Abstract
Anxiety disorders represent the most common mental disturbances in the world, and they are characterized by an abnormal response to stress. Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptor PAC1 have been proposed to have a key role in mediating the responses to stress as well as the regulation of food intake and body weight. Corticotropin-releasing factor (CRF), the major stress peptide in the brain, has been hypothesized to be involved in PACAP effects, but the reports are conflicting so far. The present study was aimed at further characterizing the behavioral effects of PACAP in rats and at determining the role of central CRF receptors. We found that intracerebroventricular PACAP treatment induced anxiety-like behavior in the elevated plus maze test and elevated intracranial self-stimulation thresholds; both of these effects were fully blocked by concurrent treatment with the CRF receptor antagonist D-Phe-CRF(12-41). Interestingly, the CRF antagonist had no effect on PACAP-induced increased plasma corticosterone, reduction of food intake, and body weight loss. Finally, we found that PACAP increased CRF levels in the paraventricular nucleus of the hypothalamus and, importantly, in the central nucleus of the amygdala, as measured by solid phase radioimmunoassay and quantitative real-time PCR. Our results strengthen the notion that PACAP is a strong mediator of the behavioral response to stress and prove for the first time that this neuropeptide has anti-rewarding (ie, pro-depressant) effects. In addition, we identified the mechanism by which PACAP exerts its anxiogenic and pro-depressant effects, via the recruitment of the central CRF system and independently from HPA axis activation.
Keywords: pituitary adenylate cyclase-activating peptide, corticotropin-releasing factor OR CRH, anxiety OR anxiolytic, anorexia OR anorexic, intracranial self-stimulation OR ICSS, amygdala
INTRODUCTION
Anxiety disorders are psychiatric conditions characterized by feelings of excessive and uncontrollable apprehension and/or fear in the absence of any specific external stimuli and are accompanied by physical, affective, and behavioral symptoms. They are the most common form of mental disorders in the United States, affecting nearly 40 million adults (Kessler et al, 2005).
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a neuropeptide belonging to the growth hormone-releasing hormone (GHRH)/secretin/glucagon/vasoactive intestinal peptides (VIP) superfamily (Sherwood et al, 2000). PACAP was originally isolated from the ovine hypothalamus and its sequence is highly conserved from fish to mammals (Miyata et al, 1989). Two fragments of PACAP exist, PACAP-38 and PACAP-27, the former representing >90% of the total peptide in brain tissue (Miyata et al, 1989; Piggins et al, 1996). PACAP exerts important biological functions as a neurohormone and neuromodulator through its G protein-coupled receptor PAC1. PACAP has 68% similarity with VIP, and both peptides are capable of binding to VPAC1, VPAC2, and PAC1 receptors (Gottschall et al, 1990); however, PAC1 shows much greater affinity for PACAP than for VIP (Kd ∼0.5 vs >500 nM) (Harmar et al, 1998).
PACAP neurons are located primarily in the paraventricular (PVN) and supraoptic nuclei of the hypothalamus. Significant amounts of PACAP cell bodies and/or fibers are also found in other hypothalamic nuclei such as the ventromedial hypothalamus, as well as various extra-hypothalamic regions, including the central nucleus of the amygdala (CeA), the bed nucleus of the stria terminalis (BNST), and nuclei of the brainstem (parabrachial nucleus, locus coeruleus, pontine nucleus, and vagal complex) (Piggins et al, 1996).
PACAP produces gross stress-like effects in rats when injected either intracerebroventricularly (i.c.v.) or site-specifically into the PVN or the CeA, and it potentiates acoustic startle when given into the BNST (Agarwal et al, 2005; Hammack et al, 2009; Legradi et al, 2007; Norrholm et al, 2005). PAC1 receptor knockout mice exhibit reduced anxiety-like behaviors (Otto et al, 2001), and recently an association between PACAP/PAC1 receptor and posttraumatic stress disorder in heavily traumatized patients has been documented (Ressler et al, 2011). Moreover, the levels of PACAP and PAC1 receptor have been reported to be altered in the brain after exposure to stressors (Hammack et al, 2009; Hannibal et al, 1999; Hannibal et al, 1995). All of this evidence suggests that the endogenous PACAP system might be a mediator of the behavioral response to stress.
Intracerebroventricular administration of PACAP provokes increases in hypophysiotropic neurohormones in the hypothalamus, such as vasopressin, GnRH, somatostatin, as well as corticotropin-releasing factor (CRF) (Grinevich et al, 1997; Kageyama et al, 2007). PACAP also induces c-Fos expression and CREB phosphorylation in CRF neurons in the PVN, raising plasma corticosterone levels (Agarwal et al, 2005; Norrholm et al, 2005). These findings, together with the observation that CRF mRNA and corticosterone levels are reduced in PACAP-deficient mice after restraint stress (Stroth and Eiden, 2010), suggest that PACAP might have a physiological role in the regulation of CRF synthesis and activity.
PACAP has been shown to induce c-Fos mRNA expression in the arcuate nucleus (Mounien et al, 2009), a brain area involved in the regulation of appetite. Moreover, PACAP (but importantly not VIP) administration dramatically reduces food intake in rats (Chance et al, 1995), mice (Mounien et al, 2009), chicks (Tachibana et al, 2003), and goldfish (Matsuda et al, 2005). The mechanism of the PACAP-induced anorectic effects is still debated, and although in rodents melanocortins have been shown to be involved (Mounien et al, 2009), in the chick and the goldfish a role for CRF has been shown (Maruyama et al, 2006; Tachibana et al, 2004).
In this paper we sought to further characterize the behavioral and endocrine effects of PACAP. Additionally, as the central administration of CRF induces behavioral and neuroendocrine effects analogous to those observed in fear and anxiety (Dunn and Berridge, 1990), a suppression of the reward system function, as well as a dramatic anorectic effect (Britton et al, 1982; Koob and Heinrichs, 1999; Morley and Levine, 1982), we tested the hypothesis that the behavioral and neuroendocrine effects of PACAP are mediated by the recruitment of the central CRF system. For this purpose we employed well-established behavioral tests, the elevated plus maze (to assess anxiety-like behavior) and the intracranial self-stimulation (ICSS, to assess the brain reward function), and we measured food intake, body weight gain, and circulating corticosterone levels after administration of PACAP with or without concurrent administration of a selective CRF receptor antagonist; finally we measured CRF tissue content by solid phase radioimmunoassay as well as CRF and CRF1 receptor gene expression by quantitative real-time PCR (qPCR) in discrete brain regions following PACAP administration.
