Abstract
Several lines of evidence suggest that neuroplasticity is impaired in depression. This study aimed to compare neuroplasticity in 23 subjects with DSM-IV major depressive episode and 23 age- and gender-matched healthy controls, using an objective test that is independent of subject effort and motivation. Neuroplasticity was assessed in the motor cortex using a brain stimulation paradigm known as paired associative stimulation (PAS), which induces transient changes in motor cortical function. Motor cortical excitability was assessed before and after PAS using single-pulse transcranial magnetic stimulation (TMS) to induce motor evoked potentials (MEPs) in a hand muscle. After PAS, MEP amplitudes significantly increased in healthy controls compared with depressed subjects (P=0.002). The functional significance of motor cortical changes was assessed using a motor learning task—a computerized version of the rotor pursuit task. Healthy controls also performed better on motor learning (P=0.02). BDNF blood levels and genotype were assayed to determine any relationship with motor cortical plasticity. However, PAS results did not correlate with motor learning, nor appear to be related to BDNF measures. The significance of these findings is that it provides one of the first direct demonstrations of reduced neuroplasticity in depressed subjects, using an objective test.
Keywords: neuroplasticity, major depressive disorder, transcranial magnetic stimulation, paired associative stimulation, motor cortex, motor learning
INTRODUCTION
The neurobiology of major depressive disorder (MDD) includes evidence for altered cortical activity (Savits and Drevets, 2009), shrinkage of neurons and glial cells, loss of dendritic complexity in the hippocampus and prefrontal cortex (PFC) (Kanner, 2004; Carlson et al, 2006), and lower levels of brain-derived neurotrophic factor (BDNF) (Sen et al, 2008). Reduction of regional volume in these affected brain areas has been confirmed in post-mortem studies (Stockmeier et al, 2004; Rajkowska et al, 1999) and shown to be greater in those with multiple episodes, longer illness duration (MacQueen et al, 2003), and increased symptom severity (Kumar et al, 1998). The pathophysiological processes that lead to these neurotrophic changes are also thought to be responsible for impaired cellular resilience and loss of neuroplasticity (Pittenger and Duman, 2008).
For example, depression is often accompanied by impairment of learning and memory processes (Landrø et al, 2001; Porter et al, 2003), which are known to require neural adaptation (Malenka and Bear, 2004). Although a finding of impaired neuroplasticity in depression may be inferred from these observations, it is also possible that reduced motivation and effort may account for these deficits. This study therefore aimed to objectively assess neuroplasticity in depressed subjects compared with age- and gender-matched controls, through the use of a noninvasive brain stimulation protocol that induces temporary neuroplastic changes independent of subject effort. A range of brain stimulation protocols have shown the capacity to induce short-lasting motor cortical plasticity (Ziemann et al, 2008). In a prior study, we compared two stimulation protocols for which robust effects have been described—paired associative stimulation (PAS) and theta burst stimulation (TBS)—and found that more consistent changes in motor cortical excitability were induced by PAS (Player et al, 2012). PAS-induced increases in cortical excitability are considered to be at least partially dependent on associative long-term potentiation (LTP) (Stefan et al, 2002), which is modulated by BDNF through both pre- and postsynaptic mechanisms (Yoshii and Constantine-Paton, 2010), known to be crucial for neuroplasticity. These mechanisms are believed to underlie motor learning and memory formation (Letzkus et al, 2007), hence providing an appropriate model for testing neuroplasticity. The PAS protocol has previously been used to demonstrate reduced synaptic change in schizophrenia (Frantseva et al, 2008) but has hitherto not been tested in depressed patients. To determine whether neuroplasticity as measured by the PAS protocol was related to functional ability in learning a motor task, subjects were also tested with a computerized version of the rotor pursuit task. A second, exploratory aspect of the study was to test if BDNF Val66Met genotype and BDNF serum levels may relate to neuroplasticity.
MATERIALS AND METHODS
Subjects
The sample consisted of 23 depressed subjects (10 males, 13 females) who met the DSM-IV criteria for a major depressive episode (20 subjects with MDD, 3 subjects with bipolar disorder), assessed using the Structured Clinical Interview for DSM Disorders IV (SCID), and 23 healthy controls, matched for gender and age (within 2 years). All were right handed as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971) and participated after providing written informed consent. The study was approved by the Human Research Ethics Committee of the University of New South Wales in accordance with the Helsinki Declaration.
Current depression severity was assessed using the Montgomery–Äsberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979). Criteria for inclusion were a MADRS of ⩾20 for MDD subjects and <6 for healthy controls (Bandelow et al, 2006). In addition, depressed subjects were required to have no change in doses of psychotropic medication for at least 4 weeks prior to the study (6 weeks if newly initiated medication). Length of current depressive episode, treatment resistance (number of failed adequate trials of antidepressant medications), and current psychotropic medication were assessed in depressed subjects. Healthy controls had no prior history of depression and were not on psychotropic medications. Other exclusion criteria were illicit drug use, alcohol abuse, musculoskeletal or neurological disorder, and electronic implants.
