Abstract
Genetic variation in a genomic region on chromosome 15q25.1, which encodes the alpha5, alpha3, and beta4 subunits of the cholinergic nicotinic receptor genes, confers risk to smoking and nicotine dependence (ND). Neural reward-related responses have previously been identified as important factors in the development of drug dependence involving ND. Applying an imaging genetics approach in two cohorts (N=487; N=478) of healthy non-smoking adolescents, we aimed to elucidate the impact of genome-wide significant smoking-associated variants in the CHRNA5–CHRNA3–CHRNB4 gene cluster on reward-related neural responses in central regions such as the striatum, orbitofrontal and anterior cingulate cortex (ACC), and personality traits related to addiction. In both samples, carriers of the rs578776 GG compared with AG/AA genotype showed a significantly lower neural response to reward outcomes in the right ventral and dorsal ACC but not the striatum or the orbitofrontal cortex. Rs578776 was unrelated to neural reward anticipation or reward magnitude. Significantly higher scores of anxiety sensitivity in GG compared with AG/AA carriers were found only in sample 1. Associations with other personality traits were not observed. Our findings suggest that the rs578776 risk variant influences susceptibility to ND by dampening the response of the ACC to reward feedback, without recruiting the striatum or orbitofrontal cortex during feedback or anticipation. Thus, it seems to have a major role in the processing of and behavioral adaptation to changing reward outcomes.
Keywords: reward, anterior cingulate cortex, genes, traits, functional imaging, adolescence
INTRODUCTION
Nicotine dependence (ND) is a complex behavior that is influenced by genetic and environmental factors (eg, Sullivan and Kendler, 1999). Identification of susceptibility factors for smoking—and thus ND—will facilitate the identification of persons at risk. Several studies have implicated the chromosomal region 15q25.1 containing the CHRNA5–CHRNA3–CHRNB4 cluster of cholinergic nicotinic receptor subunit genes in both smoking and ND (Saccone et al, 2007; Bierut et al, 2008; Thorgeirsson et al, 2008, 2010; The Tobacco and Genetics Consortium (TAG) (2010); Liu et al, 2010). A recent meta-analysis of 34 data sets of European descent smokers showed that several statistically independent loci in this region are associated with smoking behavior, including signals represented by rs578776 and rs16969968 (Saccone et al, 2010).
Previous studies have shown that exposure to nicotine results in dopamine release in the mesolimbic system (eg, Volkow et al, 2010), specifically the ventral tegmental area, that projects to the nucleus accumbens and the prefrontal cortex (Balfour et al. 2000). Already at early stages of smoking, the degree of smoking behavior and dependence, assessed by the Heaviness of Smoking Index (Robinson and Kolb, 1997), was negatively associated with the response in the prefrontal cortex during response inhibition (Borland et al, 2010). Furthermore, the response in the prefrontal cortex was significantly lower in adolescent smokers compared with non-smokers during response inhibition (Galván et al, 2011), and a working memory task in smoking abstinent adolescents (Musso et al, 2007). In addition, clinical features of smoking behaviors—cigarette dependence and number of cigarettes smoked per day—were negatively related to cortical activity during response inhibition (Galván et al, 2011).
Moreover, both development and maintenance of substance-related disorders, including ND, has been related to the dysfunctional reward processing (eg, Jacobsen et al, 2007). Functional imaging studies have demonstrated a lower blood-oxygen-level-dependent (BOLD) response to non-drug reward anticipation in the ventral striatum in adolescent smokers compared with controls (Peters et al, 2011). In ND individuals, a lower BOLD response to stimuli predicting non-drug reward (Bühler et al, 2010) and reward feedback (Martin-Sölch et al., 2001) has been observed in the ventral striatum, the orbitofrontal and the prefrontal cortex. Specifically, the anterior cingulate cortex (ACC; Fishbein et al, 2005; Perry et al, 2011) was found to be less responsive to reward feedback in healthy smokers and patients with ND.
Both, reward functions and drug dependence are associated with nicotinic acetylcholine receptors controlling dopamine release in the striatum (eg, Zhou et al, 2001). Studies in healthy individuals with a genetic risk for smoking and ND, but who do not smoke and who lack clinical ND characteristics, allow the assessment of simpler, biologically based ‘intermediate phenotypes' (Meyer-Lindenberg and Weinberger, 2006), but are scarce for variants related to CHRNA5–CHRNA3–CHRNB4. Hong et al. (2010) were the first to provide evidence in support of the hypothesis of a differential brain response in carriers of a smoking-risk genotype of this cluster, but outside the context of reward. The authors analyzed resting state functional connectivity, and found that two variants, rs16969968 and rs578776, in the CHRNA5–CHRNA3–CHRNB4 cluster were independently associated with brain circuits involving dorsal anterior cingulate and ventral striatum implicated in ND.
