Abstract
Non-selective positive allosteric modulators (PAMs) of GABAA receptors (GABAARs) are known to impair anterograde memory. The role of the various GABAAR subtypes in the memory-impairing effects of non-selective GABAAR PAMs has not been fully elucidated. The current study assessed, in rhesus monkeys, effects of modulation of α1, α2/3, and α5GABAARs on visual recognition and spatial working memory using delayed matching-to-sample (DMTS) and self-ordered spatial search (SOSS) procedures, respectively. The DMTS procedure (n=8) involved selecting a previously presented ‘sample' image from a set of multiple images presented after a delay. The SOSS procedure (n=6) involved touching a number of boxes without repeats. The non-selective GABAAR PAM triazolam and the α1GABAA preferential PAMS zolpidem and zaleplon reduced accuracy in both procedures, whereas the α5GABAA preferential PAMs SH-053-2′F-R-CH3 and SH-053-2′F-S-CH3, and the α2/3GABAA preferential PAM TPA023B were without effects on accuracy or trial completion. The low-efficacy α5GABAAR negative allosteric modulator (NAM) PWZ-029 slightly increased only DMTS accuracy, whereas the high-efficacy α5GABAAR NAMs RY-23 and RY-24 did not affect accuracy under either procedure. Finally, the slopes of the accuracy dose-effect curves for triazolam, zolpidem, and zaleplon increased with box number in the SOSS procedure, but were equivalent across DMTS delays. The present results suggest that (1) α1GABAARs, compared with α2/3 and α5GABAARs, are primarily involved in the impairment, by non-selective GABAAR PAMs, of visual recognition and visuospatial working memory in nonhuman primates; and (2) relative cognitive impairment produced by positive modulation of GABAARs increases with number of locations to be remembered, but not with the delay for remembering.
Keywords: GABAA receptor, cognition, delayed-matching-to-sample, self-ordered spatial search, benzodiazepine, rhesus monkey
INTRODUCTION
Several clinically important effects of benzodiazepines (BZs), such as the anxiolytic, sedative, and muscle-relaxing effects, occur via positive allosteric modulation of GABAA receptors (GABAARs). BZs also can produce anterograde memory impairment (Haefely et al, 1993), which is a useful effect when these drugs are used as adjuncts to surgical anesthesia, but problematic when the drugs are used for other indications (eg, Kleykamp et al, 2012; Mintzer and Griffiths, 1999b). The discovery of the heterogeneity of GABAARs, particularly those that contain BZ binding sites (ie, α1-, α2-, α3-, and α5-containing GABAARs), spurred research into the development of subtype-selective ligands that might produce clinically desired effects without unwanted side effects (Atack, 2011a, 2011b; Ator et al, 2010; Clayton et al, 2007).
Of the BZ-sensitive GABAAR subtypes, α1-, α2/3-, and α5-containing GABAARs have been implicated in cognition and memory. For example, mice with a point mutation in the gene coding for α1GABAARs were insensitive to the recall-impairing effects of the non-selective positive allosteric modulator (PAM) diazepam on passive avoidance and punishment of drinking (Rudolph et al, 1999). Also in mice, elimination of α5GABAARs improved water maze performance (Collinson et al, 2002) and mice with a point mutation in α5GABAARs displayed greater trace fear conditioning than wild-type mice (Crestani et al, 2002). In addition, in mice and rats, investigational drugs that are negative allosteric modulators (NAMs) at α5GABAARs improved performance and/or attenuated drug-induced deficits in a variety of preclinical memory assessments (Atack et al, 2006; Ballard et al, 2009; Chambers et al, 2003; Collinson et al, 2006; Dawson et al, 2006; DeLorey et al, 2001; Harris et al, 2008; Savic et al, 2008).
In contrast to the amount of work conducted on the role of GABAAR subtypes in memory in rodents, relatively little work has been done on the role of GABAAR subtypes in memory in nonhuman or human primates. In one study, an α5GABAAR NAM Ro4938581 improved object retrieval and detour performance (ORD) in cynomolgus monkeys (Ballard et al, 2009); in another study, a low-efficacy α2/3GABAAR PAM TPA023 reversed ketamine-induced deficits on a spatial delayed-response task in rhesus monkeys (Castner et al, 2010). In rhesus monkeys, ORD deficits produced by the non-selective PAM triazolam and the α1GABAAR-preferring zolpidem were blocked by an α1GABAA-preferring antagonist β-CCT, whereas an α5GABAAR-preferring antagonist XLi-093 only blocked the triazolam-induced deficits (Makaron et al, 2013). Finally, in humans, the α1GABAAR-preferring PAM zolpidem has produced an impairment in a variety of recall tasks (Kleykamp et al, 2012; Mintzer and Griffiths, 1999a.
No study has examined the effects of selective modulation of the various GABAAR subtypes within a single study. The current study investigated the role of selective modulation of α1, α2/3, and α5GABAARs on visual recognition and visuospatial working memory in rhesus monkeys. The drugs tested (see Table 1) were a GABAA PAM with non-selective binding and efficacy (triazolam), two GABAA PAMs with selective binding affinity at α1GABAARs (zolpidem and zaleplon), a GABAA PAM with selective efficacy at α2, α3, and α5GABAARs (TPA023B), two novel PAMs with preferential affinity and/or efficacy at α5GABAARs (SH-053-2′F-R-CH3 and SH-053-2′F-S-CH3), and three NAMs with selective binding affinity and varying negative efficacies at α5GABAARs (PWZ-029, RY-23, and RY-24).
Table 1. Reported Binding Affinities and Efficacies in Modulating GABA-Induced Ion Flow at GABAAR Subtypes of the Modulators Used in the Current Study.
| Drugs | Affinity or % modulation | α1 | α2 | α3 | α5 | Reference |
|---|---|---|---|---|---|---|
| Triazolam | Affinity (Ki, nM) | 0.41 | 0.32 | 1.5 | 0.42 | Smith et al (2001) |
| % Modulation at 3 μM | 132 | 255 | 270 | 165 | Sanna et al (2002) | |
| Zolpidem | Affinity (Ki, nM) | 29.6 | 160 | 380 | >10,000 | Savic et al (2010) |
| % Modulation at 100 nM | 180 | 132 | 121 | NS | Savic et al (2010) | |
| Zaleplon | Affinity (Ki, nM) | 66 | 830 | 710 | 1780 | Dämgen and Lüddens (1999) |
| % Modulationa | 113 | 208 | 362 | 109 | Sanna et al (2002) | |
| TPA023B | Affinity (Ki, nM) | 0.73 | 2.0 | 1.8 | 1.1 | Atack et al (2011) |
| % Modulationa | 4 | 43 | 67 | 45 | Atack et al (2011) | |
| SH-053-R-2′F-CH3 | Affinity (Ki, nM) | 759.1 | 948.2 | 768.8 | 95.2 | Fischer et al (2010); Savic et al (2010) |
| % Modulation at 100 nM | 111 | 124 | 125 | 183 | Fischer et al (2010) | |
| % Modulation at 1 μM | 154 | 185 | 220 | 387 | Fischer et al (2010) | |
| SH-053-S-2′F-CH3 | Affinity (Ki, nM) | 468.2 | 33.3 | 291.5 | 19.2 | Fischer et al (2010); Savic et al (2010) |
| % Modulation at 100 nM | 116 | 170 | 138 | 218 | Fischer et al (2010) | |
| % Modulation at 1 μM | 164 | 348 | 301 | 389 | Fischer et al (2010) | |
| PWZ-029 | Affinity (Ki, nM) | >300 | >300 | >300 | 38.8 | Savic et al (2008) |
| % Modulation at 1 μM | 114 | 105 | 118 | −20 | Savic et al (2008) | |
| % Modulation at 10 μM | 120 | 115 | 145 | −20 | Savic et al (2008) | |
| RY-23 | Affinity (Ki, nM) | 197 | 143 | 255 | 2.61 | Liu et al (1996) |
| % Modulationa | −37 | −50 | −52 | June et al (2001) | ||
| RY-24 | Affinity (Ki, nM) | 26.9 | 26.3 | 18.7 | 0.4 | Liu et al (1996) |
| % Modulation at 1 μM | −31 | −20.7 | 0 | −40.4 | Harris et al (2008) |
NS=nonsignificant change in current flow.