MATERIALS AND METHODS
Subjects
Male Wistar rats, weighing 301–325 g at arrival (Charles River, Wilmington, MA), were group-housed in wire-topped plastic cages in a 12-h reverse light cycle (lights off at 1100 hours) AAALAC-approved humidity- (60%) and temperature-controlled (22 °C) vivarium. Rats had access to corn-based chow (Harlan Teklad LM-485 Diet 7012) and water ad libitum at all times. The number of rats for each experiment were as follows: elevated-plus maze, n=39; corticosterone determination, n=38; intracranial self-stimulation, n=5; motor activity, n=14; food intake, n=13; CRF-like immunoreactivity measurement, n=16; qPCR, n=17. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Principles of Laboratory Animal Care, and were approved by Boston University Medical Campus Institutional Animal Care and Use Committee.
Drugs
PACAP (PACAP-38) was purchased from the American Peptide Company (Sunnyvale, CA); D-Phe-CRF(12-41) was kindly provided by Dr Jean Rivier (The Salk Institute, La Jolla, CA). Both peptides were dissolved in sterile isotonic saline in the presence of 1% bovine serum albumin, and were administered as a cocktail in a single injection. For within-subject design experiments, at least two treatment-free days were allowed between treatment days.
Intracranial Surgery and Microinfusion Procedures
The surgical procedure was performed as previously described (Iemolo et al, 2012). Rats underwent unilateral implantation of a 24-gauge stainless steel cannula (Plastics One, Roanoke, VA) under stereotaxic control (Kopf Instruments, Tujunga, CA) into the left or right lateral ventricle using the following coordinates (from bregma, in mm): AP: −1.0, ML: ±1.5, DV: −2.3 from skull, with incisor bar set at −3.3 mm below the interaural line, according to Paxinos and Watson (2007). For the ICSS experiment, only the i.c.v. coordinates had to be adjusted to allow the cannula and the electrode to fit in the same animal (AP: −1.0, ML: −3.2 with 20° vertical tilt, DV: −2.6 from skull, incisor bar set 5.0 mm above the interaural line). Pretreatment time was 30 min in all behavioral experiments, as well as in the corticosterone and the CRF-like immunoreactivity determination, and 4 h in the qPCR experiment. For further details, see Supplementary Materials and Methods.
Elevated Plus Maze Test
The elevated plus maze test was performed as previously described (Cottone et al, 2009; Sabino et al, 2009). Rats were placed individually onto the center of the maze for a 5-min period. The primary measures were the percent of open arm time (ie, 100 × open arm/total arm time), a validated index of anxiety-related behavior (Fernandes and File, 1996), and the number of closed arm entries, an index of motor activity (Cruz et al, 1994). For further details, see Supplementary Materials and Methods.
Plasma Corticosterone Measurement
Plasma levels of corticosterone were determined as previously described (Cottone et al, 2009; Fekete et al, 2011). Blood was sampled from the rat tails 30 min after drug administration and collected in tubes containing EDTA. Plasma was obtained after blood centrifugation and stored at −80 °C until levels of corticosterone-like immunoreactivity were determined using a commercially available radioimmunoassay kit, according to manufacturer's instructions (MP Biomedicals). Intra- and inter-assay coefficients of variation were <10%.
Motor Activity
Motor activity of rats was measured as described in Cottone et al (2012) using an Opto-M3 activity system (Columbus Instruments, Columbus, OH); activity was recorded by a computer using the Multi Device Interface software over a 120-min period. White noise was present.
ICSS Procedure
Surgery for electrode implantation and ICSS procedure were performed as previously described (Iemolo et al, 2012; Kenny and Markou, 2005). Rats were unilaterally implanted with a 0.125-mm diameter bipolar stainless steel electrode (Plastics One; length ∼10.5 mm) into the medial forebrain bundle at the level of the lateral hypothalamus (coordinates from bregma: AP −0.5 mm, ML +1.7 mm; DV −9.7 mm from skull; incisor bar set 5.0 mm above the interaural line). Rats were trained to lever press on a fixed ratio 1 schedule of reinforcement to obtain an electrical stimulation; once fixed ratio 1 responding was established, mean reward thresholds were assessed using a rate-independent discrete-trial current intensity procedure designed by Kornetsky et al (1979). The reward threshold is defined as the minimal current intensity able to produce a response that maintains the self-stimulation behavior. A raise in the reward threshold indicates that stimulus intensities that were previously perceived as reinforcing are no longer perceived as rewarding, reflecting a decrease in reward function. Vice versa, lowering of the reward threshold reflects increased reward function (Markou and Koob, 1991). The mean response latency is defined as the mean response latency of all trials within a session during which a positive response occurred. For more details, see Supplementary Materials and Methods.
Food Intake and Body Weight Determinations
Pre-weighed food was provided at the beginning of the dark cycle and recorded 1, 3, 6, and 24 h later. Rat body weights were assessed right before drug administration and 24 h later.
Brain Punching and qPCR
Tissue CRF and CRF1R mRNA levels were determined as previously described (Cottone et al, 2009; Sabino et al, 2011). Rats were anesthetized with isoflurane and brains were quickly removed and coronally sliced in a brain matrix; punches containing the CeA, the basolateral amygdala (BlA), the medial amygdala (MeA), and the PVN of the hypothalamus were collected on an ice-cold stage. For details on qPCR, see Supplementary Materials and Methods.
CRF-like Immunoreactivity Measurement
Tissue CRF levels were determined as previously described (Cottone et al, 2009; Zorrilla et al, 2001). Punches were collected as described above and tissue CRF-like immunoreactivity was quantified with a sensitive and specific solid-phase radioimmunoassay adapted from Zorrilla et al (2001), which followed an established procedure for peptide acid extraction. An anti-CRF serum (rC68, 1 : 200 000 titer) generously provided by Wylie Vale (The Salk Institute) was used. Sensitivity of the assay was ∼0.3 fmol/well. For further details see Supplementary Materials and Methods.
Statistical Analysis
Data from the elevated plus maze and corticosterone levels were analyzed using two-way analysis of variance (ANOVA) with PACAP and Antagonist as between-subjects factors. Motor activity was analyzed using a two-way repeated measure ANOVA with PACAP and Time as within-subject factors. ICSS data were analyzed using a two-way repeated measure ANOVA with PACAP and Antagonist as within-subject factors. One-, three-, and six-hour food intake data were analyzed using a three-way mixed design ANOVA, with Antagonist as a between-subjects factor, and PACAP and Time as within-subject factors. Twenty-four-hour food intake and body weight change were analyzed using two-way mixed design ANOVAs with Antagonist as a between-subjects factor and PACAP as a within-subject factor. Pairwise post-hoc comparisons were made using Newman–Keuls test; Student's t-test was used when comparing two groups. Significance was set at P<0.05. The software/graphic packages used were Systat 11.0, SigmaPlot 11.0, InStat 3.0, and Statistica 7.0.