Research Plan
During the same experimental day, subjects underwent blood collection for BDNF genotyping and serum measurements (n=36) (or had DNA collected by buccal swab if blood tests were refused (n=9) or declined all DNA testing (n=1)), were tested with the rotor pursuit test (to assess motor learning), and ∼45 min later completed the PAS protocol (at noon).
BDNF Genotyping and Serum Levels
Serum BDNF levels were measured by sandwich enzyme-linked immunosorbent assay (ELISA) using the PromegaEmax kit (G7610) according to the manufacturer's instructions. Subject BDNF serum levels were excluded if >2 SD from group means (n=1, healthy control; n=1, depressed).
Genomic DNA was isolated from blood or buccal swab samples using a PUREGENE DNA purification kit (QIAGEN). The BDNF SNP rs6265 (Val/Met) polymorphism was assayed by TaqMan Allele discrimination assay (cat. no. C_11592758_10, Life Technologies) analyzed with Sequence Detection Software (SDS) version 2.3 (ABI, Life Technologies).
Motor Learning Task
A computerized version of the rotor pursuit task (Life Science Associates, NY) was used. Subjects sat in front of a computer screen that featured a round red target orbiting a circular track at discrete speeds. Subjects moved a computer mouse to keep a cursor within the moving target for the trial duration (30 s per trial). Subjects completed a prebaseline block, baseline block, and five test blocks, each consisting of five trials. After each trial, feedback was given of ‘time on target' (TOT), the dependent variable of interest, measured as per cent of trial time. The testing session speed was set to have subjects perform at 25% TOT, based on performance in the prebaseline block (Schwartz et al, 1996).
Paired Associative Stimulation
During the brain stimulation protocol, subjects sat with both hands on a pillow. Electromyographic (EMG) activity was recorded through Ag/AgCl surface electrodes over the right first dorsal interosseous (FDI) muscle. EMG was amplified ( × 1000), filtered (16–1000 Hz), and digitized (2000 Hz) (Cambridge Electronics Design, UK). Motor evoked potentials (MEPs) were elicited by TMS (Magstim 200 stimulator, Magstim, UK), using a 70-mm figure-of-eight coil oriented with the handle posterolateral.
Initially, the optimal site for eliciting MEPs in right FDI was established and marked on the scalp. Resting motor threshold (RMT) was defined as the minimum stimulus intensity to evoke MEPs of ⩾50 μV in relaxed FDI in 5 of 10 consecutive trials. TMS intensity for evoking test MEPs was selected to elicit a 1-mV response. If this intensity exceeded 130% RMT, then 130% RMT was used (Table 1).
Table 1. Subject Demographic and Experimental Characteristics.
|
Group means (SDs) |
tp | ||
|---|---|---|---|
| Depressed | Healthy controls | ||
| Age (years) | 38.0 (12.8) | 38.5 (13.1) | −0.14, 0.88 |
| MADRS score | 30.7 (3.4) | 0.4 (0.7) | 41.53, <0.001* |
| Resting motor threshold (% stimulator output) | 42.2 (7.9) | 44.5 (9.9) | −0.85, 0.40 |
| Ulnar nerve stimulus threshold (mA) | 39.3 (8.5) | 39.4 (7.7) | −0.05, 0.96 |
| Testing stimulus intensity (% stimulator output) | 49.3 (10.0) | 53.3 (13.1) | 0.15, 0.26 |
| Mean number of random sensory stimuli detected (measure of attention during PAS) | 37.6 (7.1) | 37.4 (5.1) | 0.94, 0.91 |
| Motor learning task speed (r.p.m.) | 24.1 (2.2) | 24.6 (1.8) | 0.18, 0.40 |
| Baseline time on target (s) | 19.1 (7.5) | 17.0 (7.5) | −0.92, 0.36 |
| Learning (test TOT/baseline TOT)a | 1.4 (0.2) | 1.7 (0.5) | −2.48, 0.02* |
| BDNF genotype VAL/VAL | 13/23 | 14/23 | |
| MET carrier | 8/23 | 7/23 | |
| Undetermined | 2/23 | 2/23 | |
| BDNF serum level (ng/ml) | 19.4 (7.0) | 17.9 (5.9) | 0.66, 0.51 |
Abbreviations: BDNF, brain-derived neurotrophic factor; mA, milliampere; MADRS, Montgomery–Äsberg Depression Rating Scale; ng/ml, nanograms per milliliter; PAS, paired associative stimulation; r.p.m., revolutions per minute; TOT, time on target.
Learning (test TOT/baseline TOT) is the mean test block TOT divided by mean baseline TOT.*P<0.05.
The PAS25 protocol was used as previously described (Player et al, 2012). Briefly, TMS (130% RMT) to the left motor cortex was combined with electrical stimuli (200 μs duration, 300% perceptual threshold, DS7 stimulator; Digitimer, UK) to the right ulnar nerve proximal to the wrist. Two hundred pairs of stimuli (TMS and ulnar nerve) were given at 0.25 Hz over ∼13 min. In each pair, ulnar nerve stimulation preceded TMS by 25 ms. Electrical stimuli were delivered occasionally to the right index finger during PAS. Subjects counted the stimuli and reported the number at the end of the session to ensure sensory attention to the hand (Stefan et al, 2004).