Concentration not specified.
The behavioral procedures were selected from the Cambridge Neuropsychological Testing Automated Battery (CANTAB), which is a battery of tests that have been used extensively to study cognitive functioning in humans. Due to their non-verbal nature, CANTAB tests have been extended to nonhuman primates (Pearce et al, 1998; Roberts et al, 1990; Weed et al, 1999; Zurcher et al, 2010) and thereby allow direct cross-species comparisons. For the present study, a delayed matching-to-sample (DMTS) procedure, designed to measure visual recognition memory, and a self-ordered spatial search (SOSS) procedure, designed to measure visuospatial working memory, were used. DMTS and SOSS performances have been shown to be sensitive to mild cognitive impairment and Alzheimer's disease (Barbeau et al, 2004; Lange et al, 1995; Riekkinen et al, 1998; Sahakian et al, 1988), suggesting the clinical relevance of these procedures.
MATERIALS AND METHODS
Subjects
Adult male rhesus monkeys (Macaca mulatta, n=14), aged 11.8±4.10 (mean±SD) years at the start of these experiments, were singly housed, each in one section of a four-cage housing unit. All caging units were housed in the same temperature- and humidity-controlled vivarium room. Lights were on from 0700 to 02100 hours. Weights ranged between an average of 12.2 kg±1.28 at the beginning of the study and 14.5 kg±1.72 at the end of the study (∼3.8 years). Quantities of primate diet (2050 Teklad Global 20% Protein Primate Diet, Harlan Laboratories) fed once daily (139–208 g) were sufficient to permit gradual weight increase over the study. In addition, the monkeys received a piece of fresh fruit or vegetables (eg, ½ orange, ½ apple, and so on) 5 days a week. Daily feeding occurred approximately 2 h after completion of behavioral testing. Water was available at all times.
Apparatus
Experimental sessions were conducted in the home cage using custom-built mobile devices as described previously (Weed et al, 2008). Briefly, each held a computer for the control of experimental events (CANTAB software; Lafayette Industries, Lafayette, IN) and two touchscreen monitors (Intellitouch, surface acoustic wave technology, ELO TouchSystems, Menlo Park, CA, USA), which allowed two monkeys to be tested simultaneously. A pellet dispenser (BRS/VLE, Laurel, MD, or Med Associates, St Albans, VT) was used for the delivery of 190-mg food pellets (BioServ, Frenchtown, NJ).
DMTS and SOSS Procedures
Eight monkeys were trained on the DMTS procedure. Each session consisted of 24 trials with 8 trials at each of three delays (2, 30, or 300 s). The delay on each trial was selected randomly without replacement. Each trial began with presentation, on the center of the screen, of a pseudo-randomly selected sample image from a set of 600 different images (Photo Clip Art 150 000 by Hemera Technologies). A touch on the sample image within 30 s turned off the image and initiated the selected delay. After the delay, the original sample image and two other unique, randomly selected images were presented on three corners of the screen. A touch on the image that ‘matched' the original sample image produced a food pellet, followed by a 5-s period with the screen darkened. If the monkey did not touch the sample image within 30-s, did not touch one of the three choice images within 30 s, or if the monkey touched one of the two ‘non-matching' images, the trial ended without pellet delivery, followed by 10 s with the screen darkened. Sessions usually lasted about 45 min and were conducted Monday–Friday, starting at approximately 1000 or 1100 hours.
Six monkeys were trained on the SOSS procedure. Each session consisted of 54 trials, and each trial involved touchscreen presentation of a configuration of a number of small blue boxes within 16 possible screen locations (screen configured in a 4 by 4 array of possible locations). The number of boxes in the stimulus configuration varied among 2, 3, and 4 boxes (18 trials of each). Each non-repeating touch on any of the boxes produced a food pellet. If the monkey made a repeat touch or failed to make a touch within 30-s of trial onset or from the time of the previous touch, the trial ended and a 9-s period followed, during which the screen remained blank and touching the screen produced no scheduled consequence, which was followed by a new trial. If the monkey touched all the boxes without repetition, the trial ended, was defined as correct, and was followed by 5 s with the screen darkened before the next trial. Sessions generally lasted about 15–20 min and were conducted Monday–Friday, starting at approximately 1000 or 1100 hours.
Assessment of Drug Effects
Drug test sessions usually occurred on Tuesdays and Fridays if subjects had completed at least 7 of 8 trials at each delay (DMTS group) or 16 of 18 trials at each number of boxes (SOSS group) in the preceding session. Drug vehicle administration usually occurred on Thursdays. Baseline (no treatment) sessions occurred on Mondays and Wednesdays. In the DMTS group, the order of testing was RY-23 i.m. (intramuscular ), triazolam i.m., PWZ-029 i.m., SH-053-2′F-R-CH3 i.m., RY-24 i.m., zolpidem i.m., RY-23 p.o. (per os), PWZ-029 p.o., SH-053-2′F-S-CH3 p.o., TPA023B p.o., and zaleplon p.o. In the SOSS group, the order of testing was RY-23 i.m., triazolam i.m., PWZ-029 i.m., RY-24 i.m., zolpidem, RY-24 p.o. RY-23 p.o., zaleplon p.o., PWZ-029 p.o., and TPA023B p.o. Doses were studied in a pseudo-random order for each compound with the restriction that the highest two doses were studied after the other doses. Triazolam, zolpidem, SH-053-2′F-R-CH3, PWZ-029, RY-23, and RY-24 were administered via i.m. injection in the thigh at a volume of 0.2–1.5 ml. PWZ-029, RY-23, and RY-24 also were tested orally (p.o.) to extend the dose range beyond that which was feasible via the i.m. route. Zaleplon, TPA023B, and SH-053-2′F-S-CH3 only were administered p.o. due to solubility limitations. Pretreatment times were 30 min for i.m. administration of triazolam, zolpidem, PWZ-029, RY-23, RY-24, and SH-053-2′F-R-CH3 based on preliminary data collected in a subset of monkeys. Oral administration of zaleplon, TPA023B, SH-053-2′F-S-CH3, PWZ-029, RY-23, and RY-24 occurred 60 min before session start.