RESULTS
The CRF Receptor Antagonist D-Phe-CRF(12-41) Blocks PACAP-Induced Anxiety-Like Behavior in the Elevated Plus Maze
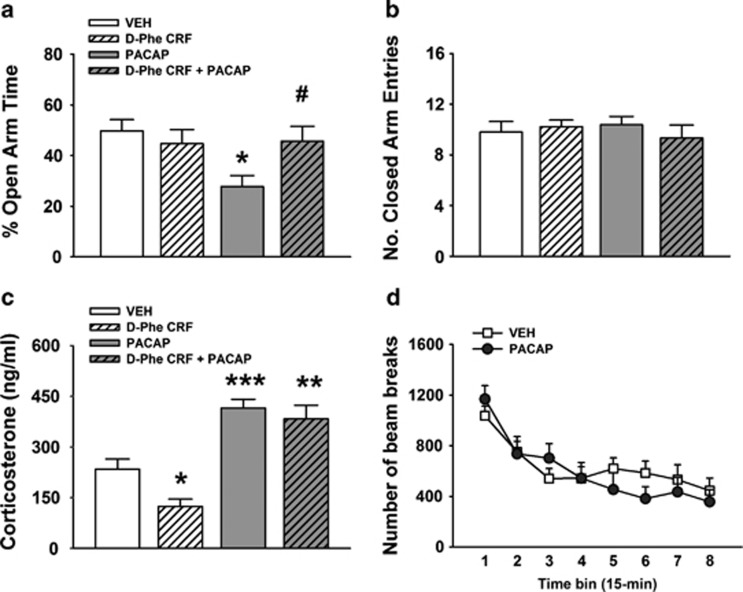
As shown in Figure 1a, i.c.v. administration of PACAP (5 μg/rat) significantly reduced the percent of open arm time, as reflected by a significant main effect of the factor PACAP (F(1,35)=4.42, P<0.05); PACAP-treated rats, indeed, spent 44% less time in the open arms compared with vehicle-treated rats. The CRF antagonist D-Phe-CRF(12–41), at a dose that had no effect on anxiety-like behavior per se (10 μg/rat), was able to fully block the PACAP-induced reduction of % open arm time, as demonstrated by a significant interaction PACAP × Antagonist (F(1,35)=5.33, P<0.05). As shown in Figure 1b, neither PACAP nor D-Phe-CRF(12-41) had any effect on the number of closed arm entries, an index of motor activity (PACAP, F(1,35)=0.15, n.s.; PACAP × Antagonist, F(1,35)=0.87, n.s.). Drug effects on the % closed arm time and on the number of open arm entries are shown in Supplementary Figure 1.
Figure 1.
Effects of i.c.v. administration of PACAP (5 μg/rat) and the CRF receptor antagonist D-Phe-CRF(12-41) (10 μg/rat) on the percentage of time spent in the open arms (a) and the number of closed arm entries (b) of an elevated plus maze test. Rats were tested 30 min after drug administration (between-subjects design). (c) The effects of the drug treatments on plasma corticosterone levels; blood samples were collected from the rat tail 30 min after drugs administration (between-subjects design). (d) The effects of i.c.v. PACAP (5 μg/rat) on motor activity immediately after drug administration (within-subject design). Data represent mean±SEM. *P<0.05, **P<0.01, ***P<0.001 vs vehicle group; #P<0.05 vs PACAP group (Newman–Keuls test).
The CRF Receptor Antagonist D-Phe-CRF(12-41) Does Not Block PACAP-Induced Adrenocortical Activation
Intracerebroventricular treatment with PACAP (5 μg/rat) caused a 77% increase in plasma levels of corticosterone 30 min after drug treatment (PACAP, F(1,34)=52.49, P<0.001) as shown in Figure 1c. Importantly, the same dose of the CRF receptor antagonist D-Phe-CRF(12-41), which blocked PACAP-induced anxiogenic-like effects (10 μg/rat), did not affect the PACAP-induced increase in corticosterone (PACAP × Antagonist, F(1,34)=1,68, n.s.). On the other hand, this same dose of D-Phe-CRF(12-41) caused a 47% decrease in plasma corticosterone levels per se (Antagonist F(1,34)=5.46, P<0.05).
PACAP does not Alter Motor Activity
As shown in Figure 1d, i.c.v. administration of PACAP (5 μg/rat) did not alter motor activity in the 2-h observation period (PACAP, F(1,12)=0.10, n.s.; PACAP × Time bin, F(7,84)=1.96, n.s.), confirming the specificity of the anxiogenic profile.
The CRF Receptor Antagonist D-Phe-CRF(12-41) Blocks PACAP-Induced Elevation of ICSS Threshold
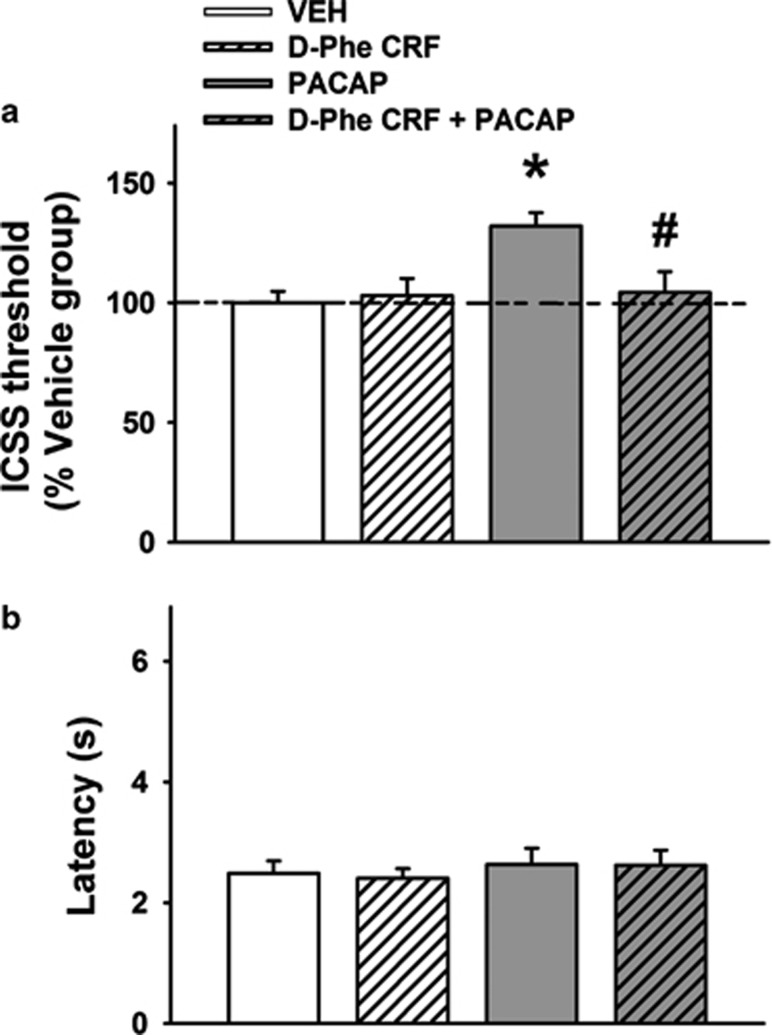
As shown in Figure 2a, i.c.v. administration of PACAP (5 μg/rat) significantly decreased the brain reward function, as reflected by a significant main effect of the factor PACAP (F(1,16)=6.14, P<0.05); PACAP-treated rats, indeed, showed a 32% elevation of the brain reward threshold compared with vehicle-treated rats. The CRF antagonist D-Phe-CRF(12-41) had no effect on ICSS threshold per se but was able to fully block the effect of PACAP, as demonstrated by a significant interaction PACAP × Antagonist (F(1,16)=5.20, P<0.05). As shown in Figure 2b, neither PACAP nor D-Phe-CRF(12-41) affected the latency to press the lever, an index of general motor activity (PACAP, F(1,16)=0.63, n.s.; PACAP × Antagonist, F(1,16)=0.02, n.s.).
