Abstract
A single dose of the short-acting NMDA antagonist ketamine produces rapid and prolonged antidepressant effects in treatment-resistant patients with major depressive disorder (MDD), which are thought to occur via restoration of synaptic connectivity. However, acute dissociative side effects and eventual fading of antidepressant effects limit widespread clinical use of ketamine. Recent studies in medial prefrontal cortex (mPFC) show that the synaptogenic and antidepressant-like effects of a single standard dose of ketamine in rodents are dependent upon activation of the mammalian target of rapamycin (mTOR) complex 1 (mTORC1) signaling pathway together with inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3), which relieves its inhibitory in influence on mTOR. Here, we found that the synaptogenic and antidepressant-like effects of a single otherwise subthreshold dose of ketamine were potentiated when given together with a single dose of lithium chloride (a nonselective GSK-3 inhibitor) or a preferential GSK-3β inhibitor; these effects included rapid activation of the mTORC1 signaling pathway, increased inhibitory phosphorylation of GSK-3β, increased synaptic spine density/diameter, increased excitatory postsynaptic currents in mPFC layer V pyramidal neurons, and antidepressant responses that persist for up to 1 week in the forced-swim test model of depression. The results demonstrate that low, subthreshold doses of ketamine combined with lithium or a selective GSK-3 inhibitor are equivalent to higher doses of ketamine, indicating the pivotal role of the GSK-3 pathway in modulating the synaptogenic and antidepressant responses to ketamine. The possible mitigation by GSK-3 inhibitors of the eventual fading of ketamine's antidepressant effects remains to be explored.
Keywords: EPSCs, mTOR, NMDA, prefrontal, spines, synaptic
INTRODUCTION
Currently available antidepressants, which block the reuptake or breakdown of monoamine neurotransmitters, have several serious limitations, including a delay of weeks or months for a therapeutic response with <50% of all patients showing full remission (Berton and Nestler, 2006; Mathew, 2008; Fekadu et al, 2009; Little, 2009). These drawbacks underline a need for new antidepressants that can produce a faster and more efficacious therapeutic response. In line with this need, recent clinical and preclinical studies demonstrate that a single, subanesthetic dose of ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist produces a rapid (within hours) and sustained antidepressant response effective in ∼70% of treatment-resistant patients with major depressive disorder (MDD) and bipolar depression (BPD) (Berman et al, 2000; Zarate et al, 2006; Diazgranados et al, 2010). The precise mechanisms underlying the rapid antidepressant actions mediated by NMDA receptor blockade are currently a research area of intense interest.
We have reported that the antidepressant-like effects of ketamine and other NMDA antagonists in animal models depend upon activation of the mammalian target of rapamycin (mTOR) complex 1 (mTORC1) and a subsequent increase in synaptogenesis in medial prefrontal cortex (mPFC). These changes are followed by increases in synaptic spine density/diameter, increases in both presynaptic (synapsin 1) and postsynaptic (PSD95 and GluR1) markers, and increased excitatory postsynaptic current (EPSC) frequency and amplitude (Li et al, 2010, 2011). These findings mesh with recent clinical data showing that in MDD there is impaired mTOR signaling (Jernigan et al, 2011) and decreased expression of synapse-related genes with a loss of synaptic spines (Kang et al, 2012). However, acute dissociative side effects and eventual fading of antidepressant responses limit widespread clinical use of ketamine. Several strategies have been proposed to address these limitations, ranging from the development of more selective NMDA receptor antagonists with reduced dissociative side effects to the addition of other antidepressant agents to limit the need for repeated ketamine dosing.
It has been reported that a combination of low, ineffective doses of ketamine (1 mg/kg) plus lithium (10 mg/kg) produces an antidepressant response in the mouse forced swim test (FST); this effect was hypothesized to be due to NMDA antagonist properties of lithium (Ghasemi et al, 2010). However, recent findings suggest that lithium may be acting through its ability to inhibit GSK-3 (Beurel et al, 2011). This inhibition can occur in two ways: directly by antagonizing Mg++ and indirectly through activation of the serine/threonine kinase Akt upstream from mTOR (Freland and Beaulieu, 2012) and lithium destabilization of a β-arrestin/Akt/PP2A/GSK-3 complex (Jope, 2011). Preclinical evidence supports the hypothesis that inhibition of GSK-3 represents a therapeutically relevant target for lithium's mood-stabilizing properties (see review by Gould and Manji (2005)). GSK-3 is notable in having high activity under resting conditions (Kaidanovich-Beilin and Woodgett, 2011) and is a major inhibitory regulator of mTOR signaling (Zoncu et al, 2011). Recent studies demonstrate that inhibitory phosphorylation of GSK-3α and GSK-3β, is required for the rapid antidepressant-like effects of ketamine (Beurel et al, 2011). Thus, inhibitors of GSK-3, by opposing tonic or phasic inhibitory influences upon mTOR, could facilitate the antidepressant effects of ketamine. In the present study, we investigated the possibility that sub-effective doses of ketamine when combined with lithium, a nonselective GSK-3 inhibitor, could mimic the synaptogenic and antidepressant effects observed with the tenfold higher effective dose of ketamine (Li et al, 2010). However, given the diverse actions of lithium (Quiroz et al, 2010), we also investigated whether a preferential GSK-3β inhibitor could mimic potentiating effects of lithium on subthreshold doses of ketamine.
MATERIALS AND METHODS
Animals and Drug Administration
Adult male Sprague–Dawley rats (Charles River Laboratories, North Franklin, CT) weighing 150–250 g were pair-housed on a 12-h light/dark cycle (lights on at 0700 hours) with food and water available ad libitum. All procedures were done in accordance with the NIH guidelines for the care and use of laboratory animals and the Yale University Institutional Animal Care and Use Committee. Drugs given in vivo were as follows: (±)-ketamine hydrochloride, Hospira (Lake Forest, IL); SB 216763, Tocris (Ellisville, MO); lithium chloride; Sigma-Aldrich (Milwaukee, WI). Drug stock solutions were dissolved in 0.9% saline immediately before use and injected intraperitoneally (i.p.).
