of DOSE, F4,48=12.45, p<0.0001) and an increase in initiation latency that was specific to the highest dose of drug used (Figure 4f, main effect of DOSE, F4,48=5.15, p<0.01). Motivation, as indexed by the latencies to collect the reward after a successful go or stop trial, was not influenced by atomoxetine in any systematic manner (Figure 4g, main effect of DOSE, F4,48=2.49, n.s.).
5-HT2C Receptor Antagonism Improves Stopping Abilities
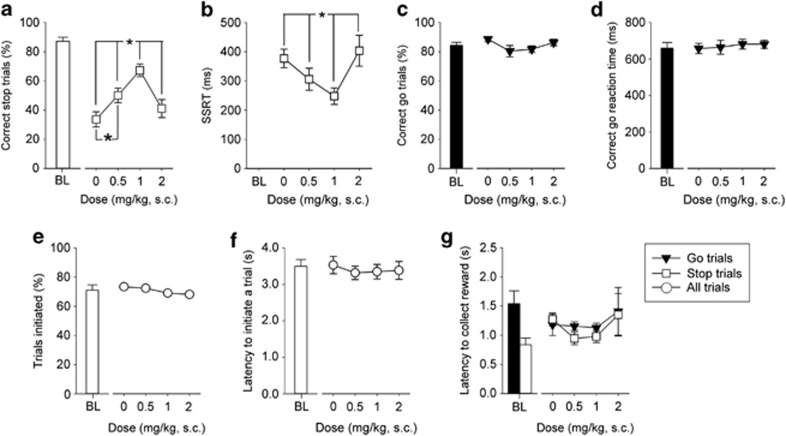
The effects of the specific 5-HT2C antagonist SB242084 on stopping and going behaviors are illustrated in Figure 5. We attempted to choose drug doses on the basis of previous work in rat models; however, at the time of writing there were no published accounts of the effects of SB242084 in rat SSRTT, so the drug doses administered were those shown to be effective in manipulating response control in the rat and mouse versions of the five-choice serial reaction time and delayed reinforcement assays (Fletcher et al, 2007; Paterson et al, 2012; Talpos et al, 2006; Winstanley et al, 2004). Within the dose range used, in sessions where the stop-signal was placed 50% into the correct go reaction time, again chosen because it was where the mice showed an average ∼50% successful stopping performance, SB242084 was effective in enhancing stopping abilities, producing progressive, dose-related increases in successful stopping (Figure 5a, main effect of DOSE, F3,36=11.52, p<0.001) paralleled by progressive reductions in SSRT (Figure 5b, main effect of DOSE, F3,36=7.40, p<0.001). In both cases, the highest 2-mg/kg dose of drug was no longer effective in altering stopping performance compared with vehicle. Pairwise comparisons confirmed that treatment with the 0.5- and 1-mg/kg doses of SB242084 significantly increased stopping (p<0.05) and reduced the SSRT (p<0.05) in comparison to administration of vehicle, and that there was no difference between the 2-mg/kg dose and vehicle. The impact of SB242084 on stopping was highly discrete with no effects, at any dose, on correct go responses (Figure 5c, main effect of DOSE, F3,36=2.33, n.s.), go reaction times (Figure 5d, main effect of DOSE, F3,36=0.41, n.s.), or on general features of task performance, such as the amount of trials initiated (Figure 5e, main effect of DOSE, F3,36=0.34, n.s.), and latencies to start a trial (Figure 5f, main effect of DOSE, F3,36=0.38, n.s.) or collect the reward (Figure 5g, main effect of DOSE, F3,36=2.41, n.s.).
Figure 5.
Effects of SB242084 on SSRTT performance. SB242084 (6-chloro-5-methyl-1-[2-(2-methylpyridyl-3-oxy)-pyrid-5-yl carbomyl] indoline, Sigma, UK) was administered (s.c.) immediately prior to testing in sessions where the stop-signal position was at 50% of the go response. Animals were given a single session with each dose of drug, based on a Latin square design, with at least 4 days of stable performance and baseline criteria between each treatment. All concentrations were calculated as free base and made up in physiological saline fresh on the day of use. Increasing doses of SB242084 up to 1 mg/kg improved stopping behavior (a) and decreased SSRT (b), whereas the highest 2 mg/kg dose was ineffective. There was no effect of SB242084 on correct go trials (c), correct go reaction time (d), the number of trials initiated (e), the latency to initiate a trial (f) or reward collection latencies (g). Baseline data (BL—mean of the five sessions immediately preceding each drug treatment session) when the stop-signal presentation was concurrent with the start of the go response (ie, at 0%) are shown for illustrative purposes and were not included in the statistical analysis. Data are mean±SEM, n=13. *p<0.05 for pairwise differences related to dose of drug.