Figure 2.
Effects of i.c.v. administration of PACAP (5 μg/rat) and the CRF receptor antagonist D-Phe-CRF(12-41) (10 μg/rat) on the brain reward function measured by (a) intracranial self-stimulation (ICSS) threshold (percentage change from vehicle-treated group). (b) How the treatments affected the latency to respond. ICSS session began immediately after drug administration (within-subject design). Data represent mean±SEM. *P<0.05 vs vehicle group; #P<0.05 vs PACAP group (Newman–Keuls test)
The CRF Receptor Antagonist D-Phe-CRF(12-41) Does Not Block PACAP-Induced Anorexia and Body Weight Loss
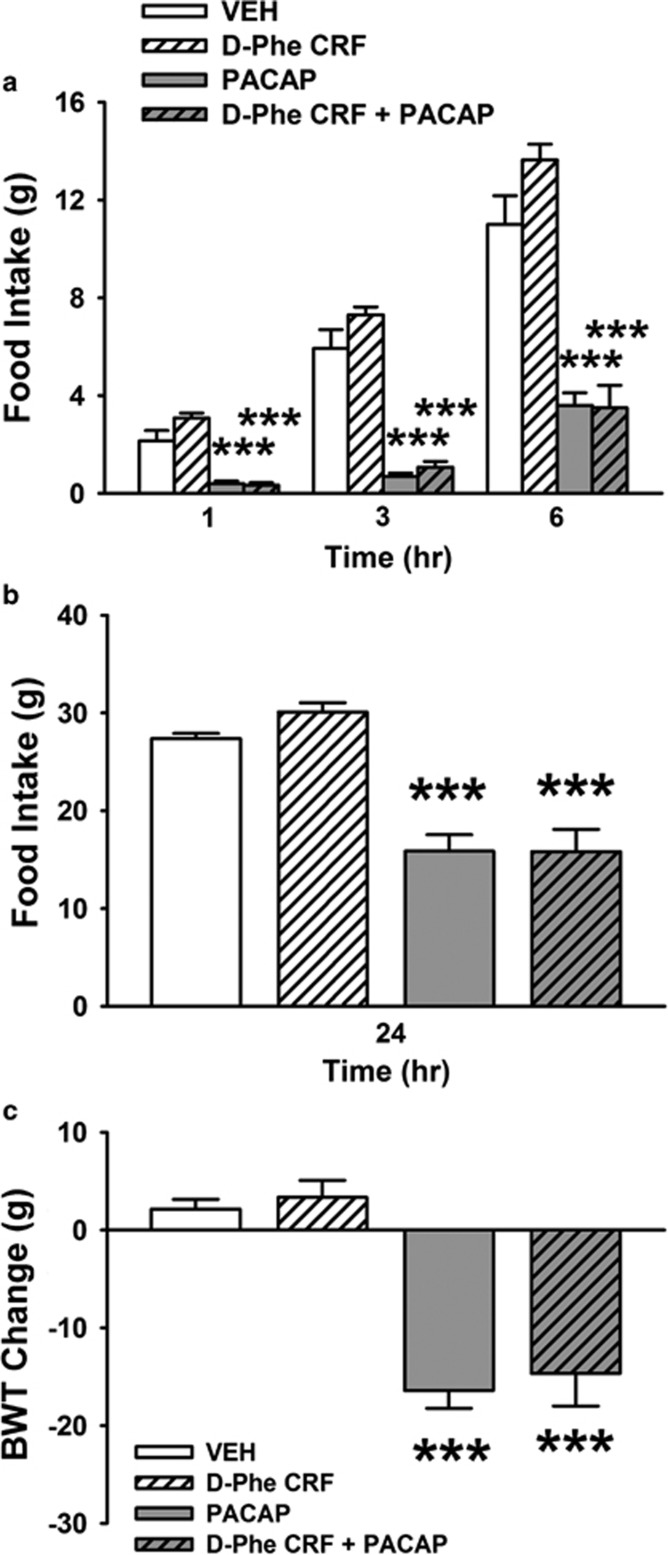
I.c.v. treatment with PACAP (5 μg/rat) significantly reduced food intake throughout the 6 h post administration, as reflected by a significant effect of PACAP (F(1,11)=120.96, P<0.001). The CRF receptor antagonist D-Phe-CRF(12-41) had no effect on food intake per se (Antagonist: F(1,11)=1.84, n.s.) nor did it affect PACAP-induced hypophagia (PACAP × Antagonist: F(1,11)=2.93, n.s.). Post-hoc comparisons revealed that PACAP reduced 1-, 3-, and 6-h food intake (−81, −88, and −67%, respectively, compared with the vehicle-treated group,), as shown in Figure 3a. The CRF receptor antagonist D-Phe-CRF(12-41) had no effect at any of the other time points, and it did not affect the anorectic effects of PACAP.
Figure 3.
