Abstract
Organ printing can be defined as layer-by-layer additive robotic biofabrication of three-dimensional functional living macrotissues and organ constructs using tissue spheroids as building blocks. The microtissues and tissue spheroids are living materials with certain measurable, evolving and potentially controllable composition, material and biological properties. Closely placed tissue spheroids undergo tissue fusion — a process that represents a fundamental biological and biophysical principle of developmental biology-inspired directed tissue self-assembly. It is possible to engineer small segments of an intraorgan branched vascular tree by using solid and lumenized vascular tissue spheroids. Organ printing could dramatically enhance and transform the field of tissue engineering by enabling large-scale industrial robotic biofabrication of living human organ constructs with “built-in” perfusable intraorgan branched vascular tree. Thus, organ printing is a new emerging enabling technology paradigm which represents a developmental biology-inspired alternative to classic biodegradable solid scaffold-based approaches in tissue engineering.
Keywords: Tissue engineering, Organ printing, Tissue spheroids, Tissue fusion, Bioreactor
1. Introduction
The ultimate goal of tissue engineering is to design and fabricate natural-like functional human tissues and organs suitable for regeneration, repair and replacement of damaged, injured or lost human organs [1–4]. The engineered tissues and organs are technically “artificial” or “man-made” based on methods of their generation, but at the same time they are also “natural-like” living tissues and organs. At least theoretically, an ideal tissue engineered human organ must eliminate, dramatically reduce, or more realistically reinvent the problem of biocompatibility [5], which is a critically important issue for any biomaterial-based approach for creating artificial organs, devices or prostheses. Without tissue engineering, living functional human organs can be produced only during natural embryonic development. How close tissue engineers can recapitulate and capture the most essential structure–function features of normal natural human tissues and organs, and how far they must try to imitate developmental histogenesis, morphogenesis and organogenesis, are still open for debate. Because of economic constraints and the intrinsic limitations of producing living tissues and organs, it is reasonable to assume that biofabrication technology will probably not allow one to create a 100% authentic copy of functional living human organs. Rather, engineering of natural-like functional vascularized tissue engineered organ constructs capable of restoring essential function is a more realistic technological goal and represent a great accomplishment if successful. Developmental processes already serve as a positive control or reference point and provide powerful insights into tissue engineering [6,7]. Furthermore, the integration of developmental biology and tissue engineering, or the so-called biomimetic approach, is not wishful thinking but rather work in progress [8]. It is interesting that one initially suggested term for the tissue engineering field was “chimeric neomorphogenesis” [9]. Thus, it is safe to predict that deep understanding, biomimicking and employing developmental mechanisms of embryonic histogenesis and organogenesis can be very beneficial for tissue engineering. The goal of this paper is to introduce and discuss a novel, rapidly emerging developmental biology-inspired paradigm of solid biodegradable scaffold-free minitissue-based biomimetic approach, or more specifically, organ printing using self-assembled tissue spheroids as a possible alternative to classic solid biodegradable scaffold-based approach in the field of tissue engineering.
2. Intrinsic limitations of biodegradable solid scaffold-based approaches in tissue engineering
Classic biodegradable solid scaffold-based approaches, that still represent a dominating conceptual framework or paradigm in tissue engineering, originated from attempts by chemical engineers to create porous scaffolds from biodegradable polymers as a temporal template-like instructive support for cell attachment and tissue neomorphogenesis [9,10]. Thus, as clearly outlined in a recent insightful review on the mechanism of biocompatibility [5], the classic solid biodegradable scaffold-based approach in tissue engineering has its roots in biomaterial science and represents an adaptation of degradable polymers used in medical devices for the purpose of engineering living tissues. However, as pointed out in the review [5], at least initially, “…the successful use of degradable materials in medical devices has, unfortunately, been extrapolated into the ‘potential’ for such materials to be used in tissue engineered products, without an understanding of the requirements and specifications for these two quite different applications”. There are several basic assumptions behind classic biodegradable solid scaffold-based tissue engineering approaches: 1) cell growth is substrate attachment-dependent; cells need a solid substrate for attachment and proliferation; 2) tissue constructs must have an organo-specific shape; a solid scaffold is essential to keep the desired shape; a tissue construct could not maintain its shape without a solid rigid scaffold; 3) the scaffold serves not only as an attachment substrate, but also as a source of inductive and instructive signals for cell differentiation, migration, proliferation and orientation; 4) the porous structure of a solid scaffold will allow optimal cell seeding, tissue construct viability, and vascularization; and 5) mechanical properties initially provided by the rigid solid scaffold after its biodegradation will be maintained by controlled neomorphogenesis of parenchymal and stromal tissue synthesized in vitro or in vivo in the tissue construct [2,9,10].
Indeed, it has been proven that cells can attach to the scaffold; the solid scaffold can maintain a tissue construct shape; functionalized scaffolds can serve as a tool for instructive morphogenetic signaling and directed cell differentiation; and, finally, cells inside porous scaffolds can maintain viability and eventually neoformed tissue can replace the biodegradable scaffold [10–12]. However, in spite of proven feasibility of solid scaffolds, this classic approach still faces some limitations and challenges [13,14]: 1) vascularization of thick tissue constructs is an unsolved issue, 2) precise placing of different multiple cell types inside 3D porous scaffolds is technologically challenging; 3) achieving organo-specific level of cell density in tissue constructs remains a big challenge; and 4) recent reports on the effect of matrix rigidity on stem cell differentiation [15] can undermine the value of solid rigid biodegradable scaffolds at least for certain tissue applications.
Another important limitation, somehow overlooked, is the fact that from a cell's point of view even three-dimensional sophisticated porous nano-structuralized scaffolds often represent a two-dimensional substrate or basically the same 2D plastic Petri dish. In essence, the nature of interactions between cells and solid scaffolds on the cellular level at least initially is basically two-dimensional. Moreover, it has been shown that collagen and other extracellular matrix molecules secreted in soluble forms are washed out from a Petri dish due to the absence of a confined space typical for real 3D tissue [16,17]. Thus, synthesized but not cross-linked yet extracellular matrix molecules could be potentially washed out from 3D porous scaffolds, too.
Another issue is biodegradability. As written in the above-cited influential review, “There is no point in designing a system that will facilitate complex tissue regeneration if that tissue is ultimately destroyed by the influx of inflammatory cells associated with degradation process or if material stimulates the immune system as it degrades and releases antigenic material [5]”. From another view, in vitro cell seeding on a solid biodegradable scaffold with sequential, relatively slow, complete scaffold biodegradation and tissue neomorphogenesis leads to laborious, expensive, time consuming and commercially unsuccessful tissue engineering technology. As Chris Mason boldly stated, “The time has come to engineer tissues and not just tissue engineer” [18]. It is also not immediately obvious how a chemically modified and functionalized 2D surface of a solid scaffold designed to biodegrade can maintain its functional activity in a 3D tissue construct in vivo [5]. Recent reviews on the problem of vascularization in tissue engineered constructs based on using biodegradable solid scaffolds also state that after two decades of intensive efforts the effective solution of this problem has not yet been found [19–21].