DISCUSSION
We report a novel assay of SSRTT performance in mice. Several aspects of task performance indicate the cross-species, translational utility of the assay. Importantly, the task was dissociable in terms of going and stopping behaviors, and as seen in people and other animal models (Aron et al, 2003; Broos et al, 2012; Chamberlain et al, 2006; Eagle and Robbins, 2003b) conformed to the prevailing ‘race' model of behavioral inhibition where the ability to cancel an ongoing motor action is dependent on competition between separate, parallel brain processes of going and stopping. Hence, as predicted, altering the position of the stop signal in the mouse model made stopping more or less difficult depending on whether it favored going or stopping processes prevailing in the race (Logan, 1994). Furthermore, consistent with the existence of discrete going and stopping processes, the effects of prefrontal damage and drugs in altering stopping behavior were highly specific with little or no impact on going behaviors.
Much evidence suggests frontal regions of brain form a key part of the circuitry controlling response inhibition (Bonelli and Cummings, 2007; Robbins et al, 2012) and the construct validity of theassay in mice was further emphasized by the effects of excitotoxic-induced damage to the mPFC. Consistent with clinical findings of dysfunction in prefrontal areas, in conditions such as ADHD (Arnsten and Rubia, 2012; Depue et al, 2010; Pliszka et al, 2006; Robbins, 2007), where the SSRTT reliably predicts inhibitory deficits, and experimental approaches in rat models (Bari et al, 2011; Eagle et al, 2008; Mar et al, 2011), lesioned animals were impaired at stopping. These effects, which may have also involved disruptions to function that were up and downstream of the lesion site, were highly selective in that they emerged when stopping was made more difficult and did not extend to learning the task, going behaviors or ancillary task measures indexing general task performance and motivation.
Another important test of the translational potential of the mouse model was to assess the effects of methylphenidate and atomoxetine, drugs used clinically in the treatment of ADHD. Our data were consistent with findings in people and previous rat models (Aron et al, 2003; Bari et al, 2009; Bari et al, 2011; Broos et al, 2012; Chamberlain et al, 2006; Eagle et al, 2007; Tannock et al, 1989) and showed that the general ability of these drugs to enhance stopping in the SSRTT extended to mice. The effects of both drugs were dose dependent with the inhibition-promoting effects being lost at higher doses, presumably as a result of a relative reduction in pharmacological selectivity. It is noteworthy that in some previous SSRTT studies the effects of methylphenidate and atomoxetine have been shown to be dependent on the speed of stopping, with the effects being confined mainly to slower stoppers (Eagle et al, 2007; Feola et al, 2000; Robinson et al, 2008a). However, we observed no statistically reliable interactions between the effects of the drugs at any dose and speed of stopping that warranted sub-analysis of group data (eg, by median split; data not shown).
Manipulations of the serotonergic system can have major effects on behavioral inhibition and our data using the mouse SSRTT provided evidence for novel and highly discrete effects of 5-HT2C receptor antagonism. To our knowledge, the finding that the specific 5-HT2C antagonist SB242084 enhances response control in the SSRTT is the first published report of such an effect. Given the behavioral selectivity and potency of SB242084, comparable to methylphenidate and atomoxetine, these data add to current debate about the therapeutic potential of 5-HT2C receptors in psychopathology (Meltzer et al, 2012; Reynolds et al, 2005), and suggest, in principle, that blockade of 5-HT2C receptors may be useful in the treatment of disorders such as ADHD, where failures of behavioral inhibition are overt; but also other disorders, such as schizophrenia, where inhibitory deficits are present but comorbid with a complex mix of other symptoms (Bonelli and Cummings, 2007; Grant and Potenza, 2012; Robbins et al, 2012).