Effects of i.c.v. administration of PACAP (5 μg/rat) and the CRF receptor antagonist D-Phe-CRF(12-41) (10 μg/rat) on cumulative food intake (a and b) and 24 h body weight change (c). Food was presented 30 min after drug administration and intake was recorded 1, 3, 6, and 24 h later (within-subject design). Data represent mean±SEM. ***P<0.001 vs vehicle group (Newman–Keuls test).
PACAP continued to have an effect on food intake 24 h after administration (−42%, PACAP: (F(1,11)=99.36, P<0.001) as shown in Figure 3b. The CRF receptor antagonist still had no effect (Antagonist: F(1,11)=0.65, n.s.; PACAP × Antagonist: (F(1,11)=1.13, n.s.).
PACAP caused dramatic body weight loss 24 h after administration (PACAP: F(1,11)=88.80, P<0.001; mean±SEM: +2.14±0.98 vs −16.42±1.81 g, vehicle vs PACAP) as shown in Figure 3c. The CRF antagonist had no effect on PACAP-induced body weight loss (Antagonist: F(1,11)=0.58, n.s.; PACAP × Antagonist: F(1,11)=0.00, n.s.).
PACAP Increases CRF mRNA Expression in the Brain
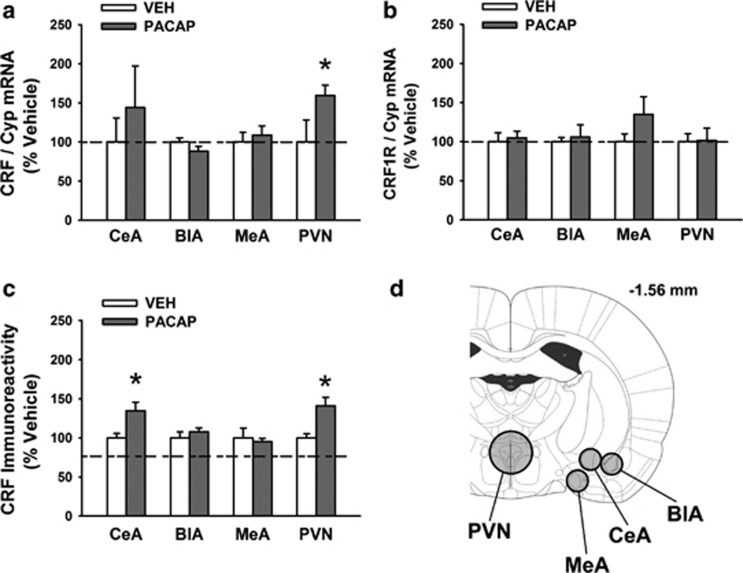
I.c.v. treatment with PACAP (5 μg/rat) significantly increased CRF mRNA expression in the PVN (59% increase, t[13]=1.82, P<0.05), as shown in Figure 4a. The treatment had no effect on CRF mRNA in the BlA (t[14]=1.46, n.s.) or the MeA (t[15]=0.51, n.s.), whereas in the CeA a nonsignificant 44% trend to an increase could be observed (t[13]=0.74, n.s.).
Figure 4.
Effects of i.c.v. administration of PACAP (5 μg/rat) on CRF mRNA expression (a), CRF1R mRNA expression (b), and CRF immunoreactivity (IR) (c) in the central nucleus of the amygdala (CeA), basolateral amygdala (BlA), medial amygdala (MeA) and paraventricular nucleus of the hypothalamus (PVN). (d) The approximate size and location of the brain punches. Rats were sacrificed either 4 h (for mRNA) or 30 min (for immunoreactivity) after drug administration. Data represent mean±SEM and are expressed as % vs vehicle group. *P<0.05 vs relative vehicle group (Student's t-test).
As shown in Figure 4b, i.c.v. PACAP administration had no effect on CRF1R mRNA expression in any of the brain areas analyzed (PVN: t[13]=0.07, n.s.; BlA: t[13]=0.34, n.s.; MeA: t[14]=1.39, n.s.; CeA: t[14]=0.36, n.s.).
PACAP Increases CRF-Like Immunoreactivity in the Brain
I.c.v. PACAP treatment caused an increase in CRF-like immunoreactivity in both the PVN (+41%, t[12]=−2.72, P<0.05) and the CeA (+35%, t[12]=−2.26, P<0.05), as shown in Figure 4c. No changes in CRF-like immunoreactivity in the BlA (t[12]=−0.83, n.s.) or the MeA (t[12]=0.44, n.s.) were observed.
DISCUSSION
The main findings of the present study were as follows: (i) i.c.v. PACAP exhibits an anxiogenic-like effect in the elevated plus maze in rats, an effect that is fully blocked by the CRF receptor antagonist D-Phe-CRF(12-41); (ii) i.c.v. PACAP raises plasma corticosterone levels but this effect is ‘not' altered by the CRF receptor antagonist; (iii) i.c.v. PACAP reduces the rewarding properties of the brain stimulation in ICSS, an effect that is blocked by the CRF receptor antagonist; (iv) i.c.v. PACAP reduces food intake and body weight gain, and this effect is ‘not' altered by the CRF receptor antagonist; (v) i.c.v. PACAP increases the levels of CRF mRNA in the PVN and the levels of CRF peptide in both the CeA and the PVN.
In the elevated plus maze test, rats treated with PACAP spent a significantly lower percent of time in the open arms compared with vehicle-treated rats (−44%); the number of entries in the closed arms, a measure of motor activity, was unaffected by the treatment, confirming that the anxiety-like behavior was not due to a general behavioral suppression. This latter finding was also confirmed by the lack of motor-suppressing effects of PACAP observed in the specific motor activity test. To the best of our knowledge, this is the first time that the anxiogenic-like effects of PACAP are shown using the gold standard test for assessing anxiety-like behavior in rodents, the elevated plus maze. Our results are in agreement with previous evidence showing that PACAP produces stress-like effects in rats such as face washing, body grooming, and wet-dog shakes when injected i.c.v. or into the PVN (Agarwal et al, 2005; Norrholm et al, 2005). Administered into the BNST, PACAP increases the startle response (Hammack et al, 2009), whereas administration into the CeA increases immobility and withdrawal from an ‘always on' electrified probe (Legradi et al, 2007). In addition, PACAP knockout and PAC1 receptor knockout mice show an anxiolytic profile (Hashimoto et al, 2001; Otto et al, 2001). Altogether, this evidence strongly indicates a critical role for the PACAP system in the behavioral response to stress.