Synaptosome Preparation and Western Blotting
Crude synaptosomes were prepared as previously described (Li et al, 2010). Briefly, the PFC of rats was dissected on ice and homogenized in 0.32 M sucrose, 20 mM Hepes (pH 7.4), 1 mM EDTA, 1 × protease inhibitor cocktail, 5 mM NaF and 1 mM NaVO3. Homogenates were centrifuged at 700 g for 10 min and supernatants were then centrifuged at 13 400 g for 10 min to obtain a pellet enriched in crude synaptosomes. Pellets were sonicated in protein lysis buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 1 mM NaVO3, 5 mM NaF and 1 × protease inhibitor cocktail and protein concentrations were measured using a BCA kit (Thermo Scientific, Waltham, MA). Proteins were separated by SDS–PAGE and then transferred to nitrocellulose or polyvinylidene difluoride membranes and blocked for 1 h in 2% BSA in PBS+0.1% Tween 20 (PBS-T). Primary antibodies for total protein kinase B (PKB or Akt), phospho-Akt (Ser473), total extracellular signal-regulated kinase (ERK), phospho-ERK (Thr202/Tyr204), total GSK-3β, phospho-GSK-3β (Ser9), total mTOR, phospho-mTOR (Ser2448), total S6K, phospho-S6K (Thr389) (all from Cell Signaling, Danvers, MA) were used. After overnight incubation with primary antibodies, membranes were washed in PBS-T (3 × , 10 min) and incubated with peroxidase-labeled secondary antibodies for 1 h. Membranes were then washed again and protein bands were detected using enhanced chemiluminescence. Membranes were then incubated in stripping buffer (Thermo Scientific) blocked in 5% milk in PBS-T and incubated with primary antibodies directed against their respective non-phosphorylated protein. Bands were quantified using densiotometric analysis with Image J software (NIH, Bethesda, MD). Data were analyzed by normalizing phospho-protein levels to total protein levels and statistical analysis conducted using a Student's t-test or a one-way ANOVA with least significant difference (LSD) post-hoc tests as appropriate. Data are expressed as fold change vs control levels.
Brain Slice Preparation and Electrophysiological Recordings
Brain slices were prepared as previously described (Liu and Aghajanian, 2008; Li et al, 2011). Briefly, one day after drug treatments, rats were anesthetized (chloral hydrate, 400 mg/kg, i.p.) and brains were removed. Coronal slices 400 μm thick were cut in ice-cold sucrose-ACSF from a block of tissue containing the mPFC with an oscillating-blade tissue slicer (Leica VT 1000S; GMI). Slices were placed in a submerged recording chamber; bath temperature was then raised slowly to 32 °C. The standard ACSF (pH 7.35), equilibrated with 95% O2/5% CO2, contained 128 mM NaCl, 3 mM KCl, 2 mM CaCl2, 2 mM MgSO4, 24 mM NaHCO3, 1.25 mM NaH2PO4, and 10 mM, D-glucose. There was recovery period of 1–2 h before recording. For the slice experiments, 5-hydroxytryptamine, creatinine sulfate (5-HT) was from Sigma (St Louis, MO) and hypocretin 2 (orexin B) was from American Peptide (Sunnyvale, CA). Both transmitters were perfused at known concentrations through a stopcock assembly.
Pyramidal neurons in layer V were patched under visual control using infrared differential interference contrast (IR/DIC) microscopy ( × 60 IR lens; Olympus, Center Valley, PA). Patch pipettes (3–5 MΩ) were pulled from glass tubing with a Flaming-Brown Horizontal Puller (Sutter, Novato, CA). The pipette solution contained: 115 mM K gluconate, 5 mM KCl, 2 mM MgCl2, 2 mM Mg-ATP, 2 mM Na2ATP, 10 mM Na2-phosphocreatine, 0.4 mM Na2GTP, and 10 mM HEPES, pH 7.33. Neurobiotin (0.3%) was added to the pipette solution to mark cells for later processing and imaging. Whole-cell recordings were made with an Axoclamp-2B amplifier (Molecular Devices, Sunnyvale, CA). The output signal was low-pass-filtered at 3 KHz and digitized at 15 kHz; data were acquired by pClamp 9.2/Digidata 1320 software (Molecular Devices). Series resistance, monitored throughout the experiment, was usually between 4 and 8 MΩ. To minimize series resistance errors, cells were discarded if series resistance rose above 10 MΩ. Postsynaptic currents were in the continuous single-electrode voltage-clamp mode (3000 Hz low-pass filter) clamped near resting potential (∼75±5 mV). Known concentrations of drugs in ACSF were applied through a stopcock arrangement (∼4 ml/min), reaching slice within 7–10 s. Electrophysiological data was displayed off-line with Clampfit software of pClamp 9.2 (Axon Instruments). Analysis of EPSCs from each 10-s block of 1-s sweeps was with MiniAnalysis software (Synaptosoft, Fort Lee, NJ). EPSP amplitude comparisons between control and test groups were analyzed by the Kolmogorov–Smirnov non-parametric test (KS test) for differences between cumulative distributions of two samples.
Spine Density and Spine Head Diameter Analysis
After completion of recording, slices were transferred to 4% paraformaldehyde (0.1 M phosphate buffer) and stored overnight at 4 °C. Slices were then processed with Streptavidin conjugated to Alexa 594 (1 : 1000) for visualization of labeled cells. Labeled neurons within layer V of anterior cingulate and prelimbic mPFC were imaged with a two-photon Ti:sapphire laser scanning system (810 nm; Mai Tai, Spectra Physics, Mountain View, CA) coupled to direct detection Radiance 2000 BioRad laser scanner (Zeiss Micromaging, Thornwood, NY) mounted on a Olympus BX50WI microscope, using a × 60 (0.9 numerical aperture) water-immersion objective. Spine analysis was sampled from the apical tuft. The length of tuft branch segments was determined within the 3D matrix of each Z-stack by using Neurolucida 10.2 (MicroBrightField, Williston, VT). Automated spine density and spine head diameter analysis was by the Autospine module of Neurolucida Explorer (version 10.2) on raw image stacks (2–5 optical sections (1 μm). Results were expressed as spine density per10 μm.