Thus, it appears that at least some of these limitations are probably intrinsic to the tissue engineering approach of using 3D porous solid biodegradable scaffolds as an instructive template. Moreover, the absence of clearly formulated pathways or roadmaps for automated, industrial scale tissue engineering using solid scaffolds is another serious reason to suggest that the search for possible alternatives is becoming a technological imperative [22]. It is becoming obvious that the minimal essential and sufficient criteria for an alternative approach — one that is technologically more advanced and potentially superior — must include enabling precise placement of cells at a high level of cell density, efficient vascularization of thick 3D tissue constructs, automated robotic large-scale industrial biofabrication, and greater reliance on developmental biology-inspired biomimetic principles of 3D tissue self-assembly, rather than on template-based tissue assembly.
3. Back to the future: developmental biology origin of minitissue-based tissue engineering concept
One of the most logical and obvious ways to look for possible alternatives to solid biodegradable scaffold-based tissue engineering approaches is to understand how tissues and organs are formed during normal embryonic development. The knowledge of developmental biology as a science can provide powerful insights for tissue engineering as a technology [6]. In this context, probably the most interesting fact is that, during embryonic development, tissues and organs are formed without any solid scaffolds. Although overall embryonic development is a relatively slow process, certain essential morphogenetic steps and events during embryonic histogenesis and organogenesis are relatively fast. The main technological and economic imperative in tissue engineering technology is a rapid tissue biofabrication. Thus, the challenge which developmental biology-inspired approach to tissue engineering is facing is the balanced combination of powerful biological insight with technological imperatives and constraints. It means that even if it is possible to recapitulate or biomimic certain essential developmental biology processes in tissue engineering technologies in order to develop commercially sound technologies, the bioassembly process must be as a minimum both accelerated and automated.
Organ printing or the biomedical application of rapid prototyping, also defined as layer-by-layer additive biomanufacturing, is an emerging transforming biomimetic technology that has potential for surpassing traditional solid scaffold-based tissue engineering [3,4]. Organ printing has certain advantages: it is an automated approach that offers a pathway for scalable, reproducible, mass production of tissue engineered products; it allows for precise simultaneous 3D positioning of several cell types; it enables creation of tissue with a high level of cell density; it can solve the problem of vascularization in thick tissue constructs; and finally organ-printing can be done in situ [4]. The ultimate goal of organ-printing technology is to fabricate 3D vascularized functional living human organ constructs suitable for clinical implantation. The conceptual framework of solid biodegradable scaffold-free tissue engineering is based on the assumption that tissues and organs are self-organizing systems, and that cells and especially microtissues can undergo biological self-assembly and self-organization without any external influence in the form of instructive, supporting and directing rigid templates or solid scaffolds.
Self-organization is defined as a process in which patterning at the global level of a system emerges solely from numerous interactions among the lower-level components of the system [23]. Self-assembly is defined as the autonomous organization of components into patterns or structures without human intervention [24]. In this context, the evolving morphological pattern in embryonic development is an emergent property of the self-assembling system, rather than a property imposed on the system by an external ordering influence [23]. The best possible examples of tissue self-organization and self-assembly are again in the field of developmental biology. An historical analysis strongly indicates that the emerging solid biodegradable scaffold-free minitissue-based biomimetic approach in tissue engineering has deep and strong roots in developmental biology. It began with Aron Moscona's pioneering works on dissociation and aggregation of cells from organ rudiments of the early chick embryo [25–27]. It continued through a series of remarkable papers with another intriguing title “Reconstruction of tissues by dissociated cells” by Malcolm Steinberg who formulated fundamental thermodynamic rules determining tissue self-assembly and developed the well validated, both in vitro and in vivo, “differential adhesion hypothesis” explaining the fluidic nature of cell sorting and tissue self-assembly [28–30]. As early as 1960, one of the world's leading developmental biologists at that time, Paul Weiss, expressed hope in his paper with the impressive title “Reconstruction of complete organs from single-cell suspension of chick embryos in advanced stages of differentiation” that his pioneering study “…will deflect at least some attention and investigative effort back towards the fundamental problems of self-organization” [31]. Thus, the early work of developmental biologists demonstrated that in vitro tissue assembly (“tissue and organ reconstruction”) from single cells or tissue aggregates is feasible. The valuable contribution of several generations of outstanding developmental biologists must be respected and appreciated. It is important from an historical perspective, but it may be even more valuable for proper future development of the tissue engineering field. Hence, this section is called “back to the future”.
The most essential element of the conceptual framework underlying the emerging minitissue-based approach in tissue engineering is based on the developmental biology-inspired assumption that 3D tissues and organs of desirable material properties and composition could be fabricated without using solid porous biodegradable synthetic or natural scaffolds. There are several partly already validated and some still not validated assumptions or arguments that taken together constitute a conceptual basis for minitissue-based approaches in tissue engineering: 1) functional macrotissues can be fabricated from functional minitissue blocks without solid scaffolds;2) scaffold-free tissue assembly and maturation can be accelerated (see corresponding section below); 3) initially strong material properties are non-essential and/or may even impede efficient organ biofabrication; 4) viability and shape of solid scaffold-free 3D tissue constructs could be maintained by other means such as a microfludics-based irrigation dripping perfusion bioreactor with removable porous tubes; 5) macrotissues and organs can be built by directed tissue self-assembly of robotically placed tissue spheroids with ‘built in” intraorgan branched vascular system (see corresponding section below). Perhaps the most compelling conceptual framework of novel solid biodegradable scaffold-free minitissue-based biomimetic tissue engineering paradigm could be presented as an integrated combination of ten basic assumptions summarized in Table 1.
Table 1.
Conceptual framework for organ-printing technology.