Drugs
PWZ-029 (methyl(8-chloro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-yl) methyl ester; Zhang et al, 1995), RY-23 (tert-Butyl 8-[(trimethylsilyl)ethynyl]-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate; Liu et al, 1996), RY-24 (tert-Butyl 8-ethynyl-5,6-dihydro-5methyl-6-oxo-4H-imidazo[1,5-a[1,4]benzodiazepine-3-carboxylate; Liu et al, 1996), and SH-053-2′F-R-CH3 ((S)-8-ethynyl-6-(2-fluorophenyl)-4-methyl-4H-2,5,10b-triaz-benzo[3]azulene-3-carboxylic acid ethyl ester; Cook et al, 2009), and SH-053-2′F-S-CH3 ((S)-8-ethynyl-6-(2-fluorophenyl)-4-methyl-4H-2,5,10b-triaz-benzo[3]azulene-3-carboxylic acid ethyl ester; Cook et al, 2009) were synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin, Milwaukee, WI. For i.m. injection, PWZ-029, RY-23, RY-24, and SH-053-2′F-R-CH3 were dissolved in 95% w/v ethanol, which was diluted with a 60:40 mixture of propylene glycol and 0.9% saline to a final concentration of 10–20% ethanol and 90–80% propylene glycol/saline mixture (prepared fresh each day), depending on doses to be tested. Triazolam (Upjohn Pharmaceuticals, Kalamazoo, MI) was dissolved in propylene glycol at 2 mg/ml (stored for up to 2 months) and then diluted with sterile water on test days to achieve the desired concentration for injection (minimum dilution of 50% sterile water). Zolpidem tartrate (Research Biochemicals International, Natick, MA) was dissolved in 0.9% saline (solutions were stored for up to 2 weeks). Zaleplon (Wyeth-Ayerst Research, Princeton, NJ) and TPA023B (6,2′-difluoro-50-[3-(1-hydroxy-1-methylethyl)imidazo[1,2-b][1,2,4]triazin-7-yl]biphenyl-2-carbonitrile; Merck, Sharp, & Dohme, Harlow, UK) only were administered orally due to solubility limitations.
For oral administration, monkeys were first habituated gradually, across days, to drinking a bitter solution consisting of increasing concentrations of quinine (up to 0.32 mg/ml) in 60 ml of Tang orange-drink solution off the tip of a 60-ml syringe. Consuming the 60 ml was followed immediately by the opportunity to drink 40 ml of unadulterated Tang (adapted from Turkkan et al, 1989). Once the consumption of the 0.32 mg/ml quinine solution was reliable, drug doses were suspended, fresh each test day, in 60 ml of a matrix of 1 g/l of Bio-Serv Agent K (Frenchtown, NJ), prepared in a blender in water that was flavored with the Tang powder.
Data Analysis
For the DMTS procedure, the percentage of correct trials in the session was calculated for individual monkeys by dividing the total number of correct trials at each delay by the total number of trials completed at that delay. The percentage of trials completed at each delay was calculated by dividing number of trials completed by total trials possible. For the SOSS procedure, the percentage of correct trials for each configuration (2, 3, and 4 boxes) was calculated for individual monkeys for each session by dividing the number of trials correct (ie, all boxes touched) divided by the number of trials in which the monkey touched at least one box. The percentage of trials completed for each number of boxes was calculated by dividing the number of trials in which the monkey touched at least one box by the number of trials possible. If a monkey did not complete three or more trials at any particular delay (DMTS) or number of boxes (SOSS) in a session, the percentage of correct trials was not calculated for that monkey. Group averages for percentage of correct trials were calculated only if three or more monkeys met the individual trial completion criterion.
Two-way repeated-measures analysis of variance (ANOVA) was used to identify statistically significant effects of drug dose and task parameter (DMTS: delay; SOSS: box number). Percentages of correct trials and trials completed were converted to proportions and arcsine square root transformed to increase normality for statistical analysis (McDonald, 2009). Post-hoc comparisons using the Holm-Sidak method were conducted to compare performance in vehicle sessions with performance following drug administration.
For drugs that affected accuracy, accuracy values for individual subjects at each dose were calculated as a percentage of the average vehicle accuracy at each parameter value for each of the two procedures. A linear model was fitted to the descending portion of the dose-response curve using log dose and the pooled individual subject data (Graphpad Prism version 5, Graphpad Software, San Diego, CA). Minimal effective doses (MED) for reducing accuracy were calculated from the linear model as the dose necessary to reduce accuracy by 15% of vehicle control. This value was selected because the drugs that significantly reduced accuracy all did so by 15% or more at each parameter. In order to facilitate comparison of potencies to reduce accuracy and trial completion, the same method was used to predict the dose required to reduce trial completion by 15%. Because trial completion did not vary across delay or number of boxes, normalization was unnecessary.
RESULTS
Control Sessions
DMTS accuracy after vehicle administration was highest after delays of 2 and 30 s and lowest after delays of 300 s (Figure 1, top panels, points above V). The percentage of trials completed did not differ as a function of delay (Figure 1, bottom panels, points above V). Similarly, SOSS accuracy following vehicle administration declined as the number of boxes in the stimulus configuration increased from 2 to 4 (Figure 2, top panels, points above V), whereas the percentage of SOSS trials completed did not differ as a function of the number of boxes (Figure 2, bottom panels, points above V).
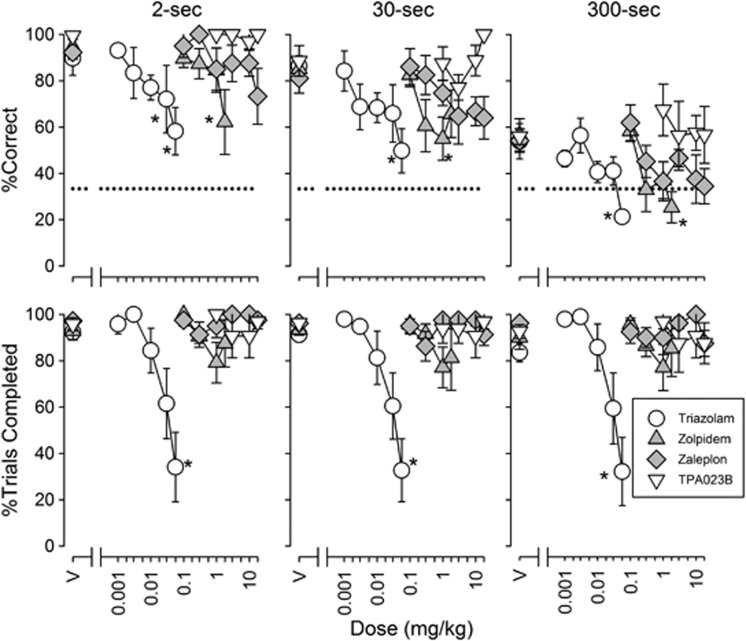
Figure 1.
Effects of triazolam (n=6), zolpidem (n=6), zaleplon (n=5), and TPA023B (n=4) on accuracy (top row) and trial completions (bottom row) for trials at each delay value in the delayed matching-to-sample (DMTS) procedure. Points above ‘V' represent results following vehicle administration. Each data point represents the mean across monkeys. Error bars represent ±1 SEM. The horizontal dashed line indicates a chance accuracy (33.3%). Statistically significant post-hoc comparisons relative to vehicle are denoted by asterisks.
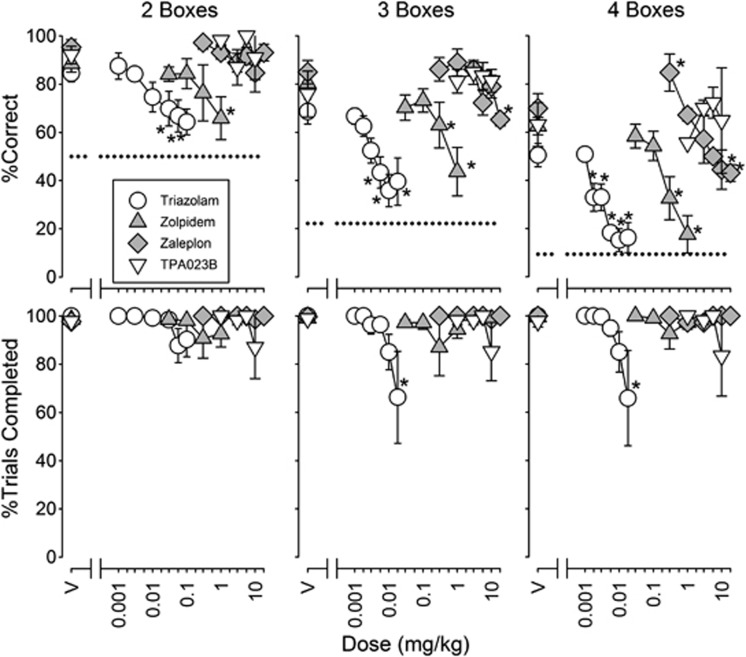
Figure 2.