Forced Swim Test
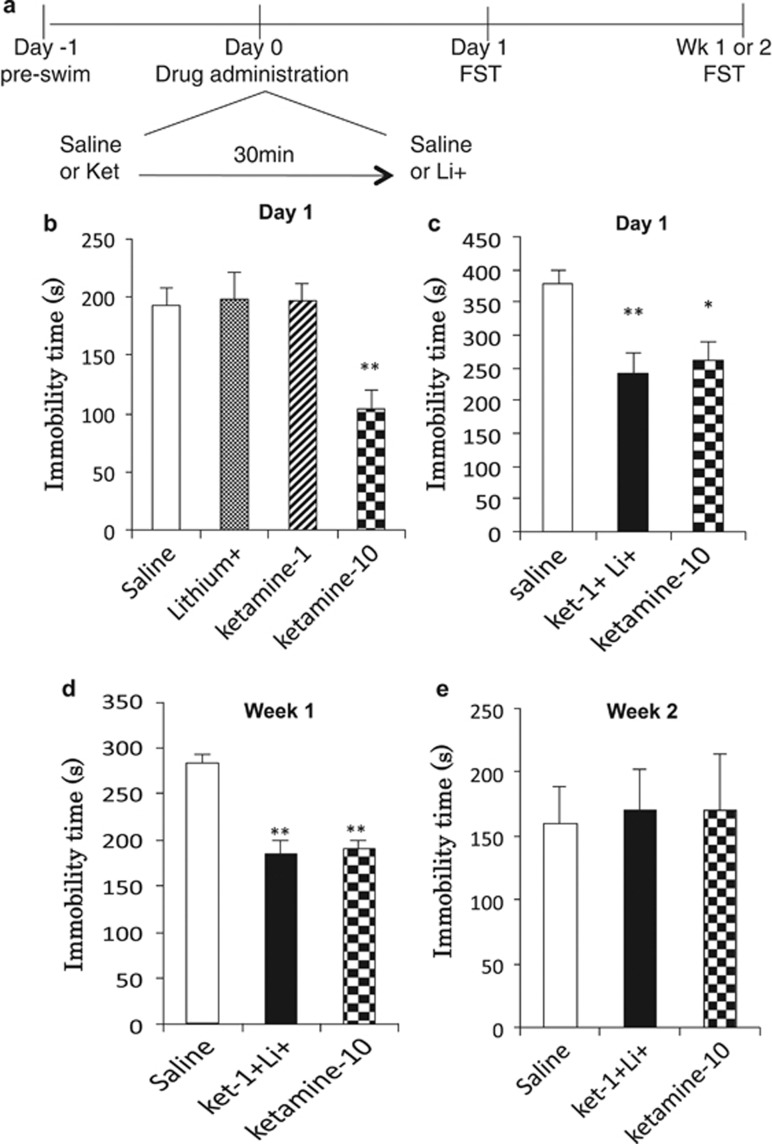
Rats were tested in a FST as previously described (Li). Briefly, rats were subjected to a 15-min pre-swim in 25 °C water in a clear Plexiglas cylinder (65 cm height, 30 cm diameter). After the pre-swim, rats were removed and dried. After 24 h, rats received i.p. injections either of vehicle, ketamine 1 mg/kg (Ket-1), lithium 10 mg/kg (Li-10), Ket-1 plus Li-10 (Ket-1/Li-10), or Ket-10 alone. Following treatment (24 h or 1 week and 2 weeks later), rats were again placed in the swimming cylinders for a 10-min test swim. All sessions were video recorded and data were analyzed by scoring total immobility time (making only the movements necessary to keep afloat) during 10-min testing period by an experimenter blind to the treatment groups. Immobility values were analyzed using a one-way analysis of variance (ANOVA) with LSD post-hoc tests as appropriate. Significance was determined at p<0.05 and data were plotted as total seconds immobile.
RESULTS
Ketamine Plus Lithium Low-Dose Combination Increases mTOR and Decreases GSK-3β Signaling in the Rat mPFC
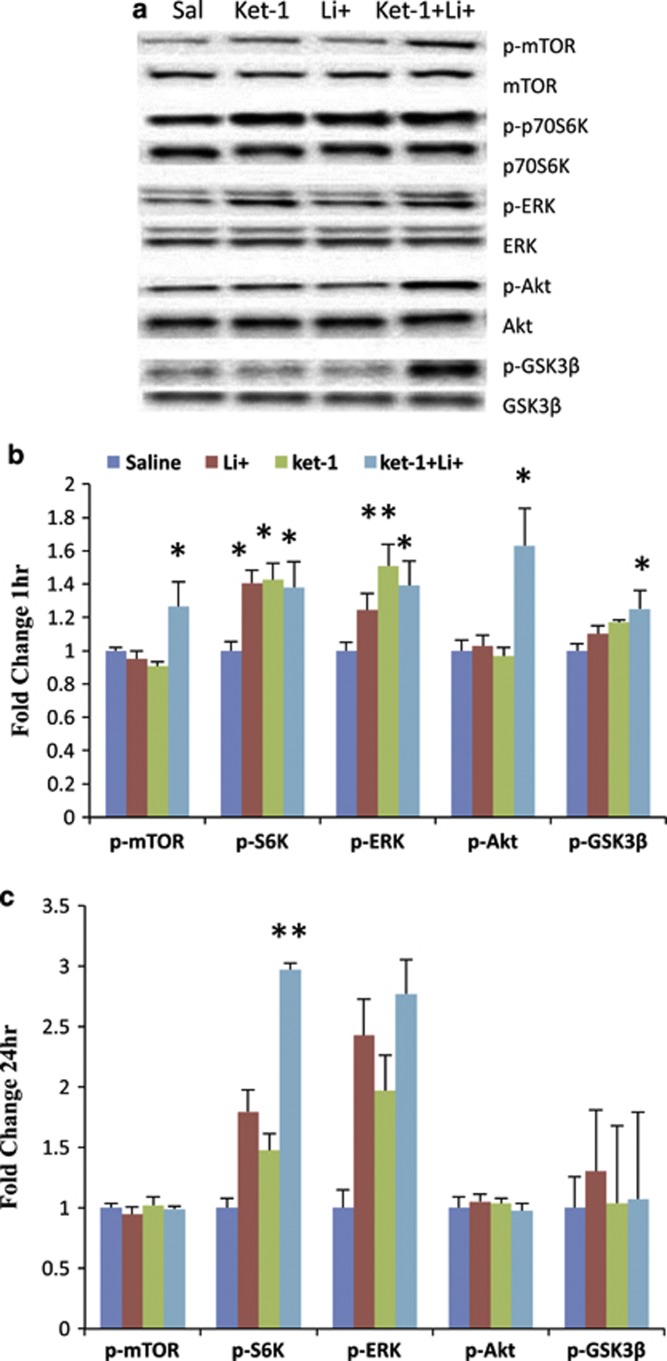
Behavioral studies have shown that when combined, non-effective doses of ketamine (1 mg/kg) and lithium chloride (10 mg/kg) have a significant short-term antidepressant-like effect in the mouse FST (Ghasemi et al, 2010). However, the underlying mechanisms of the interaction of lithium with ketamine have not been identified. Recent evidence indicates that rapid (30–60 min) activation of the mTORC1 signaling pathway (Li et al, 2010) and inhibition of the GSK-3β (Beurel et al, 2011) are both required for the antidepressant effects of ketamine. To determine whether the combination of sub-effective doses of ketamine (1 mg/kg; Ket-1) plus lithium (10 mg/kg; Li-10) are capable of regulating mTOR signaling and GSK-3β in a manner similar to an effective single dose of ketamine (10 mg/kg; Ket-10), we examined the levels of the phosphorylated forms of mTOR, S6K, GSK-3β, ERK, and Akt. In confirmation of these previous reports (Li et al, 2010; Beurel et al, 2011), western blotting analysis 1 h after Ket-10 treatment revealed significant increases in the phosphorylation of mTOR (1.38±10 fold change compared with control: t(10)=3.601, p<0.01), S6K (1.88±20 fold change: t(10)=8.098, p<0.01), ERK (2.08±13 fold change: t(10)=7.610, p<0.01), Akt (2.30±15 fold change: t(10)=6.706, p<0.01), and GSK-3β (1.37±18 fold change: t(10)=2.498, p<0.032). All changes 1 h after Ket-1/Li-10 were in the same direction as after Ket-10 (Figure 1a and b): significant increases in the phosphorylation of mTOR (1.27±15 fold change compared with control: F3,29=5.053, p<0.05), S6K (1.38±15 fold change: F3,29=6.266, p<0.05), ERK (1.39±15 fold change: F3,29=6.132, p<0.05), Akt (1.63±23 fold change: F3,29=7.403, p<0.05), and GSK-3 (1.25±11 fold change: F3,29=3.749, p<0.05) (Figure 1a and b). In contrast, at 1 h, neither Ket-1 nor Li-10 alone increased phospho-mTOR, phospho-GSK-3β; however there were increases in phospho-ERK (1.51±13 fold change compared with control: p<0.01) and phospho-S6K (1.43±10 fold change: p<0.05), and administration of Li-10 alone produced a significant increase in phospho-S6K (1.41±8 fold change: p<0.05). At 24 h after injection with the Ket-1/Li-10, there was a sustained effect in phospho-S6K (2.97±28 fold change: F3,19=10.270, p=0.0005) and a strong trend toward an increase in phospho-ERK (2.77±72 fold change: F3,19=1.885, p=0.173), no increase in phospho-mTOR (0.99±6 fold change: F3,19=0.224, p=0.878 ), phospho-Akt (0.98±2 fold change: F3,19=0.464, p=0.712) or phospho-GSK-3β (1.07±5 fold change: F3,19=1.251, p=0.325) levels (Figure 1c) was found. These data indicate that Ket-1/Li-10 treatment transiently activates the mTOR signaling pathway, inhibits GSK-3β activity, and that effects on downstream phospho-S6K is sustained for at least 24 h in the rat PFC.