|
4. Tissue spheroids as building blocks
4.1. Material properties of tissue spheroids
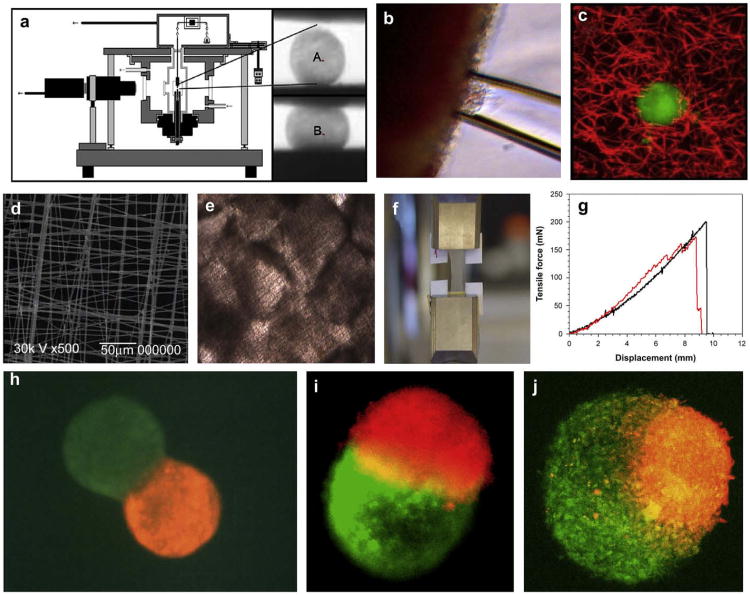
A recently published review paper with the characteristic title “Cell as a material” [32] logically implies that minitissues, and more specifically tissue spheroids, can also be considered as a material or more correctly a “living material” with certain measurable, evolving and potentially controllable material properties (Fig. 1). The most popular direct method for measuring material properties of rounded microtissues is tensiometry or controlled compression of cell aggregates between two parallel plates [33,34] (Fig. 1a). However, the requirement of ideal spherical shape is a limitation of this approach. Recently, a novel tissue aspiration method was developed (Fig. 1b), successfully tested on cushion tissue explants, that showed a desirable level of sensitivity [35]. Fluorescent microbeads have been embedded in tissue engineered constructs for studying material properties based on microbead-induced mobility [36] (Fig. 1c). Material properties of tissue constructs formed by fusion of tissue spheroids placed on very elastic and thin electrospun polyurethane scaffolds could be also measured by classic tensile test (Fig. 1d–g). Biofabrication of tissue spheroids incorporating magnetic or fluorescent microbeads is one of the most intriguing approaches to be explored for development of novel assays for studying material properties of microtissues and tissue spheroids before and after their fusion into larger tissue engineered constructs, as well as for non-destructive biomonitoring of tissue maturation in these constructs. Semi-quantitative estimation of material properties of tissue spheroids can be also based on the enveloping behavior of two adjacent tissue spheroids during the tissue fusion process [37]. Theoretically, the more cohesive tissue spheroid will be enveloped by the spheroid with lower cohesive tissue or inferior material properties (Fig. 1h–j). The fluorescent recovery after photobeaching (FRAP) method is based on the assumption that there is a certain correlation between fluorescent probe diffusion and density of extracellular matrix molecules such as collagen [38]. The estimation of electro-conductivity and electric impedance is another indirect non-invasive high-throughput approach based on similar correlations [39]. The development of a broad arsenal of highly sensitive, direct and indirect quantitative and semi-quantitative methods for the estimation of material properties of tissue spheroids and microtissue is an essential task in the development of predictive solid scaffold-free microtissue-based tissue engineering approaches (Fig. 1). This is especially true in the context of controlling the tissue fusion process, achieving a desirable level of tissue maturation, and for high-throughput screening of ‘maturogenic factors’ or factors that enhance extracellular matrix deposition (see Section 7).
Fig.1.
Methods of biomechanical testing of tissue spheroids: a) tensiometry: experimental device and changing shape of tissue spheroid before (A) and after (B) tissue compression; b) aspiration assay; c) fluorescent microbead assay; d) SEM of elastic scaffold for tensile testing; e) fused tissue spheroids attached to elastic scaffold; f) tensile testing using tissue construct fabricated from fused tissue spheroids; g) example of force–displacement relationship for tissue construct fabricated from fused tissue spheroids; h) envelopment assay – initial step of tissue spheroid fusion; i) fusion of tissue spheroid with equal level of cohesion; j) fusion of tissue spheroid with different levels of tissue cohesion – lower cohesive tissue spheroid (green) is enveloping more cohesive tissue spheroid (red).
4.2. Scalable methods of tissue spheroids' biofabrication
Tissue spheroids have been used as an in vitro 3D model system in biomedical and tumor research for several decades [40–43]. It is not surprising that an impressive arsenal of methods for the biofabrication of tissue spheroids has been eventually developed. This topic was recently systematically and comprehensively covered in excellent review paper [44]. In order to estimate what method of spheroid biofabrication is most suitable for large-scale industrial tissue engineering and for organ printing, it is essential to formulate appropriate criteria or well defined specifications. First and most important, it must be a scalable technology. For example, in order to build a human kidney we need to generate one million glomeruli and nephrons. Second, these spheroids must be maximally standardized in their size in order to make them processible or be dispensable through a bioprinter nozzle or by other means without clogging problems and their destruction. Thus, standardization of tissue spheroid size is desirable for continuous dispensing. Third, the method of tissue spheroid biofabrication must not induce significant cell injury and/or DNA damage. Fourth, the method of tissue spheroid biofabrication must not compromise the tissue spheroids' capacity for sequential tissue fusion. Finally, the biofabrication method must be flexible enough to allow generation of a diversity of tissue spheroids of complex composite structure. Thus, facing the fact that most of the existing methods are not scalable [44], it is safe to predict that development of methods for scalable biofabrication of tissue spheroids will soon became a rapidly growing field in solid scaffold-free tissue engineering.
4.3. Fluidic nature of tissue fusion process
Fusion is sometimes defined as “melting together”, which is logically implying the liquidic nature of the fusion process. Tissue fusion is a ubiquitous process during embryonic development and can be recapitulated in vitro [45]. It has been shown that the kinetics of tissue fusion of two rounded embryonic heart cushion tissue explants placed in an hanging drop fits perfectly to fusion kinetics described for two droplets of fluids [46]. Moreover, based on direct measurement of surface tension and calculation of viscosity, tissue spheroids are indeed fluidic-like structures [46]. Thus, tissue fusion is in essence a phenomenon of fluid mechanics driven by surface tension forces and can be adequately explained by physical laws and Malcolm Steinberg's “differential adhesion hypothesis” [28–30]. From another point, motile living cells, cytoskeleton and number, and redistribution and activation of cell adhesion receptors are also essential for the tissue fusion process [46,47]. The accumulation of ECM and associated restriction of cell motility and enhancing tissue cohesion in tissue spheroids [48] can change kinetics or impede the tissue spheroids' fusion process. Thus, the exact effect of accumulation of extracellular matrix and specific ECM molecules, as well as ECM remodeling on material properties of microtissues and tissue spheroids and associated modulation of the tissue fusion process, remains to be elucidated.