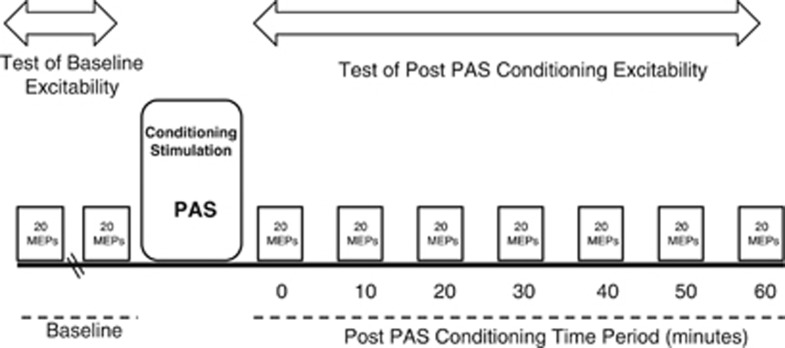
Test MEPs were recorded before and after PAS. Each testing block consisted of 20 MEPs at 0.1 Hz. Two blocks of MEPs were recorded at baseline. A block of MEPs was recorded immediately after PAS and then every 10 min for 1 h (Figure 1). During all stimulation, EMG from the FDI muscle was monitored to ensure muscle relaxation.
Figure 1.
Experimental design. Motor evoked potentials (MEPs) were elicited to measure motor cortex excitability before and after a period of conditioning stimulation, which comprised 13 min of repeated paired brain and peripheral nerve stimulation (PAS, paired associative stimulation).
Data Analysis
Independent-sample t-tests compared demographic and other experimental data between diagnostic groups: depressed (DEP) subjects and healthy controls (HC). MEP amplitudes were measured peak to peak. MEPs with preceding voluntary EMG were excluded from analysis. Amplitudes for each block of 20 MEPs were averaged. A mean baseline value was calculated from the two baseline blocks. The mean MEP values for subsequent blocks were normalized by dividing by this baseline value. For each subject, an overall (normalized) post-PAS mean MEP amplitude (averaged across all poststimulation time points) was then calculated as the primary outcome of interest.
Changes in Cortical Excitability
To examine overall change in cortical excitability after PAS, a 2 × 2 mixed ANOVA was conducted, with group (DEP or HC) as the between-subjects factor and time (baseline, post-PAS) as the repeated-measures factor. To examine the time course of change in cortical excitability after PAS, a 2 × 8 mixed ANOVA was conducted, with group (DEP or HC) as the between-subjects factor and time (baseline, 0, 10, 20, 30, 40, 50, and 60 min) as the repeated-measures factor. Planned polynomial contrasts tested for changes across time, whereas significant interactions were interpreted with tests of simple effects. As there are potential neurobiological differences between bipolar and unipolar depressed subjects, we conducted a further 2 × 2 mixed ANOVA comparing change in cortical excitability after PAS, with group (unipolar depressed or matched controls) as the between-subjects factor and time (baseline, post-PAS) as the repeated-measures factor.
Depression Severity and Episode Duration
To determine whether severity and duration of depressive symptoms had an impact upon neuroplasticity, correlations between normalized mean post-PAS MEP amplitude, MADRS score, and duration of the current depressive episode were assessed in the depressed cohort.
Motor Learning
The outcome measure for the motor learning task was the subject's TOT, which was collected for the five test blocks and one baseline block. The mean TOT values for test blocks were normalized to the baseline value by dividing the TOT for each test block by the TOT in the baseline block. The mean of all five test blocks is considered a measure of motor learning (Rajji et al, 2011). A 2 × 2 mixed ANOVA was conducted with group (DEP or HC) as the between-subjects factor and trial block (baseline, test) as the repeated-measures factor. Simple effects were conducted after any significant interactions to interpret the changes.
Effects of Disease State and BDNF Genotype on Neuroplasticity
BDNF genotype data were successfully obtained for 42 of 45 subjects tested. To examine whether the relationship between neuroplasticity (as measured by post-PAS MEPs and motor learning) and disease state was influenced by an individual's BDNF genotype, we conducted a 2 × 2 ANOVA in subjects with genotype data (n=42), with group (DEP, HC) and genotype (Val/Val, Met carrier) as between-group factors and mean post-PAS MEP amplitude as the outcome measure. In the event of a significant interaction, simple effects were conducted to interpret the differences.
Correlates of Neuroplasticity
Correlational analyses were then performed to analyze the relationship between mean post-PAS MEP amplitude and performance on the motor learning task, with serum BDNF levels for the whole cohort, and for each diagnostic Group (HC, DEP) separately. This was to determine whether neuroplasticity after PAS correlated with a measure of functional significance (motor learning) and was related to BDNF levels, a known facilitator of cellular change. Pearson's correlation was used for these analyses.