Effects of triazolam (n=6), zolpidem (n=6), zaleplon (n=4), and TPA023B (n=4) on self-ordered spatial search (SOSS) accuracy (top) and trial completion (bottom) for trials with each number of boxes in the SOSS procedure. The horizontal dotted lines in the top row of graphs represent chance accuracy on two-box (50%), three-box (22.2%), and four-box (9.38%) trials.
PAMs: Triazolam, zolpidem, zaleplon, TPA023B, SH-053-2′F-R-CH3, and SH-053-2′F-S-CH3
The non-selective high-efficacy PAM triazolam and the high-efficacy α1GABAAR-preferential PAM zolpidem reduced accuracy of DMTS performance at all delays (Figure 1; F6,18=12.8, P<0.001 and F5,25=7.22, P<0.001, respectively) with MED values of 0.004–0.012 and 0.172–0.18 mg/kg, respectively (Table 2). Zaleplon, another high-efficacy α1GABAAR-preferential PAM also reduced overall DMTS accuracy (Figure 1; F6,24=6.20, P<0.001; main effect of 18 mg/kg) with MED values of ∼4–20 mg/kg (Table 2). Triazolam dose dependently reduced the percentage of DMTS trials completed (F6,30=10.5, P<0.001), whereas zolpidem and zaleplon did not (Figure 1). TPA023B, a PAM with non-selective affinity, but selective efficacy at α2/3GABAARs, did not affect DMTS accuracy or trial completion (Figure 1). Finally, the two α5GABAAR-preferential PAMs SH-053-2′F-R-CH3 and SH-053-2′F-S-CH3 did not affect DMTS accuracy or trial completion (Table 3).
Table 2. MED Values (mg/kg) for Reducing Accuracy (Defined as 85% of Vehicle Control Accuracy Values) and for Decreasing Trial Completion by the Same Amount for Each Drug that had Statistically Significant Effects on the Relevant-Dependent Measure.
| Drugs |
DMTS delays |
||
|---|---|---|---|
| 2-s | 30-s | 300-s | |
| Accuracy | |||
| Triazolam i.m. | 0.012 (0.003–0.613) | 0.004 (0–0.023) | 0.004 (0–0.016) |
| Zolpidem i.m. | NS | 0.180 (0–0.489) | 0.172 (0.005–0.402) |
| Zaleplon p.o. | 21.7 (3.50–a) | 4.05 (0.857–518.8) | 0.891 (0.004–6.724) |
| Trial completion | |||
| Triazolam i.m. | 0.007 (0.003–0.013) | 0.006 (0.002–0.011) | 0.007 (0.003–0.013) |
| RY-23 p.o. | 1.75 (0.294–3.064) | 1.62 (0.182–2.919) | 1.22 (0.014- 2.493) |
| RY-24 i.m. | 0.022 (0.011–0.035) | 0.041 (0.024–0.056) | 0.038 (0.018–0.054) |
|
SOSS number of boxes |
|||
|---|---|---|---|
| Two boxes | Three boxes | Four boxes | |
| Accuracy | |||
| Triazolam i.m. | 0.022 (0.009–0.079) | 0.004 (0.001–0.008) | 0.002 (0.001–0.006) |
| Zolpidem i.m. | NS | 0.162 (0.004–0.358) | 0.098 (0.012–0.188) |
| Zaleplon p.o | NS | 14.2 (5.70–194) | 2.65 (1.53–4.37) |
| Trial completion | |||
| Triazolam i.m. | NS | 0.050 (a–a) | 0.049 (a–a) |
| RY-24 i.m. | 0.048 (0.024–0.069) | 0.049 (0.025–0.070) | 0.048 (0.024–0.070) |
| RY-23 p.o. | 6.038 (0.993–7.829) | 5.837 (0–7.811) | 5.954 (0.46–7.777) |
| RY-24 p.o. | 0.414 (a–a) | 0.426 (a–a) | 0.415 (a–a) |
Abbreviations: DMTS, delayed matching-to-sample; i.m., intramuscular; p.o., per os; SOSS, self-ordered spatial search.
Numbers in parentheses are 95% confidence limits (CLs).
NS=nonsignificant linear regression.
95% CL could not be calculated.
Table 3. Percentage of Trials Correct and Completed After Administration of Vehicle (‘V') and Doses (mg/kg) of SH-053-2′F-S-CH3 (n=4) and SH-053-2′F-R-CH3 (n=3) During DMTS Trials of 2-, 30-, and 300-s Delays.
| Dose |
%Trials correct (SEM) |
%Trials completed (SEM) |
||||
|---|---|---|---|---|---|---|
| 2-s | 30-s | 300-s | 2-s | 30-s | 300-s | |
| SH-053-2′F-S-CH3 (p.o.) | ||||||
| V | 93.1 (6.94) | 83.3 (2.41) | 52.8 (7.35) | 91.7 (8.33) | 91.7 (8.33) | 91.7 (8.33) |
| 3 | 91.7 (4.17) | 85.0 (2.50) | 41.7 (9.30) | 100 (0.00) | 87.5 (12.5) | 91.7 (4.17) |
| 5.6 | 93.8 (6.25) | 77.4 (12.4) | 66.7 (16.7) | 95.8 (4.17) | 91.7 (4.17) | 91.7 (8.33) |
| 10 | 95.8 (4.17) | 91.7 (8.33) | 36.3 (4.17) | 100 (0.00) | 95.8 (4.17) | 91.7 (4.17) |
| SH-053-2′F-R-CH3 (i.m.) | ||||||
| V | 89.2 (9.26) | 84.3 (6.16) | 54.2 (6.16) | 98.4 (0.90) | 99.2 (0.78) | 99.2 (0.78) |
| 0.03 | 84.4 (15.6) | 86.2 (10.1) | 56.3 (14.9) | 100 (0.00) | 96.9 (3.13) | 100 (0.00) |
| 0.3 | 84.4 (9.38) | 78.1 (10.7) | 53.6 (13.4) | 100 (0.00) | 100 (0.00) | 96.9 (3.13) |
| 1 | 78.1 (12.9) | 83.5 (6.49) | 64.3 (8.15) | 100 (0.00) | 96.9 (3.13) | 96.9 (3.13) |
| 1.8 | 87.5 (7.22) | 83.3 (16.7) | 60.7 (7.43) | 100 (0.00) | 100 (0.00) | 95.8 (4.17) |
Abbreviations: DMTS, delayed matching-to-sample; i.m., intramuscular; p.o., per os.
Triazolam, zolpidem, and zaleplon also dose dependently decreased SOSS accuracy (Figure 2; F7,35=18.7, P<0.001, F5,35=7.88, P<0.001, F6,30=5.30, P<0.001, respectively). The lowest dose of zaleplon increased SOSS accuracy on four-box trials (Figure 2, top rightmost graph). MED values for decreasing SOSS accuracy were 0.002–0.022, 0.098–0.162, and 2.65–14.2 mg/kg for triazolam, zolpidem, and zaleplon, respectively (Table 2). Triazolam decreased SOSS trials completed (Figure 2; F7,35=3.18, P=0.01) on three- and four- but, not on two-box trials (post-hoc comparisons) with a potency of ∼0.05 mg/kg. Zolpidem and zaleplon did not alter SOSS trial completion (Figure 2). TPA023B was without effect on SOSS accuracy and trial completion (Figure 2).