Figure 1.
A low-dose combination of ketamine 1 mg/kg (Ket-1) and lithium 10 mg/kg (Ket-1/Li-10) stimulates mammalian target of rapamycin (mTOR) signaling and inhibits the glycogen synthase kinase-3β (GSK-3β) in rat prefrontal cortex. Rats were administered saline, Ket-1, Li-10, or Ket-1/Li-10 and 1 h (a, b) or 24 h (c) later tissue was harvested for synaptosomal preparations. Levels of phosphorylated forms of mTOR, extracellular signal-regulated kinase (ERK), Akt, S6K, and GSK-3β were determined by western blot and were normalized to the levels of total protein. The results are expressed as fold change compared with saline control and are shown as the mean±SEM (n=5–8; *p<0.05, **p<0.01 compared with saline group; analysis of variance (ANOVA)).
The Ketamine+Lithium Low-Dose Combination Increases EPSC Frequency and Spine Density in Layer V Pyramidal Neurons of mPFC
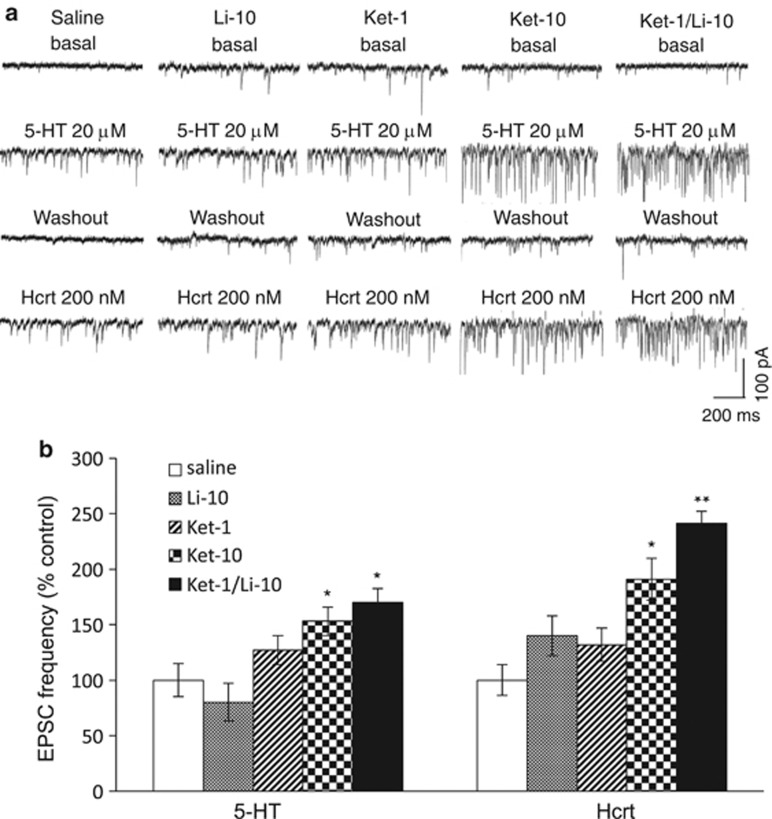
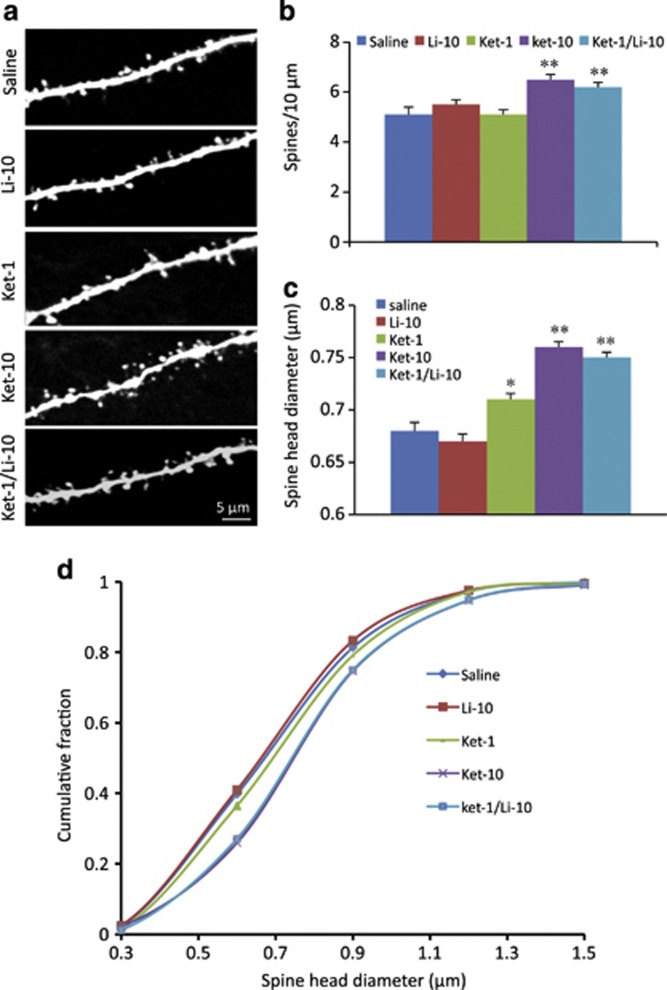
Previously, we reported that the Ket-10 dose alone increases the number and function of spine synapses in layer V pyramidal neurons of the mPFC, which is associated with its antidepressant-like actions (Li et al, 2010). Here examined if the low-dose combination Ket-1/Li-10 produces effects comparable to the Ket-10 dose on mPFC spine synapses in parallel with behavioral effects. Whole-cell patch-clamp recordings of layer V pyramidal neurons were conducted as previously described (Liu and Aghajanian 2008; Li et al, 2010). Ket-1/Li-10 was found to significantly increase the frequency of excitatory postsynaptic currents (EPSCs) induced by bath application of 5-HT or hypocretin by ∼70 and 141% above vehicle control levels (Figure 2a and b. p<0.05 and p<0.01, respectively, one-way analysis of variance+post-hoc analysis). These effects were similar to Ket-10; neither Ket-1 nor Li-10 alone had significant effects (Figure 2a and b). All treatments produced a small but statistically significant increase in the amplitude of hypocretin-induced-EPSPs compared with saline, (p<0.001, KS test); also, with the exception of Li-10 alone, all treatments increased the amplitude of 5-HT-induced EPSCS (p<0.001; KS test). However, only Ket-1/Li-10 or Ket-10 produced a robust increase in spine density in apical tuft segments of layer V pyramidal neurons in the mPFC (Figure 3a and b, p<0.01). Likewise, only Ket-1/Li-10 and Ket-10 produced a large increased spine head diameter (Figure 3a and c, p<0.01); Li-10 had no effect on spine head diameter and Ket-1 only produced a slight albeit significant increase (Figure 3a and c). The increase in spine head diameter in response to Ket-1/Li-10 or Ket-10 can be seen to produce a uniform rightward shift in cumulative fraction curves (Figure 3d; note that the curves for Ket-10 and Ket-1/Li-10 curves are virtually superimposed).
Figure 2.