RESULTS
Between-group (DEP, HC) comparison of demographic factors and baseline experimental measures demonstrated no significant difference apart from the expected greater MADRS scores (lower mood) in the depressed cohort (Table 1). In the depressed group, duration of the current episode was 27.9±20.9 months and mean number of failed antidepressants was 2.61±2.5 (current episode) and 4.17±3.1 (lifetime). Seventeen subjects in the depressed group were on psychotropic medications: selective serotonin reuptake inhibitors (n=7), atypical antipsychotics (n=6), serotonin–noradrenaline reuptake inhibitors (n=5), lithium (n=4), anticonvulsants (n=2), tricyclic antidepressants (n=2), agomelatine (n=2), and moclobemide (n=1). During the PAS protocol, depressed patients and healthy controls gave matching attention to the hand, as shown by detection of similar numbers of the stimuli delivered to the index finger at random times (Table 1).
Changes in Cortical Excitability
The 2 × 2 ANOVA showed a main effect of group (F(1, 44)=11.244, P=0.002), no significant effect of time (F(1, 44)=3.997, P=0.052), and a significant group–time interaction (F(1, 44)=11.244, P=0.002). Tests of simple effects found a significant increase in the amplitude of MEPs after PAS for the HC group (F(1, 44)=14.32, P<0.001), whereas there was no change for the DEP group (F(1, 44)=0.92, P=0.344). The 2 × 2 ANOVA comparing only subjects with unipolar depression and matched controls also showed a significant difference in main effect of group (F(1, 38)=7.748, P=0.008), no significant effect of time (F(1, 38)=3.654, P=0.063), and a significant group–time interaction (F(1, 38)=7.748, P=0.008). MEP amplitude was significantly increased after PAS for the HC group (F(1, 38)=11.02, P=0.002) but not changed for the unipolar DEP group (F(1, 38)=0.38, P=0.541).
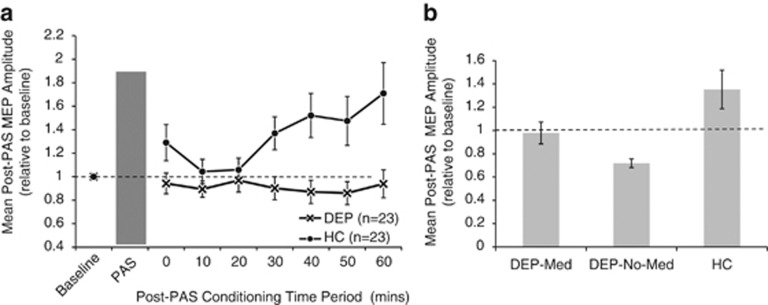
The 2 × 8 ANOVA showed a main effect of group (F(1, 44)=11.244, P=0.002), no significant effect of time (F(1, 44)=3.640, P=0.063), and a significant group–time interaction (F(1, 44)=6.089, P=0.018; Figure 2a). Simple effects analysis showed a linear increase in MEP amplitude in the healthy controls (F(1, 44)=5.44, P<0.001) but no change in the depressed cohort (F(1, 44)=0.19, P=0.987).
Figure 2.
(a) Group data (mean±SEM) showing the time course of changes in the amplitude of motor evoked potentials (MEPs) after conditioning stimulation (PAS) in depressed subjects (crosses) and healthy controls (filled circles). MEP amplitudes were normalized to baseline values for each subject. (b) Mean normalized MEP amplitudes after PAS conditioning stimulation in depressed subjects on medication (DEP-med; n=17), depressed subjects without medication (DEP-no-med; n=6), and healthy controls (HC; n=23). The seven post-PAS time points shown in panel (a) are collapsed into single group values. Means and standard errors are shown.
As 17 of 23 subjects in the depressed cohort were on psychotropic medications (16 on antidepressant medications), a further one-way ANOVA compared mean post-PAS MEP amplitude between depressed subjects on medication (DEP-med: 17), depressed subjects not on medication (DEP-no-med: 6; all unipolar depression), and healthy controls (HC: 23). This ANOVA demonstrated a main effect of group (F(2, 43)=6.446, P=0.004). Bonferroni-adjusted pair-wise analyses showed significant differences in means between healthy controls (1.35±0.52) and medicated subjects (0.98±0.39, P=0.01), and healthy controls and unmedicated subjects (0.72±0.09, P=0.035), but no difference between the two depressed groups (P=0.675; Figure 2b).
Depression Severity and Episode Duration
In the depressed cohort, there was an inverse correlation at trend level between mean poststimulation MEP amplitude and MADRS score (r=−0.364, P=0.088) but no association between mean post-PAS MEP amplitude and current episode duration (r=0.299, P=0.166).