Negative Allosteric Modulators: PWZ-029, RY-23, and RY-24
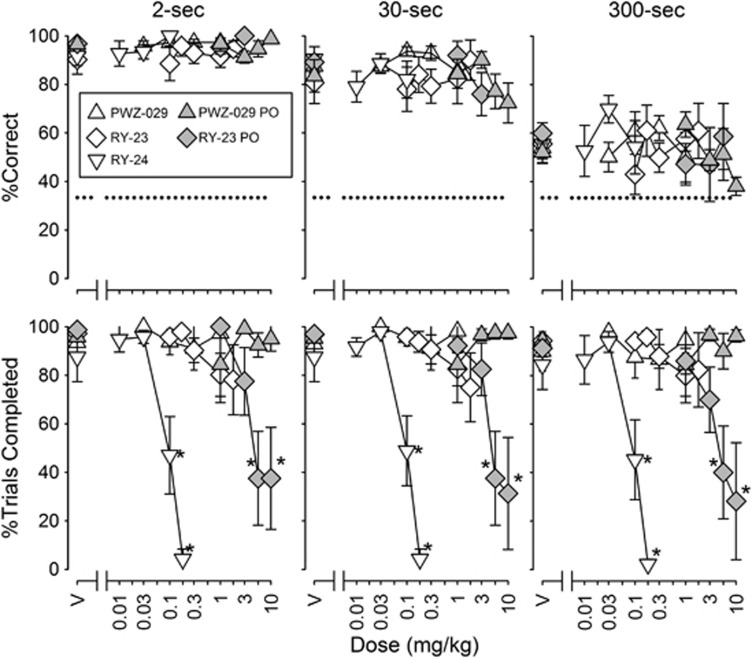
The low-efficacy α5GABAAR-selective NAM PWZ-029 (i.m.) produced a small, but statistically significant, improvement in performance (Figure 3; F5,25=3.72, P=0.012), but post-hoc tests did not reveal a dose at which the performance differed significantly from vehicle performance and when administered orally, PWZ-029 was without statistically significant effect on accuracy or trial completion (Figure 3). The high-efficacy α5GABAAR-selective NAMs RY-23 (i.m. or p.o.) and RY-24 (i.m.) were without statistically significant effects on DMTS accuracy (Figure 3, top row). Trial completions at all delays were dose dependently decreased by i.m. RY-24 (Figure 3; F4,20=7.34, P<0.001) and p.o. RY-23 (F5,10=10.22, P=0.001), but not i.m. RY-23. The MED values of i.m. RY-24 and p.o. RY-23 in reducing trial completion were 0.022–0.041 and 1.22–1.75 mg/kg, respectively (Table 2).
Figure 3.
Effects of PWZ-029 i.m. (intramuscular) (n=7) and p.o. (per os) (n=5), RY-23 i.m. (n=6) and p.o. (n=5), and RY-24 i.m. (n=6) on delayed matching-to-sample (DMTS) accuracy (top) and trial completion (bottom). Other details are as in Figure 1.
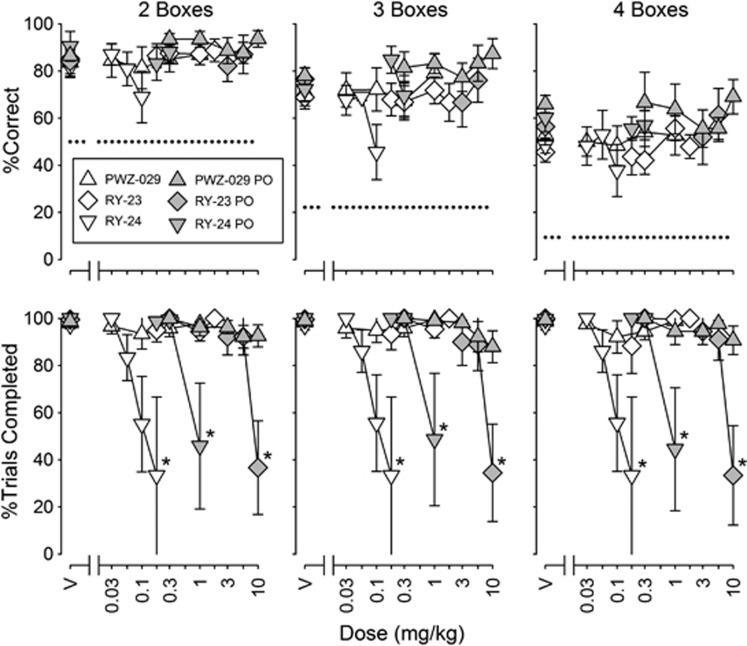
In the SOSS procedure, RY-23 (i.m. and p.o.), RY-24 (i.m. and p.o.), and PWZ-029 (i.m. and p.o.) were without significant effects on accuracy (Figure 4), despite an apparent decreasing trend in accuracy with RY-24 (i.m.) on two- and three-box trials. Trial completions at all box numbers were decreased by RY-23 (p.o.) and RY-24 (i.m. and p.o.). Decreases in SOSS trial completion were statistically significant for p.o. RY-24 and p.o. RY-23 (Figure 4; F4,12=4.65, P=0.017; and F4,16=8.05, P<0.001, respectively) and for i.m. RY-24 when the two subjects that were tested with the highest dose of 0.18 mg/kg were used in the ANOVA (F6,6=17.1, P=0.002). (Only two monkeys were tested at 0.18 mg/kg because there was⩾50% suppression of trial completion at 0.1 mg/kg for the other monkeys, and we were concerned about adverse effects if we tested the higher dose in those monkeys.) MED values for reducing SOSS trial completion were ∼0.05, 0.4, and 0.6 mg/kg for RY-24 i.m., RY-24 p.o., and RY-23 p.o. (Table 2).
Figure 4.
Effects of PWZ-029 i.m. (n=6) and p.o. (n=6), RY-23 i.m. (n=6) and p.o. (n=5), and RY-24 i.m. (n=6 except that only two monkeys were tested at the highest dose as explained in Results) and p.o. (n=4) on self-ordered spatial search (SOSS) accuracy (top) and trial completion (bottom). Other details are as in Figure 2.
Comparison of Effects of Triazolam, Zolpidem, and Zaleplon on DMTS and SOSS Performances
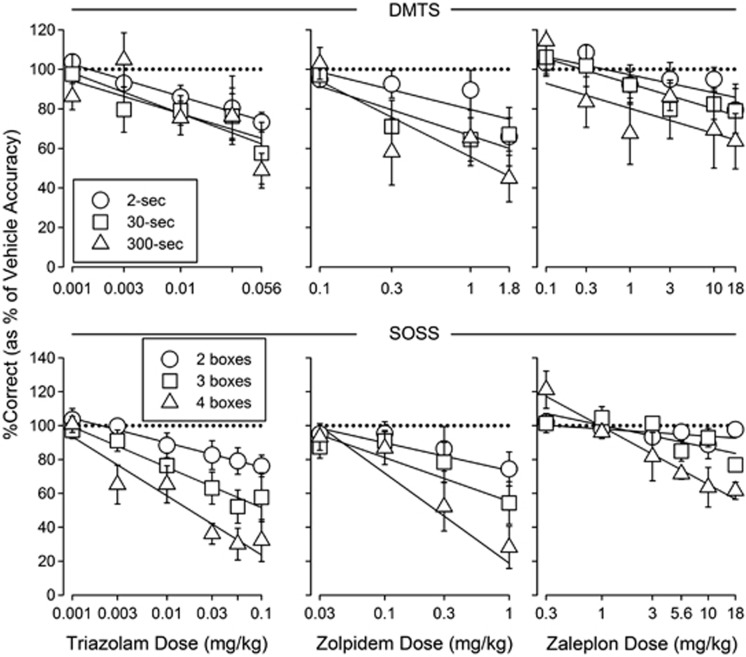
For triazolam, zolpidem, and zaleplon, the relative impairment of accuracy did not depend on DMTS delay. The slopes of the dose-response curves for reducing DMTS accuracy at each delay value were not significantly different (Figure 5, top panels). In contrast, in the SOSS procedure, relative impairment of accuracy depended on the number of locations to be remembered. The slopes of the triazolam, zolpidem, and zaleplon dose-response curves varied significantly as number of boxes increased (Figure 5, bottom panels; F2,98=4.83, P=0.01; F2,66=3.43, P=0.0384; and F2,66=12.3, P<0.001, respectively).