5. Organ printing as directed tissue self-assembly
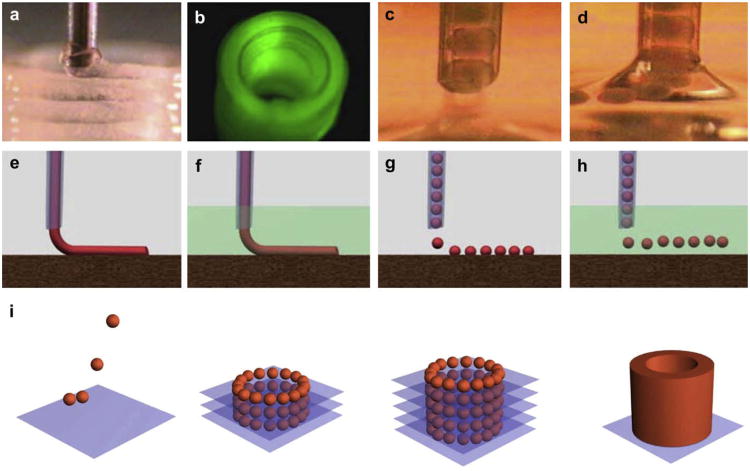
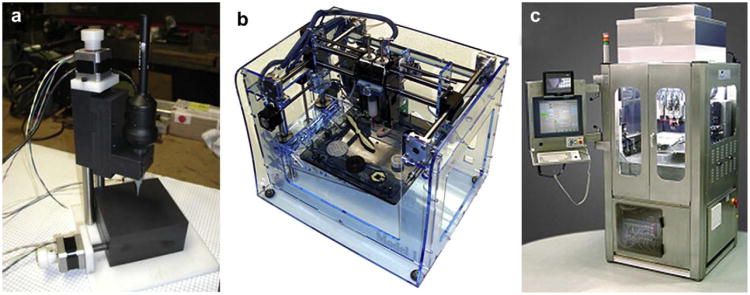
The term “directed tissue self-assembly” looks like a strange combination of words because it is basically a contradiction of terms. One can logically argue that it can be either “directed assembly” or “self-assembly”, but not both together. However, we found an even more controversial combination of words in the title of a recently published Nature paper: “Self-directed self-assembly of nanoparticle/copolymer mixtures” [49]. It is interesting that the authors of this paper use similar arguments in describing their novel approach in nanotechnology which is different from the scaffold-based approach: “Previous efforts have concentrated on using such scaffolds to spatially arrange nanoscopic elements as a strategy for tailoring the electrical, magnetic or photonic properties of the material. Recent theoretical arguments have suggested that synergistic interactions between self-organizing particles and a self-assembling matrix material can lead to hierarchically ordered structures”. Extrapolation of the last sentence in this quote provides a powerful insight into similar principles of self-assembly based scaffold-free tissue engineering. It appears that the terms “directed self-assembly” and even “self-directed self-assembly” are acceptable at least in the nanotechnology field. Self-assembly of closely placed tissue spheroids by the tissue fusion process into macrotissue constructs is a documented proven reality confirmed by different groups around the world. Direct contact of adjacent tissue spheroids in a permissive environment is an essential precondition for tissue fusion driven process of macrotissue construct self-assembly. Thus, organ printing or robotic additive biomanufacturing using precise layer-by-layer placement (“direction”) of self-assembled tissue spheroids (“bioink”) in sprayed tissue fusion permissive hydrogels (“biopaper”) is in essence an example of technological implementation of the concept of “directed tissue self-assembly” (Fig. 2). The scalability and suitability for automation with using special robotic bioprinters (Fig. 3) are probably the most attractive aspects of directed tissue self-assembly technologies.
Fig. 2.
Principles of bioprinting technology: a) bioprinter (general view); b) multiple bioprinter nozzles; c) tissue spheroids before dispensing; d) tissue spheroids during dispensing; e) continuous dispensing in air; f) continuous dispensing in fluid; g) digital dispensing in air; h) digital dispensing in fluid; i) scheme of bioassembly of tubular tissue construct using bioprinting of self-assembled tissue spheroids illustrating sequential steps of layer-by-layer tissue spheroid deposition and tissue fusion process.
Fig. 3.
Bioprinters: a) 3D dispensing Laboratory Bioprinter – ‘LBP’ (designed by Neatco, Toronto, Canada in cooperation with MUSC Bioprinting Research Center, Charleston, SC); b) 3D robotic printer – ‘Fabber’ (designed by Cornell University, USA); c) 3D robotic industrial bioprinter – ‘BioAssembly Tool’ (designed by Sciperio/nScript, Orlando, USA).
Organ-printing technology using self-assembled tissue spheroids in certain aspects is also conceptually very close to the recently invented concept of digital printing [50,51]. Conventional freeform fabrication has already been adapted for printing a variety of sophisticated 3D tissue engineered scaffolds from synthetic biodegradable polymers with sequential bioreactor-based cellularization (two step biofabrication process) making them especially suitable for fabrication of hard tissues. The continuous rapid prototyping technology based on simultaneous robotic dispensing or photopolymerization of stimuli-sensitive biomaterials containing living cells (one step biofabrication process) was also recently applied to bioprinting soft tissues. However, continuous (analog) rapid prototyping technology is usually limited to a single, homogeneous material such as hydrogels or hydrogel mixture with specific rheological and stimuli-sensitive properties ensuring non-destructive bioprocessing of living cells into 3D living tissue construct. Digital printing offers much more flexibility in selection materials for bioprinting. Digital (discrete) materials are fundamentally different from analog (continuous) materials [50,51]. Digital materials may be broken into two main classes. The first class involves accurate placement of drops of material that harden in place like droplets jetted from an inkjet system. The second class of digital materials involves assembling of prefabricated voxels. In this context, a voxel is defined as ‘a physical bit in digital matter’ [50,51]. According to the digital printing concept, ‘the physical voxel’ must passively self-align with its neighbors while being capable of self-assembly and be easy to make precisely in large quantities. Tissue spheroids could be considered as ‘spherical physical voxels’ that are relatively easy to fabricate at desirable standard size at large scale. The attractiveness of using tissue spheroids is based on their relatively easy industrial biofabrication at large scale and potential suitability for both emerging variants of bioprinting technology. In continuous (analog) bioprinting, tissue spheroids could be continuously dispensed with hydrogel. In digital bioprinting, tissue spheroids could be used as a discrete materials (‘physical voxels’) and be digitally ‘punched’ into sequentially sprayed layers of hydrogel or even be digitally self-assembled without using any hydrogels.
6. Bioprinting of an intraorgan branched vascular tree
Another potential advantage of the minitissue-based approach is its promise to solve the problem of vascularization of thick tissue constructs, arguably the most critical and still unsolved problem in tissue engineering. Recent reviews on this topic summarized the existing approaches to vascularization and stated that the optimal solution of this problem has not yet been found [19–21]. Moreover, even the term and the meaning of the word “vascularization” is basically not very well defined. For optimal perfusion, organs such as kidney need a well developed hierarchically complex branched intraorgan vascular tree. Inspired by recent progress in micro-fluidics hydrogels assumptions that effective perfusion of thick 3D macrotissues and organs are similar to assumptions that it is possible to design effective transport system by building dense network of interconnected narrow roads without highways. Thus, one can argue that a correct definition for proper vascularization of tissue engineered organs must include all essential elements of the intraorgan circulatory system and not just capillaries and micro-vessels. First, we must have an intraorgan branched vascular tree “built-in” 3D macrotissue construct, which allows perfusion of the tissue engineered organ most effectively. Organization and branching patterns of an intraorgan vascular tree must be, of course, organospecific and vasospecific. Secondly, the arteries and veins at the onset of an intraorgan vascular tree must have material properties sufficient for surgical connection by sutures with recipient large vessels. Finally, the arterial and venous vascular trees must be seamlessly connected through microvascular network and be suitable for perfusion.