Ket-1/Li-10 combination treatment increases excitatory postsynaptic current (EPSC) responses in mPFC layer V pyramidal neurons. Rats were administered Saline, Ket-1, Li-10, Ket-10, or Ket-1/Li-10 and 24 h later whole-cell recordings were conducted in mPFC slices; note that lithium was given 30 min after ketamine because preliminary data had shown that sequence produced more robust changes than the reverse order. (a) Samples of whole-cell voltage-clamp traces of 5-HT (20 μM)- and hcrt (200 nM)-induced EPSCs in the pyramidal neurons from rats treated under the described treatment conditions. (b) Frequency of 5-HT- and hypocretin-induced EPSCs. Results are the mean±SEM percent of vehicle control (F=4.61 for 5-HT and F=4.64 for hypocretin, p<0.05; post-hoc test *p<0.05 vs control, **p<0.01 vs control; n=14–18 cells from 4–7 rats).
Figure 3.
Ket-1/Li+-10 combination treatment increases spine density and spine head diameter in mPFC layer V pyramidal cells. mPFC layer V pyramidal neurons from each treatment condition (saline, Ket-1, Li-10, Ket-10, or Ket-1/Li-10) were filled passively during the recording via patch pipettes containing neurobiotin and were later stained with streptavidin conjugated to Alexa Fluor 594 and subjected to post-hoc two-photon microscopy and analysis. (a) Representative two-photon images are shown of high magnification Z-stack projections of the apical tuft branch segments (scale bar shown in bottom panel). (b) The density of spines and (c) spine head diameter were analyzed using Neurolucida/Autospine (version 10.2). The results in b and c are the mean±SEM (F=8.15 for spine density and F=16.83 for spine head diameter, p<0.05; post-hoc test *p<0.05 vs control, **p<0.01 vs control; n=10–18 cells from 4–7 rats). (d) The spine head diameter is also shown in the cumulative fraction curves. Note that curves for Ket-10 and Ket-1/Li-10 are completely superimposed and cannot be discriminated.
Spine and EPSC Responses Induced by the Low-Dose Ketamine+Lithium Combination Were Mostly Sustained For Up to 1 Week
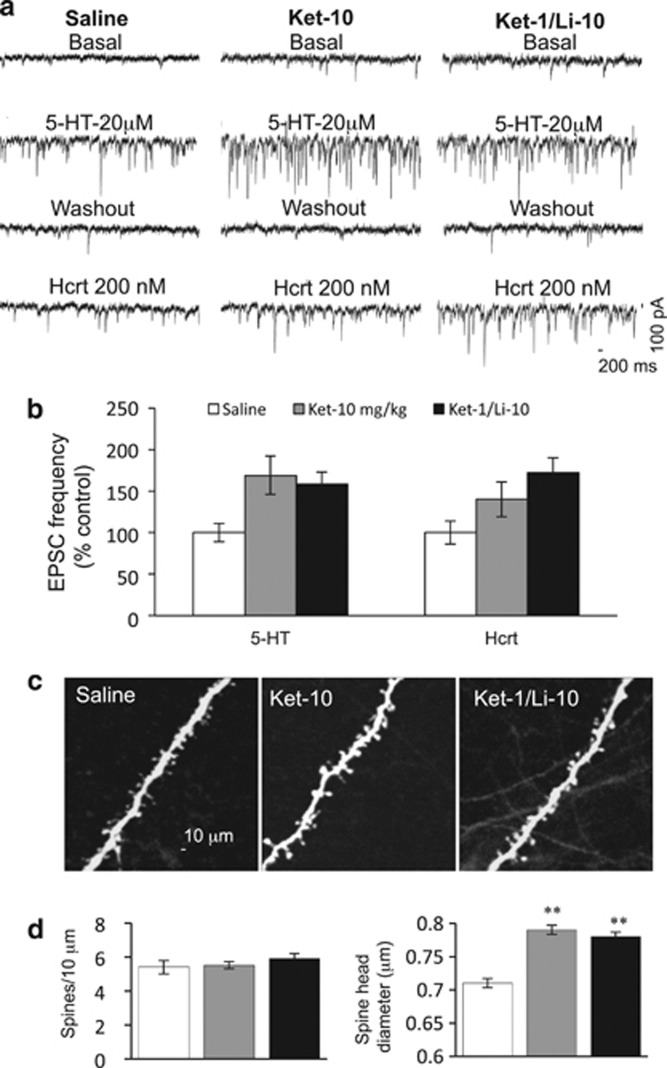
A single, subanesthetic dose of ketamine produces rapid antidepressant response in treatment-resistant patients that lasts for up to 7 days (Berman et al, 2000; Zarate et al, 2006). We therefore investigated whether the increases in EPSC and spine density/diameter after a single effective dose of ketamine (Ket-10) or the ketamine–lithium combination (Ket-1/Li-10) would be retained for a similar period in layer V pyramidal cells. We found that increased frequency of EPSCs in mPFC pyramidal cells induced by bath application of 5-HT or hypocretin were largely sustained in both the Ket-10- and Ket-1/Li-10-treated groups (Figure 4a and b). Note that incremental increases induced by bath application of 5-HT were still present in both groups but the increase in hcrt EPSC was statistically significant only for the Ket-1/Li-10 group (Figure 4a and b). Also, cumulative probability analysis showed that Ket-10 or Ket-1/Li-10 administration continued to produce statistically significant increases in 5-HT- and hcrt-induced EPSP amplitude. (KS test, p<0.001). Spine head diameter of the recorded Ket-1/Li-10 and Ket-10 cells remained significantly increased in both groups (Figure 4c and d), but the spine density returned to normal levels after 1 week (Figure 4c and d).