Figure 5.
Normalized (% of vehicle accuracy values) dose-response curves along with best-fitting straight lines for triazolam's (left), zolpidem's (middle), and zaleplon's (right) effects on delayed matching-to-sample (DMTS) (top) and self-ordered spatial search (SOSS) (bottom) accuracy. Each data point represents the mean across monkeys. Error bars represent ±1 SEM. The horizontal dotted lines are for reference and indicate 100% of vehicle accuracy. Solid lines represent best-fitting lines. Other details are as in Figures 1 and 2 for DMTS and SOSS, respectively.
DISCUSSION
The current results with triazolam are consistent with a number of studies demonstrating working memory impairment with other non-selective BZs in nonhuman primates (Baron and Wenger, 2001; Bradley and Nicholson, 1984; Myers and Hamilton, 2011; Schulze et al, 1989). Our results with subtype-selective GABAAR PAMs suggest that modulation of α1GABAARs, but not α2/3 or α5GABAARs impairs visual recognition and visuospatial working memory in nonhuman primates; and, by extension, our results suggest that cognitive impairment produced by non-selective GABAAR PAMs is primarily due to the modulation of α1GABAARs. First and foremost, zolpidem, which has virtually no affinity for α5GABAARs, and zaleplon, which has very low affinity for this subtype, produced dose-dependent reductions in DMTS and SOSS accuracy, suggesting a lack of involvement of α5GABAARs in the effects of non-selective GABAAR PAMs. Second, neither the α5GABAAR-selective PAMs (SH-053-2′F-R-CH3 and SH-053-2′F-S-CH3) nor the α5GABAAR-selective NAMs (PWZ-029, RY-23, and RY-24) affected DMTS or SOSS accuracy, even at doses of some of the NAMs that profoundly suppressed responding. Finally, the lack of effects of TPA023B, at mg/kg doses shown to produce close to 100% occupancy of α2/3GABAARs in humans, baboons, rats, and mice (Atack et al, 2011), suggests that positive modulation of α2/3GABAARs, at least at low levels, is not sufficient to produce impairments in visual recognition and visuospatial working memory. Interestingly, the lowest dose of zaleplon was associated with an improvement in SOSS accuracy on four-box trials, but this outcome was not shown with zolpidem, suggesting that such an improvement is not a general feature of low doses of non-selective or α1GABAAR-preferential PAMs. Together, these results suggest that positive allosteric modulation of α1GABAARs, compared with α2/3 and α5GABAARs, is the major contributor to visual recognition and visuospatial working memory impairment produced by non-selective GABAAR PAMs in nonhuman primates.
The lack of consistent, dose-dependent effects on DMTS and SOSS accuracy of the α5GABAAR NAMs is partially consistent with results in elderly human participants that demonstrated no effect in a paired-associates learning task at a low dose and impairment at a high dose of the α5GABAAR NAM α5IA (Atack, 2010). The lack of robust effects of the α5GABAAR NAMs on DMTS and SOSS accuracy in the present study with rhesus monkeys do not appear consistent with results in rodents that demonstrated improved performance in Morris water maze and passive avoidance procedures with a variety of α5GABAAR NAMs (Atack et al, 2006; Chambers et al, 2003, 2004; Collinson et al, 2006; Savic et al, 2008). Nor are the present results consistent with those in cynomolgus monkeys that demonstrated improved ORD performance with an α5GABAAR NAM (Ballard et al, 2009). The variables responsible for these discrepancies are not clear, but may include both task and species. Perhaps the simplest explanation is that α5GABAAR NAMs do not have pro-cognitive effects on short-term working memory performances maintained by positive reinforcement. This conclusion is consistent with the current results as well as the lack of improvement with Ro4938591 on delayed matching-to-position performance in rats (Ballard et al, 2009) and the reported pro-cognitive effects on performance in the Morris water maze and untrained behavior such as passive avoidance behavior and ORD reaching. Importantly, PWZ-029 improved novel object recognition in rats without improving Morris water maze performance (Milic et al, 2013), demonstrating that α5GABAAR NAMs do not universally improve performance in aversively motivated working memory procedures such as the Morris water maze.
In addition, the failure of the α5GABAAR PAMs SH-053-2′F-R-CH3 and SH-053-2′F-S-CH3 to affect either DMTS or SOSS performance is consistent with the results in rodents in which SH-053-2′F-R-CH3 was without effect on Morris water maze performance (Savic et al, 2010) and consistent with the failure of the α5GABAAR NAMs PWZ-029, RY-23, and RY-24 to alter DMTS or SOSS accuracy. Interestingly, a recent study demonstrated that an α5GABAAR PAM improved radial arm maze performance in aged impaired rats at doses that produced no change in the performance in young rats (Koh et al, 2012), which suggests that the effects of positive allosteric modulation of α5GABAARs may depend on the age of the subjects and/or the performance baseline.
The effects of zolpidem on DMTS accuracy compared with DMTS trial completion were more selective than those of triazolam. The ratios of the triazolam MED values for reducing accuracy and decreasing trial completion ranged from ∼0.6 to 1.8. In contrast, zolpidem was without significant effects on trial completion at doses 10-fold greater than its MED value for reducing accuracy, indicating that the selectivity of zolpidem to reduce accuracy compared with trial completion was greater than that of triazolam. Whether the effects of zolpidem on accuracy and trial completion in the SOSS procedure were more selective than those of triazolam is unclear. The selectivity of triazolam for impairing SOSS accuracy compared with suppressing SOSS trial completion, based on ratios of MED values for the two measures (∼13–25), was much higher than its selectivity in the DMTS procedure; a conservative estimate of the selectivity of zolpidem based on the highest dose tested (1 mg/kg) compared to its MED values for reducing SOSS accuracy (0.098–0.162) suggests a selectivity within that range.
The reliability of the effects of GABAAR PAMs and NAMs in the current study is demonstrated by the replication of effects across the two procedures and in the two groups of monkeys. Also, the current results demonstrate that degree of impairment produced by GABAAR PAMs varied in a load-, but not delay-dependent fashion; relative impairment increased with the number of locations to be remembered in the SOSS procedure, but did not vary with the delay for remembering a stimulus in the DMTS procedure. The delay-independent effects of triazolam are consistent with studies reporting delay-independent impairment of DMTS performance by the non-selective GABAAR PAM diazepam in humans and monkeys (Robbins et al, 1997; Schulze et al, 1989). The current study extends those findings to α1-selective GABAAR PAMs. The differential sensitivity of SOSS, but not DMTS, performance to triazolam, zolpidem, and zaleplon is consistent with the notion that the two procedures measure different aspects of working memory and suggests that positive allosteric modulation of GABAARs has a greater relative impact on spatial working memory as the number of locations to be remembered increases, but not on visual recognition working memory as the delay for remembering increases.