In the present imaging genetics study, we aimed to identify neural response to reward in target brain regions (striatum and orbitofrontal cortex and ACC), assessed by a monetary incentive delay (MID) task (eg, Knutson et al, 2001), through which genetic variation in the CHRNA5–CHRNA3–CHRNB4 region may convey risk for ND. We focused on rs16969968 and rs578776 for two reasons: (i) both these single-nucleotide polymorphisms (SNPs) showed association with neural responses in reward- and ND-related brain regions (dorsal ACC–thalamus circuits and dorsal ACC–ventral striatum) (Hong et al, 2010) and (ii) both have shown strong, statistically independent association with smoking behavior (Saccone et al, 2010). The analysis of data from a large cohort of non-smoking healthy adolescents allows the identification of robust and stable neural response patterns. In addition, adolescence is a period that is characterized by increased risky behavior that has been attributed to maturational differences in mesolimibic structures and in consequence to hyperresponsivity to reward anticipation and reward magnitude compared with adults (eg, Bjork et al, 2010). This makes this age group specifically interesting also in terms of the development of mental disorders. The study also examined the influence of these two genetic variants on traits related to nicotine use that represent personality risk factors for substance abuse/dependence (eg, Conrod et al, 1998) and might thus also significantly contribute to associations with neural reward processing.
As all individuals of our healthy sample were non-smokers, neural response patterns and traits identified in the present study may represent predisposing vulnerability factors rather than changes resulting from continuous smoking.
MATERIALS AND METHODS
Participants
For the European Multicenter Imaging Genetics (IMAGEN) study (Schumann et al, 2010), a large sample of healthy adolescents was recruited from the general populations of Germany, the United Kingdom, Ireland, and France via school visits, flyers, and resident's registration offices. A total of 487 non-smoking healthy individuals of Caucasian ancestry (231 female; mean age: 14.26 years (SD=0.30)) were included in the present analysis. Replication was performed with another 512 non-smoking Caucasian healthy individuals (220 females). Both samples partly overlap (N>300) with samples used in previous investigations (eg, Nees et al, 2012). Exclusion criteria were: any mental disorder (identified by the Development and Well-Being Assessment, DAWBA; Goodman et al, 2000), any serious medical condition, any history of head trauma, and any contraindication for magnetic resonance imaging (MRI). The study was approved by the respective local ethics committees, and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained following a detailed explanation of the study to the participants and their legal guardians. Table 1 provides an overview of the demographic, psychometric, and genotype data.
Table 1. (a) Psychometric Data and the rs578776 Genotypes Grouped in GG and AG/AA Carriers of Sample 1. (b) Psychometric Data and the rs578776 Genotypes Grouped in GG and AG/AA Carriers of Sample 2 (Replication).
| (a) |
Total sample (N=487) M (SD) |
GG group (N=255) M (SD) |
AG/AA group (N=232) M (SD) |
Inferential statistics (whole group) |
|||
|---|---|---|---|---|---|---|---|
| Male/female | Whole group | Male/female | Whole group | Male/female | Whole group | GG vs AG/AA | |
| Age (years) |
14.3 (0.3)/14 (0.2) |
14.15 (0.25) |
14.2 (0.2)/14.1 (0.2) |
14.2 (0.2) |
14.4 (0.3)/14.3 (0.4) |
14.4 (0.4) |
NS |
| Intelligencea |
99.7 (13.2)/96.6 (13.2) |
98.15 (13.2) |
102.5 (13.8)/98.5 (13.8) |
100.5 (13.8) |
98.3 (12.9)/96.2 (12.9) |
97.3 (12.9) |
NS |
| Anxiety sensitivityb |
2.3 (0.4)/2.2 (0.4) |
2.3 (0.4) |
2.3 (0.4)/2.4 (0.5) |
2.4 (0.5) |
2.3 (0.5)/2.2 (0.5) |
2.3 (0.5) |
p=0.037 |
| Sensation seekingb |
2.9 (0.5)/2.8 (0.6) |
2.9 (0.55) |
2.9 (0.5)/2.7 (0.5) |
2.8 (0.5) |
2.8 (0.5)/2.8 (0.5) |
2.8 (0.5) |
NS |
| Hopelessnessb |
1.9 (0.4)/1.8 (0.4) |
1.9 (0.4) |
1.9 (0.4)/1.9 (0.4) |
1.9 (0.5) |
1.9 (0.4)/1.8 (0.4) |
1.9 (0.4) |
NS |
| Impulsivityb | 2.4 (0.4)/2.4 (0.4) | 2.4 (0.4) | 2.4 (0.4)/2.5 (0.4) | 2.5 (0.4) | 2.5 (0.5)/2.4 (0.4) | 2.5 (0.5) | NS |
| (b) |
Total sample (N=512) M (SD) |
GG group (N=283) M (SD) |
AG/AA group (N=229) M (SD) |
Inferential statistics (whole group) |
|||
|---|---|---|---|---|---|---|---|
| Male/female | Whole group | Male/female | Whole group | Male/female | Whole group | GG vs AG/AA | |
| Age (years) |
14.65 (0.3)/14.55 (0.3) |
14.6 (0.3) |
14.6 (0.3)/14.5 (0.2) |
14.55 (0.25) |
14.7 (0.25)/14.6 (0.4) |
14.65 (0.3) |
NS |
| Intelligencea |
99.9 (12.9)/100.2 (13.2) |
100 (12.9) |
100.5 (12.8)/99 (13.3) |
99.7 (13) |
99.3 (13)/101.4 (12.5) |
100.4 (12.8) |
NS |
| Anxiety sensitivityb |
2.25 (0.45)/2.4 (0.4) |
2.3 (0.5) |
2.1 (0.5)/2.3 (0.5) |
2.2 (0.5) |
2.35 (0.4)/2.43 (0.5) |
2.4 (0.45) |
NS |
| Sensation seekingb |
2.8 (0.4)/2.9 (0.5) |
2.85 (0.5) |
2.7 (0.35)/2.8 (0.6) |
2.8 (0.5) |
2.85 (0.5)/2.9 (0.4) |
2.9 (0.5) |
NS |
| Hopelessnessb |
2 (0.55)/1.75 (0.4) |
1.85 (0.5) |
2.2 (0.7)/1.76 (0.5) |
2 (0.6) |
1.87 (0.4)/1.73 (0.3) |
1.8 (0.4) |
NS |
| Impulsivityb | 2.6 (0.5)/2.6 (0.45) | 2.55 (0.5) | 2.46 (0.4)/2.6 (0.5) | 2.5 (0.45) | 2.7 (0.55)/2.55 (0.4) | 2.6 (0.5) | NS |
Abbreviation: M, mean value.