Figure 4.
The increase of excitatory postsynaptic current (EPSC) response and spine head diameter sustained for up to 1 week after the Ket-10 or Ket-1/Li-10 combination treatment. Rats were administered saline, Ket-10, or Ket-1/Li-10 and 1 week later whole-cell recordings were conducted in slices and post-hoc two-photon microscopy sampled and analyzed. (a) Representative traces showing EPSCs recorded from mPFC layer V pyramidal neuron from rats treated under the described treatment conditions. (b) Frequency of 5-hydroxytryptamine (5-HT)- and hcrt-induced EPSCs. Results are the mean±SEM percent of vehicle control (F=5.39 for 5-HT and F=4.93 for hcrt, p<0,05; post-hoc test *p<0.05 vs control, **p<0.01 vs control; n=13–22 cells from 4–7 rats). (c) Two-photon images of apical tuft branch segments from recorded mPFC layer V pyramidal neurons filled with neurobiotin and post-hoc staining with streptavidin conjugated to Alexa 594. Scale bar shown in left panel. (d) Summary of data (n=11–12 cells) showing the spine number (top) and spine head diameter (bottom). (F=0.8 for spine density, p=0.44; F=15.68 for spine head diameter, p<0.05, post-hoc test **p<0.01 vs control).
Low-dose Ketamine+Lithium Combination Produces a Sustained Antidepressant Response in the FST
Antidepressant-like effects were assessed using the FST at a 24-h time point after drug treatments (Figure 5a). First, the effects of either Ket-1 or Li-10 alone were compared with Ket-10. Statistical analysis revealed significant effects of Ket-1/Li-10, but not Ket-1 or Li-10 on the duration of immobility in the FST (F3,27=3.920, p=0.021) (Figure 5b). Next we tested the effect of Ket-1/Li-10 compared to Ket-10 using another cohort of animals. Statistical analysis of immobility time revealed significant effects in the groups receiving Ket-1/Li-10 treatments that were similar to the group receiving Ket-10 (F2,23=7.786, p=0.003) (Figure 5c). Post-hoc analysis revealed that immobility times of Ket-1/Li-10 and Ket-10 are significantly decreased (p<0.01, p<0.05, respectively) compared with the group that received vehicle (saline) (Figure 5c). We also examined the ability of Ket-1/Li-10 and Ket-10 to produce sustained antidepressant-like effects 1 and 2 weeks after treatment. Statistical analysis revealed significant effects of both treatments on immobility time in the FST 7 days after the injection (F2,23=22.363, p<0.0001) (Figure 5d). Post-hoc analysis revealed that immobility time ketamine1/lithium10 and ketamine10 are significantly decreased (p<0.01, p<0.01, respectively) compared to the v+v group. No effects were seen 14 days after treatment (F2,23=0.029, p=0.971) (Figure 5e). Note that the same rats were tested at both the 7 and 14 day time points, and the lack of effect at the 14-day time point must be confirmed.
Figure 5.
Rapid and sustained antidepressant effects of low-dose ketamine+lithium in the FST. (a) Time sequence for drug administration and FST testing; note that on Day 0 lithium is given 30 min following ketamine. (b) The effects of Ket-1, Ket-10 and Li-10 alone on immobility time in the FST (test conducted 24 h after final injection). No significant effects of Ket-1 or Li-10 were seen. The positive control Ket-10 significantly decreased immobility time. (c) The effects of Ket-1/Li-10 compared with Ket-10 on immobility in the FST are shown. Results are compared with controls receiving vehicle+vehicle (saline). (d, e) The effects of Ket-1/Li-10 and Ket-10 were determined 7 (d) or 14 days (e) after drug injection. Antidepressant-like effects of Ket-1/Li-10 and Ket-10 were sustained for 7 but not 14 days after drug administration. Immobility times (seconds) are shown as the mean±SEM (n=4–8 per group). *p<0.05, **p<0.01 compared with saline; analysis of variance (one-way ANOVA with LSD post-hoc test).
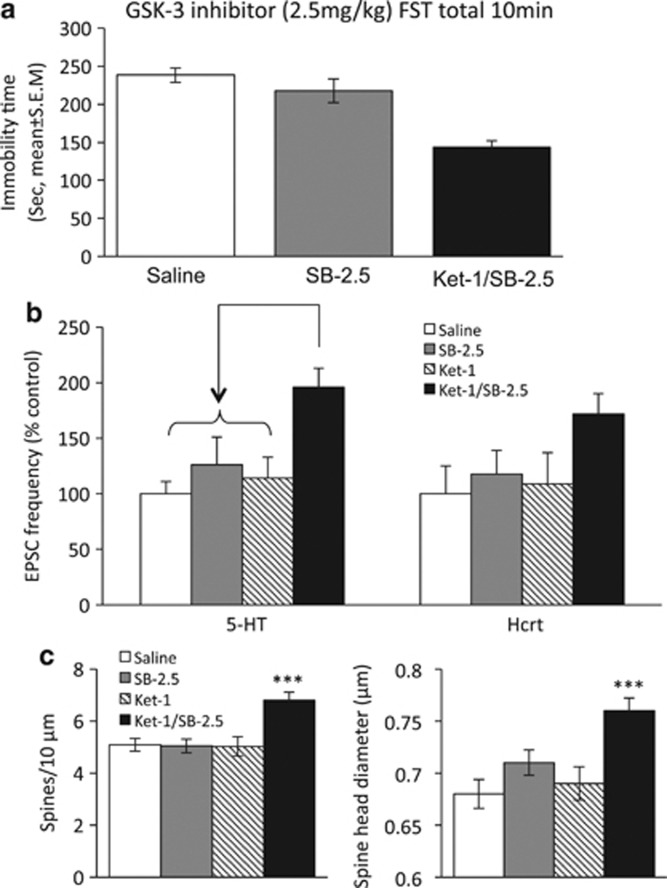
SB 216763, a Selective GSK-3 Inhibitor, Mimicked Lithium's Potentiation of Ketamine's Synaptogenic and Antidepressant-like Effects
To determine whether combined administration of a low-dose GSK-3 inhibitor and ketamine would be sufficient to evoke synaptogenesis and antidepressant effects similar to Ket-1/Li-10, we tested the effects of a combination of Ket-1 plus 2.5 mg/kg of SB 216763 (Ket-1/SB-2.5), previously shown to be ineffective by itself in reducing immobility 24 h after injection (Ma et al, 2013). Statistical analysis revealed a significant effect of the combination treatment on immobility time in the FST (F2,23=18.299, p<0.0001). Post-hoc analysis revealed that immobility time of Ket-1/SB-2.5 is significantly decreased (p<0.01) while no significant effects were seen in the group receiving SB-2.5 mg alone (Figure 6a). Ket-1/SB-2.5 administration also increased the frequency of EPSCs induced by bath application of 5-HT, a strong trend for increased hypocretin-induced EPSCs in mPFC layer V pyramidal neurons (Figure 6b). Similarly, Ket-1/SB-2.5 increased the number of spines and spinal head diameter in the apical tuft segments of layer V pyramidal neurons in the mPFC (Figure 6c and d).