Motor Learning
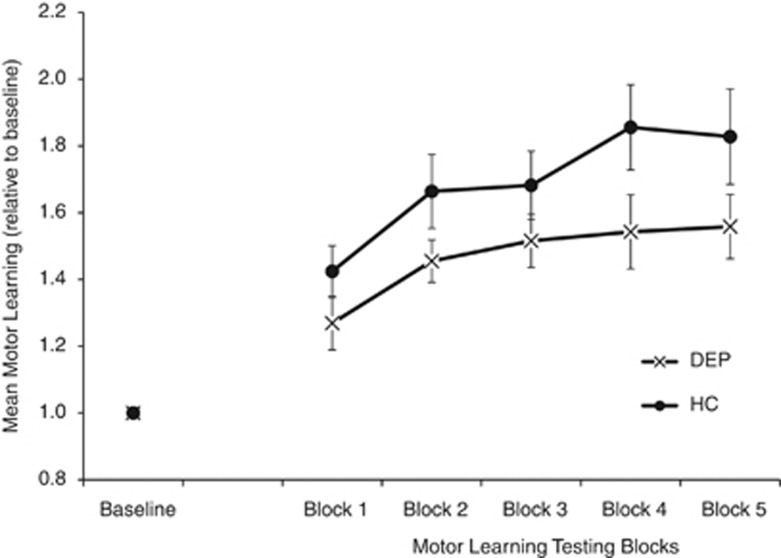
A 2 × 2 ANOVA compared motor learning between groups. Two missing values (one DEP and one HC) were replaced with the expectation maximization (Schafer and Olsen, 1998) function of the Statistical Package for the Social Sciences (SPSS) for Windows, version 20.0 (IBM Software Group, IL, USA). Motor learning was comparatively impaired in the depressed cohort shown by a significant main effect of group (F(1, 44)=5.582, P=0.023), time (F(1, 44)=85.583, P<0.001), and group–time interaction (F(1, 44)=5.582, P=0.023). Simple effects analysis showed significant increases in motor learning in both healthy controls (F(1, 44)=67.44, P<0.001) and the depressed cohort (F(1, 44)=23.73, P<0.001; Figure 3).
Figure 3.
Comparison of time on target on the rotor pursuit task in the depressed cohort (DEP, crosses) and healthy controls (HC, filled circles). Time on target in each test block was normalized to the baseline block. Mean and standard errors are shown. Increasing time on target indicates motor learning.
Effects of Disease State and BDNF Genotype on Neuroplasticity
BDNF genotype was obtained in 42 subjects (21 DEP, 21 HC). Neither the distribution of BDNF genotypes nor the BDNF serum levels differed between the groups. Furthermore, there was no difference in BDNF serum levels when subjects were divided by genotype (Val/Val vs Met carrier, 18.4±6.3 vs 18.4±7.9 ng/ml, respectively). For the 2 × 2 ANOVA (factors: group, genotype) with mean post-PAS MEP amplitude as the dependent variable, there was a main effect of group with greater post-PAS MEP amplitude in the healthy controls than in the depressed patients (F(1, 38)=8.295, P=0.006), no significant effect of genotype (F(1, 38)=3.718, P=0.061) (although there was a trend for higher mean post-PAS MEP amplitude in Met allele carriers), and no group–genotype interaction (F(1, 38)=0.178, P=0.676).
Correlates of Neuroplasticity
Mean post-PAS MEP amplitude did not correlate with motor learning (r=0.075, P=0.674, n=46) or BDNF serum levels (r=−0.007, P=0.967, n=34). However, when investigating each diagnostic group separately, there was a trend-level correlation between mean post-PAS MEP amplitude and BDNF serum levels in the depressed group (r=0.410, P=0.091, n=18).
DISCUSSION
This study clearly demonstrated a significant deficit of neuroplasticity in depressed subjects compared with age- and gender-matched healthy controls. The finding is significant as one of the first objective demonstrations of impaired neuroplasticity in depression. In this study, a physiological measure of plasticity not confounded by factors such as subjective rating and analysis, subject education levels, practice effects, or motivational factors was used, conferring clear advantages compared with cognitive or other behavioural tests previously used to assess plasticity in depression (Landrø et al, 2001; Porter et al, 2003).
In the brain stimulation protocol used, ulnar nerve stimuli were repeatedly delivered 25 ms before TMS so that each pair of stimuli arrived at the cortex at nearly the same time. Such PAS protocols result in prolonged facilitation of the excitability of the motor cortex as measured by the amplitude of MEPs elicited by TMS (Player et al, 2012; Stefan et al, 2004). Changes in cortical excitability after facilitatory PAS are thought to reflect associative LTP (Stefan et al, 2002). Thus, increases in synaptic strength are considered to underlie the increases in MEP amplitude (Thickbroom et al, 2006). Although there is limited and indirect knowledge of the processes underlying associative LTP in humans, it is believed to involve glutamate signaling and postsynaptic cell depolarization through N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methylisoxazole propionate (AMPA) receptor activation (Pittenger and Duman, 2008), which are each enhanced by BDNF (Carlson et al, 2006). Glutamate acts synergistically with BDNF and tyrosine-related kinase B (TrkB) signaling to increase NMDA activity and AMPA receptor expression (Yoshii and Constantine-Paton, 2010), both of which result in greater intracellular calcium influx. The level of postsynaptic calcium influx determines the induction of synaptic LTP or its opposite, long-term depression (LTD). Fast increases in intracellular calcium inflows lead to LTP of the synapse (Wankerl et al, 2010). In this study, MEP amplitudes were increased after PAS only in healthy controls, suggesting that the mechanisms underlying LTP induction were impaired in the motor cortex of those with depression.