GG group, carriers of the rs578776 GG genotype; AG/AA group, carriers of the rs578776 AG and AA genotype.
Wechsler Intelligence Scale for Children (WISC; 20).
Substance Use Risk Profile Scale (SURPS; 21).
Psychometric Testing
Intelligence was assessed using four subtests of the Wechsler Intelligence Scale for Children (WISC;Titze and Tewes, 2009): Block Design, Similarities, Vocabulary, and Matrix Reasoning. In addition, the traits sensation seeking, impulsivity, hopelessness, and anxiety sensitivity were assessed using the Substance Use Risk Profile Scale (SURPS; Conrod et al, 1998), implemented in Psytools software (Delosis, London, UK) in a battery that participants completed online at home.
DNA Extraction, Genotyping, and Quality Control
DNA extraction was semiautomated to ensure high quality and sufficient quantity. All samples used in the present study were genotyped within the context of the IMAGEN study (Schumann et al, 2010). Genome-wide genotyping of ∼600 000 autosomal SNPs was performed using the Illumina Quad 610 chip (Illumina, San Diego, CA, USA). DNA purification and genotyping was performed by the Centre National de Génotypage in Paris. DNA was extracted from whole-blood samples (∼10 ml) preserved in BD Vacutainer EDTA tubes (Becton Dickinson and Company, Oxford, UK) using Gentra Puregene Blood Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Genotype information was collected at 582 982 markers using the Illumina HumanHap610 Genotyping BeadChip (Illumina). SNPs with call rates of <98%, minor allele frequency <1%, or deviation from the Hardy–Weinberg equilibrium (P⩽1 × 10−4) were excluded from the analyses. Individuals with an ambiguous sex code, excessive missing genotypes (failure rate >2%), and outlying heterozygosity (heterozygosity rate of 3 SDs from the mean) were also excluded. Closely related individuals with identity-by-descent (IBD>0.1875) were filtered out before the subsequent analysis. Population stratification for the GWAS data was examined by principal component analysis (PCA) using EIGENSTRAT software (Pritchard et al, 2000). The four HapMap II populations were used as reference groups in the PCA analysis and individuals with divergent ancestry (from CEU) were also excluded (Price et al, 2006). Quality control procedure of the IMAGEN data set was done in three waves starting with N=705 in wave 1, N=1004 in wave 2, and N=394 subjects in wave 3. After quality control N=620 in wave 1, N=868 in wave 2, and N=351 in wave 3 were available.
Available SNPs in the CHRNA5–CHRNA3–CHRNB4 region were: rs578776, rs1051730, rs3743075, rs680244, rs950776, rs938682, rs3743077, rs12914385, rs11636753, rs1948, rs621849, rs8042374, rs1316971, rs6495306, rs12441998, rs12910984, and rs6495309; see Supplementary information, Supplementary Figure S5, Supplementary Table S1). As rs16969968 is not present on the Illumina Quad 610 chip used, rs1051730, which is in perfect LD in populations of European descent (r2=1.0) (https://www.broadinstitute.org/mpg/snap), was used as a proxy.
For rs578776, 255 individuals of sample 1 were GG genotype carriers, 212 heterozygous, and 20 AA genotype carriers, and in replication sample 2 283 GG, 203 heterozygous, and 26 AA genotype carriers (in Hardy–Weinberg Equilibrium: P=1.00). For rs1051730, 208 individuals in sample 1 were CC genotype carriers, 243 were heterozygous, and 36 were TT genotype carriers.
Genotypes were collapsed into two groups combining minor allele homozygous and heterozygous carriers.
MID Task
The MID task used during functional MRI is based on a reaction time task of Knutson et al. (2001). Participants had to press a button to hit a target to score a previously indicated amount of points (no win: no points, small win: 2 points, and big win: 10 points; for task details see Supplementary Information). They received 1 candy (M&Ms) for every 5 points scored at the end of the session. Previous research has shown that this adaptation induces significant activation in the respective regions of interest (ROIs) (cf., Nees et al, 2012, see Supplementary Figure S1 for task activation map). Task presentation and recording of the behavioral responses were performed using Visual Basic 2005 and NET Framework Version 2.0, as well as the visual and response grip system from Nordic Neuro Lab (NordicNeuroLab AS, Bergen, Norway).