We here demonstrate that coadministration with the CRF receptor antagonist D-Phe-CRF(12-41) blocks the anxiogenic effects of PACAP in the elevated plus maze, indicating that PACAP modulates anxiety-like behavior via the activation of central CRF receptors. CRF is one of the major stress neurotransmitters in the brain and its central administration exerts anxiogenic-like effects (Britton et al, 1982; File et al, 1988). Numerous pieces of evidence suggest a close relationship between the CRF and the PACAP systems: centrally administered PACAP increases the CRF mRNA expression in the PVN and the PAC1 receptor antagonist PACAP (6-38) decreases it (Grinevich et al, 1997); PACAP induces c-Fos expression and CREB phosphorylation in CRF neurons in the PVN, also causing an increase in plasma corticosterone levels (Agarwal et al, 2005; Norrholm et al, 2005). Interestingly, PACAP knockout mice show reduced CRF mRNA and corticosterone levels in response to restraint stress (Stroth and Eiden, 2010). Our finding that the anxiogenic effects of PACAP are mediated by CRF is an important one because it implicates that the PACAP system is located upstream of CRF, and therefore providing a potentially novel site to modulate the activity of the CRF system, which is dysregulated in stress-related disorders (Koob and Zorrilla, 2012).
Here we found that PACAP treatment causes an increase in plasma corticosterone levels. Our finding that PACAP activates the HPA axis confirms previous ones (Agarwal et al, 2005; Norrholm et al, 2005) and is consistent with the very important observation that mice lacking the gene coding for PACAP do not show HPA axis activation following restraint stress (Lehmann et al, 2012; Stroth and Eiden, 2010). Interestingly, we show that the ability of PACAP to activate the HPA axis does not require central CRF receptors, as the CRF receptor antagonist did not block the elevation in circulating corticosterone levels. Although surprising, this result is consistent with the observation that PACAP can enhance ACTH secretion ‘directly', by stimulating pituitary corticotropes independent of CRF (Koob and Heinrichs, 1999; Miyata et al, 1989). As we observed that central PACAP administration increases CRF content and synthesis in the PVN, we speculate that the blockade of PACAP-induced CRF receptor activation in the PVN may not be sufficient to block the HPA axis activation because PACAP could still act directly on pituitary cells to increase ACTH. Because the same dose of D-Phe-CRF(12-41) blocked the anxiogenic effects of PACAP in the elevated plus maze, as discussed above, we can conclude that the anxiety-like behavior induced by PACAP is likely not a direct consequence of the HPA axis activation. In agreement with our findings are the observations that the behavioral changes occurring during the stress response, which are modulated by CRF, occur independently of HPA axis activation, suggesting that CRF, acting as a neurotransmitter in extrahypothalamic brain areas, is responsible for the behavioral responses to stressors (Britton et al, 1986a; Britton et al, 1986b; Dunn and Berridge, 1990; Koob and Heinrichs, 1999; Menzaghi et al, 1994). Furthermore, it is possible that PACAP administration also increases the levels of arginine-vasopressin, which induces secretion of ACTH at the corticotropes via V1b receptors, and therefore that a concurrent blockade of CRF and arginine-vasopressin receptors may be needed to prevent the HPA-activating effects of PACAP.
Interestingly, the CRF receptor antagonist used in this study, D-Phe-CRF(12-41), significantly reduced the basal corticosterone levels per se at the dose employed here, likely blocking CRF receptors in the pituitary. Importantly, despite the reduction in basal corticosterone levels observed here, the treatment with D-Phe-CRF(12-41) was still ineffective in preventing PACAP-induced corticosterone increase. Related to this previous point, it cannot be completely ruled out that a higher dose of D-Phe-CRF(12-41) could block the HPA-activating effect of PACAP, although lower doses, ranging from 1 to 5 μg per animal vs the 10 μg used here, are usually employed in behavioral studies in rodents (Macey et al, 2000; Rodriguez de Fonseca et al, 1996; Zorrilla et al, 2002).
Within this series of experiments, a very significant finding was the discovery that PACAP increases the ICSS threshold, which clearly indicates a reduction of the rewarding impact of the brain stimulation. ICSS is a reliable behavioral paradigm in which rats self-administer rewarding electrical stimulation through electrodes implanted within the limbic system (in our case the lateral hypothalamus) (Carlezon and Chartoff, 2007; Markou and Koob, 1992). Importantly, as several conditions known to cause or contribute to depressive states in humans attenuate ICSS behavior, eg, chronic stress and drug withdrawal, increases in ICSS thresholds can be considered as signs of anhedonia, a core symptom of depression (Markou and Koob, 1991; Schulteis et al, 1995; Zacharko and Anisman, 1991); consistently, antidepressant drugs are able to attenuate ICSS threshold elevations. Therefore, the present findings raise the interesting possibility that PACAP may not only have a key role in pathological anxiety, but also contribute to the development of depressive-like symptoms in animals and humans. Importantly, we demonstrate that the PACAP-induced elevations of the ICSS thresholds are mediated by CRF receptors. This finding is consistent with the observation that CRF itself is able to elevate ICSS thresholds (Macey et al, 2000) and, again, points at an extrahypothalamic, HPA-independent mechanism of action for the behavioral effects of PACAP.
The dramatic reduction in food intake and body weight induced by PACAP in the present study confirms previous results obtained with this peptide in several species (Maruyama et al, 2006; Mounien et al, 2009; Tachibana et al, 2004), a profile not shared by VIP (Mounien et al, 2009). CRF also exerts strong anorectic effects after i.c.v. administration (Morley and Levine, 1982), which occur independently of HPA axis activation and have been proposed to be mediated by the extended amygdala (Britton et al, 1986a; Morley and Levine, 1982). Interestingly, the reduction in food intake and the body weight loss induced by PACAP were not blocked by the CRF receptor antagonist, suggesting that PACAP exerts its anorectic effect via a mechanism other than CRF. Recently, melanocortins have been proposed to be involved in this effect, as the melanocortin receptor 3–4 antagonist SHU9119 is able to revert the reduction in food intake caused by PACAP, and PACAP increases pro-opiomelanocortin expression in the hypothalamus of mice (Mounien et al, 2009). Our results conflict with the reports that in the chick and the goldfish the anorectic effect of PACAP is mediated by CRF (Maruyama et al, 2006; Tachibana et al, 2004); however, the differences between species can easily explain the discrepancy observed. Which brain regions mediate the anorectic effect of PACAP has not yet been clarified, but the ventromedial nucleus of the hypothalamus has been proposed as a candidate area (Mounien et al, 2009; Resch et al, 2011).