Figure 6.
Combination of low doses of ketamine (1 mg/kg) and the selective glycogen synthase kinase-3 (GSK-3) inhibitor SB 216763 (2.5 mg/kg) mimic the antidepressant, electrophysiological, and synaptogenesis effects of Ket-1/Li-10. (a) The effects on immobility in the FST of saline, SB-2.5 alone, and Ket-1/SB-2.5 are shown (F=18.3, p<0.05. Post-hoc test **p<0.01; n=8). (b) Ket-1/SB-2.5 increased frequency of 5-HT- but only a trend for an increased hcrt-induced excitatory postsynaptic currents (EPSCs) in mPFC layer V pyramidal neurons (F=4.7 for 5-hydroxytryptamine (5-HT), p<0.05, F=2.02 for hcrt, p>0.05; post-hoc test *p<0.05; n=17–13 cells from 4–7 rats). (c) Ket-1/SB-2.5 increased spine density and spine head diameter of the recorded neurons in mPFC. (n=8–11 cells, F=9.5 for spine density, p<0.05; F=9.12, for spine head diameter, p<0.05; post-hoc test **p<0.0, ***p<0.001). Test conducted 24 h after final injection. The results are the mean±SEM.
DISCUSSION
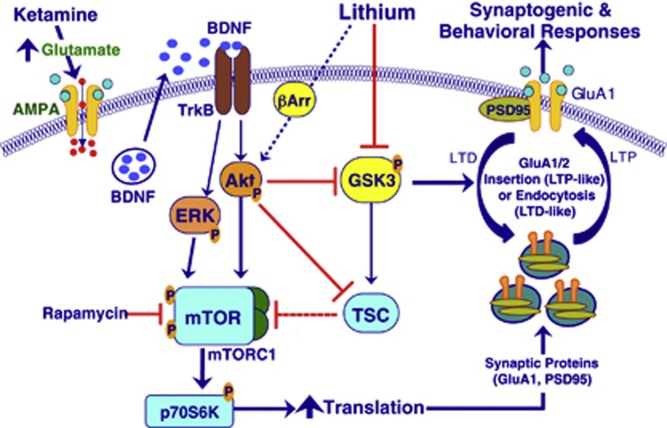
The results demonstrate that a low dose of ketamine (1 mg/kg), which had no effect by itself, when given in combination with a low dose of lithium produces changes equivalent to those of a higher dose of ketamine (10 mg/kg), namely a rapid, transient activation of the mTORC1 signaling pathway including increased phosphorylation of Akt, ERK, mTOR, and S6K (Li et al, 2010). These initial changes were followed by a sustained increase in the number and function of excitatory spines synapses (synaptogenesis) lasting up to 1 week. While low doses of ketamine or lithium when given alone did not increase mTOR or synaptogenesis, there was an increase the phosphorylation of S6K, possibly due to the engagement of different signaling pathways such as ERK that can activate S6K without modification of mTOR (Deguil et al, 2008). Nevertheless, activation of ERK and S6K in the absence of an increase mTOR signaling did not prove to be sufficient to induce a synaptogenic response. In addition to mTORC1 signaling, the low-dose combination of ketamine and lithium increased inhibitory phosphorylation of GSK-3, which would diminish its inhibitory influence on mTOR (Figure 7); this increase was transient, paralleling the transient activation of mTOR. Inhibition of GSK-3 may be critical in protecting nascent synapses from being lost through destabilization, as has been postulated for late-phase LTP (Bradley et al, 2012). GSK-3β, which can act as a negative regulator of mTORC1 by phosphorylating and activating TSC2 (Zoncu; Figure 7), is a major regulator of NMDA receptor-mediated long-term depression (LTD) (Collingridge et al, 2010).
Figure 7.
This schematic depicts how low-dose ketamine and lithium can work in concert by activating mammalian target of rapamycin (mTOR) complex 1 (mTORC1) signaling and inhibiting of glycogen synthase kinase-3β kGSK-3 β to produce robust synaptogenic and antidepressant effects equivalent to a higher effective dose alone. GSK-3 is seen acting both upstream and downstream of the BDNF/Akt/mTOR dendritic protein translation pathway. Upstream, lithium reduces GSK-3 tonic inhibitory influences upon Akt/mTORC1 signaling. Downstream from mTOR lithium is shown to reduce GSK-3-mediated long-term depression (LTD)-like synaptic deconsolidation or synaptosis. ERK, extracellular signal-regulated kinase. Blue arrows, activation; red bars, inhibition.
The prolonged ketamine-induced increase in the frequency and amplitude of 5-HT and hypocretin-induced EPSCs in layer V pyramidal cells was previously shown to be dependent upon the activation mTORC1 signaling (Li et al, 2010). Here we found that the low-dose ketamine–lithium combination, but not either alone, produced an increase in 5-HT- and hypocretin-induced EPSPs equivalent to that of the tenfold higher standard higher dose of ketamine (10 mg/kg). The responses to 5-HT and hypocretin are mediated by cortical–cortical and thalamo–cortical synapses, respectively (Lambe and Aghajanian, 2003), indicating that the combination treatment increases both types of synaptic connections. The ketamine–lithium combination also increased the density of spines on layer V mPFC pyramidal neurons as well as the number of large-diameter ‘mushroom' spines, indicative of increased spine maturation and synaptic strengthening (Yoshihara et al, 2009). Interestingly, although low-dose ketamine or lithium alone did not increase spine density, low-dose ketamine alone did induce a small increase spine head diameter, indicating that even a low-dose ketamine exhibits some tendency to increase spine maturation.