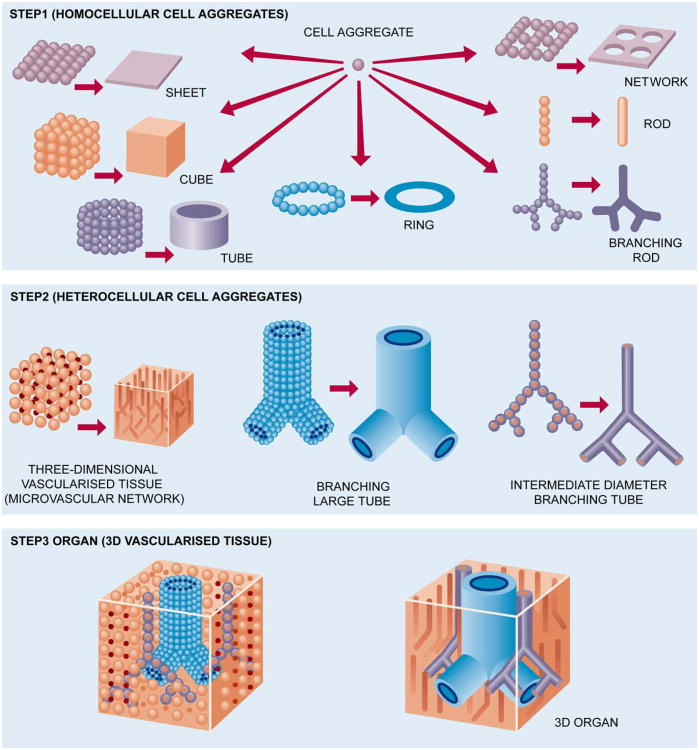
Our approach to building an intraorgan vascular tree is based on using three basic types of tissue spheroids: solid (non-lumenized) vascular tissue spheroids; mono-lumenized vascular spheroids (cyst-like spheroids with one big lumen); and histotypical micro-vascularized tissue spheroids. The principal concept of building large diameter, intermediate diameter and microvascular segments of a vascular tree or roadmap for organ printing is demonstrated in Fig. 4. The microvascular networks can be self-assembled either from single cells placed into hydrogel by vacuole accumulation [52] or from endothelial tissue spheroids [53]. Biofabrication of microvascularized histotypical tissue spheroids is also a reality [54]. It is already proven that essential parts or segments of a vascular tree can be engineered at least separately (Figs. 5–7). Moreover, it has been shown that small isolated fragments of the microvascular tree can be reunited by self-assembly in vitro and in vivo [55,56]. Thus, we can argue that building a complete intraorgan branched vascular tree with 10–12 orders of branching is technologically feasible. The proposed concept of bioengineering a natural-like intraorgan branched vascular tree from three types of self-assembled vascular tissue spheroids is specially designed for organ-printing technology. It is important to mention that the layers of vascular tissue spheroids for bioassembly of the vascular tree will be placed simultaneously with the layers of microvascularized organo-specific tissue spheroids. Thus, after finishing bioprinting, post-printed tissue fusion, and accelerated tissue maturation, the bioengineered vascular tree will be suitable for perfusion and integrated into 3D tissue or organ constructs. Bioengineering a natural-like hierarchically branched intraorgan vascular tree that can be“built in” a bioprinted 3D tissue construct represents an enormous challenge, but it is obvious that without bioengineering perfusable intraorgan branched vascular tree, it is practically impossible to print viable human organ constructs.
Fig. 4.
Roadmap for organ printing.
Fig. 5.
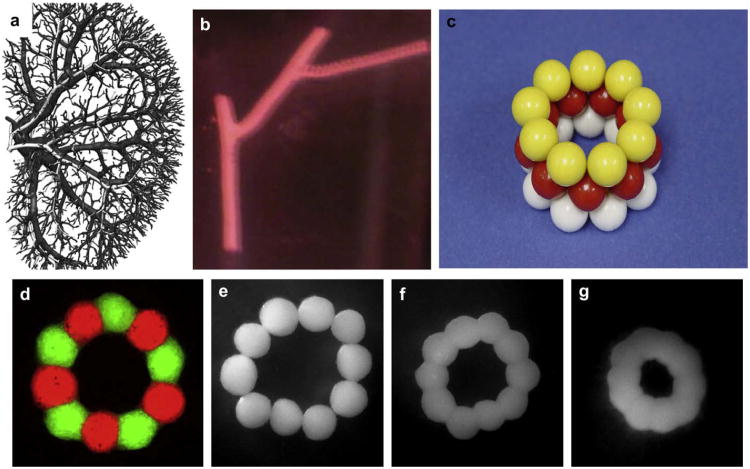
Bioprinting of segments of intraorgan branched vascular tree using solid vascular tissue spheroids: a) kidney intraorgan vascular tree; b) bioprinted segment of vascular tree; c) physical model of bioassembly of tube-like vascular tissue construct using solid tissue spheroids; d) bioassembled ring-like vascular tissue constructs of tissue spheroids fabricated from human smooth muscle cells. Tissue spheroids are labeled with green and red fluorescent stains in order to demonstrate absence of cell mixing during tissue fusion process; e–g) sequential steps of morphological evolution of ring-like vascular tissue construct during tissue fusion process.
Fig. 7.
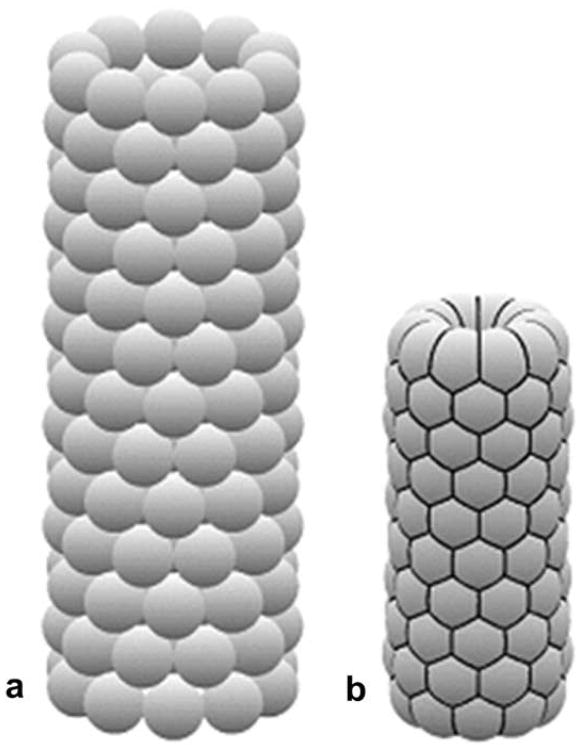
Computer simulation (using ‘Surface Evolver’ software) of morphology evolution of a tube-like vascular tissue construct during tissue fusion. The initial tube-like vascular tissue construct (a) is becoming shorter and more narrow (b). The hexagonal pattern of tissue spheroid packing is clearly demonstrated (b).
7. Concept of accelerated tissue maturation
The biomimetic concept of accelerated tissue maturation in microtissue-based approaches implies that the desirable material and biomechanical properties of engineered constructs could be rapidly achieved without using supporting biodegradable solid scaffolds. In order to achieve specified biomechanical properties of tissue engineered constructs it is essential to know in advance not only the material properties of mature adult human issues and organs, but also the developmental kinetics of tissue and organ maturation, as well as the structural determinants of material properties of specific tissues or organs at different stages of development. Although cell membrane, cytoskeleton, cell nuclei, cell-to-cell and cell-to-matrix contacts also contribute in certain degree to material properties of most living human tissues, it is obvious that any advanced accelerated tissue maturation technology must deal predominantly with enhancement of synthesis, deposition, assembly, cross-linking and intermolecular interactions of extracellular matrix molecules especially in case of organs consisting of dense connective tissues. However, stromal elements of human parenchyme organs such as kidney and liver also determine a substantial part of organ material properties. Collagen and elastin are two of the most essential structural extracellular matrix proteins in the human body that determine material properties of dense connective tissues as well as connective tissue stromal elements of parenchyme organs. Thus, accelerated tissue maturation in essence is about enhancing collagen and elastin deposition.