This novel finding of no change in MEP amplitude after a stimulation protocol known to produce facilitatory motor cortical changes supports earlier reports of impaired neuroplasticity in depression. Dysfunctional neuroplasticity has been implicated in depression-related cognitive and behavioral changes such as impaired learning and memory and inability to respond appropriately to stress or aversive stimuli (Pittenger and Duman, 2008). However, only one prior study has examined neuroplasticity in depression using an objective neurological test (Normann et al, 2007). This study used changes in visual evoked potentials (VEPs) in response to repeated visual stimuli as a means of examining changes in synaptic plasticity. Such plasticity is proposed to share common features with LTP shown in brain slices, including reliance on NMDA receptor activation. Researchers reported significant increases in VEPs in subjects after a train of patterned visual stimuli. However, increases in VEP amplitude were significantly smaller in depressed subjects compared with healthy controls (Normann et al, 2007). Impairment of synaptic plasticity in depression has also been proposed to occur in other telencephalic regions such as the hippocampus. This is evidenced by impairment in declarative memory consolidation (Porter et al, 2003). Together, these deficits in the motor cortex, sensory cortex, and hippocampus suggest a deficiency in facilitatory synaptic plasticity that is widespread and not merely in limbic and frontal regions traditionally considered central to depression.
Various mechanisms may underlie alterations in synaptic plasticity in depression. For example, the activation of synaptically localized NMDA receptors stimulates LTP, whereas excessive glutamatergic activation of extrasynaptic NMDA receptors instead may induce LTD (Papouin et al, 2012). In this study, MEPs were reduced by over 20% after PAS in approximately half of the depressed cohort, which may indicate an increased tendency for LTD in some depressed subjects. Reduction in LTP can occur through dysregulated glutamate at the synapse as a result of a number of factors. For example, glial cells not only provide metabolic support to sustain the neuron but also affect synaptic plasticity through a crucial role in the clearance and reuptake of neurotransmitters such as glutamate (Citri and Malenka, 2008). Glial cell numbers and functioning are negatively affected by chronic stress (Rajkowska et al, 1999; Pittenger and Duman, 2008), which is commonly associated with depression. Furthermore, stress also impairs LTP and facilitates LTD through increased glucocorticoid exposure, as shown in animal models of depression. These alterations in synaptic plasticity may differentially affect specific brain regions, with associated functional implications (Pittenger and Duman, 2008).
Additionally, changes in cortical plasticity require balanced excitatory and inhibitory neuronal activity (Yazaki-Sugiyama et al, 2009). Thus, weak inhibition, as may be found in depression, can prevent cortical plasticity (Fagiolini et al, 2004). In depression, in vivo magnetic resonance spectroscopy studies have found reduced cortical γ-aminobutyric acid (GABA) concentrations (Sanacora et al, 1999; Price et al, 2009) with deficits in GABA receptor-mediated inhibition (Levinson et al, 2010). Further, there is evidence that the rate-limiting enzyme for GABA synthesis (GAD67) is reduced in the cortex of individuals with bipolar disorder (Thompson et al, 2009) and hippocampus of depressed individuals (Thompson et al, 2011). Although robust changes in cortical GAD67 mRNA are not typically found in the PFC of individuals with depression (Thompson et al, 2009; Sibille et al, 2011), significant decreases in somatostatin suggest that interneuron deficits may be especially prominent in a subset of interneurons shown to directly contribute to cortical plasticity (Lazarus and Huang, 2011). As inhibitory interneuron deficits are shared among those with depression, bipolar disorder, and schizophrenia (Thompson et al, 2009, 2011; Fung et al, 2010; Hashimoto et al, 2008; Daskalakis et al, 2002), cortical plasticity deficits would also be expected to be found in multiple forms of psychopathology.
Learning is thought to rely on neuroplastic processes. A motor learning task was included in this study as a functional test of neuroplasticity. Although both healthy and depressed subjects showed significant improvement in the rotor pursuit task, motor learning was comparatively impaired in depressed subjects. The lack of correlation between motor learning and PAS test results may be considered surprising as motor learning is considered at least partially reliant on LTP in the primary motor cortex (Ziemann et al, 2004). However, very few studies have found a significant relationship between motor learning outcomes and results of brain stimulation tests of motor cortex plasticity. Thus, the impact of altered plasticity, as measured by MEP changes, on functional motor performance is unclear. There are several possible explanations for the lack of relationship. There may be only partial overlap between the cortical processes moderated by brain stimulation and those involved in motor learning protocols (Li Voti et al, 2011). For example, performance of the rotor pursuit task used in this study also involves visual and subcortical striatal systems (Rajji et al, 2011). Functional motor learning tasks also rely on subject motivation and effort, as well as strategy and planning specific to the task. Thus, poorer learning in the depressed subjects may have reflected impairment of these higher-order functions, whereas the PAS protocol directly assessed motor and sensory cortical function independent of subject effort.