The data with SB242084 provide further evidence for qualitatively distinct contributions of serotonergic transmission to response control, exemplified by the dissociable effects of systemic SB242084 on inhibitory processes mediating stopping in the SSRTT (this work), premature responding in the five-choice serial reaction time task (Fletcher et al, 2007; Robinson et al, 2008a; Winstanley et al, 2004) or choosing in the delayed reinforcement task (Paterson et al, 2012; Robinson et al, 2008a). The precise neurobiological substrates of 5-HT2C receptor antagonism in the SSRTT warrant further investigation, in the first instance using localized infusions of drug agents directly into brain. Of relevance here are recent data showing that 5-HT2C receptor function in nucleus accumbens and orbital frontal cortex has important and dissociable roles in other aspects of response inhibition as manifest in the five-choice serial reaction time task (Robinson et al, 2008b) and reversal learning (Boulougouris and Robbins, 2010), hence it will be a priority to establish the extent to which these brain regions are involved in mediating the enhanced response control following systemic SB242084 administration in the SSRTT. The involvement of 5-HT2C receptors in this and other forms of behavioral inhibition also brings into play a number of molecular mechanisms able to modify the efficacy of 5-HT2C receptor signaling in the brain. For example, the role of genes such as Snord115, which can modify 5-HT2C receptor function via effects on posttranscriptional modifications of 5-HT2C receptor pre-RNA (Kishore and Stamm, 2006), and which we have shown using mouse models in previous work, can influence aspects of response control (Doe et al, 2009). We anticipate that, in allowing exploitation of the advanced genomic and genetic tractability of mice, the development of the mouse SSRTT will be of significant utility in furthering the generation of viable translational models, and new therapeutic targets, for disorders where failures of behavioral inhibition are prominent.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We thank Clive Mann and other members of the Behavioural Neuroscience Laboratory team in Cardiff for technical support and animal husbandry.
Author contributions
TH and LSW conceived the project and wrote the paper. JBE and TH performed the experiments; ACR and MAG performed the surgery and conducted the histological processing and lesion analysis. TH and LSW analyzed the data.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Alderson RM, Rapport MD, Kofler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. J Abnorm Child Psychol. 2007;35:745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry. 2011;69:e89–e99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Balcioglu A, Ren JQ, McCarthy D, Spencer TJ, Biederman J, Bhide PG. Plasma and brain concentrations of oral therapeutic doses of methylphenidate and their impact on brain monoamine content in mice. Neuropharmacology. 2009;57:687–693. doi: 10.1016/j.neuropharm.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl) 2009;205:273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, et al. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Prog Brain Res. 2008;172:517–542. doi: 10.1016/S0079-6123(08)00925-4. [DOI] [PubMed] [Google Scholar]
- Broos N, Schmaal L, Wiskerke J, Kostelijk L, Lam T, Stoop N, et al. The relationship between impulsive choice and impulsive action: a cross-species translational study. PLoS One. 2012;7:e36781. doi: 10.1371/journal.pone.0036781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno KJ, Freet CS, Twining RC, Egami K, Grigson PS, Hess EJ. Abnormal latent inhibition and impulsivity in coloboma mice, a model of ADHD. Neurobiol Dis. 2007;25:206–216. doi: 10.1016/j.nbd.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JD, Farrow M, Silberstein RB, Stough C, Tucker A, Pipingas A. Assessing inhibitory control: a revised approach to the stop signal task. J Atten Disord. 2003;6:153–161. doi: 10.1177/108705470300600402. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–58. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Atomoxetine reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Neuropsychopharmacology. 2007;32:2011–2019. doi: 10.1038/sj.npp.1301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:e145–e157. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Depue BE, Burgess GC, Willcutt EG, Ruzic L, Banich MT. Inhibitory control of memory retrieval and motor processing associated with the right lateral prefrontal cortex: evidence from deficits in individuals with ADHD. Neuropsychologia. 2010;48:3909–3917. doi: 10.1016/j.neuropsychologia.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CM, Relkovic D, Garfield AS, Dalley JW, Theobald DE, Humby T, et al. Loss of the imprinted snoRNA mbii-52 leads to increased 5-HTR2C pre-RNA editing and altered 5HT2CR-mediated behaviour. Hum Mol Genet. 2009;18:2140–2148. doi: 10.1093/hmg/ddp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex. 2008;18:178–188. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003a;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Lesions of the medial prefrontal cortex or nucleus accumbens core do not impair inhibitory control in rats performing a stop-signal reaction time task. Behav Brain Res. 2003b;146:131–144. doi: 10.1016/j.bbr.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Tufft MR, Goodchild HL, Robbins TW. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology (Berl) 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Feola TW, de Wit H, Richards JB. Effects of d-amphetamine and alcohol on a measure of behavioral inhibition in rats. Behav Neurosci. 2000;114:838–848. doi: 10.1037/0735-7044.114.4.838. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT2A and 5-HT2C receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G.2008The Mouse Brain in Stereotaxic Coordinates3rd edn.Academic Press [Google Scholar]