In conclusion, the present results suggest a prominent role of α1GABAARs in the effects of non-selective GABAAR PAMs on visual recognition and visuospatial working memory in nonhuman primates. This finding implicates α1GABAARs in nonhuman primate working memory under unperturbed conditions; however, this conclusion is tentative given the risk associated with extrapolating from receptor function in perturbed (ie, drug-induced) conditions to receptor function in unperturbed conditions. The finding of a prominent role of α1GABAARs in the effects of non-selective GABAAR PAMs on visual recognition and visuospatial working memory further suggests that the efforts to separate sedative and working memory-impairing effects of GABAAR modulation may not be feasible because it appears that α1GABAARs are involved in both the sedative and memory-altering effects of drugs such as BZs. However, these studies suggest that positive modulators of α2/3GABAARs, which have been pursued as non-sedating anxiolytics (eg, Atack, 2011a), would have minimal impact on cognitive function. The current results do not identify underlying brain regions or circuits involved in the effects of GABAAR modulation on working memory, but future research might investigate local infusion of GABAAR modulators to determine brain region involvement. Future research also might investigate the effects of allosteric modulation of GABAAR subtypes on visual recognition and visuospatial working memory in aged monkeys or in animals with neurobiological or pharmacological impairment to determine whether the effects vary as a function of age or state of impairment.
FUNDING AND DISCLOSURE
This work was supported by NIH grants R01-AG-027798 (NAA), R01-MH-046851 (JMC), and institutional funds of the Division of Behavioral Biology, Department of Psychiatry and Behavioral Sciences, The Johns Hopkins University School of Medicine. In the past 3 years, we have received compensation for professional services as follows: Dr Soto's work has been funded by the NIH and he has received compensation, unrelated to his scientific work, for database/software consulting from the Shands Hospital at the University of Florida. Dr Ator's work has been funded by the NIH and she has received funding from Helsinn Healthcare to conduct an abuse liability evaluation of an unrelated compound. Dr Ator also has received compensation from Bristol Myers-Squibb and F Hoffman LaRoche for consulting on abuse liability evaluation. Dr Rallapalli and Dr Biawat have received funding from the NIH. Dr Clayton is an employee of Chromatic Technologies. Chromatic Technologies. provided no financial support for these studies and had no scientific involvement. Dr Cook has received funding from NIH. Dr Cook currently holds patents on several of the compounds used in the current study. Dr Weed initiated these NIH-funded studies at Johns Hopkins School of Medicine before becoming an employee of Bristol Myers-Squibb. Upon leaving Johns Hopkins, direction of the studies was under the exclusive control of Drs. Ator and Soto. Bristol Myers-Squibb provided no financial support for these studies and had no scientific involvement.
Acknowledgments
We thank Stacey Perry, Raymond Smith, and Virginia Bogdan for their expert technical assistance in conducting these studies. We also thank Jonathan L Katz, Ph.D. for numerous helpful comments and discussions on the manuscript.
References
- Atack JR. Preclinical and clinical pharmacology of the GABAA receptor alpha5 subtype-selective inverse agonist alpha5IA. Pharmacol Ther. 2010;125:11–26. doi: 10.1016/j.pharmthera.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Atack JR. GABAA receptor subtype-selective modulators. I. alpha2/alpha3-selective agonists as non-sedating anxiolytics. Curr Top Med Chem. 2011a;11:1176–1202. doi: 10.2174/156802611795371350. [DOI] [PubMed] [Google Scholar]
- Atack JR. GABAA receptor subtype-selective modulators. II. alpha5-selective inverse agonists for cognition enhancement. Curr Top Med Chem. 2011b;11:1203–1214. doi: 10.2174/156802611795371314. [DOI] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABAA receptors. Neuropharmacology. 2006;51:1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Atack JR, Hallett DJ, Tye S, Wafford KA, Ryan C, Sanabria-Bohorquez SM, et al. Preclinical and clinical pharmacology of TPA023B, a GABAA receptor α2/α3 subtype-selective partial agonist. J Psychopharmacol. 2011;25:329–344. doi: 10.1177/0269881109354928. [DOI] [PubMed] [Google Scholar]
- Ator NA, Atack JR, Hargreaves RJ, Burns HD, Dawson GR. Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at alpha1 and alpha2/3 subtypes. J Pharmacol Exp Ther. 2010;332:4–16. doi: 10.1124/jpet.109.158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard TM, Knoflach F, Prinssen E, Borroni E, Vivian JA, Basile J, et al. RO4938581, a novel cognitive enhancer acting at GABAA alpha5 subunit-containing receptors. Psychopharmacology (Berl) 2009;202:207–223. doi: 10.1007/s00213-008-1357-7. [DOI] [PubMed] [Google Scholar]
- Barbeau E, Didic M, Tramoni E, Felician O, Joubert S, Sontheimer A, et al. Evaluation of visual recognition memory in MCI patients. Neurology. 2004;62:1317–1322. doi: 10.1212/01.wnl.0000120548.24298.db. [DOI] [PubMed] [Google Scholar]
- Baron SP, Wenger GR. Effects of drugs of abuse on response accuracy and bias under a delayed matching-to-sample procedure in squirrel monkeys. Behav Pharmacol. 2001;12:247–256. doi: 10.1097/00008877-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Bradley CM, Nicholson AN. Activity of the chloro- and triazolo-benzodiazepines. Behavioural studies in the monkey (Macaca mulatta) Neuropharmacology. 1984;23:327–331. doi: 10.1016/0028-3908(84)90195-3. [DOI] [PubMed] [Google Scholar]
- Castner SA, Arriza JL, Roberts JC, Mrzljak L, Christian EP, Williams GV. Reversal of ketamine-induced working memory impairments by the GABAAalpha2/3 agonist TPA023. Biol Psychiatry. 2010;67:998–1001. doi: 10.1016/j.biopsych.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Broughton HB, Collinson N, Cook S, Dawson GR, et al. Identification of a novel, selective GABA(A) alpha5 receptor inverse agonist which enhances cognition. J Med Chem. 2003;46:2227–2240. doi: 10.1021/jm020582q. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Carling RW, Collinson N, Cook SM, Dawson GR, et al. An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABAA alpha5 receptors with cognition enhancing properties. J Med Chem. 2004;47:5829–5832. doi: 10.1021/jm040863t. [DOI] [PubMed] [Google Scholar]
- Clayton T, Chen JL, Ernst M, Richter L, Cromer BA, Morton CJ, et al. An updated unified pharmacophore model of the benzodiazepine binding site on gamma-aminobutyric acid(a) receptors: correlation with comparative models. Curr Med Chem. 2007;14:2755–2775. doi: 10.2174/092986707782360097. [DOI] [PubMed] [Google Scholar]
- Collinson N, Atack JR, Laughton P, Dawson GR, Stephens DN. An inverse agonist selective for alpha5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology (Berl) 2006;188:619–628. doi: 10.1007/s00213-006-0361-z. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JM, Zhou H, Huang S, Sarma PVVS, Zhang C.2009. Stereospecific anxiolytic and anticonvulsant agents with reduced muscle-relaxant, sedative-hypnotic and ataxic effects. US Patent 7,681,958.