BDNF mediates changes in synaptic plasticity and can further influence structural changes that can be found within minutes of LTP-inducing stimulation of synapses in rat brain slices (Tanaka et al, 2008). Although decreased BDNF serum levels are reported in MDD, we found no differences in BDNF serum levels in depressed subjects compared with healthy controls. However, 16 of the depressed subjects were taking antidepressant medications, which have been shown to increase serum concentrations of BDNF (Sen et al, 2008). Similarly, no relationship was seen between synaptic plasticity (as assessed by PAS) and serum BDNF levels in the whole study sample. In terms of BDNF genotype, we did not confirm a prior report that Val/Val homozygotes demonstrated greater MEP amplitude after PAS (Cheeran et al, 2008).
There are three important limitations to this study. First, the majority of depressed subjects were on antidepressant medications. This is important because there is some experimental evidence that antidepressant medications may affect neuroplasticity, although these studies found that they tended to enhance rather than reduce plasticity (Normann et al, 2007; Rocher et al, 2004). Further analysis of medicated and non-medicated depressed subjects found no difference in MEP changes after PAS between these groups, although both differed significantly from healthy controls. However, as the number of subjects not on psychotropic medications was small, this study could not resolve the degree to which the presence of medications may have influenced results. Inspection of the results suggests that the presence of antidepressant medications increased the variability in neuroplasticity measured in depressed subjects (Figure 2b), which may have confounded exploration of secondary outcomes, that is, the relationship between neuroplasticity, motor learning, and BDNF levels.
A second limitation is that neuroplasticity was measured only once in the depressed cohort, and this was while they were symptomatic. A second measurement during remission would enable us to evaluate if impaired neuroplasticity is a state or trait phenomenon and may assist in elucidating the underlying mechanisms. The suggestion that impaired neuroplasticity is a state phenomenon is supported by improvements in indirect measures of neuroplasticity, such as learning and memory, while patients are in remission (Gallgher et al, (2007) and also after antidepressant treatment in humans and in animal models of depression (Pittenger and Duman, 2008).
A third limitation is that neuroplasticity was assessed in the motor cortex, which is not considered the primary site of cerebral dysfunction in depression. This study used the PAS protocol, as it provides an accessible physiological measure of plasticity that is not confounded by factors such as subject motivation or prior learning and experience. Moreover, it supports other studies that have found abnormalities in motor cortical function in depressed subjects (Shajahan et al, 1999; Loo et al, 2008; Levinson et al, 2010). It is possible that motor cortical abnormalities may reflect global pathophysiological disturbances in depression.
In conclusion, this is one of the first studies to demonstrate reduced neuroplasticity in depression, using a test of experimentally induced plasticity. Findings also suggested that motor learning is impaired.
FUNDING AND DISCLOSURE
This study was supported by an Australian Post-Graduate Award (APA), awarded to Michael J Player, and by a University of New South Wales Major Research Equipment and Infrastructure Grant, awarded to Colleen Loo's research program. Janet Taylor was supported by a fellowship from the National Health and Medical Research Council of Australia. Cynthia Shannon Weickert's work was supported by the Schizophrenia Research Institute (utilizing infrastructure funding from the NSW Ministry of Health and the Macquarie Group Foundation), the University of New South Wales, and Neuroscience Research Australia. In the last 3 years, Colleen Loo has received honoraria from Astra-Zeneca and Pfizer as an invited speaker at psychiatry conferences. The talks were unrelated to the topic of this paper, and the authors declare no potential conflict of interest.
Footnotes
AUTHOR CONTRIBUTIONS
Colleen Loo, Janet Taylor, and Michael Player conceived and designed the overall study. Cyndi Weickert designed and conducted BDNF study components. All authors were involved in data collection, analysis, and interpretation of the data. Michael Player, Colleen Loo, Janet Taylor, Cyndi Weickert, and Angelo Alonzo drafted the article. All authors revised the article critically for intellectual content and have approved the final version.