The low-dose ketamine–lithium combination also produced a robust and relatively prolonged antidepressant-like effect in the rat FST, comparable to that of a single effective 10 mg/kg dose of ketamine (Li et al, 2010). These results resemble a recent study showing a combination of low doses of ketamine and lithium, which had no effect alone, significantly decreased immobility time of mice in the FST (Ghasemi et al, 2010). However, in the latter study, behavioral testing was conducted only 30 min after drug administration rather than at later times. Similarly, while selective GSK-3 inhibitors produce antidepressant-like activity when given alone (Gould et al, 2004; Kaidanovich-Beilin et al, 2004), only short-term behavioral tests (30 min) were conducted in these studies. A more recent study reported that after selective GSK-3 inhibition alone, antidepressant effects were not observed after 24 h or 1 week (Ma et al, 2013). The latter results are consistent with our present finding that single acute doses of lithium or the selective GSK-3 inhibitor SB 216763 are not sufficient to produce a more prolonged phase of antidepressant-like effects. In contrast, treatment with combined ketamine-SB 216763 was clearly effective at 24 h; later time points remain to be tested.
Figure 7 illustrates how combined low-dose ketamine and lithium can interact in the activation of mTORC1 signaling and inhibition of GSK-3β to produce synaptogenic and antidepressant effects. First, ketamine is shown to activate BDNF-dependent Akt/mTOR signaling; these initial activations occur within ∼30 min, followed by an increase in synaptic protein translation within 2 h (Li et al, 2010). Interactions between ketamine and lithium can occur partly upstream of mTOR where both stimulate Akt, albeit by different mechanisms. Also, both ketamine and lithium by activating Akt, increase inhibitory phosphorylation of GSK-3 and, via the tuberous sclerosis complex (TSC) and Rheb (not shown), can diminish its tonic inhibitory influence on mTORC1 signaling. Lithium can also inhibit GSK-3 directly, resulting in disinhibition of Akt via destabilization of its complex with β-arrestin (Jope, 2011). Downstream of mTOR, the initiation of activity-dependent dendritic protein translation (Hoeffer and Klann, 2010) is seen to promote synthesis and insertion of synaptic proteins (eg, GluA1 and PSD95) into the postsynaptic density of dendritic spines (Li et al, 2010). At the synapse, inhibition of GSK-3 is shown to favor synaptic homeostasis by protecting spines from premature LTD-like deconsolidation (Bradley et al, 2012). As the model would predict, knock-in mice lacking serine sites for inhibitory phosphorylation of GSK-3 are more susceptible to chronic stress-induced depressive-like behaviors (Polter et al, 2010). The model also predicts that inhibition of GSK-3 would mitigate the synaptic and depressive deficits caused by chronic stress; this conjecture remains to be tested.
Finally, although the synaptogenic and antidepressant effects of a single dose of ketamine are relatively prolonged, clinical and preclinical studies show that fading of antidepressant responses tends to occur after 1 or 2 weeks in both MDD and BPD. Postmortem and genetic studies suggest that basal GSK-3 activity may be increased in mood disorders (Jope, 2011), possibly contributing to deficits in synaptic connectivity that eventually develop in MDD (Duman and Aghajanian, 2012). Thus, given its high level of basal activity, GSK-3 would seem a prime interventional target for preventing the fading of ketamine's antidepressant effects. However, in the studies on patients with BPD, where high levels of lithium were present throughout the testing period, ketamine's therapeutic effects faded even more rapidly than previously observed in MDD (Diazgranados et al, 2010; Zarate et al, 2012). These results suggest that sustained clinical levels of lithium paradoxically may interfere with the pivotal role of GSK-3 in the dynamics of maintaining homeostatic balance between synaptic gain and loss. Figure 7 illustrates how a persistent block of GSK-3 would remove tonic inhibition from mTOR, potentially negating its physiological role in transient, activity-dependent dendritic protein translation. This is exemplified by late-phase LTP, which requires transient inhibition of LTD for stabilization of nascent synapses that otherwise would be lost through the process of GSK-3-mediated destabilization or synaptosis (Bradley et al, 2012). Clearly, in an effort to stabilize connectivity gains achieved with either single or repeat treatments with low dose ketamine, further studies will be necessary to determine the appropriate schedule for administering lithium or selective GSK-3 inhibitors.
FUNDING AND DISCLOSURE
This work was supported by National Institutes of Health Grant MH093897 and the State of Connecticut. The authors declare no conflict of interest
References
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CA, Peineau S, Taghibiglou C, Nicolas CS, Whitcomb DJ, Bortolotto ZA, et al. A pivotal role of GSK-3 in synaptic plasticity. Front Mol Neurosci. 2012;5:13. doi: 10.3389/fnmol.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- Deguil J, Perault-Pochat MC, Chavant F, Lafay-Chebassier C, Fauconneau B, Pain S. Activation of the protein p7OS6K via ERK phosphorylation by cholinergic muscarinic receptors stimulation in human neuroblastoma cells and in mice brain. Toxicol Lett. 2008;182:91–96. doi: 10.1016/j.toxlet.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekadu A, Wooderson SC, Markopoulo K, Donaldson C, Papadopoulos A, Cleare AJ. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J Affect Disord. 2009;116:4–11. doi: 10.1016/j.jad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Freland L, Beaulieu JM. Inhibition of GSK3 by lithium, from single molecules to signaling networks. Front Mol Neurosci. 2012;5:14. doi: 10.3389/fnmol.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi M, Raza M, Dehpour AR. NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. J Psychopharmacol. 2010;24:585–594. doi: 10.1177/0269881109104845. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30:1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci. 2011;4:16. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Woodgett JR. GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci. 2011;4:40. doi: 10.3389/fnmol.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slice. Neuron. 2003;40:139–150. doi: 10.1016/s0896-6273(03)00598-1. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little A. Treatment-resistant depression. Am Fam Physician. 2009;80:167–172. [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XC, Dang YH, Jia M, Ma R, Wang F, Wu J, et al. Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice. PLoS One. 2013;8:e56053. doi: 10.1371/journal.pone.0056053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ. Treatment-resistant depression: recent developments and future directions. Depress Anxiety. 2008;25:989–992. doi: 10.1002/da.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz JA, Machado-Vieira R, Zarate CA, Jr, Manji HK. Novel insights into lithium's mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62:50–60. doi: 10.1159/000314310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, De Roo M, Muller D. Dendritic spine formation and stabilization. Curr Opin Neurobiol. 2009;19:146–153. doi: 10.1016/j.conb.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2011;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]