MRI Acquisition
Scanning was performed with a 3T whole-body MRI system at each of the eight IMAGEN assessment sites (London, Nottingham, Dublin, Mannheim, Dresden, Berlin, Hamburg, and Paris). The scanners were obtained from various manufacturers (Siemens, Philips, General Electric, and Bruker). We acquired 40 slices in descending order (2.4 mm, 1 mm gap) using a gradient-echo T2*-weighted sequence (EPI): TR=2200 ms, TE=30 ms, in-plane resolution of 64 × 64 pixels. The plane of acquisition was tilted to the anterior–posterior commissure line. For anatomical reference, a 3D magnetization prepared gradient-echo sequence of the whole brain was obtained with TR=6.8 ms and TE=3.2 ms, in accordance with the ADNI protocol (http://www.loni.ucla.edu/ADNI/Cores/index.shtml). To ensure comparability of MRI data acquired on the different scanners, a set of parameters that were compatible with all scanners were used and kept constant across all sites, eg, those directly affecting image contrast or signal-to-noise (cf., Schumann et al, 2010).
Data Analysis
Analyses of variance and Bonferroni-corrected t-tests (p<0.05) for psychometric and behavioral data (reaction times, correct vs incorrect responses) of the MID task were performed using the Predictive Analytic Software (PASW, SPSS, Chicago, IL) for Windows version 18.0.1. Whenever the assumption of homogeneity of variances was violated, we applied the Greenhouse–Geisser adjustment and corrected degrees of freedom are reported. The fMRI data were analyzed with Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, University College London, UK) using slice time correction, spatially realignment, non-linearly warping on the MNI space (custom EPI template (53 × 63 × 46 voxels) based on an average of the mean images of 400 adolescents), resampling at a resolution of 3 × 3 × 3 mm3, and smoothing (isotropic Gaussian kernel: 5 mm full-width at half-maximum). First-level statistics involved modeling ‘reward anticipation' and ‘reward feedback' as predictor variables within the context of the general linear model. This was performed on a voxel by voxel basis, with an AR noise model against a design matrix, and 18 additional columns for estimated movements (3 translational, 3 rotations, 3 quadratic, 3 cubic translations, 3 translations shifted 1TR before, and 3 shifted 1TR later). For both anticipation and feedback, magnitudes of no win, small win, and big win, as well as information on hit (response within the correct time window) vs missed (response outside the correct time window) trials, were entered in a parametric design, and study center was included as a covariate.
The individual contrast images were entered in a second-level random-effects analysis (full flexible procedure of SPM8), and a non-sphericity correction was performed. A significance level of p<0.05 was selected (familywise-error (FWE)-corrected), with a minimum cluster size of 10 voxels. With a ROI approach using Wake Forest University PickAtlas v3.0.3 (Tzourio-Mazoyer et al, 2002), we focused on previously identified target regions (ROIs: orbitofrontal, ACC, and striatum (nucleus accumbens/putamen/caudate nucleus)) (Peters et al, 2011; Hong et al, 2010; Kelley and Berridge, 2002). Whole-brain analysis was performed to uncover further significant associations between rs578776 and neural BOLD response. Furthermore, interactions of BOLD response with traits and behavioral response to reward were applied using a regression approach implemented in SPM8. All reported results are corrected for multiple comparisons.
Imaging center effects were controlled for using research center as a covariate as well as a ‘dropping one site approach' (Friedman et al, 2008), which did not result in different findings.
Previous findings by Hong et al. (2010) revealed an association of rs578776 and dorsal ACC–thalamus circuits, as well as an association of rs16969968 and dorsal ACC–ventral striatum interaction. We applied a functional connectivity analysis for rs578776 and rs1051730 (as a proxy for rs16969968). We used the time course from the dorsal ACC (seed region) and determined regions whose time series of activation exhibit significant covariance with the seed region (psychophysiological interaction analysis;Friston et al, 1997), but could not find significant interactions.
RESULTS
Behavioral Performance During Reward Processing
For both rs578776 and rs1051730 in the MID task, no significant difference in reaction times to no win, small, or big win was observed between the genotype groups. Independent of genotype, reaction times were significantly shorter in response to big vs small vs no win (data not shown). No significant differences in the percentage of correct responses within each condition were observed between the genotype groups.
Brain Response in the Striatum, Orbitofrontal and ACC to Reward
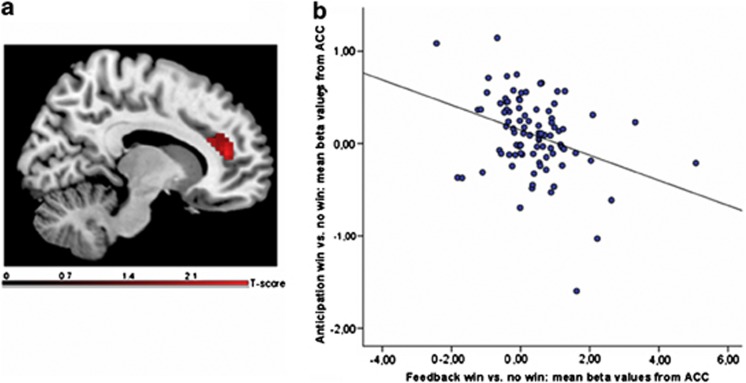
For rs578776, we found no significant BOLD response differences in the ventral and dorsal striatum and the orbitofrontal cortex between GG and AG/AA genotype groups, neither for reward anticipation nor for feedback (Supplementary Figure S2). However, a significant lower BOLD response in the right ventral and dorsal ACC (Brodmann area 24, 32) was observed during reward feedback, independent of magnitude (hit win vs no win), in GG compared with AG/AA genotype carriers (x=6, y=41, z=−10, k=30; t=3.48, p=0.008; FWE-corrected, see Figure 1b). Significant differences were neither observed for missed trials of reward feedback nor for reward anticipation. To show that this finding represents less response to reward rather than deviations in reward absence, we additionally compared the neural response with the no win condition between the genotype groups. This did not result in any significant effect. However, in carriers of the GG genotype, the BOLD response in the right ACC to reward anticipation (hit, but not missed trials) significantly predicted the BOLD response in this region to reward feedback (F(1,210)=8.700; r=−0.200; p=0.004, see Figure 1a). These findings could be replicated in sample 2 (F(1,259)=4.103; r=−253; p=0.047; x=6, y=44, z=19, k=10; t=2.32, p=0.049; FWE-corrected). No association was found between rs1051730 and neural responses to reward.