References
- Bandelow B, Baldwin DS, Dolberg OT, Andersen HF, Stein DJ. What is the threshold for symptomatic response and remission for major depressive disorder, panic disorder, social anxiety disorder, and generalized anxiety disorder. J Clin Psychiatry. 2006;67:1428–1434. doi: 10.4088/jcp.v67n0914. [DOI] [PubMed] [Google Scholar]
- Carlson PJ, Singh JB, Zarate CA, Jr, Drevets WC, Manji HK. Neural circuitry and neuroplasticity in mood disorders: Insights for novel therapeutic targets. NeuroRx. 2006;3:22–41. doi: 10.1016/j.nurx.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002;59:347–354. doi: 10.1001/archpsyc.59.4.347. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy J-M, Löw K, Möhler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, Fitzgerald PB, Chen R, Moller B, Daigle M, Daskalakis ZJ. Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill learning. Cereb Cortex. 2008;18:990–996. doi: 10.1093/cercor/bhm151. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Gallgher P, Robinson LJ, Gray JM, Porter RJ, Young AH. Neurocognitive function following remission in major depressive disorder: Potential objective marker of response. Aus N Z J Psychiatry. 2007;41:54–61. doi: 10.1080/00048670601057734. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner A. Structural MRI changes of the brain in depression. Clin EEG Neurosci. 2004;35:46–52. doi: 10.1177/155005940403500111. [DOI] [PubMed] [Google Scholar]
- Kumar A, Jin Z, Bilker W, Udupa J, Gottlieb G. Late-onset minor and major depression: Early evidence for common neuroanatomical substrates detected by using MRI. Proc Natl Acad Sci U S A. 1998;95:7654–7658. doi: 10.1073/pnas.95.13.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrø NI, Stiles TC, Sletvold H. Neuropsychological function in nonpsychotic unipolar major depression. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:233–240. [PubMed] [Google Scholar]
- Lazarus MS, Huang ZJ. Distinct maturation profiles of perisomatic and dendritic targeting GABAergic interneurons in the mouse primary visual cortex during the critical period of ocular dominance plasticity. J Neurophysiol. 2011;106:775–787. doi: 10.1152/jn.00729.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. Does spike timing-dependent synaptic plasticity underlie memory formation. Clin Exp Pharmacol Physiol. 2007;34:1070–1076. doi: 10.1111/j.1440-1681.2007.04724.x. [DOI] [PubMed] [Google Scholar]
- Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ. Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry. 2010;67:458–464. doi: 10.1016/j.biopsych.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Li Voti P, Conte A, Suppa A, Iezzi E, Bolonga M, Aniello MS, et al. Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Exp Brain Res. 2011;212:91–99. doi: 10.1007/s00221-011-2700-5. [DOI] [PubMed] [Google Scholar]
- Loo CK, Sachdev P, Mitchell PB, Gandevia SC, Mahli GS, Todd G, et al. A study using transcranial magnetic stimulation to investigate motor mechanisms in psychomotor retardation in depression. Int J Neuropsychopharmacol. 2008;11:935–946. doi: 10.1017/S1461145708008821. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Normann C, Schmitz D, Furmaier A, Doing C, Bach M. Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry. 2007;62:373–380. doi: 10.1016/j.biopsych.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Player MJ, Taylor JL, Alonzo A, Loo CK. Paired associative stimulation increases motor cortex excitability more effectively than theta-burst stimulation. Clin Neurophysiol. 2012;123:2220–2226. doi: 10.1016/j.clinph.2012.03.081. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Gallgher P, Thompson JM, Young AH. Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry. 2003;182:214–220. doi: 10.1192/bjp.182.3.214. [DOI] [PubMed] [Google Scholar]
- Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA,, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: Relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajji TK, Liu SK, Frantseva MV, Mulsant BH, Thoma J, Chen R, et al. Exploring the effect of inducing long-term potentiation in the human motor cortex on motor learning. Brain Stimul. 2011;4:137–144. doi: 10.1016/j.brs.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: Effects of antidepressants. Cereb Cortex. 2004;14:224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Savits J, Drevets WC. Bipolar and major depressive disorder: Neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J, Olsen M. Multiple imputation for multivariate missing-data problems: A data analyst's perspective. Multivar Behav Res. 1998;33:545–571. doi: 10.1207/s15327906mbr3304_5. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Rosse RB, Veazey C, Deutsch SI. Impaired motor skill learning in schizophrenia: Implications for corticostriatal dysfunction. Biol Psychiatry. 1996;39:241–248. doi: 10.1016/0006-3223(95)00130-1. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajahan PM, Glabus MF, Gooding PA, Shah PJ, Ebmeier KP. Reduced cortical excitability in depression. Impaired post-exercise motor facilitation with transcranial magnetic stimulation. Br J Psychiatry. 1999;174:449–454. doi: 10.1192/bjp.174.5.449. [DOI] [PubMed] [Google Scholar]
- Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Edwards DJ, Mastaglia FL. Repetitive paired-pulse TMS at I-wave periodicity markedly increases corticospinal excitability: A new technique for modulating synaptic plasticity. Clin Neurophysiol. 2006;117:61–66. doi: 10.1016/j.clinph.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatric Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36:195–203. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wankerl K, Weise D, Gentner R, Rumpf JJ, Classen J. L-type voltage-gated Ca2+ channels: A single molecular switch for long-term potentiation/long-term depression-like plasticity and activity-dependent metaplasticity in humans. J Neurosci. 2010;30:6197–6204. doi: 10.1523/JNEUROSCI.4673-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Kang S, Câteau H, Fukai T, Hensch TK. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature. 2009;462:218–221. doi: 10.1038/nature08485. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Ilić TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, et al. Consensus: Motor cortex plasticity protocols. Brain Stim. 2008;1:164–182. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]