In the absence of a solid scaffold, biofabricated 3D macrotissue constructs must undergo a rapid process of tissue maturation or fluid–solid transition in order to maintain their shape, composition and integrity. Traditional relatively slow physical, chemical and genetic approaches for tissue maturation — typical for biodegradable solid scaffold-based tissue engineering — is not well suited for requirements of rapid tissue maturation in a scaffold-free minitissue self-assembly based approach. New innovative approaches must be explored. Rapid polymerization of photo-sensitive or stimuli-sensitive polymers is probably the most logical option [57], but increased hydrogel stiffness can interfere with the tissue fusion process [58]. Designing hybrid bioprinter-processible tissue spheroids with rigid internal microscaffolds, or using biodegradable macroporous microcarriers [59] in combination with exploring “jamming effect” [60] or rapid transition from fluid state (spheroids' suspension) to solid state (spheroids' jamming) as a function of spheroid packing density, could be potentially fruitful approaches.
Although these two approaches conceptually can rapidly enhance material properties of tissue constructs, they represent a compromise between solid scaffold-based and microtissue-based approaches and do not fit well to the definition of true biological process-based accelerated tissue maturation. The development of irrigation dripping tripled perfusion bioreactor with temporal non-biodegradable removable porous tubes (Fig. 8) can also help to maintain fragile tissue constructs and “buy” time necessary for post-processing tissue fusion, remodeling and maturation, and also can be used for delivery of “maturogens” [4]. Another interesting approach is based on using so-called ‘sacrificial’ hydrogels. The removal of hyaluronan by hyaluronidase and sequential tissue condensation is a critical step in embryonic chondrogenesis [61]. It has been shown that removal of hyaluronic acid by hyauronidase enhances material properties of embryonic heart cushion tissue explants [35]. The recently discovered, strong enhancing effect of “molecular crowding” or “excluding volume effect” on collagen deposition [16,17] opens a very promising approach for accelerated tissue maturation. The recruitment of circulated soluble forms of extracellular matrix molecules such as fibronectin and collagen type 1 can also be employed as an effective rapid method of accelerated tissue maturation. Soluble variants of ECM molecules could directly contribute to accelerated extracellular matrix assembly and deposition. For example, it has been shown that the addition of soluble fibronectin and collagen type 1 increases tissue cohesion [62–64]. Finally, emerging methods of nanoassembly and self-assembly of extracellular matrix molecules are another possible approaches [65]. Thus, development of effective “maturogenic cocktails” and innovative technologies of rapid tissue maturation including methods of rapid self-assembly of extracellular matrices is extremely challenging, but feasible and important goals.
Fig. 8.
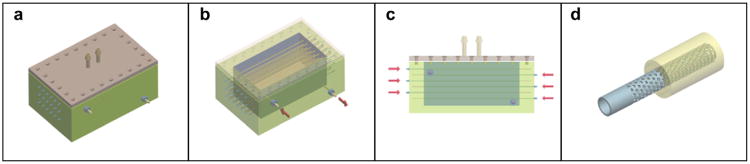
Scheme of the design of irrigation dripping tripled perfusion bioreactor: a) general view; b) view with opened lid; c) sectional view; d) design of removable microporous non-biodegradable tube.
8. Conclusions and perspectives
The emerging microtissue-based approach emphasizes the increasing recognition of the value of fundamental developmental biology expertise in tissue engineering, which is extending far beyond the already generally accepted fundamental role of research in stem cell biology and regenerative biology in advancing tissue engineering. Thus, it is safe to state that the ongoing integration of developmental biology and tissue engineering is already a work in progress. However, it will be also fundamentally wrong to assume that microtissue-based approaches do not have any technological challenges. It is obvious that large-scale biofabrication of tissue spheroids; development of continuous and digital industrial bioprinters; robotic bioprinting of 3D macrotissues and living organ constructs using microtissues; bioengineering of a perfusable intraorgan branched vascular tree “built-in” these 3D macrotissue constructs; development of microfluidic-inspired irrigation dripping tripled perfusion bioreactor with removable non-biodegradable porous tubes and accelerated tissue maturation technologies are serious technological challenges that need to be systematically addressed in order to facilitate successful development of a microtissue-based approach. The minitissue-based approach also demands synthesis of more sophisticated soft natural-like biomaterials and extracellular matrices such as bioprocessible and biomimetic stimuli-sensitive functional hydrogels [4]. The most attractive feature of the microtissue-based approach is that it is suitable for industrial scale robotic and automated biofabrication of human tissue and organs. The rapidly evolving microtissue-based biomimetic approach employing developmental biology-inspired principles of directed tissue self-assembly is a potentially transforming and superior technology and, thus, represents an emerging paradigm which, if successfully developed, can dramatically enhance our technological arsenal and significantly advance the tissue engineering field. Finally, the fact that growing number of research groups are employing solid scaffold-free microtissue-based approach provides certain optimism for further exploring and development of this new direction in tissue engineering [66–70].
Fig. 6.
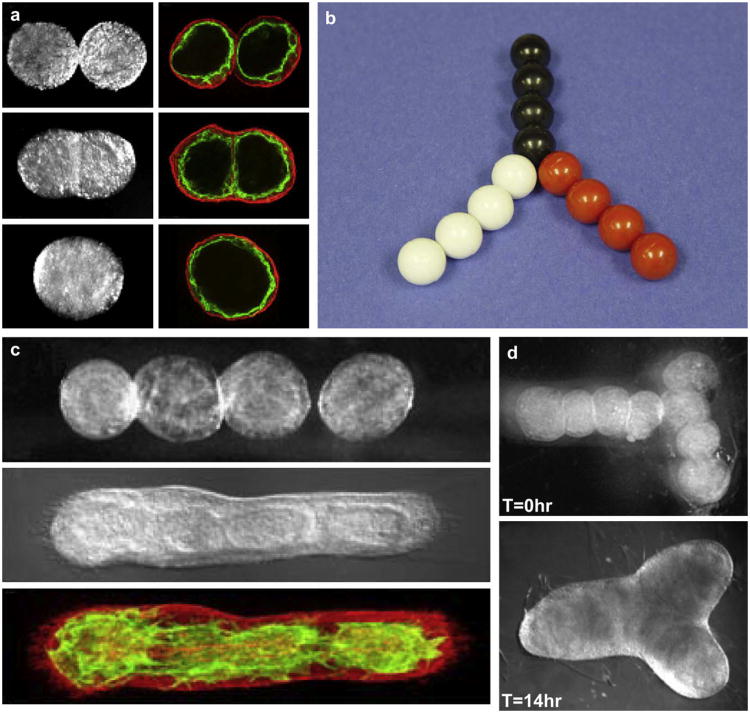
Bioprinting of segments of intraorgan branched vascular tree using uni-lumenal vascular tissue spheroids: a) fusion of uni-lumenal vascular tissue spheroids in hanging drop; b) physical model of fabrication of branched vascular segment from uni-lumenal vascular tissue spheroids; c) sequential steps of tissue fusion of vascular tissue spheroids placed in collagen type 1 hydrogel; d) fabrication branched vascular segments from uni-lumenal vascular tissue spheroids in collagen type 1 hydrogel (before and after tissue fusion process).