Figure 1.
(a) Lower response in the right anterior cingulate cortex (x=6, y=41, z=−10, k=30; t=3.48, p=0.008; FWE-corrected) during reward feedback (hit win vs no win) in carriers of the rs578776 GG compared with AG/AA genotype (sample 1); (b) Regression analysis of the response in the anterior cingulate cortex (extracted mean beta values) during reward anticipation and reward feedback (hit win vs no win): prediction of feedback response from response during anticipation (F(1,210)=8.700; r=−,200; p=0.004; sample 1).
We found no significant difference in associations of rs578776 and rs1051730 with neural reward responses between male and female participants.
Brain Response to Reward Derived From Whole-Brain Analysis
We found feedback- but not anticipation-related effects of rs578776 on BOLD response in neural response clusters (p<0.05; FWE-corrected) involving the following brain circuits: precentral gyrus, postcentral gyrus, precuneus, lingual gyrus, inferior frontal gyrus, superior temporal gyrus, inferior occipital gyrus, and hippocampus (lower response in GG compared with AG/AA genotype carriers; see Supplementary Figures S3 and S4). These findings could be replicated for clusters involving the following regions: precentral gyrus, precuneus, superior temporal gyrus, postcentral gyrus, and hippocampus. No association was found between rs1051730 and whole-brain responses to reward.
Trait Risk Factors
For rs578776, higher scores for anxiety sensitivity were found in the GG compared with AG/AA genotype carriers (p=0.037; mean (SD) GG: 2.32 (0.46), mean (SD) AG/AA: 2.24 (0.45), see Table 1a). However, no significant association was observed between this trait and neural reward-related responses. Using anxiety sensitivity as a regressor of no interest, we did not find significant differences in the striatal response for GG compared with AG/AA genotype carriers, but significant differences in the ACC in response to reward feedback was also present. Significant differences in anxiety sensitivity could not be found in sample 2 (Table 1b).
We found no significant association of rs578776 and rs1051730 with sensation seeking, impulsivity, or hopelessness in either sample, and no significant difference in rs578776 and rs1051730 and trait associations between male and female participants. Finally, no significant associations were observed between personality and behavioral responses to reward.
DISCUSSION
The aim of the present imaging genetics study was to analyze the effects of two of the most widely studied SNPs in the CHRNA5–CHRNA3–CHRNB4 gene cluster—rs578776 and rs16969968—on neural reward-related intermediate phenotypes (‘reward-anticipation' and ‘feedback-processing') previously implicated in smoking and ND (eg, Peters et al, 2011; Bühler et al, 2010) and the role of addiction-related traits using a large cohort of non-smoking healthy adolescents. These hypothesis-driven analyses related to previously identified target brain regions revealed that rs578776 was associated with BOLD response in the ACC, but not in the striatum or orbitofrontal cortex. Carriers of the GG compared with AG/GG genotype displayed lower BOLD responses in the right ACC (ventral, dorsal parts) during reward feedback, but not anticipation, and independent of reward magnitude. This effect was only present for hits, but neither for missed trials nor for the no win condition indicating that the dampened reward response in the GG genotype group most likely represents reduced response to reward and is not related to or affected by the absence of reward. In addition, carriers of the GG compared with AG/AA genotype showed higher anxiety sensitivity scores, yet only in sample 1 and not in the replication sample. No significant interaction of anxiety sensitivity with neural reward-related responses and no significant association of rs578776 with traits other than anxiety sensitivity were observed.
Previous studies have clearly identified the ventral striatum (specifically the nucleus accumbens), ventral pallidum, anterior cingulate, and orbitofrontal cortex being associated with reward processing (eg, Belin and Everitt, 2008). These regions are involved in encoding the hedonic value of an event, and assumed to have a role in attention, reward expectancy, and incentive motivation (eg, Meyer-Lindenberg and Weinberger, 2006). The nucleus accumbens was additionally involved in drug self-administration (eg, Hikosaka et al, 2008). Moreover, a dissociation of brain regions related to different reward aspects such as anticipation and feedback has been reported (Knutson et al, 2001). For example, Robinson and Berridge (2000) differentiated ‘wanting' (anticipatory component) and ‘liking' (outcome component) of reward. While the nucleus accumbens has a major role in ‘wanting', the ventral striatum, anterior and posterior cingulate, and mesial prefrontal cortex are primarily involved in ‘liking' (Knutson et al, 2001).