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, et al. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dämgen K, Lüddens H. Zaleplon displays a selectivity to recombinant GABAA receptors different from zolipdem, zopiclone and benzodiazepines. Neurosci Res Commun. 1999;25:139–148. [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, et al. An inverse agonist selective for alpha5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther. 2006;316:1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Lin RC, McBrady B, He X, Cook JM, Lameh J, et al. Influence of benzodiazepine binding site ligands on fear-conditioned contextual memory. Eur J Pharmacol. 2001;426:45–54. doi: 10.1016/s0014-2999(01)01199-2. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Licata SC, Edwankar RV, Wang ZJ, Huang S, He X, et al. Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology. 2010;59:612–618. doi: 10.1016/j.neuropharm.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefely WE, Martin JR, Richards JG, Schoch P. The multiplicity of actions of benzodiazepine receptor ligands. Can J Psychiatry. 1993;38 (Suppl 4:S102–S108. [PubMed] [Google Scholar]
- Harris D, Clayton T, Cook J, Sahbaie P, Halliwell RF, Furtmuller R, et al. Selective influence on contextual memory: physiochemical properties associated with selectivity of benzodiazepine ligands at GABAA receptors containing the alpha5 subunit. J Med Chem. 2008;51:3788–3803. doi: 10.1021/jm701433b. [DOI] [PubMed] [Google Scholar]
- June HL, Harvey SC, Foster KL, McKay PF, Cummings R, Garcia M, et al. GABA(A) receptors containing (alpha)5 subunits in the CA1 and CA3 hippocampal fields regulate ethanol-motivated behaviors: an extended ethanol reward circuitry. J Neurosci. 2001;21:2166–2177. doi: 10.1523/JNEUROSCI.21-06-02166.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleykamp BA, Griffiths RR, McCann UD, Smith MT, Mintzer MZ. Acute effects of zolpidem extended-release on cognitive performance and sleep in healthy males after repeated nightly use. Exp Clin Psychopharmacol. 2012;20:28–39. doi: 10.1037/a0025237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Rosenzweig-Lipson S, Gallagher M. Selective GABA(A) alpha5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology. 2012;64:145–152. doi: 10.1016/j.neuropharm.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KW, Sahakian BJ, Quinn NP, Marsden CD, Robbins TW. Comparison of executive and visuospatial memory function in Huntington's disease and dementia of Alzheimer type matched for degree of dementia. J Neurol Neurosurg Psychiatry. 1995;58:598–606. doi: 10.1136/jnnp.58.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Hu RJ, Zhang P, Skolnick P, Cook JM. Synthesis and pharmacological properties of novel 8-substituted imidazobenzodiazepines: high-affinity, selective probes for alpha 5-containing GABAA receptors. J Med Chem. 1996;39:1928–1934. doi: 10.1021/jm950887n. [DOI] [PubMed] [Google Scholar]
- Makaron L, Moran CA, Namjoshi O, Rallapalli S, Cook JM, Rowlett JK. Cognition-impairing effects of benzodiazepine-type drugs: role of GABAA receptor subtypes in an executive function task in rhesus monkeys. Pharmacol Biochem Behav. 2013;104:62–68. doi: 10.1016/j.pbb.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JH.2009Handbook of Biological Statistics2nd ednSparky House Publishing: Baltimore, MD, USA [Google Scholar]
- Milic M, Timic T, Joksimovic S, Biawat P, Rallapalli S, Divljakovic J, et al. PWZ-029, an inverse agonist selective for alpha(5) GABAA receptors, improves object recognition, but not water-maze memory in normal and scopolamine-treated rats. Behav Brain Res. 2013;241:206–213. doi: 10.1016/j.bbr.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Selective effects of zolpidem on human memory functions. J Psychopharmacol. 1999a;13:18–31. doi: 10.1177/026988119901300103. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Triazolam and zolpidem: effects on human memory and attentional processes. Psychopharmacology (Berl) 1999b;144:8–19. doi: 10.1007/s002130050971. [DOI] [PubMed] [Google Scholar]
- Myers TM, Hamilton LR. Delayed match-to-sample performance in African green monkeys (Chlorocebus aethiops sabaeus): effects of benzodiazepine, cholinergic, and anticholinergic drugs. Behav Pharmacol. 2011;22:814–823. doi: 10.1097/FBP.0b013e32834d6292. [DOI] [PubMed] [Google Scholar]
- Pearce PC, Crofts HS, Muggleton NG, Scott EA. Concurrent monitoring of EEG and performance in the common marmoset: a methodological approach. Physiol Behav. 1998;63:591–599. doi: 10.1016/s0031-9384(97)00494-0. [DOI] [PubMed] [Google Scholar]
- Riekkinen M, Soininen H, Riekkinen P, Sr, Kuikka J, Laakso M, Helkala EL, et al. Tetrahydroaminoacridine improves the recency effect in Alzheimer's disease. Neuroscience. 1998;83:471–479. doi: 10.1016/s0306-4522(97)00400-4. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A, et al. Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacology (Berl) 1997;134:95–106. doi: 10.1007/s002130050430. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, Everitt BJ, Jones GH, Sirkia TE, Wilkinson J, et al. The effects of excitotoxic lesions of the basal forebrain on the acquisition, retention and serial reversal of visual discriminations in marmosets. Neuroscience. 1990;34:311–329. doi: 10.1016/0306-4522(90)90142-q. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, et al. 1988A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson's disease Brain 111)Pt 3):695–718. [DOI] [PubMed] [Google Scholar]
- Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, et al. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol. 2002;451:103–110. doi: 10.1016/s0014-2999(02)02191-x. [DOI] [PubMed] [Google Scholar]
- Savic MM, Clayton T, Furtmuller R, Gavrilovic I, Samardzic J, Savic S, et al. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABA(A) receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats. Brain Res. 2008;1208:150–159. doi: 10.1016/j.brainres.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic MM, Majumder S, Huang S, Edwankar RV, Furtmuller R, Joksimovic S, et al. Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at alpha1 plus alpha5 versus alpha2 plus alpha3 subunits account for dissimilarities in behavioral effects in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:376–386. doi: 10.1016/j.pnpbp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze GE, Slikker W, Jr., Paule MG. Multiple behavioral effects of diazepam in rhesus monkeys. Pharmacol Biochem Behav. 1989;34:29–35. doi: 10.1016/0091-3057(89)90348-1. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, et al. Effect of alpha subunit on allosteric modulation of ion channel function in stably expressed human recombinant gamma-aminobutyric acid(A) receptors determined using (36)Cl ion flux. Mol Pharmacol. 2001;59:1108–1118. doi: 10.1124/mol.59.5.1108. [DOI] [PubMed] [Google Scholar]
- Turkkan JS, Ator NA, Brady JV, Craven KA.1989Beyond chronic catheterization in laboratory primatesIn: Segal EF (ed).Housing, Care and Psychological Wellbeing of Captive and Laboratory Primates Noyes Publications: Park Ridge, NJ, USA; 305–324. [Google Scholar]
- Weed MR, Bryant R, Perry S. Cognitive development in macaques: attentional set-shifting in juvenile and adult rhesus monkeys. Neuroscience. 2008;157:22–28. doi: 10.1016/j.neuroscience.2008.08.047. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, et al. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhang W, Liu R, Harris B, Skolnick P, Cook JM. Synthesis of novel imidazobenzodiazepines as probes of the pharmacophore for ‘diazepam-insensitive' GABAA receptors. J Med Chem. 1995;38:1679–1688. doi: 10.1021/jm00010a013. [DOI] [PubMed] [Google Scholar]
- Zurcher NR, Rodriguez JS, Jenkins SL, Keenan K, Bartlett TQ, McDonald TJ, et al. Performance of juvenile baboons on neuropsychological tests assessing associative learning, motivation and attention. J Neurosci Methods. 2010;188:219–225. doi: 10.1016/j.jneumeth.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]