- Grant JE, Potenza MN.(eds) (2012The Oxford Handbook of Impulse Control Disorders Oxford University Press [Google Scholar]
- Griffin WC, 3rd, McGovern RW, Bell GH, Randall PK, Middaugh LD, Patrick KS. Interactive effects of methylphenidate and alcohol on discrimination, conditioned place preference and motor coordination in C57BL/6J mice. Psychopharmacology (Berl) 2013;225:613–625. doi: 10.1007/s00213-012-2849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Gubner NR, Wilhelm CJ, Mitchell SH, Grandy DK. D4 receptor deficiency in mice has limited effects on impulsivity and novelty seeking. Pharmacol Biochem Behav. 2008;90:387–393. doi: 10.1016/j.pbb.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RV, Craig AT.1995Introduction to Mathematical Statistics5th edn.Macmillan: New York [Google Scholar]
- Humby T, Laird FM, Davies W, Wilkinson LS. Visuo-spatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur J Neurosci. 1999;11:2813–2823. doi: 10.1046/j.1460-9568.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- Humby T, Wilkinson LS. Assaying dissociable elements of behavioural inhibition and impulsivity: translational utility of animal models. Curr Opin Pharmacol. 2011;11:534–539. doi: 10.1016/j.coph.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Humby T, Wilkinson LS, Dawson G.2005Assaying aspects of attention and impulse control in mice using the 5-choice serial reaction time task Curr Protoc Neurosci Chapter 8Unit 8.5H. [DOI] [PubMed] [Google Scholar]
- Kishore S, Stamm S. Regulation of alternative splicing by snoRNAs. Cold Spring Harb Symp Quant Biol. 2006;71:329–334. doi: 10.1101/sqb.2006.71.024. [DOI] [PubMed] [Google Scholar]
- Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T. Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem. 2010;114:259–270. doi: 10.1111/j.1471-4159.2010.06750.x. [DOI] [PubMed] [Google Scholar]
- Lambourne SL, Humby T, Isles AR, Emson PC, Spillantini MG, Wilkinson LS. Impairments in impulse control in mice transgenic for the human FTDP-17 tauV337M mutation are exacerbated by age. Hum Mol Genet. 2007;16:1708–1719. doi: 10.1093/hmg/ddm119. [DOI] [PubMed] [Google Scholar]
- Logan GD.1994On the ability to inhibit thought and action: a users' guide to the stop signal paradigmIn Dagenbach D, Carr TH (eds).Inhibitory Processes in Attention, Memory, and Language Academic Press: San Diego; 189–239. [Google Scholar]
- Mar AC, Walker AL, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci. 2011;31:6398–6404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Massey BW, Horiguchi M. Serotonin receptors as targets for drugs useful to treat psychosis and cognitive impairment in schizophrenia. Curr Pharm Biotechnol. 2012;13:1572–1586. doi: 10.2174/138920112800784880. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Wetzler C, Hackett A, Hanania T. Impulsive action and impulsive choice are mediated by distinct neuropharmacological substrates in rat. Int J Neuropsychopharmacol. 2012;15:1473–1487. doi: 10.1017/S1461145711001635. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R, 3rd, Xiong J, et al. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am J Psychiatry. 2006;163:1052–1060. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Templeman LA, Zhang ZJ. The role of 5-HT2C receptor polymorphisms in the pharmacogenetics of antipsychotic drug treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1021–1028. doi: 10.1016/j.pnpbp.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Dalley JW, Theobald DE, Glennon JC, Pezze MA, Murphy ER, et al. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology. 2008b;33:2398–2406. doi: 10.1038/sj.npp.1301636. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, et al. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008a;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, et al. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Swann AC. Mechanisms of impulsivity in bipolar disorder and related illness. Epidemiol Psichiatr Soc. 2010;19:120–130. [PubMed] [Google Scholar]
- Talpos JC, Wilkinson LS, Robbins TW. A comparison of multiple 5-HT receptors in two tasks measuring impulsivity. J Psychopharmacol. 2006;20:47–58. doi: 10.1177/0269881105056639. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol. 1989;17:473–491. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol. 2011;164:1301–1321. doi: 10.1111/j.1476-5381.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.