Reinforcement learning theories (eg, Robinson and Berridge, 2000) suggest that the ACC is involved in learning from feedback, eg, related to contingencies in the external environment in response to reward. This is thought to guide future behavior (Holroyd and Coles, 2002), eg, through rapid behavioral adaptations to immediate reward (Pfabigan et al, 2011). Specifically, the dorsal part of the ACC has also been implicated in this learning process (Amiez et al, 2006; Palomero-Gallagher et al, 2008; Bush et al, 2002). Moreover, ACC response to reward, assessed by a probabilistic reversal learning task, has also been positively associated with self-reported high extrinsic and negatively with intrinsic motivation (Linke et al, 2010). These data were mainly acquired in choice conditions, though the MID task does not probe learning. But interestingly, lower ACC BOLD response to non-drug compared with drug-related rewards has previously been shown in ND individuals outside a direct learning context, but to reward predicting cues (Bühler et al, 2010). In addition, a negative correlation between subjective craving and right dorsal ACC response to a cigarette reward during a cognitive reappraisal procedure was found in smokers (Zhao et al, 2012). Our present results can be interpreted in light of these findings in that homozygous risk allele compared with AG/AA genotype carriers may show less extrinsic motivation and thus reduced neural response to non-drug reward—a pattern characteristic for smoking and ND (eg, Bühler et al, 2010; Peters et al, 2011). Our findings indicate reduced recruitment of brain regions that may be necessary for successful adaptation to the environment.
The ventral striatum has also been associated with smoking and ND (Peters et al, 2011; Bühler et al, 2010). A recent study with 86 individuals from the IMAGEN study identified lower ventral striatal response to reward anticipation in adolescent smokers compared with controls (Peters et al, 2011). Furthermore, the ventral striatum–dorsal ACC circuit has been shown to be associated with rs16969968 in CHRNA5 (Hong et al, 2010). Prompted by these findings and the fact that adolescence is characterized by increased risk taking that may be specifically mediated through the ventral striatum (eg, Galvan et al, 2006), we analyzed associations between the ventral striatal response to reward and rs578776. However, this resulted in no significant findings suggesting that variation in different polymorphisms of the CHRNA5–CHRNA3–CHRNB4 gene cluster manifest in different neural circuits. Both rs16969968 and rs578776 were shown to be associated with smoking (Hong et al, 2010), but rs578776 represents an independent smoking-related signal in the CHRNA5–CHRNA3–CHRNB4 cluster (Bierut et al, 2008), not related to the ventral striatum (Hong et al, 2010). We note that rs680244, which in populations of European descent is in strong LD with another statistically independent association with smoking and also with messenger RNA expression levels of CHRNA5 (Wang et al, 2009; Smith et al, 2011), showed no association with the reward-related responses tested here. The present findings confirm the independence of ventral striatal recruitment during reward anticipation and feedback from rs578776, here in a sample of healthy adolescents. In the study of Peters et al. (2011), smokers reported only a limited exposure to tobacco and the authors concluded that reward system hyposensitivity could be a neural risk factor for ND. We found no significant differences in ventral striatal response in non-smoking individuals with a smoking-risk genotype. A possible explanation is that in contrast to the hypothesis of Peters et al. (2011), even mild but continuous smoking induces changes in neural functioning, at least in the striatum. Longitudinal studies will be necessary to determine whether ventral striatal hypoactivation is a risk factor or a consequence of nicotine consumption.
Several traits, in particular sensation seeking, impulsivity, anxiety sensitivity, and hopelessness, have been reported to contribute to vulnerability to drug dependence (eg, Malmberg et al, 2010). In the present study, carriers of the GG compared with AG/AA genotype showed higher trait anxiety sensitivity scores. This is consistent with previous reports that individuals with higher anxiety sensitivity scores are more likely to use drugs to cope with distress (eg, Hearon et al, 2011). Several studies have shown that anxiety sensitivity is related to important aspects of smoking behavior, such as less success during smoking cessation attempts (Zvolensky and Bernstein, 2005), and smoking to reduce negative affect (eg, Leyro et al, 2008). However, as in the present study both genotype groups showed only moderate scores, and low variance, it is speculative to consider this trait a risk phenotype that is influenced by genetic variation. This was underlined by the fact that the findings on anxiety sensitivity could not be replicated. No significant association was found between rs578776 and other traits associated with nicotine use (eg, Doran et al, 2004; Peters et al, 2011).
Several studies reported significant correlations between ACC response to reward and anxiety (eg, Britton and Rauch, 2008). We did not find any significant association between reward-related ACC response and anxiety sensitivity, neither in carriers of the GG nor AG/AA genotype. This is not surprising, as an association between ACC and anxiety was mainly observed in emotion processing (Klumpp et al, 2011). Patients with anxiety disorders (eg, social phobia and generalized anxiety disorder) showed an increased BOLD response in prefrontal regions (including the ACC) to angry faces (eg, Monk et al., (2008)). In contrast, clinically non-anxious individuals with higher trait anxiety have been reported to display a less pronounced ACC response to both angry and ambiguous affective (angry and happy) facial expressions compared with ambiguous gender stimuli (Monk et al., (2008)). In addition, in a 4-year longitudinal study, a bias toward negative emotion (indicated by enhanced recognition of angry faces) was identified as significant predictor for nicotine use initiation in adolescents (Ernst et al, 2010). Prompted by these findings, we additionally analyzed genotype-specific effects on neural angry face processing, which had been assessed in the IMAGEN study using a face task (see Schneider et al, 2011), but found no significant associations. Moreover, and to further elucidate the functional specificity of rs578776, we analyzed the interaction between rs578776 and motor response inhibition on a neural level (using a stop signal task; see Whelan et al, 2012), as greater reward-delay discounting has been reported to be a measure of impulsivity in adolescent smokers (Peters et al, 2011). Significant associations could also not be found.