Acknowledgments
Work was funded by NSF FIBR and MUSC Bioprinting Research Center grants, P20-RR1-16434 from the NCRR and P20-RR1-6461 from the SC IDeA Network of Biomedical Research Excellence.
Footnotes
Editor's Note: This paper is one of a newly instituted series of scientific articles that provide evidence-based scientific opinions on topical and important issues in biomaterials science. They have some features of an invited editorial but are based on scientific facts, and some features of a review paper, without attempting to be comprehensive. These papers have been commissioned by the Editor-in-Chief and reviewed for factual, scientific content by referees.
References
- 1.Griffith LG, Naughton G. Tissue engineering – current challenges and expanding opportunities. Science. 2002;295:1009–14. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21:157–61. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 4.Mironov V, Kasyanov V, Drake C, Markwald RR. Organ printing: promises and challenges. Regen Med. 2008;3:93–103. doi: 10.2217/17460751.3.1.93. [DOI] [PubMed] [Google Scholar]
- 5.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–53. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Marga F, Neagu A, Kosztin I, Forgacs G. Developmental biology and tissue engineering. Birth Defects Res C Embryo Today. 2007;81:320–8. doi: 10.1002/bdrc.20109. [DOI] [PubMed] [Google Scholar]
- 7.Steer DL, Nigam SK. Developmental approaches to kidney tissue engineering. Am J Physiol Renal Physiol. 2004;286:F1–7. doi: 10.1152/ajprenal.00167.2003. [DOI] [PubMed] [Google Scholar]
- 8.Ingber DE, Mow VC, Butler D, Niklason L, Huard J, Mao J, et al. Tissue engineering and developmental biology: going biomimetic. Tissue Eng. 2006;12:3265–83. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 9.Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perez-Atayde A, Langer R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J Pediatr Surg. 1988;23:3–9. doi: 10.1016/s0022-3468(88)80529-3. [DOI] [PubMed] [Google Scholar]
- 10.Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues – state of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12:107–24. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 11.Ma PX, Elisseeff J, editors. Scaffolding in tissue engineering. CRC; 2005. [Google Scholar]
- 12.Hutmacher DW, Schantz JT, Lam CX, Tan KC, Lim TC. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med. 2007;1:245–60. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- 13.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103:2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langer R. Tissue engineering: perspectives, challenges, and future directions. Tissue Eng. 2007;13:1–2. doi: 10.1089/ten.2006.0219. [DOI] [PubMed] [Google Scholar]
- 15.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Lareu RR, Arsianti I, Subramhanya HK, Yanxian P, Raghunath M. In vitro enhancement of collagen matrix formation and crosslinking for applications in tissue engineering: a preliminary study. Tissue Eng. 2007;13:385–91. doi: 10.1089/ten.2006.0224. [DOI] [PubMed] [Google Scholar]
- 17.Lareu RR, Subramhanya KH, Peng Y, Benny P, Chen C, Wang Z, et al. Collagen matrix deposition is dramatically enhanced in vitro when crowded with charged macromolecules: the biological relevance of the excluded volume effect. FEBS Lett. 2007;581:2709–14. doi: 10.1016/j.febslet.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Mason C. The time has come to engineer tissues and not just tissue engineer. Regen Med. 2006;1:303–6. doi: 10.2217/17460751.1.3.303. [DOI] [PubMed] [Google Scholar]
- 19.Lokmic Z, Mitchell GM. Engineering the microcirculation. Tissue Eng Part B Rev. 2008;14:87–103. doi: 10.1089/teb.2007.0299. [DOI] [PubMed] [Google Scholar]
- 20.Moon JJ, West JL. Vascularization of engineered tissues: approaches to promote angio-genesis in biomaterials. Curr Top Med Chem. 2008;8:300–10. doi: 10.2174/156802608783790983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434–41. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Mason C. Regenerative medicine 2.0. Regen Med. 2007;2:11–8. doi: 10.2217/17460751.2.1.11. [DOI] [PubMed] [Google Scholar]
- 23.Camazine S, Deneubourg JL, Franks NR, Sneyd J, Theraulaz G, Bonabeau E, editors. Self-organization in biological systems. Princeton University Press; 2001. [Google Scholar]
- 24.Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002;295:2418–21. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 25.Moscona A, Moscona H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J Anat. 1952;86:287–301. [PMC free article] [PubMed] [Google Scholar]
- 26.Moscona AA. Sci Am. Vol. 200. passim; 1959. Tissues from dissociated cells; pp. 132–4. [DOI] [PubMed] [Google Scholar]
- 27.Weiss P, Moscona A. Type-specific morphogenesis of cartilages developed from dissociated limb and scleral mesenchyme in vitro. J Embryol Exp Morphol. 1958;6:238–46. [PubMed] [Google Scholar]
- 28.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278:255–63. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev. 2007;17:281–6. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg MS. Reconstruction of tissues by dissociated cells: some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science. 1963;141:401–8. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- 31.Weiss P, Taylor AC. Reconstitution of complete organs from single-cell suspensions of chick embryos in advanced stages of differentiation. Proc Natl Acad Sci U S A. 1960;46:1177–85. doi: 10.1073/pnas.46.9.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasza KE, Rowat AC, Liu J, Angelini TE, Brangwynne CP, Koenderink GH, et al. The cell as a material. Curr Opin Cell Biol. 2007;19:101–7. doi: 10.1016/j.ceb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Forgacs G, Foty RA, Shafrir Y, Steinberg MS. Viscoelastic properties of living embryonic tissues: a quantitative study. Biophys J. 1998;74:2227–34. doi: 10.1016/S0006-3495(98)77932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foty RA, Pfleger CM, Forgacs G, Steinberg MS. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122:1611–20. doi: 10.1242/dev.122.5.1611. [DOI] [PubMed] [Google Scholar]
- 35.Butcher JT, McQuinn TC, Sedmera D, Turner D, Markwald RR. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ Res. 2007;100:1503–11. doi: 10.1161/CIRCRESAHA.107.148684. [DOI] [PubMed] [Google Scholar]