The present study showed that i.c.v. PACAP administration causes an increase of CRF expression (both mRNA and immunoreactivity, ie, peptide tissue content) in the PVN. The increased levels of CRF that we observed in the PVN are in agreement with the increased circulating corticosterone levels, and strongly suggest that PACAP activates the HPA axis by increasing both the synthesis and release of CRF (although this is likely not the only mechanism). Our result is also in agreement with previous studies showing increased CRF mRNA levels in the PVN after i.c.v. PACAP administration (Grinevich et al, 1997). Thus, once PACAP is released after stress exposure, it would act on PAC1 receptors of PVN CRF neurons, increasing intracellular cAMP levels and protein kinase A pathway signaling, inducing the synthesis of CRF.
Noteworthy, the present study suggests that PACAP-induced HPA axis activation is likely not responsible for the PACAP-induced anxiogenic effects. We can, therefore, hypothesize that the activation of CRF receptors in brain areas other than the hypothalamus is responsible for the ability of PACAP to induce anxiety-like behavior. Obvious extrahypothalamic candidates are the brain regions part of the extended amygdala, where the administration of CRF produces anxiogenic effects independently of the HPA axis. Indeed, we found PACAP treatment to increase CRF-like immunoreactivity in the CeA, a very novel and significant finding. Together with the shell of the nucleus accumbens and the BNST, the CeA is part of the extended amygdala, a circuitry crucial for the response to stress, fear, and anxiety. The CeA in particular is believed to be the primary output regulating fear responses, the release of glucocorticoids, and the autonomic nervous system (Davis, 1992). CeA neurons are also considered to be the major source of CRF that can act locally or in the BlA and the BNST (Britton et al, 1986b). Stressful or fearful events cause an increase of CRF levels in the CeA (Kalin et al, 1994; Merlo Pich et al, 1995), and intra-CeA CRF receptor antagonist administration attenuates the behavioral response to stress and the aversive consequences of drug withdrawal (Heinrichs et al, 1995; Heinrichs et al, 1992; Rassnick et al, 1993). The observation that high densities of PACAP fibers as well as medium/high densities of PAC1 receptors are present in the CeA (Hannibal, 2002; Piggins et al, 1996) suggests that PACAP in this brain area may have an important role in stress-related behaviors, perhaps through the modulation of CRF release and/or synthesis, as our present data strongly suggest. Interestingly, PAC1 receptor gene expression has been shown to increase in the whole amygdala of rats during the consolidation of fear in a fear-conditioning model (Ressler et al, 2011), further strengthening a putative role of the amygdala PACAP-PAC1 system in anxiety and fear.
Although a significant increase in CRF peptide immunoreactivity was detected in the CeA, only a trend toward an increase (+44%) in the mRNA was observed. This finding can be interpreted as PACAP having an effect exclusively on the release, but not the synthesis of CRF, as rats were sacrificed 30 min after PACAP administration for the CRF-like immunoreactivity, which is likely not enough time for new peptide to be synthesized ex novo (however, effects on synthesis or metabolism of CRF cannot be completely ruled out based on the present data alone). Alternatively, the time window at which the CRF mRNA was increased may have been missed. Indeed, in the present study rats were sacrificed 4 h after PACAP administration for the mRNA level measurement. Although 4 h is a very typical time point for maximal detection of gene expression changes (Grinevich et al, 1997; Li et al, 1996), it is still possible that an increase in CRF mRNA occurred earlier. In support of this hypothesis is the observation that CRF mRNA levels in the CeA, but not the PVN, are found to be increased already 60–90 min after the onset of different types of stressors (Funk et al, 2006; Makino et al, 1999), suggesting that CRF synthesis in the CeA following stressors may occur at an earlier time point compared to the PVN.
In summary, our results suggest that PACAP induces anxiety-like behavior and pro-depressant effects, independently of the HPA axis activation, by increasing the levels of CRF in the CeA. The CRF system therefore is an immediate downstream target and mediator of PACAP. Conversely, the anorectic and body weight loss-inducing effects of PACAP seem to occur independently of CRF via other mechanisms. Our data provide novel insights into this neuropeptide system as a mechanism for modulating anxiety-like behavior, and may ultimately lead to a new class of therapeutic agents for the treatment of anxiety-related and mood disorders.
FUNDING AND DISCLOSURE
This work was made possible by grant numbers MH093650, MH091945, AA016731, DA030425, and DA023680 from the National Institute of Mental Health (NIMH), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), and the National Institute on Drug Abuse (NIDA); by the Peter Paul Career Development Professorship (PC); and by Boston University's Undergraduate Research Opportunities Program (UROP). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The authors declare no conflict of interest.
Acknowledgments
We thank Stephen St Cyr, Arturo M Escajeda and Patrick De Souza for technical assistance, and Tamara Zeric for technical and editorial assistance.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res. 2005;138:45–57. doi: 10.1016/j.molbrainres.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci. 1982;31:363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- Britton DR, Varela M, Garcia A, Rosenthal M. Dexamethasone suppresses pituitary-adrenal but not behavioral effects of centrally administered CRF. Life Sci. 1986a;38:211–216. doi: 10.1016/0024-3205(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Britton KT, Lee G, Dana R, Risch SC, Koob GF. Activating and ‘anxiogenic' effects of corticotropin releasing factor are not inhibited by blockade of the pituitary-adrenal system with dexamethasone. Life Sci. 1986b;39:1281–1286. doi: 10.1016/0024-3205(86)90189-x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Chance WT, Thompson H, Thomas I, Fischer JE. Anorectic and neurochemical effects of pituitary adenylate cyclase activating polypeptide in rats. Peptides. 1995;16:1511–1516. doi: 10.1016/0196-9781(95)02048-9. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, et al. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci USA. 2009;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Wang X, Park JW, Valenza M, Blasio A, Kwak J, et al. Antagonism of sigma-1 receptors blocks compulsive-like eating. Neuropsychopharmacology. 2012;37:2593–2604. doi: 10.1038/npp.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses. Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zhao Y, Szucs A, Sabino V, Cottone P, Rivier J, et al. Systemic urocortin 2, but not urocortin 1 or stressin 1-A, suppresses feeding via CRF2 receptors without malaise and stress. Br J Pharmacol. 2011;164:1959–1975. doi: 10.1111/j.1476-5381.2011.01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- File SE, Johnston AL, Baldwin HA. Anxiolytic and anxiogenic drugs: changes in behaviour and endocrine responses. Stress Med. 1988;4:221–230. [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Gottschall PE, Tatsuno I, Miyata A, Arimura A. Characterization and distribution of binding sites for the hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide. Endocrinology. 1990;127:272–277. doi: 10.1210/endo-127-1-272. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Fournier A, Pelletier G. Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on corticotropin-releasing hormone (CRH) gene expression in the rat hypothalamic paraventricular nucleus. Brain Res. 1997;773:190–196. doi: 10.1016/s0006-8993(97)01011-1. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, et al. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol. 2002;453:389–417. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Jessop DS, Fahrenkrug J, Harbuz MS, Larsen PJ. PACAP gene expression in neurons of the rat hypothalamo-pituitary-adrenocortical axis is induced by endotoxin and interleukin-1beta. Neuroendocrinology. 1999;70:73–82. doi: 10.1159/000054461. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Mikkelsen JD, Fahrenkrug J, Larsen PJ. Pituitary adenylate cyclase-activating peptide gene expression in corticotropin-releasing factor-containing parvicellular neurons of the rat hypothalamic paraventricular nucleus is induced by colchicine, but not by adrenalectomy, acute osmotic, ether, or restraint stress. Endocrinology. 1995;136:4116–4124. doi: 10.1210/endo.136.9.7649120. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T, et al. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP) Proc Natl Acad Sci USA. 2001;98:13355–13360. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995;6:74–80. [PubMed] [Google Scholar]
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Iemolo A, Valenza M, Tozier L, Knapp CM, Kornetsky C, Steardo L, et al. Withdrawal from chronic, intermittent access to a highly palatable food induces depressive-like behavior in compulsive eating rats. Behav Pharmacol. 2012;23:593–602. doi: 10.1097/FBP.0b013e328357697f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama K, Hanada K, Iwasaki Y, Sakihara S, Nigawara T, Kasckow J, et al. Pituitary adenylate cyclase-activating polypeptide stimulates corticotropin-releasing factor, vasopressin and interleukin-6 gene transcription in hypothalamic 4B cells. J Endocrinol. 2007;195:199–211. doi: 10.1677/JOE-07-0125. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Takahashi LK, Chen FL. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci. 2005;25:6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Update on corticotropin-releasing factor pharmacotherapy for psychiatric disorders: a revisionist view. Neuropsychopharmacology. 2012;37:308–309. doi: 10.1038/npp.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch Gen Psychiatry. 1979;36:289–292. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, Diamond DM. Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plast. 2007;2007:79102. doi: 10.1155/2007/79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Mustafa T, Eiden AM, Herkenham M, Eiden LE. PACAP-deficient mice show attenuated corticosterone secretion and fail to develop depressive behavior during chronic social defeat stress. Psychoneuroendocrinology. 2012;38:702–715. doi: 10.1016/j.psyneuen.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Grinevich V, Fournier A, Pelletier G. Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on gonadotropin-releasing hormone and somatostatin gene expression in the rat brain. Brain Res Mol Brain Res. 1996;41:157–162. doi: 10.1016/0169-328x(96)00086-1. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Koob GF, Markou A. CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain Res. 2000;866:82–91. doi: 10.1016/s0006-8993(00)02229-0. [DOI] [PubMed] [Google Scholar]
- Makino S, Shibasaki T, Yamauchi N, Nishioka T, Mimoto T, Wakabayashi I, et al. Psychological stress increased corticotropin-releasing hormone mRNA and content in the central nucleus of the amygdala but not in the hypothalamic paraventricular nucleus in the rat. Brain Res. 1999;850:136–143. doi: 10.1016/s0006-8993(99)02114-9. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Miura T, Uchiyama M, Shioda S, Matsuda K. Relationship between anorexigenic action of pituitary adenylate cyclase-activating polypeptide (PACAP) and that of corticotropin-releasing hormone (CRH) in the goldfish, Carassius auratus. Peptides. 2006;27:1820–1826. doi: 10.1016/j.peptides.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Maruyama K, Nakamachi T, Miura T, Uchiyama M, Shioda S. Inhibitory effects of pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) on food intake in the goldfish, Carassius auratus. Peptides. 2005;26:1611–1616. doi: 10.1016/j.peptides.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Rassnick S, Heinrichs S, Baldwin H, Pich EM, Weiss F, et al. The role of corticotropin-releasing factor in the anxiogenic effects of ethanol withdrawal. Ann N Y Acad Sci. 1994;739:176–184. doi: 10.1111/j.1749-6632.1994.tb19819.x. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS. Corticotrophin releasing factor, grooming and ingestive behavior. Life Sci. 1982;31:1459–1464. doi: 10.1016/0024-3205(82)90007-8. [DOI] [PubMed] [Google Scholar]
- Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, et al. Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology. 2009;34:424–435. doi: 10.1038/npp.2008.73. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Das M, Legradi G. Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN) Regul Pept. 2005;128:33–41. doi: 10.1016/j.regpep.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res. 2001;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.2007The Rat Brain6th edn.Academic Press: Orlando, FL [Google Scholar]
- Piggins HD, Stamp JA, Burns J, Rusak B, Semba K. Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. J Comp Neurol. 1996;376:278–294. doi: 10.1002/(SICI)1096-9861(19961209)376:2<278::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Resch JM, Boisvert JP, Hourigan AE, Mueller CR, Yi SS, Choi S. Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1625–R1634. doi: 10.1152/ajpregu.00334.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, et al. Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1996;276:56–64. [PubMed] [Google Scholar]
- Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, et al. Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology. 2011;36:1207–1218. doi: 10.1038/npp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L, Jr., Steardo L, et al. The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology. 2009;34:1482–1493. doi: 10.1038/npp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci USA. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21:619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2010;165:1025–1030. doi: 10.1016/j.neuroscience.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana T, Saito ES, Takahashi H, Saito S, Tomonaga S, Boswell T, et al. Anorexigenic effects of pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide in the chick brain are mediated by corticotrophin-releasing factor. Regul Pept. 2004;120:99–105. doi: 10.1016/j.regpep.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Saito S, Tomonaga S, Takagi T, Saito ES, Boswell T, et al. Intracerebroventricular injection of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibits feeding in chicks. Neurosci Lett. 2003;339:203–206. doi: 10.1016/s0304-3940(03)00017-x. [DOI] [PubMed] [Google Scholar]
- Zacharko RM, Anisman H. Stressor-induced anhedonia in the mesocorticolimbic system. Neurosci Biobehav Rev. 1991;15:391–405. doi: 10.1016/s0149-7634(05)80032-6. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Schulteis G, Ormsby A, Klaassen A, Ling N, McCarthy JR, et al. Urocortin shares the memory modulating effects of corticotropin-releasing factor (CRF): mediation by CRF1 receptors. Brain Res. 2002;952:200–210. doi: 10.1016/s0006-8993(02)03345-0. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.