Finally, whole-brain analysis resulted in reward feedback-related lower BOLD response in the precentral gyrus, precuneus, superior temporal gyrus, postcentral gyrus, and hippocampus in GG compared with AG/AA genotype carriers. The dorsal prefrontal cortex, thalamus, and hippocampus have an important role in reward regulation (Gardner, 2011). There is evidence that motivation enhances memory formation by hippocampal binding (eg, Wittmann et al, 2005). Thus, GG genotype carriers might form less detailed information about (non-drug) reward, specifically in situations where they should have clear expectancies about reward outcome. This might prevent homozygous risk allele carriers to adapt their behavior accordingly, and to profit from an optimal handling of reward. Recruitment of the precentral gyrus during reward was recently found in a study by Addicott et al. (2012). Smokers in a satiated condition compared with abstinent smokers showed increased precentral gyrus response to reward anticipation. The precentral gyrus seems thus being associated with reward sensitivity and our findings that homozygous risk allele carriers recruited the precentral gyrus to a lesser extent than A-allele carriers might be interpreted as reduced sensitivity to (non-drug) reward in individuals being genetically at risk for smoking and ND.
One limitation of the present study is that we did not assess reward salience in our adolescent sample. Thus, we cannot explicitly rule out that M&Ms used as reward in the MID task may have held more value for some participants than others and that this reward salience may additionally have affected our results. The specific role of reward salience might be an interesting and important issue, and should be addressed in future studies.
The present study identified an association between a nicotinic acetylcholine receptor α3 subunit variant (rs578776) and reward-related neural response in a large cohort of healthy non-smoking adolescents. Rs578776 lies in the 3′ untranslated region of CHRNA3 and to date, no evidence for functional relevance of the G/A exchange in rs578776 or a polymorphism in strong linkage disequilibrium with it have been described. None of the further tested variants in this region showed a similar association. The present findings suggest that rs578776 in CHRNA3 or other hitherto unidentified underlying variation may display functional specificity with respect to smoking- and ND-related intermediate phenotypes. Sequencing of this region may help to identify causal variation that then can be used to corroborate our findings in further samples.
FUNDING AND DISCLOSURE
TB served in an advisory or consultancy role for Bristol-Myers Squibb, Develco Pharma, Lilly, Medice, Novartis, Shire, and Viforpharma. The present work is unrelated to the above grants and relationships. JG has received research funding from the German Federal Ministry of Education and Research, research funding from AstraZeneca, Eli Lilly & Co, Janssen-Cilag, and Bristol-Myers Squibb, and speaker fees from AstraZeneca, Janssen-Cilag, and Bristol-Myers Squibb. AH has received research funding from the German Research Foundation and the Bernstein Center for Computational Neuroscience Berlin (German Federal Ministry of Education and Research), Eli Lilly & Company, Janssen-Cilag, and Bristol-Myers Squibb. AH has received Speaker Honoraria from Janssen-Cilag, Johnson & Johnson, Lilly, Pfizer, and Servier.
Acknowledgments
Support for this study was provided by the IMAGEN project, which receives research funding from the European Community's Sixth Framework Program (LSHM-CT-2007-037286) and coordinated project ADAMS (242257), as well as the UK-NIHR-Biomedical Research Centre for Mental Health, the MRC-Addiction Research Cluster ‘Genomic Biomarkers', and the MRC program grant ‘Developmental pathways into adolescent substance abuse' (93558). This research was also supported by the German Ministry of Education and Research (BMBF grant # 01EV0711). During the past 3 years, GJB has received honoraria for teaching from General Electric Medical Systems. This manuscript reflects the views of the authors only, and the Community is not liable for any use that may be made of the information contained therein.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Addicott MA, Baranger DA, Kozink RV, Smoski MJ, Dichter GS, McClernon FJ. Smoking withdrawal is associated with increases in brain activation during decision making and reward anticipation: a preliminary study. Psychopharmacology (Berl) 2012;219:563–573. doi: 10.1007/s00213-011-2404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex. 2006;16:1040–1055. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour DJK, Wright AE, Benwell MEM, Birrell CE. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res. 2000;113:73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R, Yong HH, O'Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country study. Nicotine Tob Res. 2010;12 (Suppl:45–50. doi: 10.1093/ntr/ntq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Rauch SL.2008Neuroanatomy and neuroimaging of anxiety disordersIn: Anthony MM, Stein MB (eds)Oxford Handbook of Anxiety and Related Disorders Oxford University Press: USA; p.97 [Google Scholar]
- Bühler M, Vollstädt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, et al. Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biol Psychiatry. 2010;67:745–752. doi: 10.1016/j.biopsych.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Pihl RO, Vassileva J. Differential sensitivity to alcohol reinforcement in groups of men at risk for distinct alcoholism subtypes. Alcohol Clin Exp Res. 1998;22:585–597. doi: 10.1111/j.1530-0277.1998.tb04297.x. [DOI] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D, Pergadia M, Richmond M. Impulsivity and smoking relapse. Nicotine Tobacco Res. 2004;6:641–647. doi: 10.1080/14622200410001727939. [DOI] [PubMed] [Google Scholar]