- 36.Leung LY, Tian D, Brangwynne CP, Weitz DA, Tschumperlin DJ. A new microrheometric approach reveals individual and cooperative roles for TGF-beta1 and IL-1beta in fibroblast-mediated stiffening of collagen gels. FASEB J. 2007;21:2064–73. doi: 10.1096/fj.06-7510com. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci U S A. 1994;91:206–9. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braeckmans K, Peeters L, Sanders NN, De Smedt SC, Demeester J. Three-dimensional fluorescence recovery after photobleaching with the confocal scanning laser microscope. Biophys J. 2003;85:2240–52. doi: 10.1016/s0006-3495(03)74649-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartholoma P, Impidjati, Reininger-Mack A, Zhang Z, Thielecke H, Robitzki A. A more aggressive breast cancer spheroid model coupled to an electronic capillary sensor system for a high-content screening of cytotoxic agents in cancer therapy: 3-dimensional in vitro tumor spheroids as a screening model. J Biomol Screen. 2005;10:705–14. doi: 10.1177/1087057105277841. [DOI] [PubMed] [Google Scholar]
- 40.Friedrich J, Ebner R, Kunz-Schughart LA. Experimental anti-tumor therapy in 3-D: spheroids – old hat or new challenge? Int J Radiat Biol. 2007;83:849–71. doi: 10.1080/09553000701727531. [DOI] [PubMed] [Google Scholar]
- 41.Kloss D, Fischer M, Rothermel A, Simon JC, Robitzki AA. Drug testing on 3D in vitro tissues trapped on a microcavity chip. Lab Chip. 2008;8:879–84. doi: 10.1039/b800394g. [DOI] [PubMed] [Google Scholar]
- 42.Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R. The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen. 2004;9:273–85. doi: 10.1177/1087057104265040. [DOI] [PubMed] [Google Scholar]
- 43.Mueller-Klieser W. Three-dimensional cell cultures: from molecular mechanisms to clinical applications. Am J Physiol Cell Physiol. 1997;273:C1109–23. doi: 10.1152/ajpcell.1997.273.4.C1109. [DOI] [PubMed] [Google Scholar]
- 44.Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3(9-10):1172–84. doi: 10.1002/biot.200700228. Review. [DOI] [PubMed] [Google Scholar]
- 45.Perez-Pomares JM, Foty RA. Tissue fusion and cell sorting in embryonic development and disease: biomedical implications. Bioessays. 2006;28:809–21. doi: 10.1002/bies.20442. [DOI] [PubMed] [Google Scholar]
- 46.Jakab K, Damon B, Marga F, Doaga O, Mironov V, Kosztin I, et al. Relating cell and tissue mechanics: implications and applications. Dev Dyn. 2008;237:2438–49. doi: 10.1002/dvdy.21684. [DOI] [PubMed] [Google Scholar]
- 47.Dean DM, Morgan JR. Cytoskeletal-mediated tension modulates the directed self-assembly of microtissues. Tissue Eng Part A. 2008;14(12):1989–97. doi: 10.1089/ten.tea.2007.0320. [DOI] [PubMed] [Google Scholar]
- 48.Neagu A, Jakab K, Jamison R, Forgacs G. Role of physical mechanisms in biological self-organization. Phys Rev Lett. 2005;95:178104. doi: 10.1103/PhysRevLett.95.178104. [DOI] [PubMed] [Google Scholar]
- 49.Lin Y, Boker A, He J, Sill K, Xiang H, Abetz C, et al. Self-directed self-assembly of nanoparticle/copolymer mixtures. Nature. 2005;434:55–9. doi: 10.1038/nature03310. [DOI] [PubMed] [Google Scholar]
- 50.Hiller J, Lipson H. Methods of parallel voxel manipulation for 3D digital printing. Proceedings of the 18th solid freeform fabrication symposium; 2007; pp. 200–11. [Google Scholar]
- 51.Hiller J, Lipson H. Design and analysis of digital materials for physical 3D voxel printing. Rapid Prototyping J. in press. [Google Scholar]
- 52.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–6. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 53.Alajati A, Laib AM, Weber H, Boos AM, Bartol A, Ikenberg K, et al. Spheroid-based engineering of a human vasculature in mice. Nat Methods. 2008;5:439–45. doi: 10.1038/nmeth.1198. [DOI] [PubMed] [Google Scholar]
- 54.Kelm JM, Moritz W, Schmidt D, Hoerstrup SP, Fussenegger M. In vitro vascularization of human connective microtissues. Methods Mol Med. 2007;140:153–66. doi: 10.1007/978-1-59745-443-8_9. [DOI] [PubMed] [Google Scholar]
- 55.Hoying JB, Boswell CA, Williams SK. Angiogenic potential of microvessel fragments established in three-dimensional collagen gels. In Vitro Cell Dev Biol Anim. 1996;32:409–19. doi: 10.1007/BF02723003. [DOI] [PubMed] [Google Scholar]
- 56.Shepherd BR, Chen HY, Smith CM, Gruionu G, Williams SK, Hoying JB. Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler Thromb Vasc Biol. 2004;24:898–904. doi: 10.1161/01.ATV.0000124103.86943.1e. [DOI] [PubMed] [Google Scholar]
- 57.Liu Tsang V, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, et al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007;21:790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- 58.Jakab K, Neagu A, Mironov V, Markwald RR, Forgacs G. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc Natl Acad Sci U S A. 2004;101:2864–9. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang Q, Zhou Y, Chen F, Tan W. Preparing engineered tissues in vitro by macroporous microcarriers. Chin J Biotechnol. 2008;24:74–82. [Google Scholar]
- 60.Lu PJ, Zaccarelli E, Ciulla F, Schofield AB, Sciortino F, Weitz DA. Gelation of particles with short-range attraction. Nature. 2008;453:499–503. doi: 10.1038/nature06931. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, Toole BP, Dealy CN, Kosher RA. Hyaluronan in limb morphogenesis. Dev Biol. 2007;305:411–20. doi: 10.1016/j.ydbio.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson EE, Foty RA, Corbett SA. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol Biol Cell. 2004;15:973–81. doi: 10.1091/mbc.E03-07-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu Q, Norman JT, Shrivastav S, Lucio-Cazana J, Kopp JB. In vitro models of TGF-beta-induced fibrosis suitable for high-throughput screening of antifibrotic agents. Am J Physiol Renal Physiol. 2007;293:F631–40. doi: 10.1152/ajprenal.00379.2006. [DOI] [PubMed] [Google Scholar]
- 64.Robinson EE, Zazzali KM, Corbett SA, Foty RA. Alpha5beta1 integrin mediates strong tissue cohesion. J Cell Sci. 2003;116:377–86. doi: 10.1242/jcs.00231. [DOI] [PubMed] [Google Scholar]
- 65.Mironov V, Kasyanov V, Markwald RR. Nanotechnology in vascular tissue engineering: from nanoscaffolding towards rapid vessel biofabrication. Trends Biotechnol. 2008;26:338–44. doi: 10.1016/j.tibtech.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 66.Dean DM, Napolitano AP, Youssef J, Morgan JR. Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. FASEB J. 2007;21:4005–12. doi: 10.1096/fj.07-8710com. [DOI] [PubMed] [Google Scholar]
- 67.Handschel JG, Depprich RA, Kubler NR, Wiesmann HP, Ommerborn M, Meyer U. Prospects of micromass culture technology in tissue engineering. Head Face Med. 2007;3:4. doi: 10.1186/1746-160X-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kelm JM, Fussenegger M. Microscale tissue engineering using gravity-enforced cell assembly. Trends Biotechnol. 2004;22:195–202. doi: 10.1016/j.tibtech.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Layer PG, Robitzki A, Rothermel A, Willbold E. Of layers and spheres: the reaggregate approach in tissue engineering. Trends Neurosci. 2002;25:131–4. doi: 10.1016/s0166-2236(00)02036-1. [DOI] [PubMed] [Google Scholar]
- 70.Lin RZ, Chu WC, Chiang CC, Lai CH, Chang HY. Magnetic reconstruction of three-dimensional tissues from multicellular spheroids. Tissue Eng Part C Methods. 2008;14:197–205. doi: 10.1089/ten.tec.2008.0061. [DOI] [PubMed] [Google Scholar]