- Ernst M, Luckenbaugh DA, Moolchan ET, Temple VA, Jenness J, Korelitz KE, et al. Decision-making and facial emotion recognition as predictors of substance-use initiation among adolescents. Addict Behav. 2010;35:286–289. doi: 10.1016/j.addbeh.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Fishbein DH, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, et al. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain Res Cogn Brain Res. 2005;23:119–136. doi: 10.1016/j.cogbrainres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Friedman L, Stern H, Brown GG, Mathalon DH, Turner J, Glover GH, et al. Test-retest and between-site reliability in a multicenter fMRI study. Hum Brain Mapp. 2008;29:958–972. doi: 10.1002/hbm.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A, Poldrack RA, Baker CM, McGlennen KM, London ED. Neural correlates of response inhibition and cigarette smoking in late adolescence. Neuropsychopharmacology. 2011;36:970–978. doi: 10.1038/npp.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL. Addiction and brain reward and antireward pathways. Adv Psychosom Med. 2011;30:22–60. doi: 10.1159/000324065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The development and well-being assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–655. [PubMed] [Google Scholar]
- Hearon BA, Calkins AW, Halperin DM, McHugh RK, Murray HW, Otto MW. Anxiety sensitivity and illicit sedative use among opiate-dependent women and men. Am J Drug Alcohol Abuse. 2011;37:43–47. doi: 10.3109/00952990.2010.535581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Bromberg-Martin E, Hong S, Matsumoto M. New insights on the subcortical representation of reward. Curr Opin Neurobiol. 2008;18:203–208. doi: 10.1016/j.conb.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. Dorsal anterior cingulate cortex integrates reinforcement history to guide voluntary behavior. Psychol Rev. 2002;109:679–709. doi: 10.1016/j.cortex.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci USA. 2010;107:13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology. 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Ho SS, Taylor SF, Phan KL, Abelson JL, Liberzon I. Trait anxiety modulates anterior cingulate activation to threat interference. Depress Anxiety. 2011;28:194–201. doi: 10.1002/da.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyro TM, Zvolensky MJ, Vujanovic A, Bernstein A. Anxiety sensitivity and smoking motives and outcome expectancies among adult daily smokers: replication and extension. Nicotine Tobacco Res. 2008;10:985–994. doi: 10.1080/14622200802097555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke J, Kirsch P, King AV, Gass A, Hennerici MG, Bongers A, et al. Motivational orientation modulates the neural response to reward. Neuroimage. 2010;49:2618–2625. doi: 10.1016/j.neuroimage.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SD, Muglia P, Middleton L, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42:436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg M, Overbeek G, Monshouwer K, Lammers J, Vollebergh WA, Engels RC. Substance use risk profiles and associations with early substance use in adolescence. J Behav Med. 2010;33:474–485. doi: 10.1007/s10865-010-9278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sölch C, Magyar S, Künig G, Missimer J, Schultz W, Leenders KL. Changes in brain activation associated with reward processing in smokers and nonsmokers. A positron emission tomography study. Exp Brain Res. 2001;139:278–286. doi: 10.1007/s002210100751. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso F, Bettermann F, Vucurevic G, Stoeter P, Konrad A, Winterer G. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology. 2007;191:159–169. doi: 10.1007/s00213-006-0499-8. [DOI] [PubMed] [Google Scholar]
- Nees F, Tzschoppe J, Patrick CJ, Vollstädt-Klein S, Steiner S, Poustka L, et al. Determinants of early alcohol use in healthy adolescents: the differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology. 2012;37:986–995. doi: 10.1038/npp.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol. 2008;508:906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, et al. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, et al. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Pfabigan DM, Alexopoulos J, Bauer H, Sailer U. Manipulation of feedback expectancy and valence induces negative and positive reward prediction error signals manifest in event-related brain potentials. Psychophysiology. 2011;48:656–664. doi: 10.1111/j.1469-8986.2010.01136.x. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;Suppl 2:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefronatal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, et al. 2010Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD PLoS Genet 6piie1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Menz MM, Miedl SF, et al. Boys do it the right way: sex-dependent amygdala lateralization during face processing in adolescents. Neuroimage. 2011;56:1847–1853. doi: 10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- Smith RM, Alachkar H, Papp AC, Wang D, Mash DC, Wang JC, et al. Nicotinic α5 receptor subunit mRNA expression is associated with distant 5' upstream polymorphisms. Eur J Hum Genet. 2011;19:76–83. doi: 10.1038/ejhg.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1:S51–S57. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- The Tobacco and Genetics Consortium (TAG) Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P., Rafnar T, Wiste A, Magnusson KT, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Fink JM, Amin N, Geller F, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze I, Tewes U. [Messung der Intelligenz bei Kindern mit dem HAWIK-R] Bern, Göttingen, Toronto, Seattle; 2009. pp. 13–21. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Cruchaga C, Saccone NL, Bertelsen S, Liu P, Budde JP, et al. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet. 2009;18:3125–3135. doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Düzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Zhao LY, Tian J, Wang W, Qin W, Shi J, Li Q, et al. The role of dorsal anterior cingulate cortex in the regulation of craving by reappraisal in smokers. PLoS One. 2012;7:e43598. doi: 10.1371/journal.pone.0043598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bernstein A. Cigarette smoking and panic psychopathology. Curr Direct Psychol Sci. 2005;14:301–305. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.